Anthocyanins Isolated from Vitis coignetiae Pulliat Enhances Cisplatin Sensitivity in MCF-7 Human Breast Cancer Cells through Inhibition of Akt and NF-κB Activation
Abstract
1. Introduction
2. Results
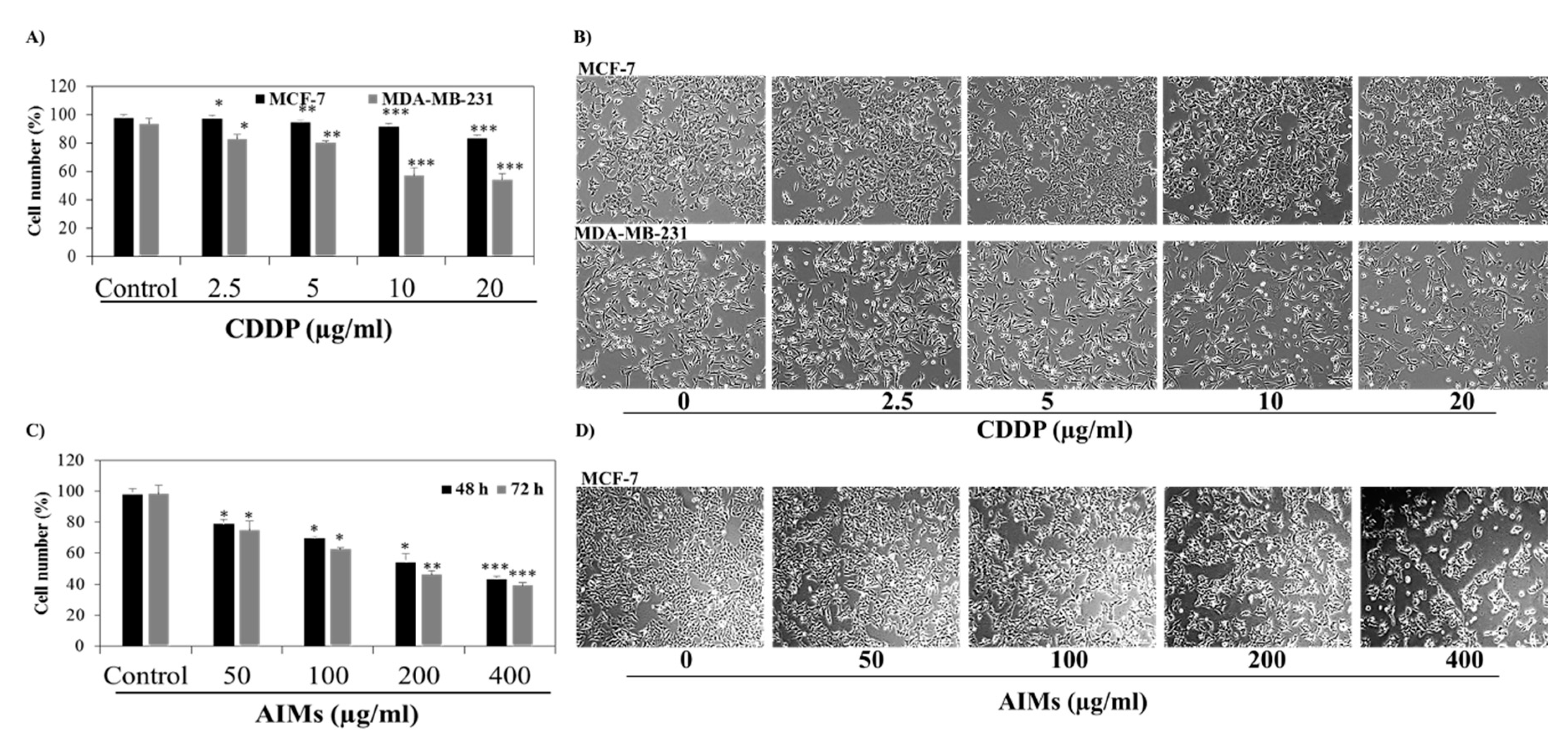
2.1. MCF-7 Cells Were More Resistant to CDDP Than MDA-MB-231 Cells, and Anthocyanins Isolated from Vitis coignetiae Pulliat (AIMs) Induced Anti-Proliferative Effects
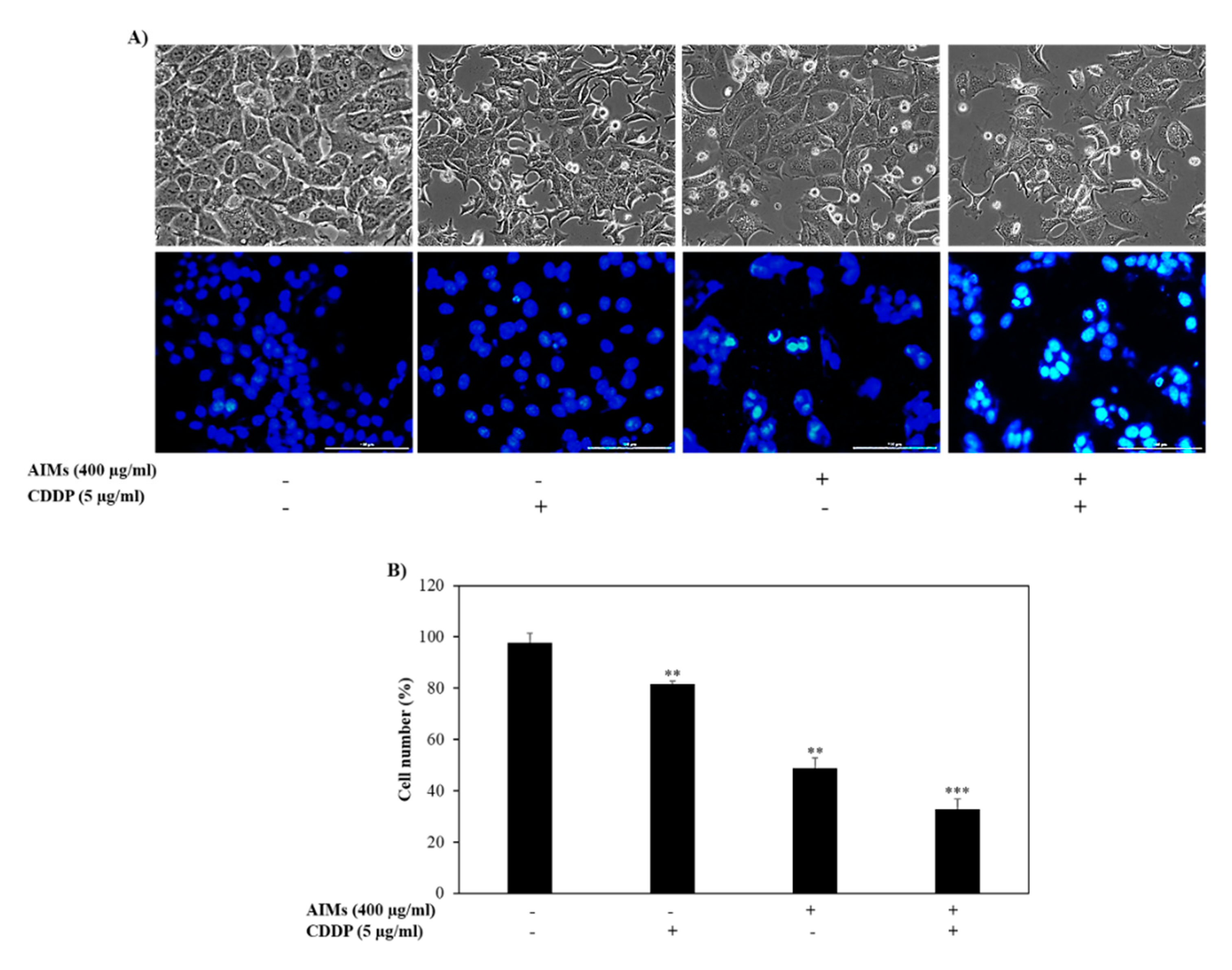
2.2. AIMs Induced a Synergistic Effect on Cell Death of MCF-7 Cells with Co-Treatment of CDDP
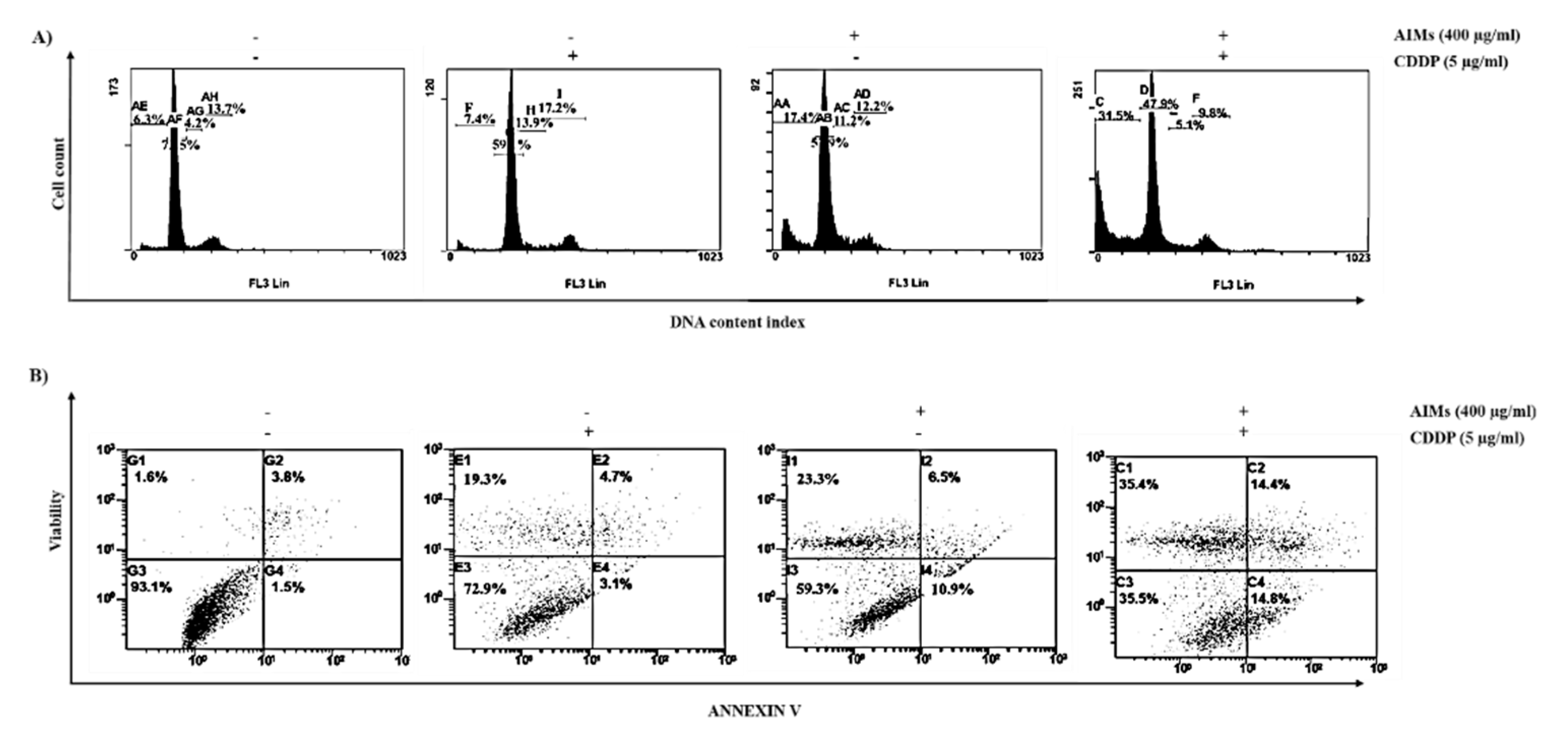
2.3. AIMs Enhanced CDDP Efficacy in MCF-7 Cells That Showed Relative CDDP Resistance
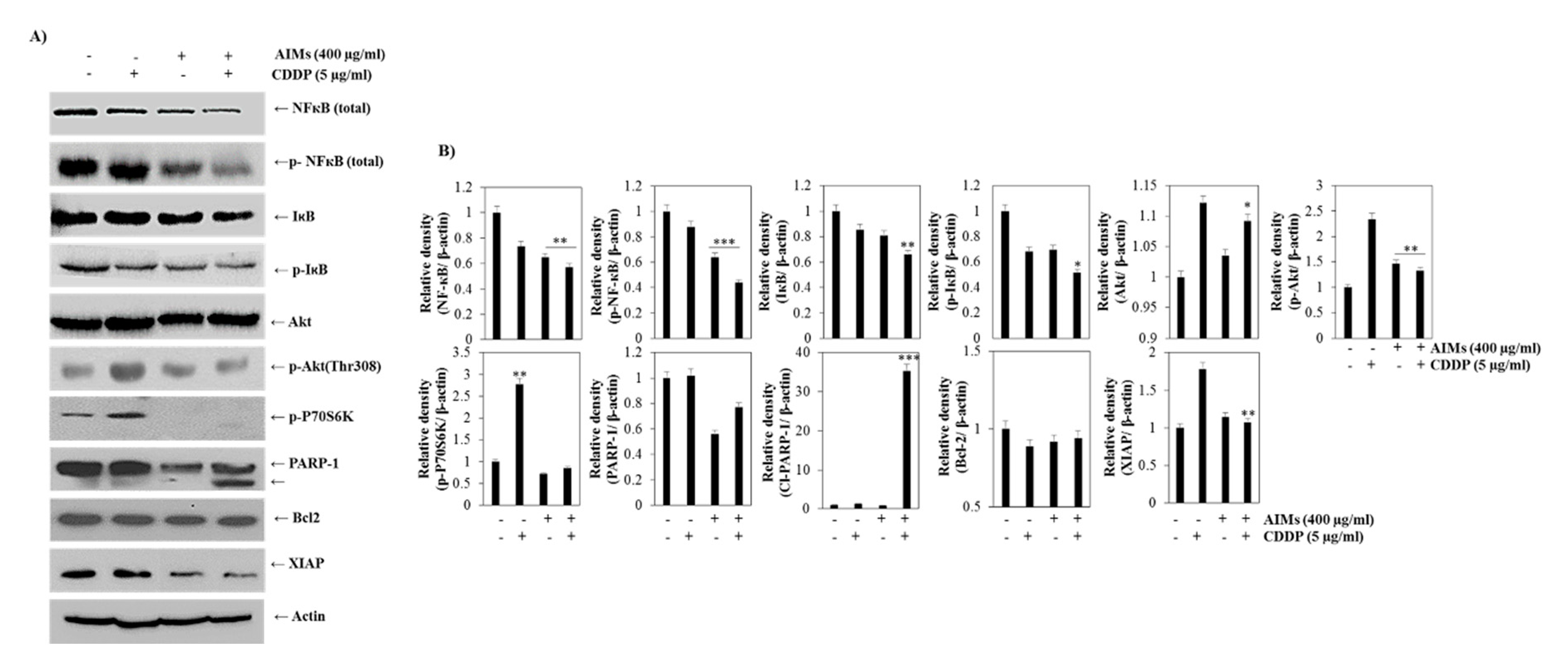
2.4. AIMs Enhanced CDDP Efficacy by Inhibiting NF-κB and Akt Activation in MCF-7 Cells That Showed Relative CDDP Resistance
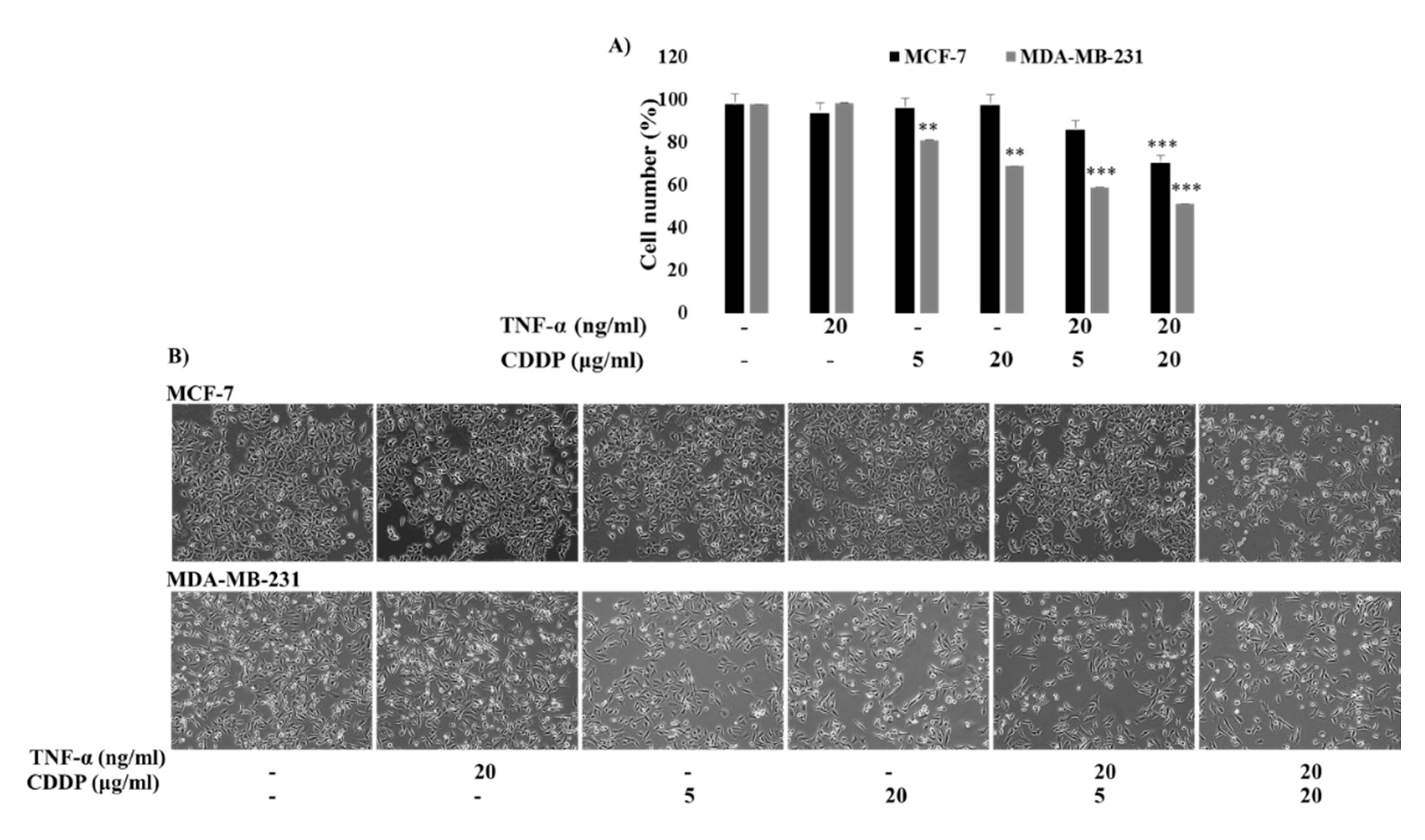
2.5. TNF-α Enhanced the CDDP Sensitivity of Both MCF-7 and MDA-MB-231 Cells, But the Intensity Was Different between Them; MCF-7 Cells Are Still Less Sensitive to the Combination Treatment of TNF-α and CDDP
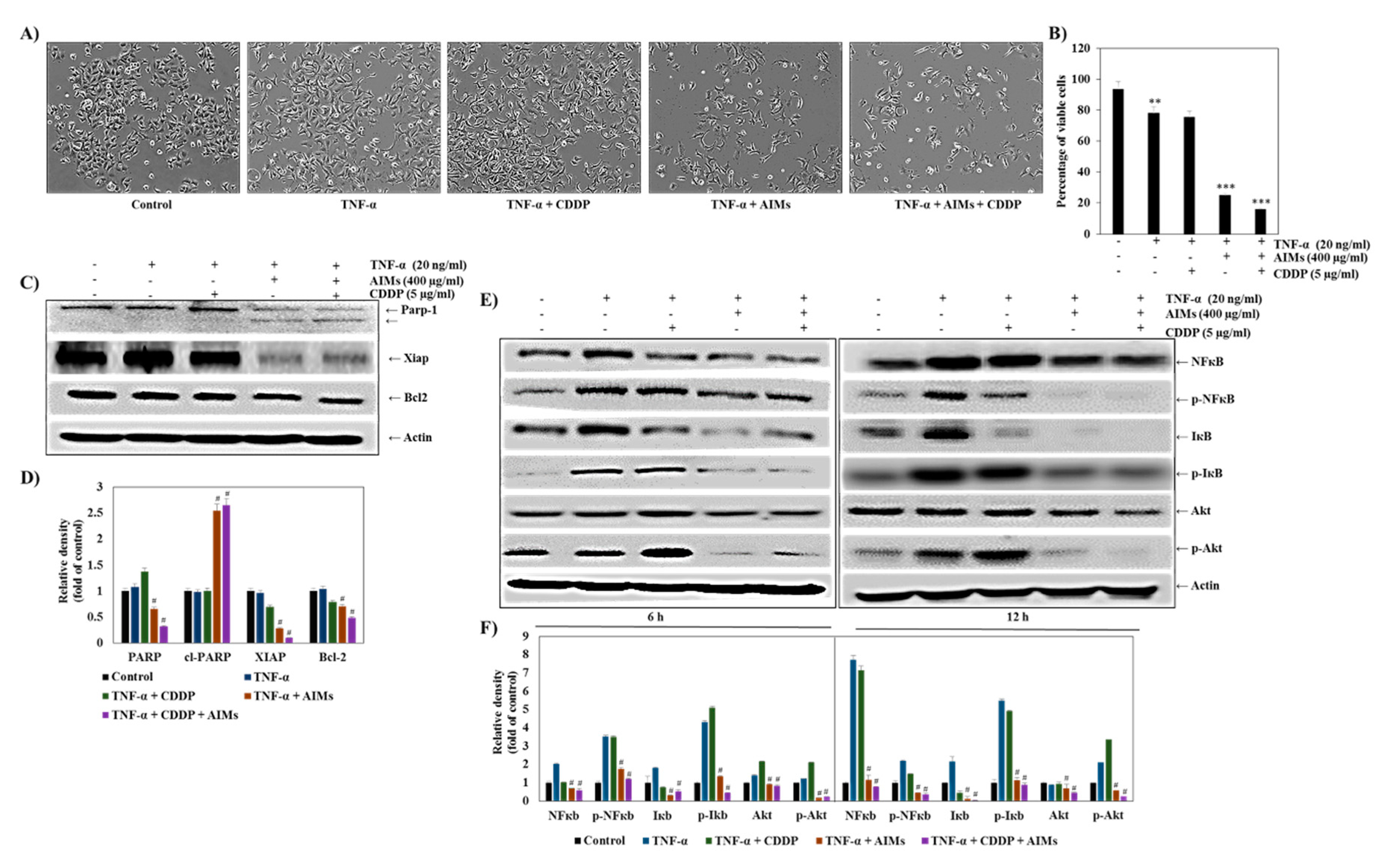
2.6. AIMs Significantly Enhanced the Effects of TNF-α Alone and Combination Treatment of TNF-α and CDDP
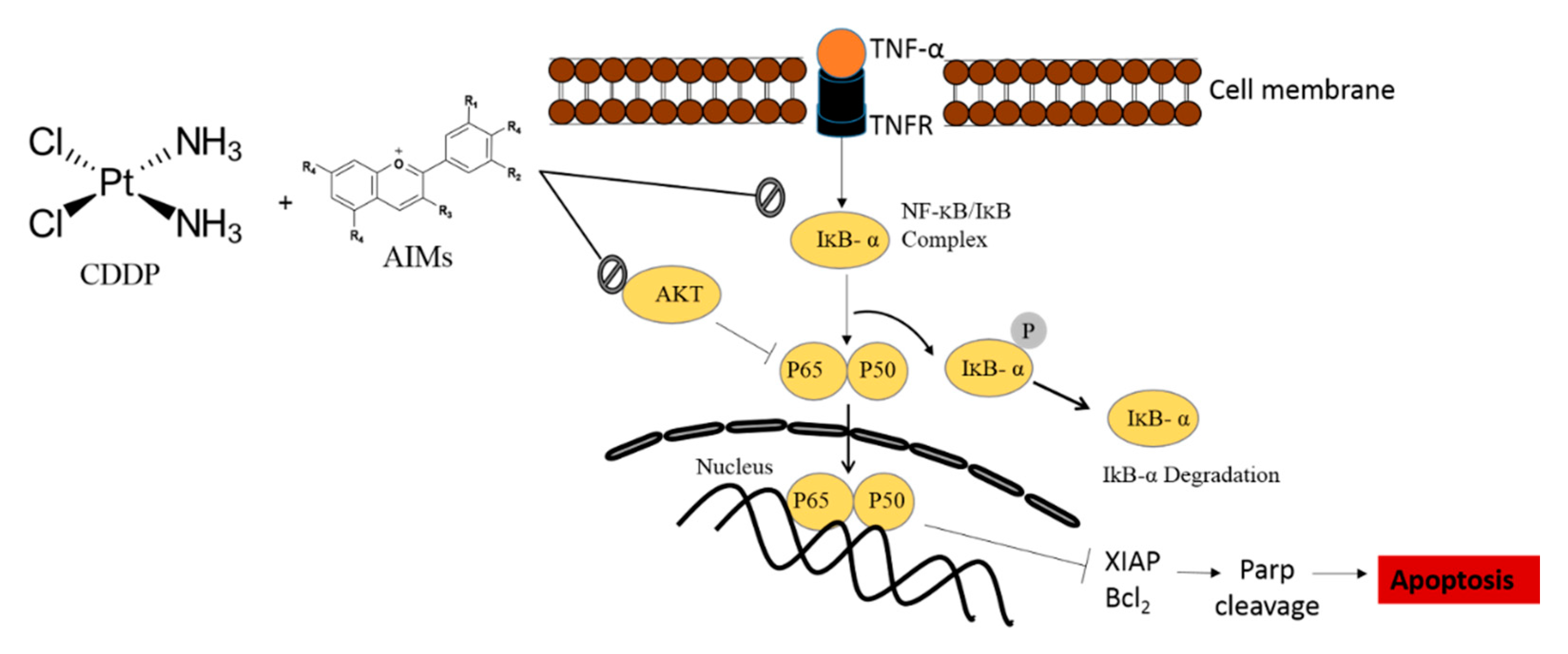
3. Discussion
4. Material and Methods
4.1. Cell Culture and Chemicals
4.2. AIM Preparation
4.3. Trypan Blue Exclusion Assay
4.4. DAPI Staining
4.5. Cell Cycle Analysis through Flow Cytometry (PI Staining)
4.6. Apoptosis Analysis through Annexin V and PI Staining
4.7. Western Blot Analysis
4.8. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Zaheer, S.; Shah, N.; Maqbool, S.A.; Soomro, N.M. Estimates of past and future time trends in age-specific breast cancer incidence among women in Karachi, Pakistan: 2004–2025. BMC Public Health 2019, 19, 1001. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.-W.; Won, Y.-J.; Kong, H.-J.; Lee, E.S. Cancer Statistics in Korea: Incidence, Mortality, Survival, and Prevalence in 2016. Cancer Res. Treat. 2019, 51, 417–430. [Google Scholar] [CrossRef] [PubMed]
- Housman, G.; Byler, S.; Heerboth, S.; Lapinska, K.; Longacre, M.; Snyder, N.; Sarkar, S. Drug resistance in cancer: An overview. Cancers 2014, 6, 1769–1792. [Google Scholar] [CrossRef] [PubMed]
- Van der Jeught, K.; Xu, H.C.; Li, Y.J.; Lu, X.B.; Ji, G. Drug resistance and new therapies in colorectal cancer. World J. Gastroenterol. 2018, 24, 3834–3848. [Google Scholar] [CrossRef]
- Fichtinger-Schepman, A.M.; van der Veer, J.L.; den Hartog, J.H.; Lohman, P.H.; Reedijk, J. Adducts of the antitumor drug cis-diamminedichloroplatinum(II) with DNA: Formation, identification, and quantitation. Biochemistry 1985, 24, 707–713. [Google Scholar] [CrossRef]
- Zwelling, L.A.; Anderson, T.; Kohn, K.W. DNA-protein and DNA interstrand cross-linking by cis- and trans-platinum(II) diamminedichloride in L1210 mouse leukemia cells and relation to cytotoxicity. Cancer Res. 1979, 39, 365–369. [Google Scholar]
- Zhuo, W.; Wang, Y.; Zhuo, X.; Zhang, Y.; Ao, X.; Chen, Z. Knockdown of Snail, a novel zinc finger transcription factor, via RNA interference increases A549 cell sensitivity to cisplatin via JNK/mitochondrial pathway. Lung Cancer 2008, 62, 8–14. [Google Scholar] [CrossRef]
- Johnson, S.W.; Laub, P.B.; Beesley, J.S.; Ozols, R.F.; Hamilton, T.C. Increased platinum-DNA damage tolerance is associated with cisplatin resistance and cross-resistance to various chemotherapeutic agents in unrelated human ovarian cancer cell lines. Cancer Res. 1997, 57, 850–856. [Google Scholar]
- Kumar Biswas, S.; Huang, J.; Persaud, S.; Basu, A. Down-regulation of Bcl-2 is associated with cisplatin resistance in human small cell lung cancer H69 cells. Mol. Cancer 2004, 3, 327–334. [Google Scholar]
- Siddik, Z.H. Cisplatin: Mode of cytotoxic action and molecular basis of resistance. Oncogene 2003, 22, 7265–7279. [Google Scholar] [CrossRef]
- Cojocneanu Petric, R.; Braicu, C.; Raduly, L.; Zanoaga, O.; Dragos, N.; Monroig, P.; Dumitrascu, D.; Berindan-Neagoe, I. Phytochemicals modulate carcinogenic signaling pathways in breast and hormone-related cancers. Oncotargets Ther. 2015, 8, 2053–2066. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.P.; Li, S.; Chen, Y.M.; Li, H.B. Natural Polyphenols for Prevention and Treatment of Cancer. Nutrients 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Koide, T.; Kamei, H.; Hashimoto, Y.; Kojima, T.; Hasegawa, M. Antitumor effect of hydrolyzed anthocyanin from grape rinds and red rice. Cancer Biother. Radiopharm. 1996, 11, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.W.; Gong, C.C.; Song, H.F.; Cui, Y.Y. Effects of anthocyanins on the prevention and treatment of cancer. Br. J. Pharmacol. 2017, 174, 1226–1243. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.Y.; Lee, W.S.; Lu, J.N.; Kang, M.H.; Ryu, C.H.; Kim, G.Y.; Kang, H.S.; Shin, S.C.; Choi, Y.H. Induction of apoptosis in human colon cancer HCT-116 cells by anthocyanins through suppression of Akt and activation of p38-MAPK. Int. J. Oncol. 2009, 35, 1499–1504. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.W.; Lee, W.S.; Kim, M.J.; Lu, J.N.; Kang, M.H.; Kim, H.G.; Kim, D.C.; Choi, E.J.; Choi, J.Y.; Lee, Y.K.; et al. Characterization of a profile of the anthocyanins isolated from Vitis coignetiae Pulliat and their anti-invasive activity on HT-29 human colon cancer cells. Food Chem. Toxicol. 2010, 48, 903–909. [Google Scholar] [CrossRef]
- Thévenod, F.; Friedmann, J.M.; Katsen, A.D.; Hauser, I.A. Up-regulation of multidrug resistance P-glycoprotein via nuclear factor-kappaB activation protects kidney proximal tubule cells from cadmium- and reactive oxygen species-induced apoptosis. J. Biol. Chem. 2000, 275, 1887–1896. [Google Scholar] [CrossRef]
- Ruan, H.Y.; Masuda, M.; Ito, A.; Umezawa, K.; Nakashima, T.; Yasumatsu, R.; Kuratomi, Y.; Yamamoto, T.; Weinstein, I.B.; Komune, S. Effects of a novel NF-kappaB inhibitor, dehydroxymethylepoxyquinomicin (DHMEQ), on growth, apoptosis, gene expression, and chemosensitivity in head and neck squamous cell carcinoma cell lines. Head Neck 2006, 28, 158–165. [Google Scholar] [CrossRef]
- Mabuchi, S.; Ohmichi, M.; Nishio, Y.; Hayasaka, T.; Kimura, A.; Ohta, T.; Saito, M.; Kawagoe, J.; Takahashi, K.; Yada-Hashimoto, N.; et al. Inhibition of NFkappaB increases the efficacy of cisplatin in in vitro and in vivo ovarian cancer models. J. Biol. Chem. 2004, 279, 23477–23485. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Yang, W.; Soares, J.; Greninger, P.; Edelman, E.J.; Lightfoot, H.; Forbes, S.; Bindal, N.; Beare, D.; Smith, J.A.; Thompson, I.R.; et al. Genomics of Drug Sensitivity in Cancer (GDSC): A resource for therapeutic biomarker discovery in cancer cells. Nucleic Acids Res. 2013, 41, D955–D961. [Google Scholar] [CrossRef] [PubMed]
- Nagata, S. Apoptosis by death factor. Cell 1997, 88, 355–365. [Google Scholar] [CrossRef]
- Yde, C.W.; Issinger, O.G. Enhancing cisplatin sensitivity in MCF-7 human breast cancer cells by down-regulation of Bcl-2 and cyclin D1. Int. J. Oncol. 2006, 29, 1397–1404. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.; Bushmeyer, S.M.; Witt, P.L.; Jordan, V.C.; Borden, E.C. Effects of type I and II interferons on cultured human breast cells: Interaction with estrogen receptors and with tamoxifen. Cancer Res. 1989, 49, 2698–2702. [Google Scholar] [PubMed]
- Eichholtz-Wirth, H.; Sagan, D. IkappaB/NF-kappaB mediated cisplatin resistance in HeLa cells after low-dose gamma-irradiation is associated with altered SODD expression. Apoptosis Int. J. Program. Cell Death 2000, 5, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wu, M.Y.; Jiang, M.; Zhi, Q.; Bian, X.; Xu, M.D.; Gong, F.R.; Hou, J.; Tao, M.; Shou, L.M.; et al. TNF-α sensitizes chemotherapy and radiotherapy against breast cancer cells. Cancer Cell Int. 2017, 17, 13. [Google Scholar] [CrossRef]
- Donato, N.J.; Klostergaard, J. Distinct stress and cell destruction pathways are engaged by TNF and ceramide during apoptosis of MCF-7 cells. Exp. Cell Res. 2004, 294, 523–533. [Google Scholar] [CrossRef]
- Paramanantham, A.; Kim, M.J.; Jung, E.J.; Nagappan, A.; Yun, J.W.; Kim, H.J.; Shin, S.C.; Kim, G.S. Pretreatment of Anthocyanin from the Fruit of Vitis coignetiae Pulliat Acts as a Potent Inhibitor of TNF-α Effect by Inhibiting NF-κB-Regulated Genes in Human Breast Cancer Cells. Molecules 2020, 25. [Google Scholar] [CrossRef]
- Yeh, P.Y.; Yeh, K.H.; Chuang, S.E.; Song, Y.C.; Cheng, A.L. Suppression of MEK/ERK signaling pathway enhances cisplatin-induced NF-kappaB activation by protein phosphatase 4-mediated NF-kappaB p65 Thr dephosphorylation. J. Biol. Chem. 2004, 279, 26143–26148. [Google Scholar] [CrossRef]
- Gilmore, T.D. Introduction to NF-kappaB: Players, pathways, perspectives. Oncogene 2006, 25, 6680–6684. [Google Scholar] [CrossRef]
- Thakur, B.; Ray, P. Cisplatin triggers cancer stem cell enrichment in platinum-resistant cells through NF-κB-TNFα-PIK3CA loop. J. Exp. Clin. Cancer Res. 2017, 36, 164. [Google Scholar] [CrossRef]
- Lu, J.N.; Lee, W.S.; Yun, J.W.; Kim, M.J.; Kim, H.J.; Kim, D.C.; Jeong, J.H.; Choi, Y.H.; Kim, G.S.; Ryu, C.H.; et al. Anthocyanins from Vitis coignetiae Pulliat Inhibit Cancer Invasion and Epithelial-Mesenchymal Transition, but These Effects Can Be Attenuated by Tumor Necrosis Factor in Human Uterine Cervical Cancer HeLa Cells. Evid.-Based Complementary Altern. Med. Ecam 2013, 2013, 503043. [Google Scholar] [CrossRef]
- Li, Y.; Ellis, K.-L.; Ali, S.; El-Rayes, B.F.; Nedeljkovic-Kurepa, A.; Kucuk, O.; Philip, P.A.; Sarkar, F.H. Apoptosis-Inducing Effect of Chemotherapeutic Agents Is Potentiated by Soy Isoflavone Genistein, a Natural Inhibitor of NF-κB in BxPC-3 Pancreatic Cancer Cell Line. Pancreas 2004, 28, e90–e95. [Google Scholar] [CrossRef] [PubMed]
- Dan, H.C.; Cooper, M.J.; Cogswell, P.C.; Duncan, J.A.; Ting, J.P.; Baldwin, A.S. Akt-dependent regulation of NF-{kappa}B is controlled by mTOR and Raptor in association with IKK. Genes Dev. 2008, 22, 1490–1500. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.N.; Lee, W.S.; Nagappan, A.; Chang, S.H.; Choi, Y.H.; Kim, H.J.; Kim, G.S.; Ryu, C.H.; Shin, S.C.; Jung, J.M.; et al. Anthocyanins From the Fruit of Vitis coignetiae Pulliat Potentiate the Cisplatin Activity by Inhibiting PI3K/Akt Signaling Pathways in Human Gastric Cancer Cells. J. Cancer Prev. 2015, 20, 50–56. [Google Scholar] [CrossRef]
- Han, S.Y.; Choung, S.Y.; Paik, I.S.; Kang, H.J.; Choi, Y.H.; Kim, S.J.; Lee, M.O. Activation of NF-kappaB determines the sensitivity of human colon cancer cells to TNFalpha-induced apoptosis. Biol. Pharm. Bull. 2000, 23, 420–426. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lu, J.N.; Lee, W.S.; Kim, M.J.; Yun, J.W.; Jung, J.H.; Yi, S.M.; Jeong, J.H.; Kim, H.J.; Choi, Y.H.; Kim, G.S.; et al. The inhibitory effect of anthocyanins on Akt on invasion and epithelial-mesenchymal transition is not associated with the anti-EGFR effect of the anthocyanins. Int. J. Oncol. 2014, 44, 1756–1766. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10. [Google Scholar] [CrossRef]
- Leiherer, A.; Mündlein, A.; Drexel, H. Phytochemicals and their impact on adipose tissue inflammation and diabetes. Vasc. Pharmacol. 2013, 58, 3–20. [Google Scholar] [CrossRef]
- Siddiqui, A.M.; Cui, X.; Wu, R.; Dong, W.; Zhou, M.; Hu, M.; Simms, H.H.; Wang, P. The anti-inflammatory effect of curcumin in an experimental model of sepsis is mediated by up-regulation of peroxisome proliferator-activated receptor-gamma. Crit. Care Med. 2006, 34, 1874–1882. [Google Scholar] [CrossRef]
- Eigler, A.; Sinha, B.; Hartmann, G.; Endres, S. Taming TNF: Strategies to restrain this proinflammatory cytokine. Immunol. Today 1997, 18, 487–492. [Google Scholar] [CrossRef]
- Chen, X.; Shu, Y.; Li, W.; Yin, Y. TNF-alpha-induced metastasis gene changes in MCF-7 cells. J. Nanjing Med. Univ. 2008, 22, 366–371. [Google Scholar] [CrossRef]
- Huang, M.; Wei, H.; Ling, R.; Lv, Y. Opposite effects of TNF0± on proliferation via ceramide in MDA-MB-231 and MCF-7 breast cancer cell lines. Int. J. Clin. Exp. Med. 2018, 11, 9239–9247. [Google Scholar]
- Beg, A.A.; Baltimore, D. An essential role for NF-kappaB in preventing TNF-alpha-induced cell death. Science 1996, 274, 782–784. [Google Scholar] [CrossRef]
- Van Antwerp, D.J.; Martin, S.J.; Kafri, T.; Green, D.R.; Verma, I.M. Suppression of TNF-alpha-induced apoptosis by NF-kappaB. Science 1996, 274, 787–789. [Google Scholar] [CrossRef]
- Wang, C.Y.; Mayo, M.W.; Baldwin, A.S., Jr. TNF- and cancer therapy-induced apoptosis: Potentiation by inhibition of NF-kappaB. Science 1996, 274, 784–787. [Google Scholar] [CrossRef]
- Correia, M.; Cravo, M.; Marques-Vidal, P.; Grimble, R.; Dias-Pereira, A.; Faias, S.; Nobre-Leitao, C. Serum concentrations of TNF-alpha as a surrogate marker for malnutrition and worse quality of life in patients with gastric cancer. Clin. Nutr. 2007, 26, 728–735. [Google Scholar] [CrossRef]
- Tas, F.; Duranyildiz, D.; Argon, A.; Oguz, H.; Camlica, H.; Yasasever, V.; Topuz, E. Serum levels of leptin and proinflammatory cytokines in advanced-stage non-small cell lung cancer. Med. Oncol. 2005, 22, 353–358. [Google Scholar] [CrossRef]
- Lee, Y.K.; Lee, W.S.; Kim, G.S.; Park, O.J. Anthocyanins are novel AMPKalpha1 stimulators that suppress tumor growth by inhibiting mTOR phosphorylation. Oncol. Rep. 2010, 24, 1471–1477. [Google Scholar] [CrossRef]
- Boivin, D.; Blanchette, M.; Barrette, S.; Moghrabi, A.; Beliveau, R. Inhibition of cancer cell proliferation and suppression of TNF-induced activation of NFkappaB by edible berry juice. Anticancer Res. 2007, 27, 937–948. [Google Scholar]
- Yeh, C.T.; Yen, G.C. Induction of apoptosis by the Anthocyanidins through regulation of Bcl-2 gene and activation of c-Jun N-terminal kinase cascade in hepatoma cells. J. Agric. Food Chem. 2005, 53, 1740–1749. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Tsoy, I.; Park, J.M.; Chung, J.I.; Shin, S.C.; Chang, K.C. Anthocyanins from soybean seed coat inhibit the expression of TNF-α-induced genes associated with ischemia/reperfusion in endothelial cell by NF-κB-dependent pathway and reduce rat myocardial damages incurred by ischemia and reperfusion in vivo. FEBS Lett. 2006, 580, 1391–1397. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paramanantham, A.; Kim, M.J.; Jung, E.J.; Kim, H.J.; Chang, S.-H.; Jung, J.-M.; Hong, S.C.; Shin, S.C.; Kim, G.S.; Lee, W.S. Anthocyanins Isolated from Vitis coignetiae Pulliat Enhances Cisplatin Sensitivity in MCF-7 Human Breast Cancer Cells through Inhibition of Akt and NF-κB Activation. Molecules 2020, 25, 3623. https://doi.org/10.3390/molecules25163623
Paramanantham A, Kim MJ, Jung EJ, Kim HJ, Chang S-H, Jung J-M, Hong SC, Shin SC, Kim GS, Lee WS. Anthocyanins Isolated from Vitis coignetiae Pulliat Enhances Cisplatin Sensitivity in MCF-7 Human Breast Cancer Cells through Inhibition of Akt and NF-κB Activation. Molecules. 2020; 25(16):3623. https://doi.org/10.3390/molecules25163623
Chicago/Turabian StyleParamanantham, Anjugam, Min Jeong Kim, Eun Joo Jung, Hye Jung Kim, Seong-Hwan Chang, Jin-Myung Jung, Soon Chan Hong, Sung Chul Shin, Gon Sup Kim, and Won Sup Lee. 2020. "Anthocyanins Isolated from Vitis coignetiae Pulliat Enhances Cisplatin Sensitivity in MCF-7 Human Breast Cancer Cells through Inhibition of Akt and NF-κB Activation" Molecules 25, no. 16: 3623. https://doi.org/10.3390/molecules25163623
APA StyleParamanantham, A., Kim, M. J., Jung, E. J., Kim, H. J., Chang, S.-H., Jung, J.-M., Hong, S. C., Shin, S. C., Kim, G. S., & Lee, W. S. (2020). Anthocyanins Isolated from Vitis coignetiae Pulliat Enhances Cisplatin Sensitivity in MCF-7 Human Breast Cancer Cells through Inhibition of Akt and NF-κB Activation. Molecules, 25(16), 3623. https://doi.org/10.3390/molecules25163623








