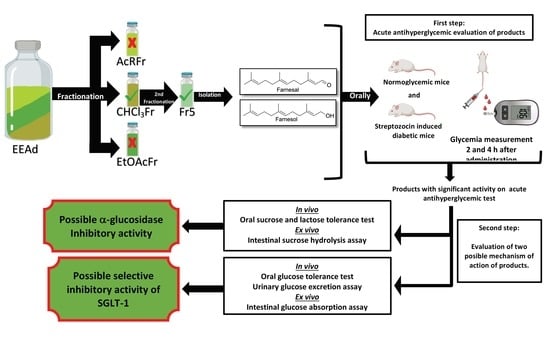
Antihyperglycemic Effects of Annona diversifolia Safford and Its Acyclic Terpenoids: α-Glucosidase and Selective SGLT1 Inhibitiors
Abstract
:1. Introduction
2. Results
2.1. In Vivo Assays
2.1.1. Acute Antihyperglycemic Activity of Ethanolic Extract from A. diversifolia and Its Products
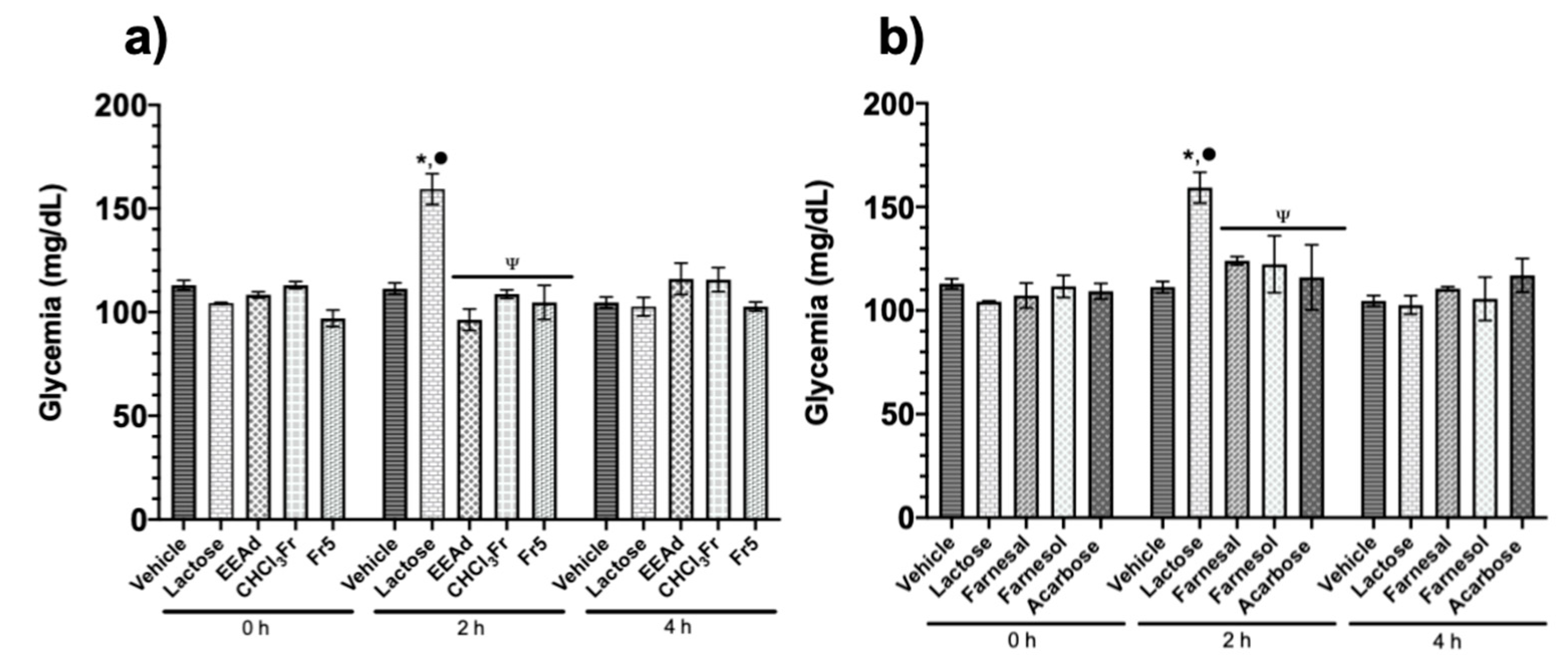
2.1.2. Oral Sucrose and Lactose Tolerance Test of Ethanolic Extract from A. diversifolia and Its Products
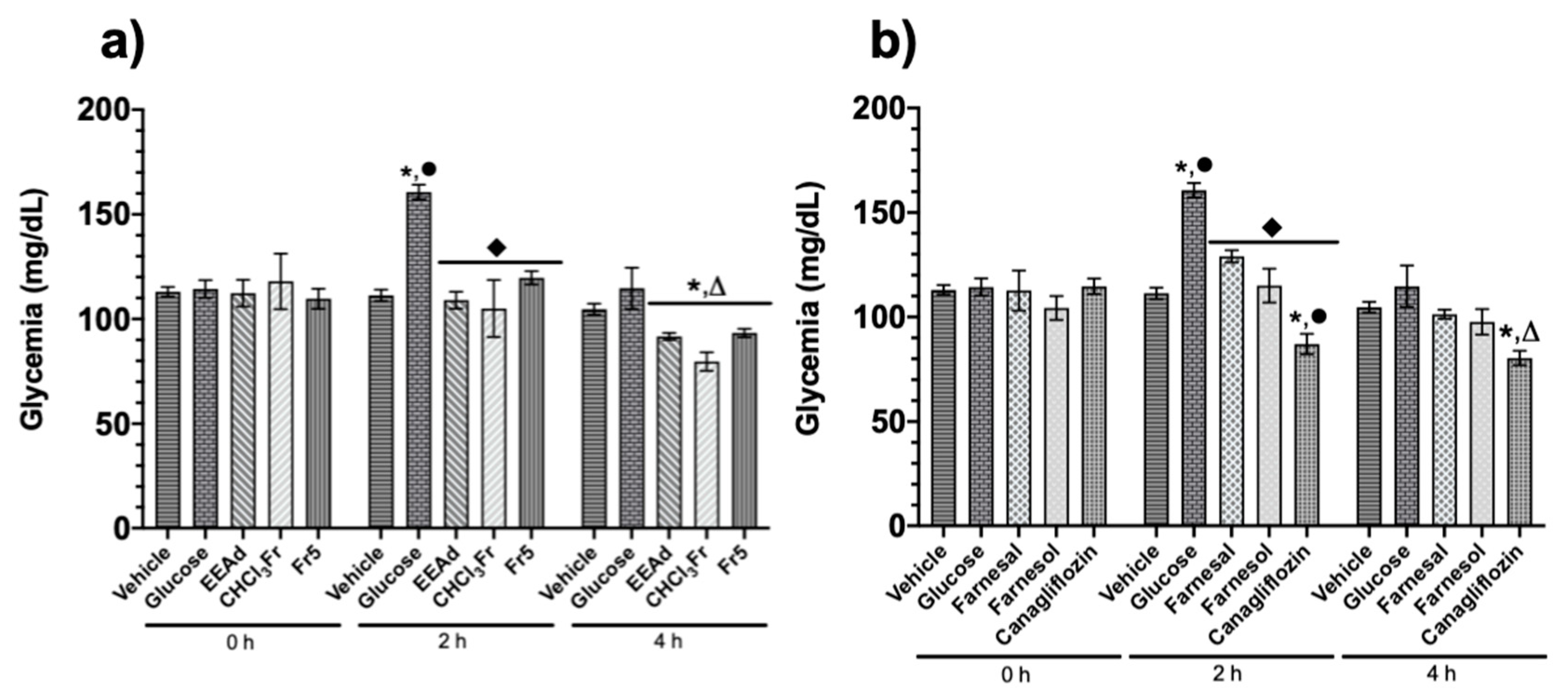
2.1.3. Oral Glucose Tolerance Test of Ethanolic Extract from A. diversifolia and Its Products
2.2. Ex Vivo Assays
2.2.1. Intestinal Sucrose Hydrolysis Inhibition Test of Ethanolic Extract from A. diversifolia and Its Products
2.2.2. Intestinal Glucose Absorption Inhibition Test of Ethanolic Extract from A. diversifolia and Its Products
2.3. Urinary Glucose Excretion Assay of Ethanolic Extract from A. diversifolia and Its Products
3. Discussion
4. Materials and Methods
4.1. General Information
4.2. Plant Materials
4.3. Extraction, Isolation and Identification of Farnesol and Farnesal
4.4. Animals
4.5. In Vivo Assays
4.5.1. Induction of Experimental Type 2 Diabetes
4.5.2. Acute Antihyperglycemic Activity of Ethanolic Extract from A. diversifolia and Its Products
4.5.3. Oral Sucrose and Lactose Tolerance Test of Ethanolic Extract from A. diversifolia and Its Products
4.5.4. Oral Glucose Tolerance Test of Ethanolic Extract from A. diversifolia and Its Products
4.6. Ex Vivo Assays
4.6.1. Intestinal Sucrose Hydrolysis Inhibition Assay of Ethanolic Extract from A. diversifolia and Its Products
4.6.2. Intestinal Glucose Absorption Inhibition Assay of Ethanolic Extract from A. diversifolia and Its Products
4.7. Urinary Glucose Excretion Assay of Ethanolic Extract from A. diversifolia and Its Products
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas. Available online: https://www.diabetesatlas.org/upload/resources/material/20200302_133352_2406-IDF-ATLAS-SPAN-BOOK.pdf (accessed on 14 November 2019).
- Georgiou, P.; Johnston, D. Guest Editorial Biomedical and Health Informatics for Diabetes. J. Biomed. Health Inf. 2016, 20, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Key Facts. Diabetes. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 23 March 2020).
- American Diabetes Association. Diabetes Mellitus, Diagnostic and Classification. Available online: http://archives.diabetes.org/es/informacion-basica-de-la-diabetes/?loc=globalnav (accessed on 24 March 2020).
- American Diabetes Association. Diabetes Mellitus, Diagnostic and Classification. Available online: https://www.diabetes.org/diabetes/type-2?loc=db-slabnav (accessed on 24 March 2020).
- Alam, U.; Asghar, O.; Azmi, S.; Malik, R. General aspects of diabetes mellitus. Handb. Clin. Neurol. 2014, 126, 211–222. [Google Scholar] [PubMed]
- Chaudhury, A.; Duvoor, C.; Dendi, V.; Kraleti, S.; Chada, A.; Ravilla, R.; Marco, A.; Shekhawat, N.; Montales, M.; Kuriakose, K.; et al. Clinical review of antidiabetic drugs: Implications for type 2 diabetes mellitus management. Front. Endocrinol. 2017, 8, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nanjan, M.; Mohammed, M.; Prashantha, K.; Chandrasekar, M. Thiazolidinediones as antidiabetic agents: A critical review. Bioorg. Chem. 2018, 77, 548–567. [Google Scholar] [CrossRef] [PubMed]
- Viollet, B.; Guigas, B.; Garcia, N.; Leclerc, J.; Foretz, M.; Andreelli, F. Mecanismos celulares y moleculares de metformina: Una vision general. Clin. Sci. (Lond) 2012, 6, 253–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singla, R.; Singh, R.; Dubey, A. Important aspects of post-prandial antidiabetic drug, Acarbose. Curr. Top. Med. Chem. 2016, 16, 2625–2633. [Google Scholar] [CrossRef]
- Panai, S.; Akira, O.; Herman, K.; Volker, V. Sodium glucose cotransporter SGLT1 as a therapeutic target in diabetes mellitus. Expert Opin. Ther. Targets 2016, 20, 1109–1125. [Google Scholar]
- Valdes, M.; Calzada, F.; Mendieta-Wejebe, J. Structure-activity relationship study of acyclic terpenes in blood glucose levels: Potential α-glucosidase and sodium glucose cotransporter (SGLT-1) inhibitors. Molecules 2019, 24, 4020. [Google Scholar] [CrossRef] [Green Version]
- Lehman, A.; Hornby, P. Intestinal SGLT1 in metabolic health and disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 310, G887–G898. [Google Scholar] [CrossRef] [Green Version]
- Furman, B. Acarbose. In Elsevier Strathclyde Institute of Pharmacy and biomedical Sciences, 1st ed.; Elsevier: Glasgow, UK, 2017; pp. 1–3. [Google Scholar]
- Galasko, G. Insulin, Oral Hypoglycemics, and Glucagon. In Pharmacology and Therapeutics for Dentistry, 7th ed.; Mosby: Omaha, NE, USA, 2017; pp. 437–445. [Google Scholar]
- Lee, B.; Rose, D.; Lin, A.; Quezad-Calvillo, R.; Nichols, B.; Hamaker, B. Contribution of the individual small intestinal α-glucosidases on digestion of unusual α-linked glycemic disaccharides. J. Agric. Food Chem. 2016, 64, 6487–6494. [Google Scholar] [CrossRef]
- Deeks, E.; Scheen, A. Canagliflozin: A review in type 2 diabetes. Drugs 2017, 77, 1577–1592. [Google Scholar] [CrossRef] [PubMed]
- Rosak, C.; Mertes, G. Critical evaluation of the role of acarbose in the treatment of diabetes: Patient considerations. Diabetes Metab. Syndr. Obes. 2012, 5, 357–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakher, H.; Chang, T.; Tan, M.; Mahaffey, K. Canagliflozin review-safety and efficacy profile in patients with T2D M. Diabetes Metab. Syndr. Obes. 2019, 12, 209–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhenhua, Y.; Zhang, W.; Feng, F.; Zhang, Y.; Kang, W. α-Glucosidase inhibitors isolated from medicinal plants. Food Sci. Human Wellnes 2014, 3, 136–174. [Google Scholar]
- Ramírez, G.; Zavala, M.; Pérez, J.; Zamilpa, A. In Vitro Screening of medicinal plants used in México as antidiabetics with glucosidase and lipase inhibitory activities. Evid.-Based Complement. Alternat. Med. 2012, 2012, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Chavez-Silva, F.; Cerón-Romero, L.; Arias-Durán, L.; Navarrete-Vázquez, G.; Almazán-Pérez, J.; Román-Ramos, R.; Ramírez-Ávila, G.; Perea-Arango, I.; Villalobos-Molina, R.; Estrada-Soto, S. Antidiabetic effect of Achillea millefollium through multitarget interactions: α-glucosidases inhibition, insulin sensitization and insulin secretagogue activities. J. Ethnopharmacol. 2017, 212, 1–7. [Google Scholar] [CrossRef]
- Alam, F.; Shafique, Z.; Amjad, S.; Asad, M. Enzymes inhibitors from natural sources with antidiabetic activity: A review. Phytother. Res. 2018, 33, 41–54. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Traditional, Complementary and Integrative Medicine. Available online: https://www.who.int/health-topics/traditional-complementary-and-integrative-medicine#tab=tab_1 (accessed on 22 April 2020).
- Ibáñez-Camacho, R.; Meckes, M.; Mellado, V. The hypoglucemic effect of Opuntia streptacantha studied in differential animal experimental models. J. Ethnopharmacol. 1983, 7, 175–181. [Google Scholar] [CrossRef]
- Pérez, G.; Ocegueda, A.; Muñoz, L.; Ávila, A.; Morrow, W. A study of the hypoglycemic effect of some mexican plants. J. Ethnopharmacol. 1984, 3, 253–262. [Google Scholar] [CrossRef]
- Esquivel-Gutierrez, E.; Noriega-Cisneros, R.; Bello-González, M.; Saavedra-Molina, A.; Salgado-Garciglia, R. Plantas utilizadas en la medicina tradicional mexicana con propiedades antidiabéticas y antihipertensivas. Biológicas Julio 2012, 14, 45–52. [Google Scholar]
- Kaleem, M.; Asif, M.; Ahmed, Q.; Bano, B. Antidiabetic and antioxidant activity of Annona squamosa extract in streptozotocin-induced diabetic rats. Singapore Med. J. 2006, 47, 670–675. [Google Scholar] [PubMed]
- Kumar, R.; Narayan, A.; Murthy, P.; Chandra, R.; Tanton, V.; Watal, G. Hypoglycemic and antidiabetic effect of ethanolic extract of leaves of Annona squamosa L. in experimental animals. J. Ethnopharmacol. 2005, 99, 75–81. [Google Scholar]
- Tripathi, R.; Tripathi, Y. Insulin secreting and α-glucosidase inhibitory activity of hexane extract of Annona squamosa Linn. in streptozotocin (STZ) induced diabetic rats. Indian J. Exp. Biol. 2014, 52, 623–629. [Google Scholar]
- Shirwaikar, A.; Rajendran, K.; Dinesh, C.; Bodla, R. Antidiabetic activity of aqueous leaf extract of Annona squamosa in streptozotocin-nicotinamide type 2 diabetic rats. J. Ethnopharmacol. 2004, 91, 171–175. [Google Scholar] [CrossRef]
- Benatti, A.; Carnevalli, N.; Rodriguez, R.; Machado, M.; Maria da silva, N.; Salmen, F. Annona muricata Linn. leaf as a source of antioxidant compounds with in vitro antidiabetic and inhibitory potential against α-amylase, α-glucosidase, lipase, non-enzymatic glycation and lipid peroxidation. Biomed. Pharmacother. 2018, 100, 83–92. [Google Scholar]
- Calzada, F.; Solares-Pascasio, J.; Ordoñez-Razo, R.; Velazquez, C.; Barbosa, E.; García-Hernández, N.; Mendez-Luna, D.; Correa Basurto, J. Antihyperglycemic activity of the leaves from Annona cherimola Miller and rutin on alloxan-induced diabetic rats. Pharmacognosy Res. 2017, 9, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Jamkhande, P.; Wattamwal, A. Annona reticulata Linn. (Bullock’s heart): Plant profile, phytochemistry and pharmacological properties. J. Tradit. Complement. Med. 2015, 5, 144–152. [Google Scholar] [CrossRef] [Green Version]
- Wen, W.; Lin, Y.; Ti, Z. Antidiabetic, antihyperlipidemic, antioxidant, anti-inflamatory activities of ethanolic seed extract of Annona reticulata L. in streptozotocin inducen diabetic rats. Front. Endocrinol. 2019, 10, 1–15. [Google Scholar] [CrossRef]
- Calzada, F.; Valdes, M.; García-Hernández, N.; Velázquez, C.; Barbosa, E.; Bustos-Brito, C.; Quijano, L.; Pina-Jimenez, E.; Mendieta-Wejebe, J. Antihyperglycemic activity of the leaves from Annona diversifolia Safford. and farnesol on normal and alloxan-induced diabetic mice. Phcog. Mag. 2019, 15, S5–S11. [Google Scholar]
- Carballo, A.; Martínez, A.; González-Trujano, M.; Pellicer, F.; Ventura-Martínez, R.; Días-Reval, M.; López-Muñoz, F. Antinociceptive activity of Annona diversifolia Saff. leaf extracts and palmitone as bioactive compound. Pharmocol. Biochem. Behav. 2010, 95, 6–12. [Google Scholar] [CrossRef]
- Calzada, F.; Ramirez-Santos, J.; Valdes, M.; Garcia-Hernandez, N.; Pina-Jiménez, E.; Ordoñez-Razo, R. Evaluation of acute oral toxicity, brine shrimp lethality, and antilymphoma activity of geranylgeraniol and Annona macroprophylata leaf extracts. Rev. Bras. Farm. 2020, 30, 301–304. [Google Scholar] [CrossRef]
- González-Trujano, M.; Navarrete, A.; Reyes, B.; Cedillo-Portugal, E.; Hong, E. Anticonvulsivant properties and bio-guided Isolation of palmitone from leaves of Annona diversifolia. Planta Med. 2001, 67, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Angeles-López, G.; González-Trujano, M.; Déciga-Cámpos, M.; Ventura-Martínez, R. Neuroprotective evaluation of Tilia americana and Annona diversifolia in the neuronal damage induced by intestinal ischemia. Neurochem. Res. 2013, 38, 1632–1640. [Google Scholar] [CrossRef] [PubMed]
- González-Trujano, M.; Tapia, E.; López-Meraz, L.; Navarrete, A.; Reyes-Ramírez, A.; Martínez, A. Anticonvulsant effect of Annona diversifolia Saff. and palmitone on penicillin-induced convulsive activity. A behavioral and EEG study in rats. Epilepsia 2006, 47, 1810–1817. [Google Scholar]
- De la Cruz-Chacón, I.; González-Esquinca, A.; Fefer, P.; Jiménez, L. Liriodenine, early antimicrobial defence in Annona Divers. J. Biosci Z. Nat. C (ZNC) 2011, 66, 377–384. [Google Scholar]
- Shile-Guzmán, M.; García-Carrancá, A.; González-Esquinca, A. In vitro and in vivo antiproliferative activity of laherradurin and cherimolin-2 of Annona diversifolia Saff. Phytother. Res. 2009, 23, 1128–1133. [Google Scholar] [CrossRef]
- González-Trujano, M.; López-Meraz, L.; Reyes-Ramírez, A.; Aguillón, M.; Martínez, A. Effect of repeated administration of Annona diversifolia Saff. (ilama) extracts and palmitone on rat amígdala kindking. Epilepsy Behav. 2009, 16, 590–595. [Google Scholar]
- González-Trujano, M.; Martínez, A.; Reyes-Ramírez, A.; Reyes-Trejo, B.; Navarrete, A. Palmitone isolated from Annona diversifolia induces an anxiolytic-like effect in mice. Planta Med. 2006, 72, 703–707. [Google Scholar] [CrossRef]
- Brindis, F.; González-Trujano, M.; González-Andrade, M.; Aguirre-Hernández, E.; Villalobos-Molina, A. Aqueous Extract of Annona macroprophyllata A Potential ∝-Glucosidase Inhibitor. BioMed Res. Int. 2013, 2013, 1–6. [Google Scholar]
- Grunberger, G. Should side effects influence the selection of antidiabetic therapier in type 2 diabetes? Curr. Diab. Rep. 2017, 17, 1–12. [Google Scholar] [CrossRef]
- Montoya, G.; Rendón, A.; Aranjo, M. Identificación y diferenciación de monosacáridos y disacáridos diasteroméricos no derivatizados por ESI-IT-MS/MS. In Vitae, Revista De La Facultad De Química Farmacéutica; Universidad de Antioquia: Medellín, Colombia, 2010; Volume 17, pp. 37–44. [Google Scholar]
- Ortiz-Andrade, R.; García-Jiménez, S.; Castillo-España, P.; Ramírez-Avila, G.; Villalobos-Molina, R.; Estrada-Soto, S. α-Glucosidase inhibitory activity of the methanolic extract from Tournefortia hartwegina: An antihyperglycemic agent. J. Ethnopharmacol. 2007, 109, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Katsuno, K.; Fujimori, Y.; Takemura, Y.; Hiratochi, M.; Itoh, F.; Komatsu, Y.; Fujikuram, H.; Isaji, M. Sergiflozin, a novel selective inhibitor of low-affinity sodium glucose cotransporter (SGLT2), validates the critical role of SGLT2 in renal glucose reabsorption and modulates plasma glucose level. J. Pharmacol. Exp. Ther. 2007, 320, 323–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escalada, F. Fisiología del GLP-1 y su papel en la fisiopatología de la diabetes mellitus tipo 2. Med. Clin. 2014, 143, 2–7. [Google Scholar] [CrossRef]
- Nakamura, K.; Yamagishi, S.; Matsui, T.; Inoue, H. Acarbose, an alpha-glucoisdase inhibitor, improves insulin resistance in fructose-fed rats. Drugs Exp. Clin. Res. 2005, 31, 155–159. [Google Scholar]
- Hamada, Y.; Nagasaki, H.; Fuchigami, M.; Furuta, S.; Seino, Y.; Nakamura, J.; Oiso, Y. The alpha-glucosidase inhibitor miglitol affects bile acid metabolism and amelioratesobesity and insulin resistance in diabetic mice. Metabolism 2013, 62, 734–742. [Google Scholar] [CrossRef]
- Norma Oficial Mexicana (NOM). Especificaciones técnicas Para la Producción, Cuidado y Uso de los Animales de Laboratorio. Available online: http://www.ibt.unam.mx/computo/pdfs/bioterio.NOM-062.pdf (accessed on 30 April 2020).
- Hsu, J.; Wu, C.; Hung, C.; Wang, C.; Huang, H. Myrciaria cauliflora extract improves diabetic nephropathy via suppression of oxidative stress and inflammation in streptozotocin-nicotinamide mice. J. Food Drug Anal. 2016, 24, 730–737. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the compounds are available from the authors (Dr. Miguel Valdés and Dr. Fernando Calzada). |



| Treatment | Glycemia (mg/dL) | ||
|---|---|---|---|
| 0 h | 2 h | 4 h | |
| NM Control | 137.3 ± 4.7 | 131.8 ± 2.4 | 134.8 ± 2.1 |
| NM + EEAd | 138.7 ± 8.5 | 122 ± 6.6 | 128 ± 9 |
| NM + CHCl3Fr | 142 ± 10.9 | 133.7 ± 4.9 | 124.7 ± 4 |
| NM + AcRFr | 141 ± 5.4 | 167.7 ± 7.3 *,⬪ | 175.3 ± 10.2 *,⬪⬪ |
| NM + EtOAcFr | 141 ± 2 | 171 ± 8.1 *,⬪ | 167.3 ± 5.1 *,⬪⬪ |
| NM + Fr5 | 148.3 ± 4 | 134.1 ± 4.8 | 145.7 ± 10.9 |
| NM + Farnesal | 143.7 ± 6.1 | 133.7 ± 3.6 | 128.3 ± 2.9 |
| NM + Farnesol | 136 ± 6.6 | 130.7 ± 5.2 | 128 ± 5.5 |
| NM + Acarbose | 137.7 ± 3.6 | 130.7 ± 2.3 | 125.3 ± 2.9 |
| NM + Canagliflozin | 140.8 ± 2.9 | 106.8 ± 2.2 *,⬪ | 95.2 ± 0.4 *,⬪⬪ |
| NM + Glibenclamide | 144.7 ± 2.6 | 95.7 ± 1.1 *,⬪ | 84.6 ± 2.8 *,⬪⬪ |
| NM + Pioglitazone | 140.3 ± 3.8 | 109 ± 1.9 *,⬪ | 123.7 ± 1.3 * |
| NM + Metformin | 139.3 ± 1.3 | 125 ± 3.4 | 142 ± 0.8 |
| SID2 Control | 331 ± 14.4 | 353.7 ± 5.7 | 336 ± 2.9 |
| SID2 + EEAd | 366.3 ± 18.4 | 221 ± 35.5 *,Ψ | 324 ± 17.9 |
| SID2 + CHCl3Fr | 336.7 ± 21.6 | 230.3 ± 13.3 *,Ψ | 287.7 ± 5.6 *, ΨΨ |
| SID2 + AcRFr | 354.3 ± 12.4 | 398 ± 23.5 *,Ψ | 439 ± 14.2 *,ΨΨ |
| SID2 + EtOAcFr | 337.8 ± 3.6 | 377 ± 3.1 *,Ψ | 365.5 ± 11.1 *,ΨΨ |
| SID2 + Fr5 | 350.7 ± 26 | 213.5 ± 35.5 *,Ψ | 209 ± 12.6 *,ΨΨ |
| SID2 + Farnesal | 337.3 ± 13.1 | 268 ± 12.2 *,Ψ | 226 ± 8 *,ΨΨ |
| SID2 + Farnesol | 339.7 ± 10.3 | 255.3 ± 23.1 *,Ψ | 337 ± 7.2 |
| SID2 + Acarbose | 335.8 ± 8.7 | 255.5 ± 20.5 *,Ψ | 323.3 ± 11.6 |
| SID2 + Canagliflozin | 367.3 ± 8.4 | 157.7 ± 31.5 *,Ψ | 102 ± 11.5 *,ΨΨ |
| SID2 + Glibenclamide | 357 ± 7.5 | 254.3 ± 3.3 *,Ψ | 234.3 ± 19.3 *,ΨΨ |
| SID2 + Pioglitazone | 335.7 ± 8.2 | 259.7 ± 7.9 *,Ψ | 248.3 ± 4.4 *,ΨΨ |
| SID2 + Metformin | 338.7 ± 13.5 | 275 ± 7.6 * | 266.7 ± 7.2 *,ΨΨ |
| Treatment | Glucose (mg/dL) | Glucose (mg/dL) | % of Inhibition | CE50 |
|---|---|---|---|---|
| 0 h | 2 h | |||
| Sucrose (15%) | 0 ± 0 | 89.3 ± 17.5 | - | - |
| EEAd [200 µg/mL] | 0 ± 0 | 63.6 ± 7.5 * | 28.70 | 565.6 µg/mL |
| EEAd [400 µg/mL] | 0 ± 0 | 52.33 ± 10 * | 41.39 | |
| EEAd [800 µg/mL] | 0 ± 0 | 33 ± 4 * | 63.04 | |
| CHCl3Fr [200 µg/mL] | 0 ± 0 | 107 ± 6.4 | 0 | 662.2 µg/mL |
| CHCl3Fr [400 µg/mL] | 0 ± 0 | 65.6 ± 2.9 * | 26.46 | |
| CHCl3Fr [800 µg/mL] | 0 ± 0 | 33 ± 7.6 * | 63.04 | |
| Fr 5 [200 µg/mL] | 0 ± 0 | 85.3 ± 5.8 | 4.41 | 590.4 µg/mL |
| Fr 5 [400 µg/mL] | 0 ± 0 | 59 ± 4.4 * | 33.93 | |
| Fr 5 [800 µg/mL] | 0 ± 0 | 25.66 ± 2.1 * | 71.25 | |
| Farnesal [200 µM] | 0 ± 0 | 93 ± 9.8 | 0 | 682.9 µM |
| Farnesal [400 µM | 0 ± 0 | 64.6 ± 8.8 | 27.58 | |
| Farnesal [800 µM] | 0 ± 0 | 36 ± 2.5 * | 59.68 | |
| Farnesol [200 µM] | 0 ± 0 | 84.33 ± 2.6 | 5.56 | 802.2 µM |
| Farnesol [400 µM] | 0 ± 0 | 67.33 ± 1.76 | 24.59 | |
| Farnesol [800 µM] | 0 ± 0 | 45.6 ± 1.2 * | 48.86 | |
| Acarbose [200 µM] | 0 ± 0 | 36.3 ± 4 * | 59.31 | 187.8 µM |
| Acarbose [400 µM] | 0 ± 0 | 11.33 ± 0.89 * | 87.30 | |
| Acarbose [800 µM] | 0 ± 0 | 6.66 ± 0.33 * | 90.29 | |
| Treatment | Glucose (mg/dL) | Glucose (mg/dL) | % of Inhibition | CE50 |
|---|---|---|---|---|
| 0 h | 1 h | |||
| Glucose (5%) | 0 ± 0 | 217.3 ± 8.7 | - | - |
| EEAd [200 µg/mL] | 0 ± 0 | 202.6 ± 5.2 | 6.73 | 1059.9 µg/mL |
| EEAd [400 µg/mL] | 0 ± 0 | 162.3 ± 30 | 25.29 | |
| EEAd [800 µg/mL] | 0 ± 0 | 138.6 ± 5.2 * | 36.18 | |
| CHCl3Fr [200 µg/mL] | 0 ± 0 | 308.6 ± 25.9 | 0 | 783.5 µg/mL |
| CHCl3Fr [400 µg/mL] | 0 ± 0 | 182.3 ± 6.2 * | 16.09 | |
| CHCl3Fr [800 µg/mL] | 0 ± 0 | 105 ± 2.8 * | 51.67 | |
| Fr 5 [200 µg/mL] | 0 ± 0 | 255.6 ± 11.7 | 0 | 539.9 µg/mL |
| Fr 5 [400 µg/mL] | 0 ± 0 | 114 ± 7.7 * | 47.57 | |
| Fr 5 [800 µg/mL] | 0 ± 0 | 51 ± 1.52 * | 76.53 | |
| Farnesal [200 µM] | 0 ± 0 | 311 ± 11.06 | 0 | 1211.8 µM |
| Farnesal [400 µM] | 0 ± 0 | 255.3 ± 14 | 6.46 | |
| Farnesal [800 µM] | 0 ± 0 | 152 ± 35.6 * | 30.05 | |
| Farnesol [200 µM] | 0 ± 0 | 398.6 ± 39.8 | 0 | 372.3 µM |
| Farnesol [400 µM] | 0 ± 0 | 86.3 ± 20.5 * | 60.26 | |
| Farnesol [800 µM] | 0 ± 0 | 112.3 ± 20.6 * | 48.30 | |
| Canagliflozin [200 µM] | 0 ± 0 | 237.6 ± 22 | 0 | 763.0 µM |
| Canagliflozin [400 µM] | 0 ± 0 | 112.3 ± 14.1 * | 48.30 | |
| Canagliflozin [800 µM] | 0 ± 0 | 119 ± 8.5 * | 45.23 | |
| Position | Farnesal (1) | Farnesol (2) | ||
|---|---|---|---|---|
| δH, mult. (J in Hz) | δC, Type | δH mult. (J in Hz) | δC, Type | |
| 1 | 9.97, d (8.04) | 59.14, CH | 4.1, d (8) | 59.14, CH2 |
| 2 | 5.86, dd, (8.1, 4.3) | 124.5, CH | 5.37, t (8) | 124.5, CH |
| 3 | - | 136.18, C | - | 136.18, C |
| 4 | 2.01, t (3.2) | 35.27, CH2 | 1.97, s | 35.27, CH2 |
| 5 | 2.15, t (1.44) | 32.35, CH2 | 1.97, s | 32.35, CH2 |
| 6 | 5.07, s | 124.66, CH | 5.4, s | 124.66, CH |
| 7 | - | 135.48, C | - | 135.48, C |
| 8 | 2.15, t (1.44) | 28.11, CH2 | 1.97, s | 28.11, CH2 |
| 9 | 2.15, t (1.44) | 26.54, CH2 | 1.97, s | 26.54, CH2 |
| 10 | 5.07, s | 125.14, CH | 5.1, s | 125.14, CH |
| 11 | - | 135.36, C | - | 135.36, C |
| 12 | 1.58, d (3.49) | 16.13, CH3 | 1.55, s | 16.13, CH3 |
| 13 | 1.96, t (1.58) | 25.82, CH3 | 1.68, s | 25.82, CH3 |
| 14 | 1.66, d (5.51) | 23.57, CH3 | 1.68, s | 23.57, CH3 |
| 15 | 1.58, d (3.49) | 19.89, CH3 | 1.68, s | 19.89, CH3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valdés, M.; Calzada, F.; Mendieta-Wejebe, J.E.; Merlín-Lucas, V.; Velázquez, C.; Barbosa, E. Antihyperglycemic Effects of Annona diversifolia Safford and Its Acyclic Terpenoids: α-Glucosidase and Selective SGLT1 Inhibitiors. Molecules 2020, 25, 3361. https://doi.org/10.3390/molecules25153361
Valdés M, Calzada F, Mendieta-Wejebe JE, Merlín-Lucas V, Velázquez C, Barbosa E. Antihyperglycemic Effects of Annona diversifolia Safford and Its Acyclic Terpenoids: α-Glucosidase and Selective SGLT1 Inhibitiors. Molecules. 2020; 25(15):3361. https://doi.org/10.3390/molecules25153361
Chicago/Turabian StyleValdés, Miguel, Fernando Calzada, Jessica Elena Mendieta-Wejebe, Verenice Merlín-Lucas, Claudia Velázquez, and Elizabeth Barbosa. 2020. "Antihyperglycemic Effects of Annona diversifolia Safford and Its Acyclic Terpenoids: α-Glucosidase and Selective SGLT1 Inhibitiors" Molecules 25, no. 15: 3361. https://doi.org/10.3390/molecules25153361
APA StyleValdés, M., Calzada, F., Mendieta-Wejebe, J. E., Merlín-Lucas, V., Velázquez, C., & Barbosa, E. (2020). Antihyperglycemic Effects of Annona diversifolia Safford and Its Acyclic Terpenoids: α-Glucosidase and Selective SGLT1 Inhibitiors. Molecules, 25(15), 3361. https://doi.org/10.3390/molecules25153361