Bioactive Compounds, Antioxidant Activity, and Antinutritional Content of Legumes: A Comparison between Four Phaseolus Species
Abstract
1. Introduction
2. Results
2.1. Physical Characteristics of Phaseolus Species
2.2. Proximate Composition of Bean Seeds
2.3. Essential Amino Acids
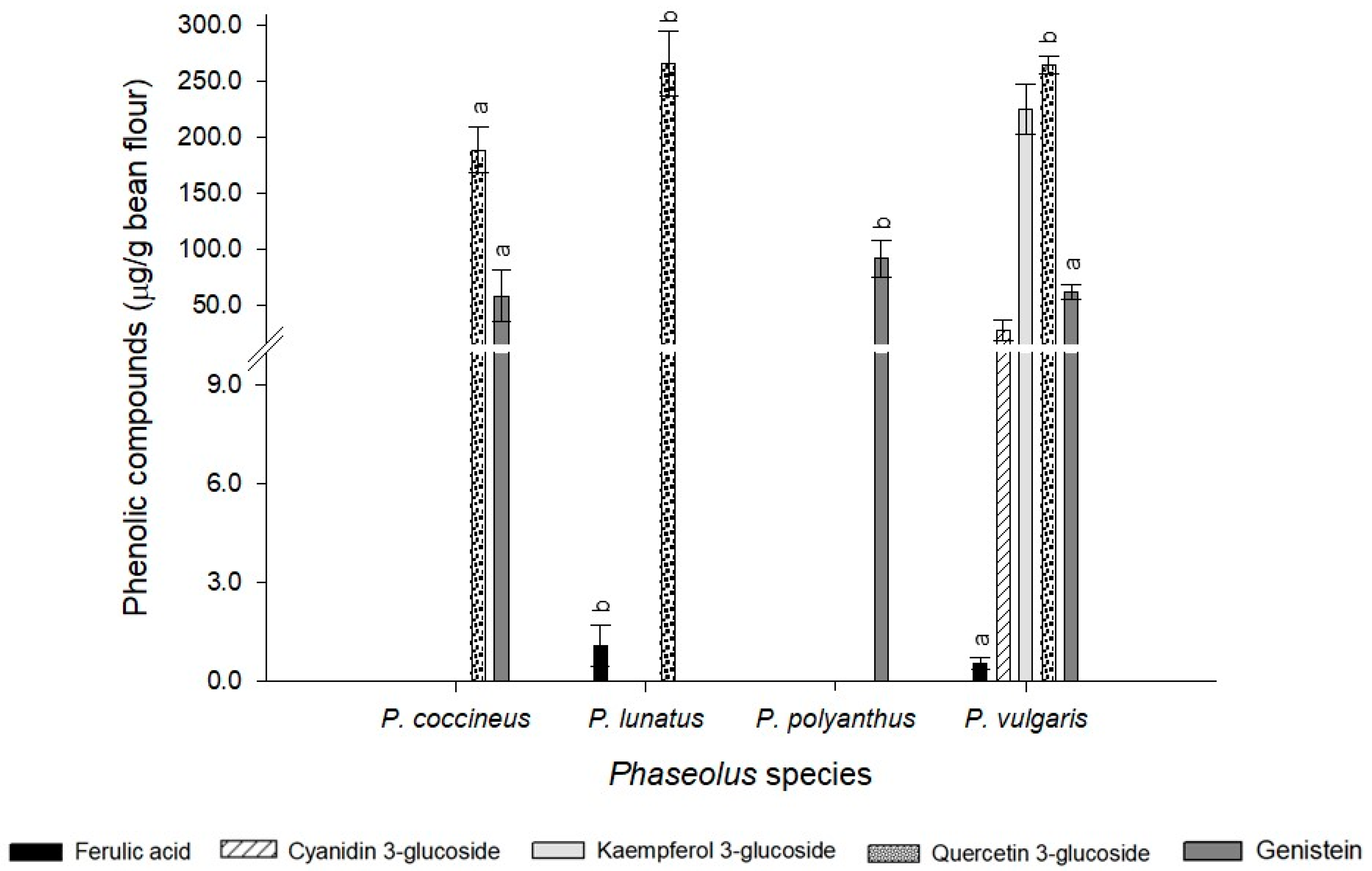
2.4. Phenolic Compounds and Antioxidant Activity
2.4.1. Total Phenols
2.4.2. Flavonoids
2.4.3. Condensed Tannins
2.4.4. Anthocyanins
2.4.5. Antioxidant Activity
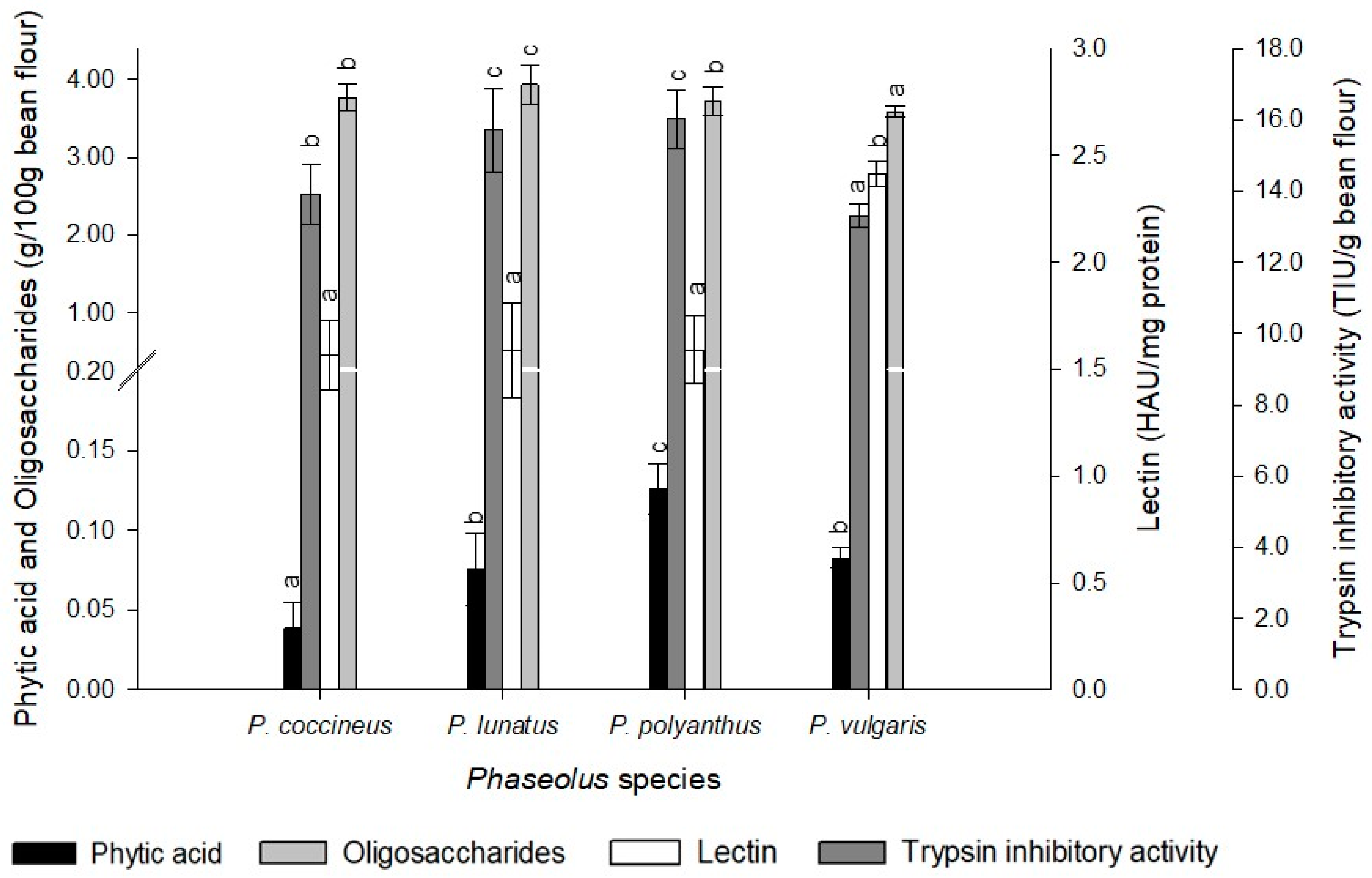
2.5. Antinutritional Components
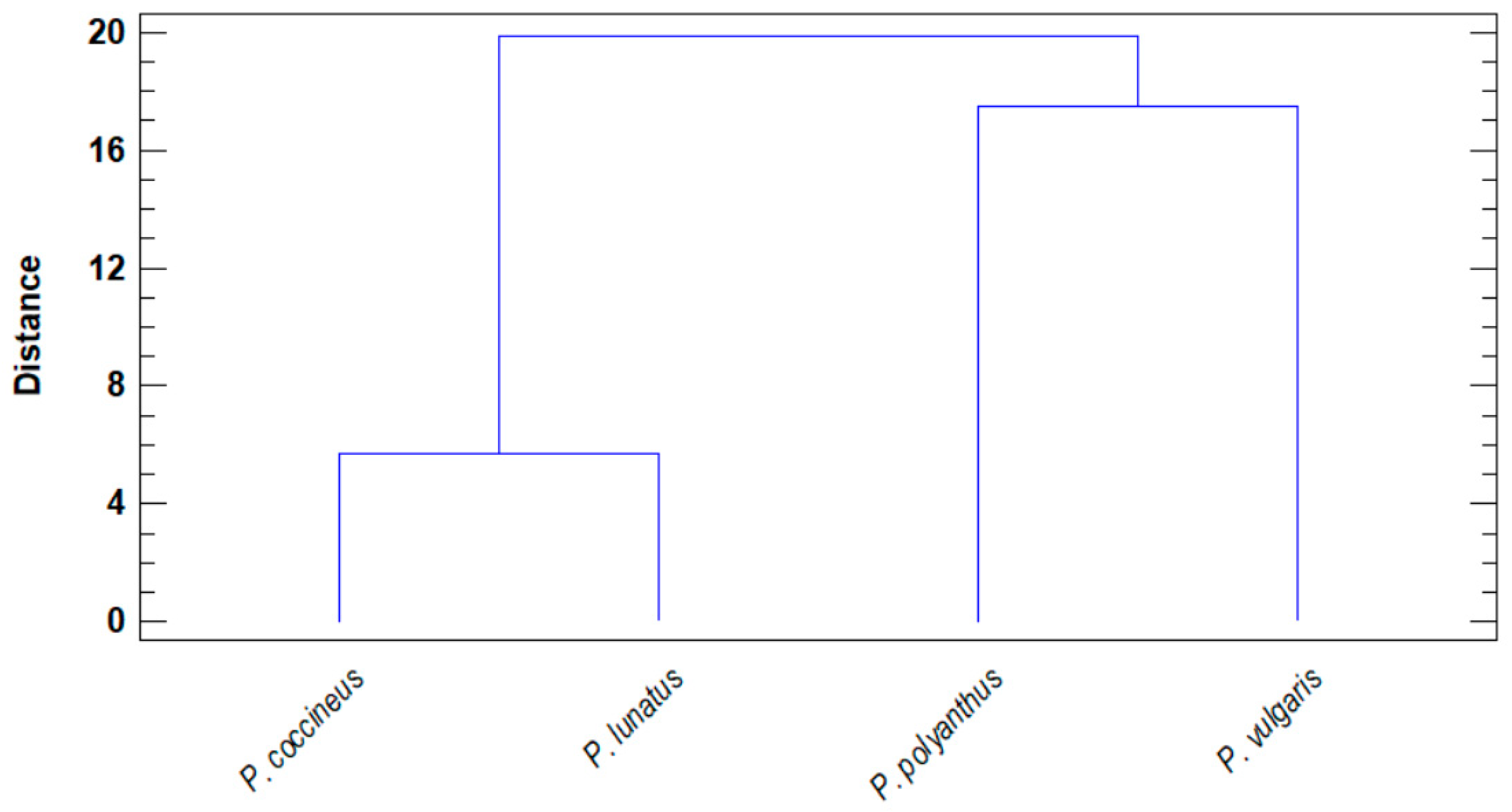
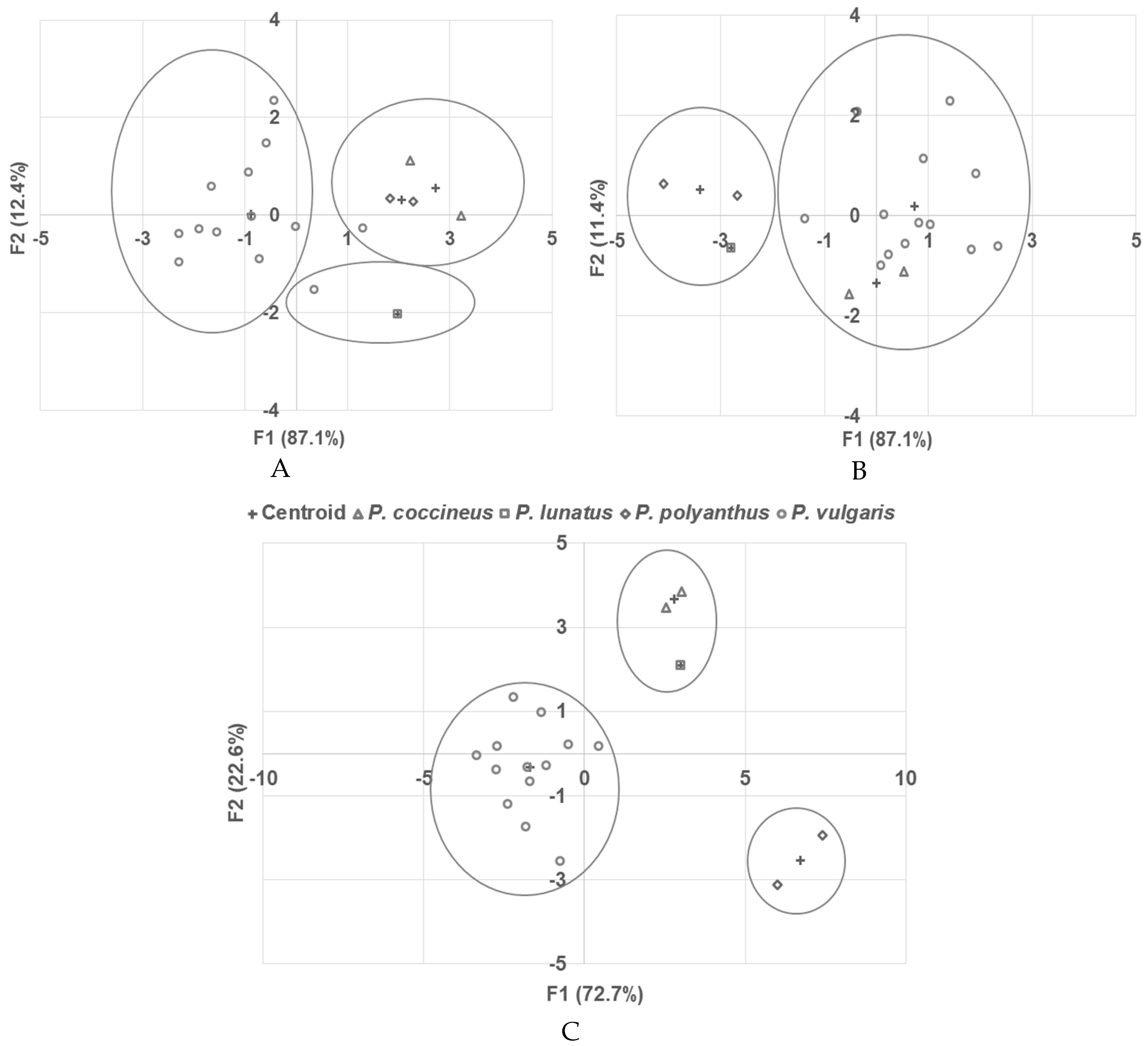
2.6. Discriminant Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Physicochemical Analysis
4.2.1. Bean Size Determination
4.2.2. Bean Color Evaluation
4.2.3. Proximate Composition Analysis of Bean Seeds
4.2.4. Total Amino Acid Analysis
4.3. Phenolic Compounds
4.3.1. Extraction of Phenolic Compounds
4.3.2. Determination of Total Phenols
4.3.3. Determination of Flavonoids
4.3.4. Determination of Condensed Tannins
4.3.5. Determination of Anthocyanins
4.3.6. Quantification of Phenolic Compounds by UHPLC
4.4. Determination of Antioxidant Activity
4.4.1. ABTS Assay (2,2′-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) Diammonium Salt)
4.4.2. DPPH Assay (2, 2 Diphenyl-1-picrylhydrazyl)
4.5. Antinutritional Compounds
4.5.1. Phytic Acid Assay
4.5.2. Oligosaccharide Assay
4.5.3. Determination of Lectin Activity
4.5.4. Determination of Trypsin Inhibitory Activity
4.6. Statisdical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Romero-Arenas, O.; Damián-Huato, M.; Rivera-Tapia, J.A.; Báez-Simón, A.; Huerta-Lara, M.; Cabrera-Huerta, E. The nutritional value of Beans (Phaseolus vulgaris L.) and its importance for feeding of rural communities in Puebla-Mexico. Int. Res. J. Biological Sci. 2013, 2, 59–65. [Google Scholar]
- Chávez-Mendoza, C.; Sánchez, E. Bioactive compounds from Mexican varieties of the common bean (Phaseolus vulgaris): Implications for health. Molecules 2017, 22, 1360. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Alonso, L.G.; Lygin, A.; Widholm, J.M.; Valverde, M.E.; Paredes-Lopez, O. Polyphenols in wild and weedy Mexican common beans (Phaseolus vulgaris L.). J. Agric. Food Chem. 2006, 54, 4436–4444. [Google Scholar] [CrossRef]
- Juárez-López, B.A.; Aparicio-Fernández, X. Polyphenolics concentration and antiradical capacity of common bean varieties (Phaseolus vulgaris L.) after thermal treatment. In Food Science and Food Biotechnology Essentials: A Contemporary Perspective, 1st ed.; Nevárez-Moorllón, G.V., Ortega-Rivas, E., Eds.; Asociación Mexicana de Ciencia de Los Alimentos, A.C.: Durango, Mexico, 2012; pp. 25–33. [Google Scholar]
- Luthria, D.L.; Pastor-Corrales, M.A. Phenolic acid content of fifteen dry edible beans (Phaseolus vulgaris L.) varieties. J. Food Compos. Anal. 2006, 19, 205–211. [Google Scholar] [CrossRef]
- Mojica, L.; Meyer, A.; Berhow, M.A.; González de Mejia, E. Bean cultivars (Phaseolus vulgaris L.) have similar high antioxidant capacity, in vitro inhibition of α-amylase and α-glucosidase while diverse phenolic composition and concentration. Food Res. Int. 2015, 69, 38–48. [Google Scholar] [CrossRef]
- Huber, K.; B Brigide, P.; Bretas, E.B.; Canniatti-Brazaca, S.G. Phenolic Acid, flavonoids and antioxidant activity of common brown beans (Phaseolus vulgaris L.) before and after cooking. J. Nutr. Food Sci. 2016, 6, 551. [Google Scholar] [CrossRef]
- Akond, G.M.; Khandaker, L.; Berthold, J.; Gates, L.; Peters, K.; Delong, H.; Hossain, K. Anthocyanin, total polyphenols and antioxidant activity of common bean. Am. J. Food Technol. 2011, 6, 385–394. [Google Scholar]
- Batista, K.A.; Prudencio, S.H.; Fernandes, K.F. Changes in the functional properties and antinutritional factors of extruded hard-to-cook common beans (Phaseolus vulgaris, L.). J. Food Sci. 2010, 75, C286–C290. [Google Scholar] [CrossRef]
- De Mejia, E.G.; Guzmán-Maldonado, S.H.; Acosta-Gallegos, J.A.; Reynoso-Camacho, R.; Ramírez-Rodríguez, E.; Pons-Hernández, J.L.; González-Chavira, M.M.; Castellanos, J.Z.; Kelly, J.D. Effect of cultivar and growing location on the trypsin inhibitors, tannins, and lectins of common beans (Phaseolus vulgaris L.) grown in the semiarid highlands of Mexico. J. Agric. Food Chem. 2003, 51, 5962–5966. [Google Scholar] [CrossRef]
- McGuire, R.G. Reporting of objective color measurements. HortScience 1992, 27, 1254–1255. [Google Scholar] [CrossRef]
- Hernández-López, V.M.; Vargas-Vázquez, M.L.P.; Muruaga-Martínez, J.S.; Hernández-Delgado, S.; Mayek-Pérez, N. Origin, domestication and diversification of common beans, advances and perspectives. Rev. Fitotec. Mex. 2013, 36, 95–104. [Google Scholar]
- Singh, S.P.; Gepts, P.; Debouck, D.G. Races of common bean (Phaseolus vulgaris, Fabaceae). Econ. Bot. 1991, 45, 379–396. [Google Scholar] [CrossRef]
- Sinkovic, L.; Pipan, B.; Sinkovic, E.; Meglic, V. Morphological seed characterization of common (Phaseolus vulgaris L.) and runner (Phaseolus coccineus L.) bean germplasm: A Slovenian gene bank example. BioMed Res. Int. 2019, 6376948. [Google Scholar] [CrossRef]
- Schmit, V.; Debouck, D.G. Observations on the origin of Phaseolus polyanthus Greenman. Econ. Bot. 1991, 45, 345–364. [Google Scholar] [CrossRef]
- Akillioglu, H.G.; Karakaya, S. Changes in total phenol, total flavonoids, and antioxidant activities of common beans and pinto beans after soaking, cooking and in vitro digestion process. Food Sci. Biotechnol. 2010, 19, 633–639. [Google Scholar] [CrossRef]
- Bernardino-Nicanor, A.; Acosta-García, G.; Güemes-Vera, N.; Montañez-Soto, J.L.; de Los Ángeles Vivar-Vera, M.; González-Cruz, L. Fourier transform infrared and Raman spectroscopic study of the effect of the thermal treatment and extraction methods on the characteristics of Ayocote bean starches. J. Food Sci. Technol. 2017, 54, 933–943. [Google Scholar] [CrossRef]
- Alvarado-López, A.N.; Gómez-Oliván, L.M.; Heredia, J.B.; Baeza-Jiménez, R.; García-Galindo, H.S.; Lopez-Martinez, L.X. Nutritional and bioactive characteristics of Ayocote bean (Phaseolus coccineus L.): An underutilized legume harvested in Mexico. Cy TA J. Food 2019, 17, 199–206. [Google Scholar] [CrossRef]
- Holecek, M.; Vodenicarovova, M. Effects of histidine supplementation on amino acid metabolism in rats. Physiol. Res. 2020, 69, 99–111. [Google Scholar] [CrossRef]
- Xu, B.J.; Chang, S.K.C. A comparative study on phenolic profiles and antioxidant activities of legumes as affected by extraction solvents. J. Food Sci. 2007, 72, 159–166. [Google Scholar] [CrossRef]
- Capistrán-Carabarin, A.; Aquino-Bolaños, E.N.; García-Díaz, Y.D.; Chávez-Servia, J.L.; Vera-Guzmán, A.M.; Carrillo-Rodríguez, J.C. Complementarity in Phenolic Compounds and the Antioxidant Activities of Phaseolus coccineus L. and P. vulgaris L. Landraces. Foods 2019, 8, 295. [Google Scholar] [CrossRef]
- Díaz, A.M.; Caldas, G.V.; Blair, M.W. Concentrations of condensed tannins and anthocyanins in common bean seed coats. Food Res. Inter. 2010, 43, 595–601. [Google Scholar] [CrossRef]
- Díaz-Batalla, L.; Widholm, J.M.; Fahey, G.C.; Castaño-Tostado, E.; Paredes-López, O. Chemical components with health implications in wild and cultivated Mexican common bean seeds (Phaseolus vulgaris L.). J. Agric. Food Chem 2006, 54, 2045–2052. [Google Scholar] [CrossRef]
- Choung, M.G.; Choi, B.R.; An, Y.N.; Chu, Y.H.; Cho, Y.S. Anthocyanin Profile of Korean cultivated kidney bean (Phaseolus vulgaris L.). J. Agric. Food Chem. 2003, 51, 7040–7043. [Google Scholar] [CrossRef] [PubMed]
- Giusti, F.; Capuano, E.; Sagratini, G.; Pellegrini, N. A comprehensive investigation of the behaviour of phenolic compounds in legumes during domestic cooking and in vitro digestion. Food Chem. 2019, 285, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Beninger, C.W.; Hosfield, G.L. Antioxidant activity of extracts, condensed tannin fractions, and pure flavonoids from Phaseolus vulgaris L. seed coat color genotypes. J. Agric. Food Chem. 2003, 51, 7879–7883. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aal, E.S.M.; Hucl, P. A rapid method for quantifying total anthocyanins in blue aleurone and purple pericarp wheats. Cereal Chem. 1999, 76, 350–354. [Google Scholar] [CrossRef]
- Tovar-Pérez, E.G.; Guerrero-Becerra, L.; Lugo-Cervantes, E. Antioxidant activity of hydrolysates and peptide fractions of glutelin from cocoa (Theobroma cacao L.) seed. CyTA J. Food 2017, 15, 489–496. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Samtiya, M.; Aluko, R.E.; Dhewa, T. Plant food anti-nutritional factors and their reduction strategies: An overview. Food Prod. Process. Nutr. 2020, 2, 6. [Google Scholar] [CrossRef]
- E Lacerda, R.R.; do Nascimiento, E.S.; de Lacerda, J.T.; Pinto, L.D.; Rizzi, C.; Bezerra, M.M.; Pinto, I.R.; Filho, S.M.; Pinto, V.P.; Filho, G.C.; et al. Lectin from sedes of a Brazilian lima bean variety (Phaseolus lunatus L. var. cascabel) presentes antioxidant, antitumour and gastroprotective activities. Int. J. Biol. Macromol. 2017, 95, 1072–1081. [Google Scholar] [CrossRef]
- Carbas, B.; Machado, N.; Oppolzer, D.; Ferreira, L.; Queiroz, M.; Brites, C.; Rosa, E.A.; Barros, A.I. Nutrientes, Antinutrients, Phenolic Composition, and Antioxidant Activity of Common Bean Cultivars and their Potential for Food Applications. Antioxidants 2020, 9, 186. [Google Scholar] [CrossRef]
- Cruz-Bojórquez, R.M.; Coop-Gamas, F.Y.; Cárdenas-García, S.; Ávila-Escalante, M.L. Perception of body image in Maya Adolescents and its Relationship with Body Dissatisfaction and nutritional status. J. Nutr. Food Sci. 2019, 9, 756. [Google Scholar] [CrossRef]
- AOAC, Association of Analytical Communities. Official Methods of Analysis of AOAC International, 16th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 1995. [Google Scholar]
- AOAC, Association of Analytical Communities. Official Methods of Analysis Amino Acids Analysis, 17th ed.; Association of Analytical Communities: Gaithersburg, MD, USA, 2006. [Google Scholar]
- Broadhurst, R.B.; Jones, W.T. Analysis of condensed tannins using acidified vanillin. J. Sci. Food Agric. 1978, 29, 788–794. [Google Scholar] [CrossRef]
- Sun, B.; Ricardo-da-Silva, J.M.; Spranger, I. Critical factors of vanillin assay for catechins and proanthocyanidins. J. Agric. Food Chem. 1998, 46, 4267–4274. [Google Scholar] [CrossRef]
- De Mejia, E.G.; Hankins, C.N.; Paredes-Lopez, O.; Shannon, L.M. The lectins and lectin-like proteins of tepary beans (Phaseolus acutifolius) and tepary-common bean (Phaseolus vulgaris) hybrids. J. Food Biochem. 1990, 14, 117–126. [Google Scholar] [CrossRef]
- Kakade, M.L.; Rackis, J.J.; McGhee, J.E.; Puski, G. Determination of trypsin inhibitor activity of soy products: A collaborative analysis of an improved procedure. Cereal Chem. 1974, 51, 376–382. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |


| Sample | *L | c | h | Length (mm) | Width (mm) | Thickness (mm) | Weigh (g) | Seed Coat Color |
|---|---|---|---|---|---|---|---|---|
| CH-01 P.v. | 27.97 ± 3.32 b–d | 20.89 ± 0.12 e,f | 0.50 ± 0.02 b,c | 14.4 ± 1.2 e–g | 5.9 ± 0.5 a–c | 4.3 ± 0.5 a–d | 43.4 ± 0.4 c–e | Red |
| CH-02 P.v. | 15.01 ± 0.34 a | 1.92 ± 0.05 b | 1.22 ± 0.05 c,d | 9.2 ± 1.1 a,b | 4.9 ± 0.6 a | 3.2 ± 0.5 a | 18.6 ± 0.6 a | Black (violet) |
| CH-03 P.v. | 17.50 ± 0.44 a | 1.21 ± 0.66 a,b | −1.48 ± 0.08 a | 8.4 ± 1.1 a | 5.0 ± 0.6 a | 3.3 ± 0.4 a,b | 20.2 ± 0.2 a | Black (blue) |
| CH-04 P.v. | 27.37 ± 0.19 bc | 20.02 ± 0.20 e | 0.55 ± 0.01 b,c | 9.4 ± 0.9 a,b | 5.7 ± 0.5 a,b | 4.5 ± 0.6 a–e | 31.0 ± 0.5 a–c | Red |
| CH-05 P.v. | 17.73 ± 0.26 a | 0.11 ± 0.06 a | 0.58 ± 0.78 b,c | 11.5 ± 1.2 b–e | 6.1 ± 0.6 a–d | 3.7 ± 0.6 a–c | 29.3 ± 0.9 a,b | Black (violet) |
| CH-06 P.p. | 23.75 ± 0.71 b | 28.47 ± 0.77 h | 0.73 ± 0.01 b–d | 12.9 ± 1.1 c–f | 10.1 ± 0.9 g | 6.5 ± 0.7 g–i | 81.9 ± 0.4 f,g | Yellow |
| CH-07 P.c. | 26.51 ± 0.45 b,c | 11.92 ± 0.16 c | −0.06 ± 0.06 b | 16.3 ± 1.4 g | 10.5 ± 1.0 g | 5.8 ± 0.8 e–h | 88.5 ± 1.3 g | Purple |
| CH-08 P.v. | 69.43 ± 0.81 g | 14.71 ± 0.12 d | 1.48 ± 0.01 d | 10.3 ± 1.2 a–c | 6.4 ± 0.6 a–e | 4.7 ± 0.7 b–f | 37.2 ± 0.7 b–d | White |
| CH-09 P.v. | 24.39 ± 0.16 b | 22.13 ± 0.62 f,g | 0.48 ± 0.01 b,c | 8.6 ± 0.7 a | 4.7 ± 0.6 a | 3.5 ± 0.4 a,b | 20.4 ± 0.2 a | Red |
| CH-10 P.v. | 16.12 ± 0.32 a | 0.91 ± 0.10 a,b | −1.47 ± 0.05 a | 12.6 ± 1.2 c–e | 7.9 ± 0.7 d,e | 4.6 ± 0.5 a–f | 40.9 ± 3.3 b–e | Black (blue) |
| CH-11 P.p. | 18.89 ± 0.12 a | 23.13 ± 0.41 g | 0.59 ± 0.0 1 b,c | 13.4 ± 1.1 d–f | 10.5 ± 0.7 g | 7.4 ± 0.6 i | 73.0 ± 2.0 f | Red |
| CH-12 P.v. | 39.06 ± 0.36 e | 21.89 ± 0.07 f,g | 1.09 ± 0.01 c,d | 10.3 ± 0.8 a–c | 8.0 ± 0.7 e,f | 6.1 ± 0.9 f–i | 43.3ׅ ± 3.1 c–e | Brown |
| CH-13 P.v. | 15.30 ± 0.38 a | 0.88 ± 0.13 a,b | −1.43 ± 0.03 a | 10.7 ± 0.8 a–d | 6.1 ± 0.5 a–d | 4.5 ± 0.4 a–e | 21.1 ± 0.1 a | Black (blue) |
| CH-14 P.v. | 25.53 ± 0.16 b | 20.06 ± 0.35 e | 0.37 ± 0.01 b,c | 10.1 ± 0.8 a–c | 7.4 ± 0.6 b–e | 5.8 ± 0.5 d–h | 30.5 ± 0.2 a,b | Red |
| CH-15 P.v. | 15.79 ± 0.44 a | 0.76 ± 0.12 a,b | −1.44 ± 0.02 a | 10.7 ± 0.9 a-d | 7.2 ± 0.5 b–e | 5.1 ± 0.4 c–g | 28.3 ± 03 a,b | Black (blue) |
| CH-16 P.c. | 30.62 ± 0.08 c,d | 13.80 ± 0.06 d | 0.04 ± 0.01 b | 15.6 ± 1.3 f,g | 11.0 ± 1.0 g | 6.9 ± 0.7 h,i | 75.7 ± 1.9 f | Purple |
| CH-17 P.l. | 31.92 ± 0.32 d | 28.33 ± 0.22 h | 0.55 ± 0.01 b,c | 13.7 ± 1.1 e–g | 9.8 ± 0.6 f,g | 4.5 ± 0.3 a–e | 44.9 ± 0.2 d,e | Pink |
| CH-18 P.v. | 49.23 ± 1.26 f | 21.63 ± 0.42 e–g | 1.00 ± 0.02 c,d | 16.4 ± 1.0 g | 7.6 ± 0.4 c–e | 5.9 ± 0.4 e–i | 50.8 ± 0.5 e | Brown |
| Amino Acid (mg/100 g Dry Beans) | Phaseolus Species | |||
|---|---|---|---|---|
| P. coccineus | P. lunatus | P. polyanthus | P. vulgaris | |
| Histidine | 10.66 ± 0.57 a | 14.02 ± 0.00 a | 24.08 ± 0.32 b | 40.08 ± 0.39 c |
| Arginine | 157.71 ± 0.38 a | 127.88 ± 0.00 a | 151.84 ± 0.27 a | 380.98 ± 0.39 b |
| Threonine | 15.32 ± 0.62 a | 15.36 ± 0.0 a,b | 28.22 ± 0.51 c | 19.08 ± 0.42 b |
| Lysine | 14.33 ± 0.63 a,b | 12.80 ± 0.00 a | 14.84 ± 0.49 a,b | 16.70 ± 0.37 b |
| Valine | 15.18 ± 0.62 a,b | 14.84 ± 0.00 a,b | 27.20 ± 0.52 c | 19.07 ± 0.42 b |
| Isoleucine | 4.51 ± 0.59 a | 5.27 ± 0.00 a | 8.70 ± 0.67 c | 7.24 ± 0.46 b |
| Leucine | 12.72 ± 0.14 a | 19.09 ± 0.00 b | 20.01 ± 0.09 b | 19.18 ± 0.18 b |
| Phenylalanine | 9.14 ± 0.62 a,b | 8.68 ± 0.00 a,b | 12.08 ± 0.68 b | 10.51 ± 0.48 a,b |
| Methionine | 1.75 ± 0.76 a | 2.35 ± 0.00 a,b | 2.56 ± 0.91 a | 3.34 ± 0.57 b |
| Tryptophan | 14.13 ± 0.38 a | 12.77 ± 0.00 a | 15.14 ± 0.30 a | 48.19 ± 0.53 b |
| Sample | TPH | FLV | ANT | TAN | PHY | OSD | LET | TRY | %ARSA | %DRSA |
|---|---|---|---|---|---|---|---|---|---|---|
| CH-01 | 1043.3 ± 13.2 d,e | 5.7 ± 0.2 a | 0.69 ± 0.06 a | 1.42 ± 0.08 a,b | 96.1 ± 0.5 a–e | 3.21 ± 0.17 a–c | 1.74 | 14.7 ± 0.5 d–g | 90.9 ± 0.0 a,b | 92.0 ± 0.0 c–f |
| CH-02 | 661.2 ± 27.8 b–d | 6.4 ± 0.3 a,b | 3.60 ± 0.16 b | 1.62 ± 0.11 a,b | 76.1 ± 0.6 a–e | 2.96 ± 0.07 a,b | 2.05 | 10.02 ± 0.25 a | 61.9 ± 0.3 a,b | 91.8 ± 0.0 c–f |
| CH-03 | 550.1 ± 0.5 a–c | 5.9 ± 0.2 a | 2.82 ± 0.06 b | 3.83 ± 0.04 d,e | 49.1 ± 0.5 a–d | 3.00 ± 0.04 a,b | 1.62 | 15.6 ± 2.3 e–g | 80.5 ± 0.2 a,b | 94.0 ± 0.1 e,f |
| CH-04 | 861.1 ± 0.8 c,d | 11.7 ± 0.1 a–c | 0.50 ± 0.05 a | 3.60 ± 0.04 d | 144.7 ± 0.3 d,e | 2.70 ± 0.04 a | 2.00 | 13.0 ± 0.7 b–e | 56.7 ± 0.1 a | 92.5 ± 0.0 d–f |
| CH-05 | 607.6 ± 0.9 b–d | 7.6 ± 0.2 a–c | 6.22 ± 0.05 c | 5.00 ± 0.03 e,f | 158.2 ± 0.5 e | 3.47 ± 0.05 b–e | 1.88 | 14.3 ± 0.9 c–g | 96.3 ± 01 b | 95.0 ± 0.0 f |
| CH-06 | 660.5 ± 21.9 b–d | 1.7 ± 0.2 a | 0.19 ± 0.05 a | 0.87 ± 0.02 a | 130.2 ± 0.2 c–f | 4.44 ± 0.03 g,h | 1.59 | 16.2 ± 0.5 g | 63.6 ± 0.1 a,b | 84.2 ± 0.0 a,b |
| CH-07 | 627.4 ± 3.5 b–d | 1.8 ± 0.1 a | 0.26 ± 0.23 a | 1.34 ± 0.02 a,b | 29.8 ± 0.6 a,b | 3.86 ± 0.08 d–h | 1.63 | 14.3 ± 0.1 c–g | 57.4 ± 0.2 a | 85.3 ± 0.0 a–d |
| CH-08 | 114.9 ± 0.7 a | 1.1 ± 0.3 a | ND | 0.53 ± 0.06 a | 114.2 ± 0.9 a–e | 3.83 ± 0.13 d–g | 2.12 | 11.5 ± 0.1 a,b | 89.5 ± 0.1 a,b | 85.6 ± 0.0 a–d |
| CH-09 | 1364.5 ± 0.5 e | 19.9 ± 0.5 c | 0.59 ± 0.04 a | 3.40 ± 0.02 c,d | 34.9 ± 0.8 a–c | 4.37 ± 0.07 g,h | 2.36 | 14.2 ± 2.2 c–g | 88.9 ± 0.3 a,b | 94.6 ± 0.02 f |
| CH-10 | 787.5 ± 17.0 b–d | 18.6 ± 0.2 b,c | 9.42 ± 0.03 d | 5.57 ± 0.02 f | 24.1 ± 0.4 a | 3.74 ± 0.04 c–f | 2.12 | 14.3 ± 0.5 c–f | 80.6 ± 0.1 a,b | 87.0 ± 0.1 b–e |
| CH-11 | 595.8 ± 24.0 b–d | 1.6 ± 0.2 a | 0.10 ± 0.09 a | 0.85 ± 0.01 a | 121.8 ± 0.1 b–e | 3.01 ± 0.09 a,b | 1.59 | 15.9 ± 0.1 f,g | 65.5 ± 0.3 a,b | 85.0 ± 0.0 a–c |
| CH-12 | 573.8 ± 12.5 a–d | 4.2 ± 0.3 a | 0.22 ± 0.03 a | 0.82 ± 0.06 a | 33.2 ± 0.4 a,b | 3.40 ± 0.07 b–d | 2.10 | 15.5 ± 1.3 e–g | 70.0 ± 0.2 a,b | 82.9 ± 0.0 a,b |
| CH-13 | 846.5 ± 16.8 c,d | 8.7 ± 0.4 a–c | 5.70 ± 0.09 c | 4.92 ± 0.02 e,f | 96.8 ± 0.9 a–e | 4.48 ± 0.04 h | 1.96 | 15.5 ± 1.4 e–g | 70.0 ± 0.2 a,b | 82.9 ± 0.0 a,b |
| CH-14 | 343.8 ± 0.9 a,b | 5.1 ± 0.2 a | 0.43 ± 0.07 a | 0.59 ± 0.01 a | 64.9 ± 0.4 a–e | 4.07 ± 0.05 e–h | 1.78 | 11.9 ± 1.1 a–c | 76.7 ± 0.1 a,b | 86.5 ± 0.1 b–d |
| CH-15 | 567.1 ± 1.9 a–d | 5.6 ± 0.1 a | 5.30 ± 0.09 c | 2.18 ± 0.50 b,c | 73.1 ± 0.2 a–e | 4.24 ± 0.05 f–h | 1.68 | 13.4 ± 0.3 b–f | 79.0 ± 0.1 a,b | 94.8 ± 0.1 f |
| CH-16 | 506.0 ± 18.3 a–c | 1.4 ± 0.1 a | 0.27 ± 0.07 a | 1.70 ± 0.03 a,b | 47.2 ± 0.6 a–c | 3.69 ± 0.16 c–f | 1.50 | 13.5 ± 1.3 b–g | 63.9 ± 0.2 a,b | 78.9 ± 0.6 a |
| CH-17 | 662.0 ± 20.1 b–d | 2.2 ± 0.1 a | 0.050 ± 0.14 a | 0.90 ± 0.02 a,b | 75.3 ± 0.3 a–e | 3.94 ± 0.06 d–h | 1.59 | 15.7 ± 0.8 f,g | 87.5 ± 0.0 a,b | 82.1 ± 0.1 a,b |
| CH-18 | 451.9 ± 28.2 a–c | 1.2 ± 0.1 a | 0.10 ± 0.11 a | 0.80 ± 0.04 a | 111.4 ± 0.5 a–e | 3.18 ± 0.04 a–c | 1.54 | 12.2 ± 1.5 a–d | 96.9 ± 0.0 b | 98.2 ± 0.4 f |
| Correlation | r | p-Value |
|---|---|---|
| Flavonoids and Anthocyanins | 0.9891 | 0.0109 |
| Flavonoids and %DRSA | 0.9525 | 0.0475 |
| Flavonoids and Lectin | 0.9975 | 0.0025 |
| Tannins and Anthocyanins | 0.9511 | 0.0489 |
| Anthocyanins and %DRSA | 0.9609 | 0.0391 |
| Anthocyanins and Lectin | 0.9959 | 0.0041 |
| %DRSA and Lectin | 0.9669 | 0.0331 |
| Sample | Phaseolus Species | Common Name | Origin | Picture |
|---|---|---|---|---|
| CH-01 | P. vulgaris | Pinto Colorado (Tzirin chenek) | Tenejapa |  |
| CH-02 | P. vulgaris | Negro Cubano | Villaflores |  |
| CH-03 | P. vulgaris | Negro graceño | Villaflores |  |
| CH-04 | P. vulgaris | Pie de Paloma | Teopisca |  |
| CH-05 | P. vulgaris | Negro de vaina blanca | Teopisca |  |
| CH-06 | P. polyanthus | Ibes | Tenejapa |  |
| CH-07 | P. coccineus | Botil | Tenejapa |  |
| CH-08 | P. vulgaris | Blanco | Teopisca |  |
| CH-09 | P. vulgaris | Rojo | Chenalho |  |
| CH-10 | P. vulgaris | Negro de vaina morada | Teopisca |  |
| CH-11 | P. polyanthus | Ibes rojo | Chenalho |  |
| CH-12 | P. vulgaris | Bayo | Teopisca |  |
| CH-13 | P. vulgaris | Negro | Chenalho |  |
| CH-14 | P. vulgaris | Regadillo | Teopisca |  |
| CH-15 | P. vulgaris | Negro | Pantelho |  |
| CH-16 | P. coccineus | Botil | Chamula |  |
| CH-17 | P. lunatus | Patachete | Teopisca |  |
| CH-18 | P. vulgaris | Barreton | Teopisca |  |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alcázar-Valle, M.; Lugo-Cervantes, E.; Mojica, L.; Morales-Hernández, N.; Reyes-Ramírez, H.; Enríquez-Vara, J.N.; García-Morales, S. Bioactive Compounds, Antioxidant Activity, and Antinutritional Content of Legumes: A Comparison between Four Phaseolus Species. Molecules 2020, 25, 3528. https://doi.org/10.3390/molecules25153528
Alcázar-Valle M, Lugo-Cervantes E, Mojica L, Morales-Hernández N, Reyes-Ramírez H, Enríquez-Vara JN, García-Morales S. Bioactive Compounds, Antioxidant Activity, and Antinutritional Content of Legumes: A Comparison between Four Phaseolus Species. Molecules. 2020; 25(15):3528. https://doi.org/10.3390/molecules25153528
Chicago/Turabian StyleAlcázar-Valle, Montserrat, Eugenia Lugo-Cervantes, Luis Mojica, Norma Morales-Hernández, Heidy Reyes-Ramírez, Jhony Navat Enríquez-Vara, and Soledad García-Morales. 2020. "Bioactive Compounds, Antioxidant Activity, and Antinutritional Content of Legumes: A Comparison between Four Phaseolus Species" Molecules 25, no. 15: 3528. https://doi.org/10.3390/molecules25153528
APA StyleAlcázar-Valle, M., Lugo-Cervantes, E., Mojica, L., Morales-Hernández, N., Reyes-Ramírez, H., Enríquez-Vara, J. N., & García-Morales, S. (2020). Bioactive Compounds, Antioxidant Activity, and Antinutritional Content of Legumes: A Comparison between Four Phaseolus Species. Molecules, 25(15), 3528. https://doi.org/10.3390/molecules25153528








