Protein and Polysaccharide-Based Fiber Materials Generated from Ionic Liquids: A Review
Abstract
1. Introduction
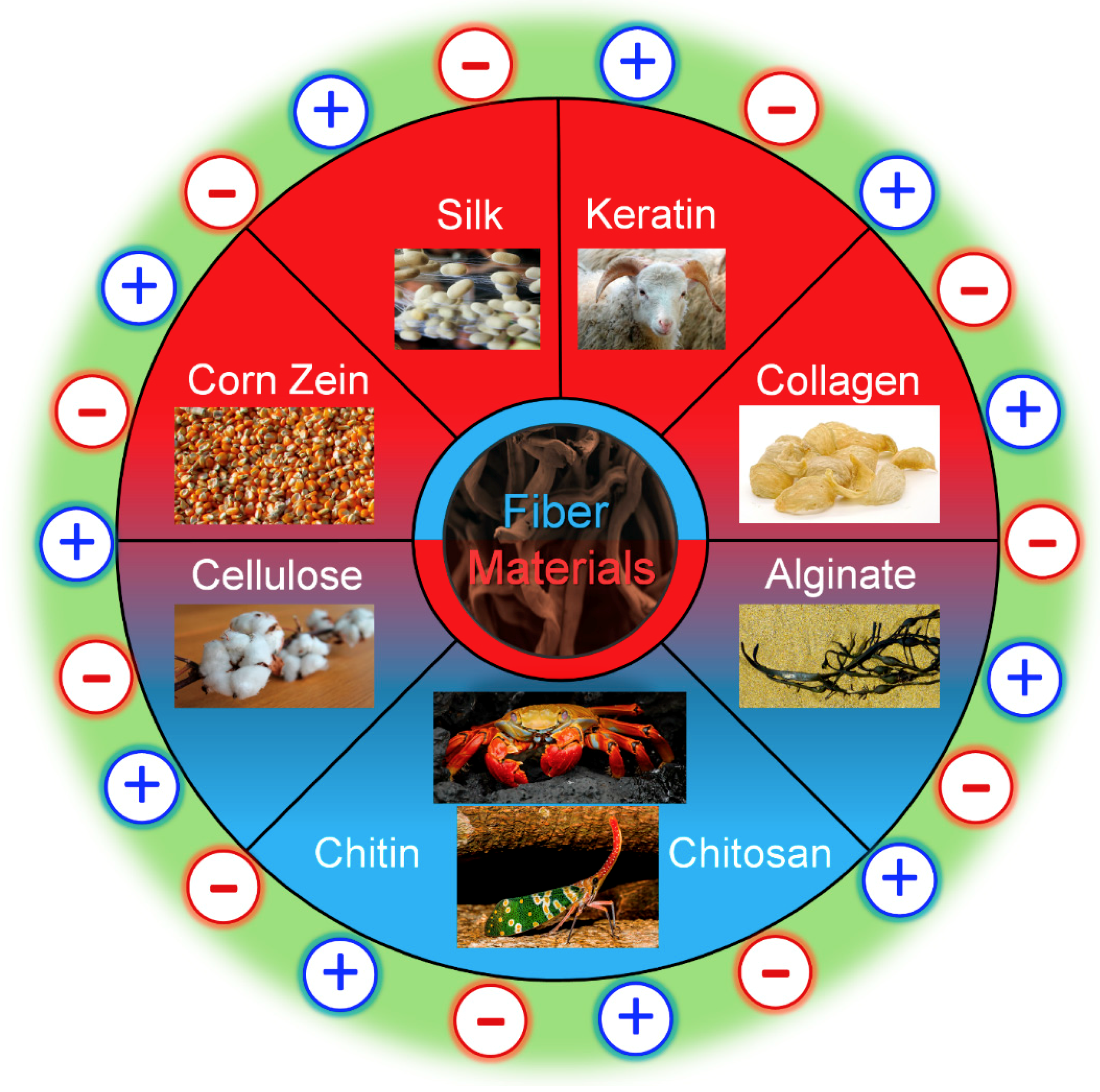
2. Typical Biopolymers from Proteins and Polysaccharides
2.1. Protein Materials
2.1.1. Silk Proteins
2.1.2. Keratin Proteins
2.1.3. Soy Proteins
2.1.4. Collagen
2.1.5. Elastin
2.1.6. Corn Zein
2.1.7. Reflectin
2.2. Polysaccharide Materials
2.2.1. Starch
2.2.2. Cellulose
2.2.3. Chitin
2.2.4. Chitosan
2.2.5. Alginate
2.3. Protein and Polysaccharide Based Composite Materials
3. Fabrication Methods to Control Fiber Formation
3.1. Solvents Useful for Biopolymer Dissolution
3.1.1. Ionic Liquids as Solvents
3.1.2. Other Potential Solvents
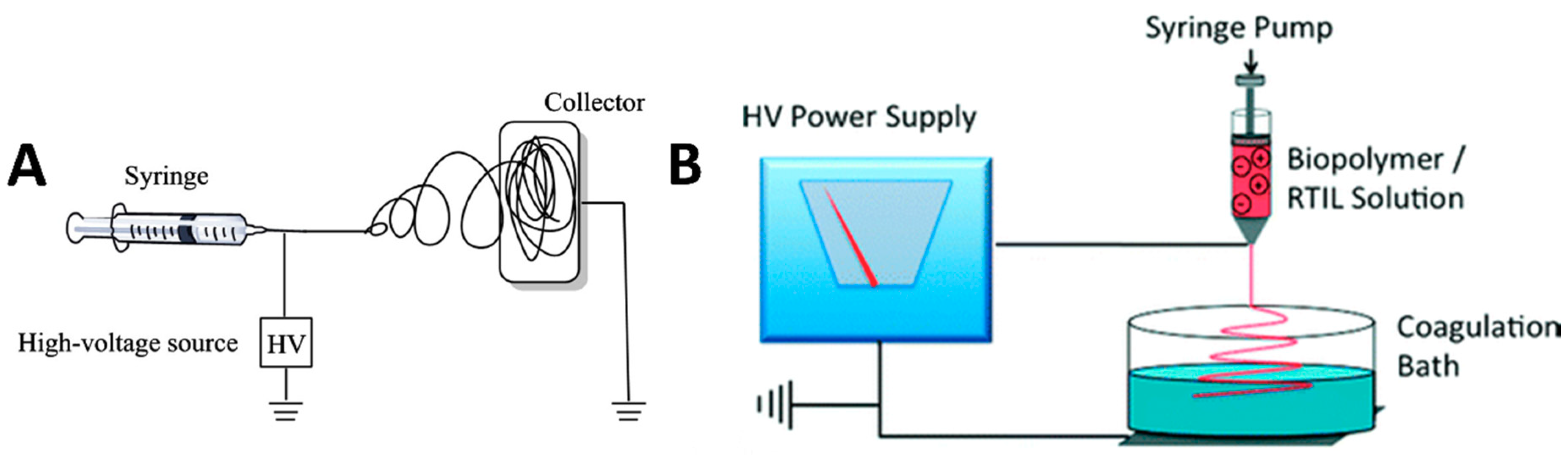
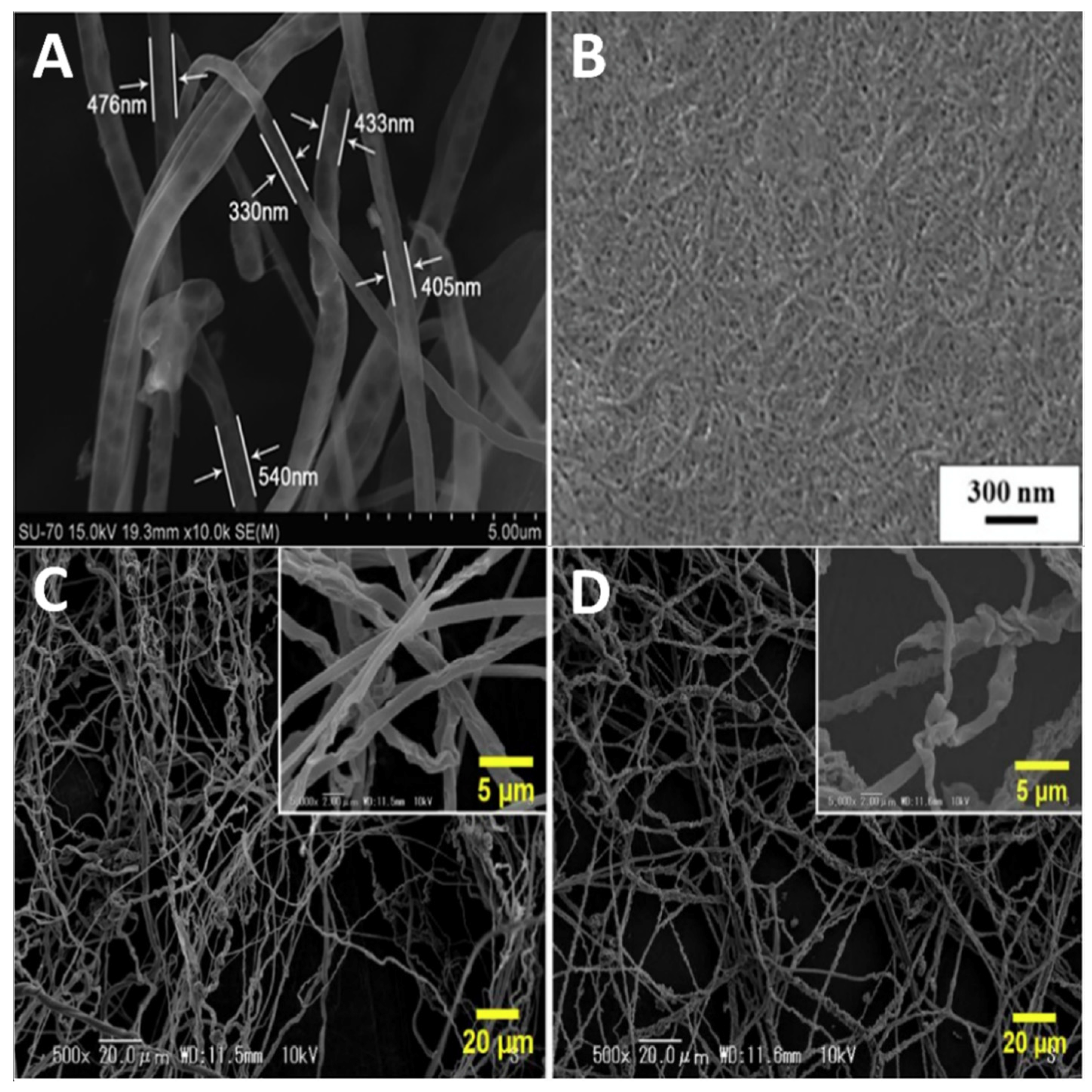
3.2. Fiber Spinning Methods
3.2.1. Electrospinning
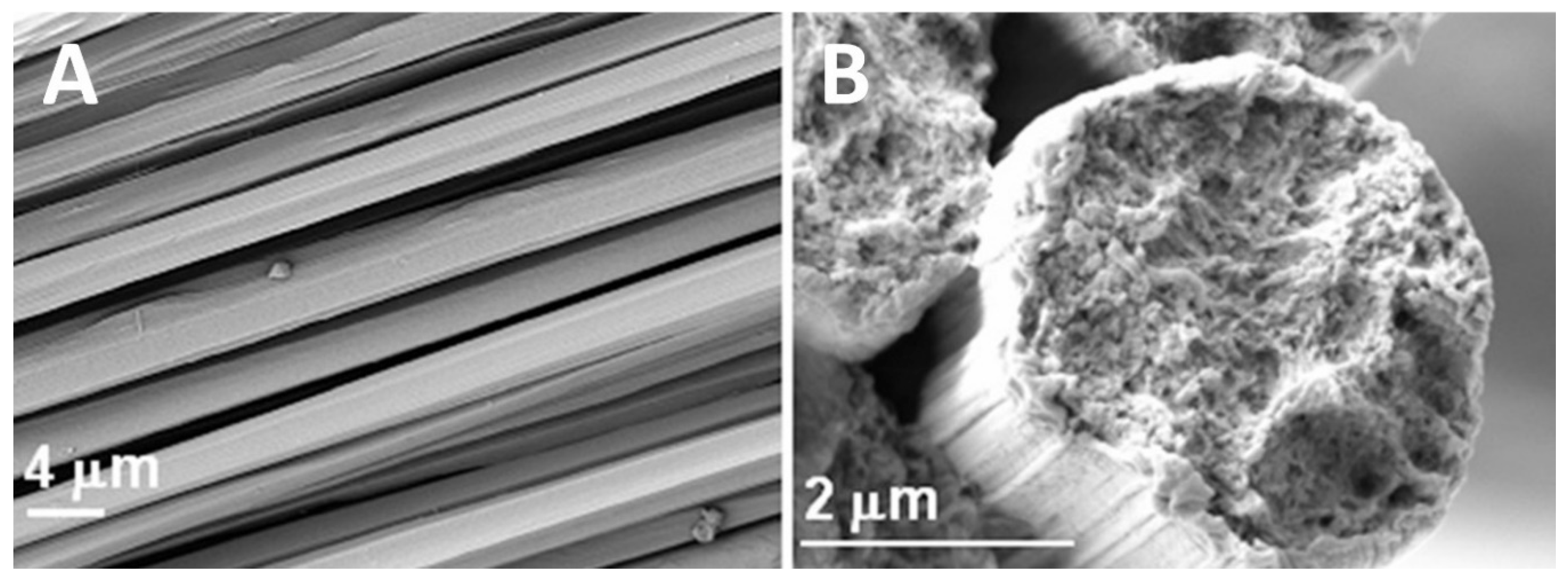
3.2.2. Wet Spinning/Dry-Jet Wet Spinning
3.2.3. Phase Separation
4. Impact of Coagulation and Ions on Material Structure
4.1. Chemical Coagulation
4.1.1. Water
4.1.2. Methanol and Other Organic Solvents
4.1.3. H2O2 Coagulation
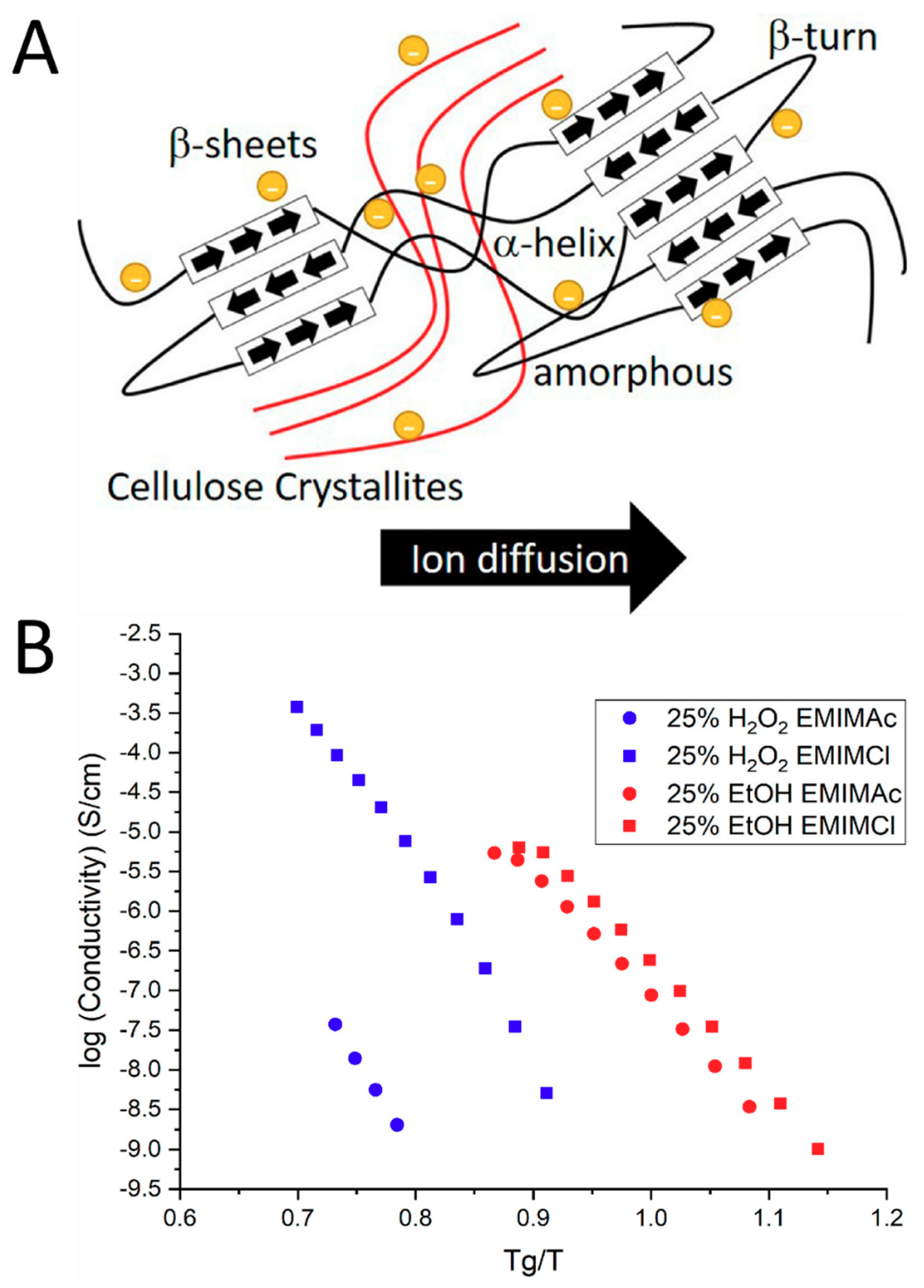
4.2. Effect of Ionic Liquid Ions on Fiber Structure and Ion Conductivity
5. Novel Applications of Biopolymer-Based Fibers from Ionic Liquids
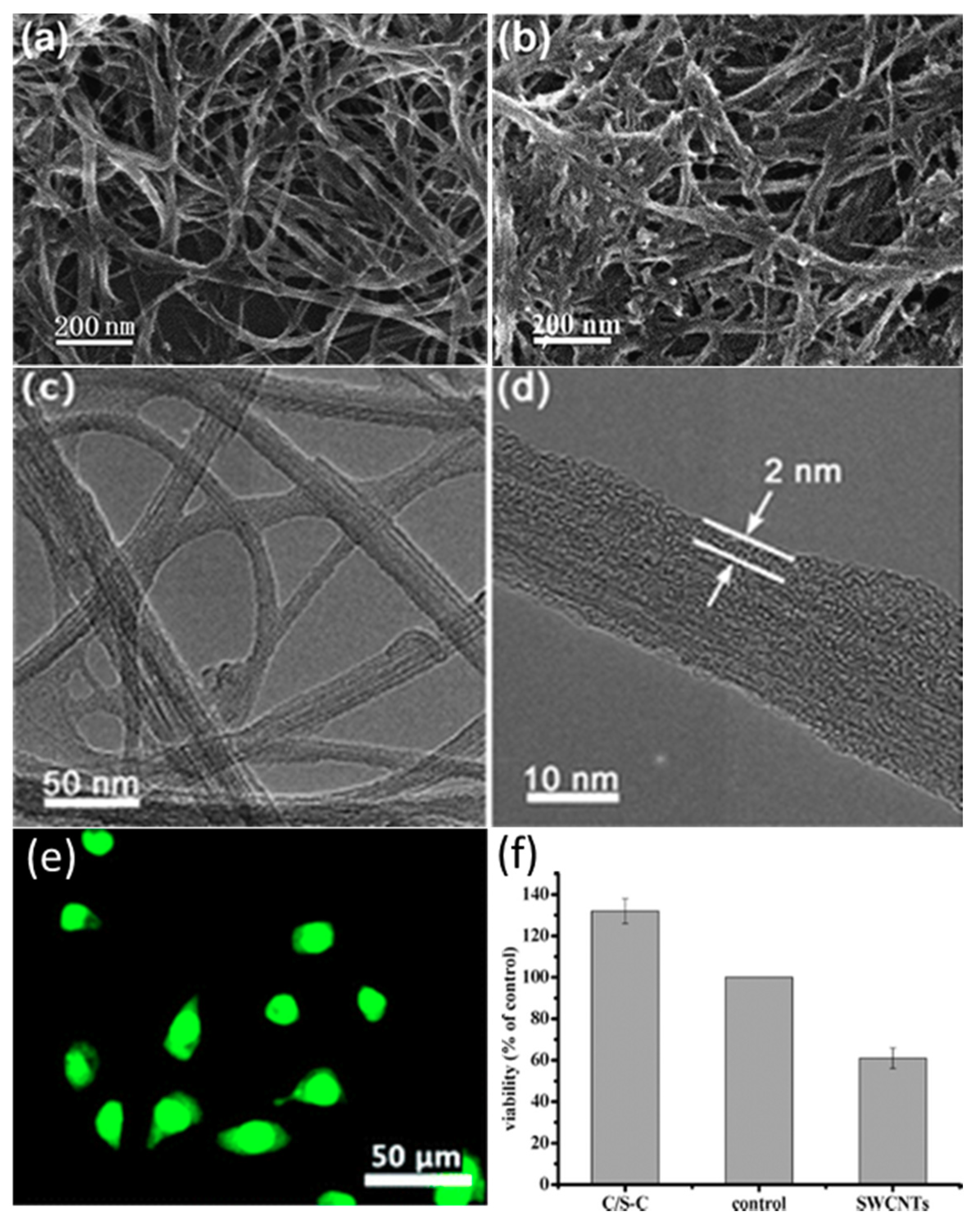
5.1. Tissue Regeneration
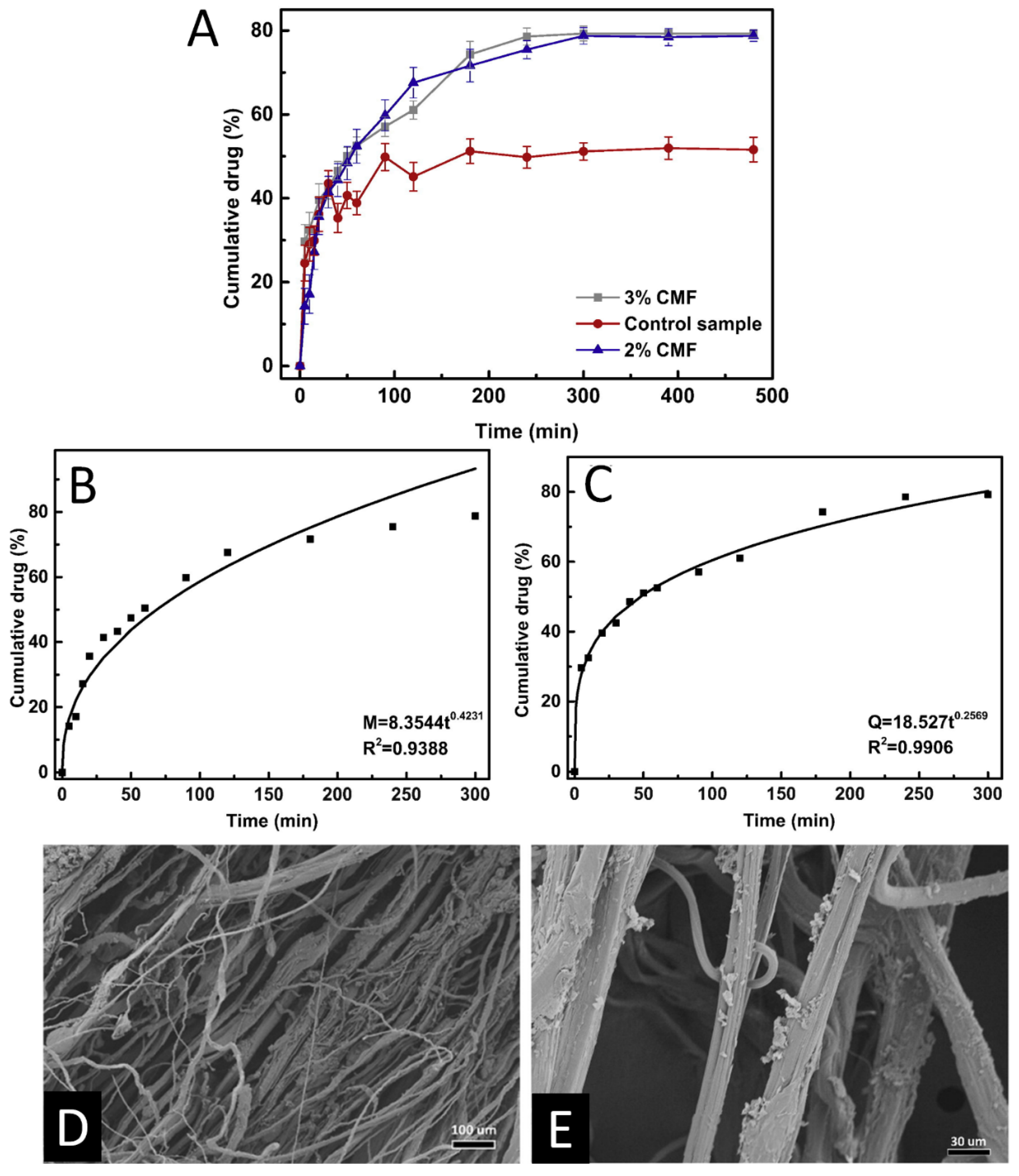
5.2. Drug Delivery
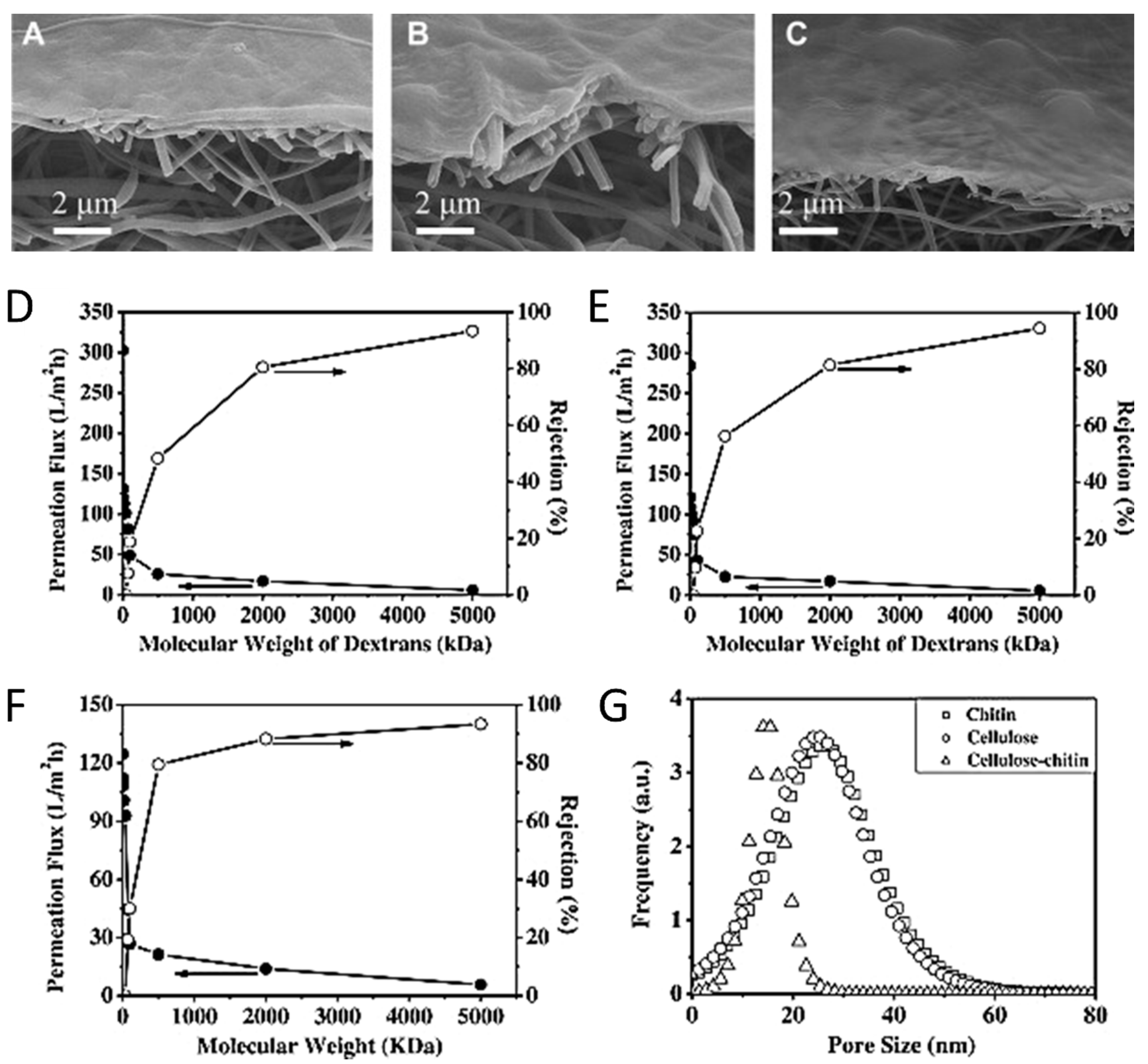
5.3. Water Purification
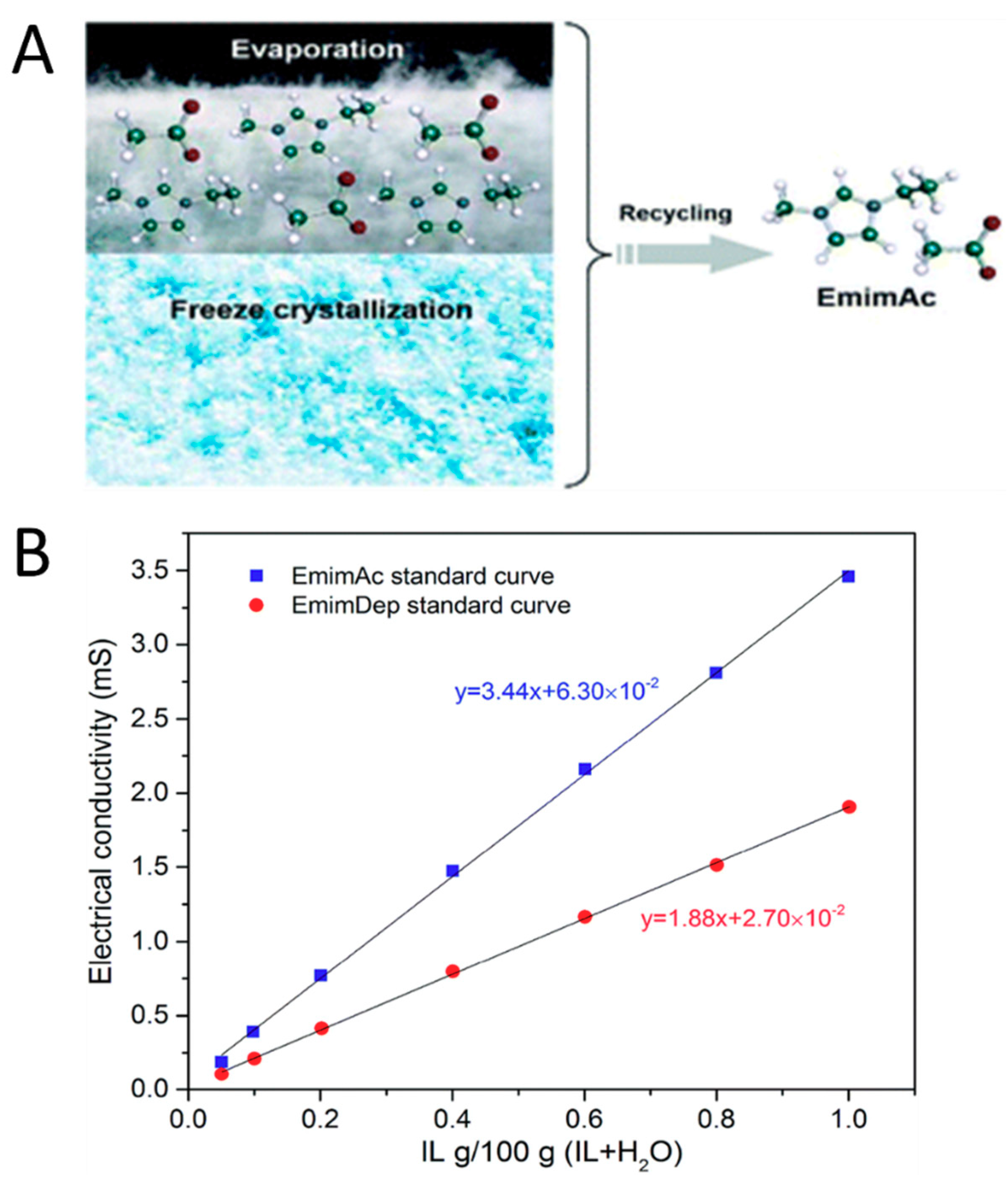
5.4. Recycling of Ionic Liquid
5.5. Electromechanical Actuators
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mohan, T.; Čas, A.; Bračič, M.; Plohl, O.; Vesel, A.; Rupnik, M.; Zemljič, L.F.; Rebol, J. Highly Protein Repellent and Antiadhesive Polysaccharide Biomaterial Coating for Urinary Catheter Applications. ACS Biomater. Sci. Eng. 2019, 5, 5825–5832. [Google Scholar] [CrossRef]
- David, K. Advances and challenges with fibrous protein biomaterial designs. Front. Bioeng. Biotechnol. 2016, 4. [Google Scholar] [CrossRef]
- Kalia, S.; Sabaa, M.W. (Eds.) Polysaccharide Based Graft Copolymers, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Cortajarena, A.L.; Grove, T.Z. (Eds.) Protein-Based Engineered Nanostructures, 1st ed.; Springer International Publishing: Cham, Swizerland, 2016. [Google Scholar]
- Toita, S.; Morimoto, N.; Akiyoshi, K. Functional cycloamylose as a polysaccharide-based biomaterial: Application in a gene delivery system. Biomacromolecules 2010, 11, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kotsuchibashi, Y.; Liu, Y.; Narain, R. Temperature-responsive hyperbranched amine-based polymers for solid-liquid separation. Langmuir ACS J. Surf. Colloids 2014, 30, 2360–2368. [Google Scholar] [CrossRef] [PubMed]
- Villmow, T.; Pegel, S.; Pötschke, P.; Heinrich, G. Polymer/carbon nanotube composites for liquid sensing: Model for electrical response characteristics. Polymer 2011, 52, 2276–2285. [Google Scholar] [CrossRef]
- Villmow, T.; John, A.; Pötschke, P.; Heinrich, G. Polymer/carbon nanotube composites for liquid sensing: Selectivity against different solvents. Polymer 2012, 53, 2908–2918. [Google Scholar] [CrossRef]
- Leipzig, N.D.; Wylie, R.G.; Kim, H.; Shoichet, M.S. Differentiation of neural stem cells in three-dimensional growth factor-immobilized chitosan hydrogel scaffolds. Biomaterials 2011, 32, 57–64. [Google Scholar] [CrossRef]
- Hayden, R.S.; Quinn, K.P.; Alonzo, C.A.; Georgakoudi, I.; Kaplan, D.L. Quantitative characterization of mineralized silk film remodeling during long-term osteoblast–osteoclast co-culture. Biomaterials 2014, 35, 3794–3802. [Google Scholar] [CrossRef]
- Hubbell, J.A.; Chilkoti, A. Chemistry. Nanomaterials for drug delivery. Science 2012, 337, 303–305. [Google Scholar] [CrossRef]
- Mottaghitalab, F.; Farokhi, M.; Shokrgozar, M.A.; Atyabi, F.; Hosseinkhani, H. Silk fibroin nanoparticle as a novel drug delivery system. J. Control. Release 2015, 206, 161–176. [Google Scholar] [CrossRef]
- Yewale, C.; Baradia, D.; Vhora, I.; Misra, A. Proteins: Emerging carrier for delivery of cancer therapeutics. Expert Opin. Drug Deliv. 2013, 10, 1429–1448. [Google Scholar] [CrossRef] [PubMed]
- Pattamaprom, C.; Hongrojjanawiwat, W.; Koombhongse, P.; Supaphol, P.; Jarusuwannapoo, T.; Rangkupan, R. The Influence of Solvent Properties and Functionality on the Electrospinnability of Polystyrene Nanofibers. Macromol. Mater. Eng. 2006, 291, 840–847. [Google Scholar] [CrossRef]
- Wu, X.; Wang, L.; Yu, H.; Huang, Y. Effect of solvent on morphology of electrospinning ethyl cellulose fibers. J. Appl. Polym. Sci. 2005, 97, 1292–1297. [Google Scholar] [CrossRef]
- Lu, C.; Chen, P.; Li, J.; Zhang, Y. Computer simulation of electrospinning. Part I. Effect of solvent in electrospinning. Polymer 2006, 47, 915–921. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, F.; Gu, Z.; Ma, Q.; Hu, X. Exploring the Structural Transformation Mechanism of Chinese and Thailand Silk Fibroin Fibers and Formic-Acid Fabricated Silk Films. Int. J. Mol. Sci. 2018, 19, 3309. [Google Scholar] [CrossRef] [PubMed]
- Fox, D.; Fylstra, P.; Hanley, M.; Henderson, W.A.; Trulove, P.C.; Bellayer, S.; Gilman, J.; De Long, H.C. The Preparation and Characterization of Bombyx Mori Silk Nanocomposites Using Ionic Liquids; ECS Transactions; ECS: Fairfax, VA, USA, 2007. [Google Scholar]
- Park, T.-J.; Jung, Y.J.; Choi, S.-W.; Park, H.; Kim, H.; Kim, E.; Lee, S.H.; Kim, J.H. Native chitosan/cellulose composite fibers from an ionic liquid via electrospinning. Macromol. Res. 2011, 19, 213–215. [Google Scholar] [CrossRef]
- Viswanathan, G.; Murugesan, S.; Pushparaj, V.; Nalamasu, O.; Ajayan, P.M.; Linhardt, R.J. Preparation of Biopolymer Fibers by Electrospinning from Room Temperature Ionic Liquids. Biomacromolecules 2006, 7, 415–418. [Google Scholar] [CrossRef]
- Meli, L.; Miao, J.; Dordick, J.S.; Linhardt, R.J. Electrospinning from room temperature ionic liquids for biopolymer fiber formation. Green Chem. 2010, 12, 1883–1892. [Google Scholar] [CrossRef]
- Lee, M.; Choi, U.H.; Colby, R.H.; Gibson, H.W. Ion Conduction in Imidazolium Acrylate Ionic Liquids and their Polymers. Chem. Mater. 2010, 22, 5814–5822. [Google Scholar] [CrossRef]
- Stanton, J.; Xue, Y.; Pandher, P.; Malek, L.; Brown, T.; Hu, X.; Salas-de la Cruz, D. Impact of ionic liquid type on the structure, morphology and properties of silk-cellulose biocomposite materials. Int. J. Biol. Macromol. 2018, 108, 333–341. [Google Scholar] [CrossRef]
- Mahmood, H.; Moniruzzaman, M.; Yusup, S.; Welton, T. Ionic liquids assisted processing of renewable resources for the fabrication of biodegradable composite materials. Green Chem. 2017, 19, 2051–2075. [Google Scholar] [CrossRef]
- Blessing, B.; Trout, C.; Morales, A.; Rybacki, K.; Love, S.A.; Lamoureux, G.; O’Malley, S.M.; Hu, X.; Salas-de la Cruz, D. Morphology and ionic conductivity relationship in silk/cellulose biocomposites. Polym. Int. 2019, 68, 1580–1590. [Google Scholar] [CrossRef]
- Blessing, B.; Trout, C.; Morales, A.; Rybacki, K.; Love, S.A.; Lamoureux, G.; O’Malley, S.M.; Hu, X.; Salas-de la Cruz, D. The Impact of Composition and Morphology on Ionic Conductivity of Silk/Cellulose Bio-Composites Fabricated from Ionic Liquid and Varying Percentages of Coagulation Agents. Int. J. Mol. Sci. 2020, 21, 4695. [Google Scholar] [CrossRef] [PubMed]
- Luzio, A.; Canesi, E.; Bertarelli, C.; Caironi, M. Electrospun Polymer Fibers for Electronic Applications. Materials 2014, 7, 906–947. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.-L.; Xu, Q.; Lu, Z.; Wang, J.-Y. Cell adhesion on zein films under shear stress field. Colloids Surf. B Biointerfaces 2013, 111, 479–485. [Google Scholar] [CrossRef]
- Dhyani, V.; Singh, N. Controlling the cell adhesion property of silk films by graft polymerization. ACS Appl. Mater. Interfaces 2014, 6, 5005–5011. [Google Scholar] [CrossRef]
- Sutherland, T.D.; Young, J.H.; Weisman, S.; Hayashi, C.Y.; Merritt, D.J. Insect silk: One name, many materials. Annu. Rev. Entomol. 2010, 55, 171–188. [Google Scholar] [CrossRef]
- Extracellular fibrous proteins: The silks. Compr. Biochem. 1968, 26, 475–558.
- Florkin, M. Comparative Biochemistry V4: A Comprehensive Treatise; Elsevier Science: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Rudall, K.M.; Kenchington, W. Arthropod Silks: The Problem of Fibrous Proteins in Animal Tissues. Annu. Rev. Entomol. 1971, 16, 73–96. [Google Scholar] [CrossRef]
- Slotta, U.; Tammer, M.; Kremer, F.; Koelsch, P.; Scheibel, T. Structural Analysis of Spider Silk Films. Supramol. Chem. Spec. Issue Supramol. Biochem. Assembl. 2006, 18, 465–471. [Google Scholar] [CrossRef]
- Hu, X.; Raja, W.K.; An, B.; Tokareva, O.; Cebe, P.; Kaplan, D.L. Stability of Silk and Collagen Protein Materials in Space. Sci. Rep. 2013, 3, 3428. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wu, S.; Jian, M.; Xie, J.; Xu, L.; Yang, X.; Zheng, Q.; Zhang, Y. Silk nanofibers as high efficient and lightweight air filter. Nano Res. 2016, 9, 2590–2597. [Google Scholar] [CrossRef]
- Cohen-Karni, T.; Jeong, K.J.; Tsui, J.H.; Reznor, G.; Mustata, M.; Wanunu, M.; Graham, A.; Marks, C.; Bell, D.C.; Langer, R.; et al. Nanocomposite gold-silk nanofibers. Nano Lett. 2012, 12, 5403–5406. [Google Scholar] [CrossRef] [PubMed]
- Dinis, T.M.; Vidal, G.; Jose, R.R.; Vigneron, P.; Bresson, D.; Fitzpatrick, V.; Marin, F.; Kaplan, D.L.; Egles, C. Complementary effects of two growth factors in multifunctionalized silk nanofibers for nerve reconstruction. PLoS ONE 2014, 9, e109770. [Google Scholar] [CrossRef] [PubMed]
- Wenk, E.; Merkle, H.P.; Meinel, L. Silk fibroin as a vehicle for drug delivery applications. J. Control. Release 2011, 150, 128–141. [Google Scholar] [CrossRef]
- Reeves, A.R.D.; Spiller, K.L.; Freytes, D.O.; Vunjak-Novakovic, G.; Kaplan, D.L. Controlled release of cytokines using silk-biomaterials for macrophage polarization. Biomaterials 2015, 73, 272–283. [Google Scholar] [CrossRef]
- Asakura, T.; Miller, T. Biotechnology of Silk; Springer: Dordrecht, The Netherlands, 2013. [Google Scholar]
- Chun, H.J.; Park, K.; Kim, C.H.; Khang, G. Novel Biomaterials for Regenerative Medicine; Springer: Singapore, 2018. [Google Scholar]
- Yin, Z.; Jian, M.; Wang, C.; Xia, K.; Liu, Z.; Wang, Q.; Zhang, M.; Wang, H.; Liang, X.; Liang, X.; et al. Splash-Resistant and Light-Weight Silk-Sheathed Wires for Textile Electronics. Nano Lett. 2018, 18, 7085. [Google Scholar] [CrossRef]
- Kim, D.-H.; Kim, Y.-S.; Amsden, J.; Panilaitis, B.; Kaplan, D.L.; Omenetto, F.G.; Zakin, M.R.; Rogers, J.A. Erratum: “Silicon electronics on silk as a path to bioresorbable, implantable devices”. Appl. Phys. Lett. 2009, 95, 133701. [Google Scholar] [CrossRef]
- Dae-Hyeong, K.; Jonathan, V.; Jason, J.A.; Jianliang, X.; Leif, V.; Yun-Soung, K.; Justin, A.B.; Bruce, P.; Eric, S.F.; Diego, C.; et al. Dissolvable films of silk fibroin for ultrathin conformal bio-integrated electronics. Nat. Mater. 2010, 9, 511. [Google Scholar]
- Phillips, D.M.; Drummy, L.F.; Naik, R.R.; Long, H.C.D.; Fox, D.M.; Trulove, P.C.; Mantz, R.A. Regenerated silk fiber wet spinning from an ionic liquid solution. J. Mater. Chem. 2005, 15, 4206–4208. [Google Scholar] [CrossRef]
- Shen, X. Regeneration Spider Silk Fiber Based on Ionic Liquid and Preparation Method of Regeneration Spider Silk Fiber. China Patent CN104818539A, 5 August 2015. [Google Scholar]
- Yao, Y.; Mukuze, K.S.; Zhang, Y.; Wang, H. Rheological behavior of cellulose/silk fibroin blend solutions with ionic liquid as solvent. Cellulose 2014, 21, 675–684. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, Q.; Wen, J.; Chen, X.; Shao, Z. Preparation and characterization of transparent silk fibroin/cellulose blend films. Polymer 2013, 54, 5035–5042. [Google Scholar] [CrossRef]
- Aluigi, A.; Corbellini, A.; Rombaldoni, F.; Mazzuchetti, G. Wool-derived keratin nanofiber membranes for dynamic adsorption of heavy-metal ions from aqueous solutions. Text. Res. J. 2013, 83, 1574–1586. [Google Scholar] [CrossRef]
- Lee, C.-H.; Yun, Y.J.; Cho, H.; Lee, K.S.; Park, M.; Kim, H.Y.; Son, D.I. Environment-friendly, durable, electro-conductive, and highly transparent heaters based on silver nanowire functionalized keratin nanofiber textiles. J. Mater. Chem. C 2018, 6, 7847–7854. [Google Scholar] [CrossRef]
- Atri, H.; Bidram, E.; Dunstan, D.E. Reconstituted Keratin Biomaterial with Enhanced Ductility. Materials 2015, 8, 7472–7485. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Gong, J.; Fan, X.; Wang, P.; Wang, Q.; Qiu, Y. Transglutaminase-modified wool keratin film and its potential application in tissue engineering. Eng. Life Sci. 2013, 13, 149–155. [Google Scholar] [CrossRef]
- Feng, Y.; Borrelli, M.; Meyer-Ter-Vehn, T.; Reichl, S.; Schrader, S.; Geerling, G. Epithelial Wound Healing on Keratin Film, Amniotic Membrane and Polystyrene In Vitro. Curr. Eye Res. 2014, 39, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Reichl, S.; Müller-Goymann, C.C. Keratin film made of human hair as a nail plate model for studying drug permeation. Eur. J. Pharm. Biopharm. 2011, 78, 432–440. [Google Scholar]
- Ramirez, D.O.S.; Carletto, R.A.; Tonetti, C.; Giachet, F.T.; Varesano, A.; Vineis, C. Wool keratin film plasticized by citric acid for food packaging. Food Packag. Shelf Life 2017, 12, 100–106. [Google Scholar] [CrossRef]
- Murrell, D.F.; Trisnowati, N.; Miyakis, S.; Paller, A.S. The Yin and the Yang of Keratin Amino Acid Substitutions and Epidermolysis Bullosa Simplex. J. Investig. Dermatol. 2011, 131, 1787–1790. [Google Scholar] [CrossRef]
- Chen, J.; Vongsanga, K.; Wang, X.; Byrne, N. What Happens during Natural Protein Fibre Dissolution in Ionic Liquids. Materials 2014, 7, 6158–6168. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, D. Preparation of regenerated wool keratin films from wool keratin–ionic liquid solutions. J. Appl. Polym. Sci. 2013, 127, 2648–2653. [Google Scholar] [CrossRef]
- Wool, R.; Sun, X.S. Bio-Based Polymers and Composites; Elsevier Science: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Liu, X.; Souzandeh, H.; Zheng, Y.; Xie, Y.; Zhong, W.-H.; Wang, C. Soy protein isolate/bacterial cellulose composite membranes for high efficiency particulate air filtration. Compos. Sci. Technol. 2017, 138, 124–133. [Google Scholar] [CrossRef]
- Kang, H.-J.; Kim, S.-J.; You, Y.-S.; Lacroix, M.; Han, J. Inhibitory effect of soy protein coating formulations on walnut (Juglans regia L.) kernels against lipid oxidation. LWT—Food Sci. Technol. 2013, 51, 393–396. [Google Scholar] [CrossRef]
- Peles, Z.; Binderman, I.; Berdicevsky, I.; Zilberman, M. Soy protein films for wound-healing applications: Antibiotic release, bacterial inhibition and cellular response. J. Tissue Eng. Regen. Med. 2013, 7, 401–412. [Google Scholar] [CrossRef]
- Chuysinuan, P.; Pengsuk, C.; Lirdprapamongkol, K.; Techasakul, S.; Svasti, J.; Nooeaid, P. Enhanced Structural Stability and Controlled Drug Release of Hydrophilic Antibiotic-Loaded Alginate/Soy Protein Isolate Core-Sheath Fibers for Tissue Engineering Applications. Fibers Polym. 2019, 20, 1–10. [Google Scholar] [CrossRef]
- Noshad, M.; Mohebbi, M.; Koocheki, A.; Shahidi, F. Microencapsulation of vanillin by spray drying using soy protein isolate–maltodextrin as wall material. Flavour Fragr. J. 2015, 30, 387–391. [Google Scholar] [CrossRef]
- Wu, R.L.; Wang, X.L.; Wang, Y.Z.; Bian, X.C.; Li, F. Cellulose/soy protein isolate blend films prepared via room-temperature ionic liquid. Ind. Eng. Chem. Res. 2009, 48, 7132–7136. [Google Scholar] [CrossRef]
- Li, C.; He, M.; Tong, Z.; Li, Y.; Sheng, W.; Luo, L.; Tong, Y.; Yu, H.; Huselstein, C.; Chen, Y. Construction of biocompatible regenerated cellulose/SPI composite beads using high-voltage electrostatic technique. RSC Adv. 2016, 6, 52528–52538. [Google Scholar] [CrossRef]
- Brennan, A.B.; Kirschner, C.M.; Kirschner, C.M. Bio-Inspired Materials for Biomedical Engineering; John Wiley & Sons: Hoboken, NJ, USA; Incorporated: Somerset, NJ, USA, 2014. [Google Scholar]
- Meng, Z.; Zheng, X.; Tang, K.; Liu, J.; Ma, Z.; Zhao, Q. Dissolution and regeneration of collagen fibers using ionic liquid. Int. J. Biol. Macromol. 2012, 51, 440–448. [Google Scholar] [CrossRef]
- Pei, Y.; Chu, S.; Zheng, Y.; Zhang, J.; Liu, H.; Zheng, X.; Tang, K. Dissolution of Collagen Fibers from Tannery Solid Wastes in 1-Allyl-3-methylimidazolium Chloride and Modulation of Regenerative Morphology. ACS Sustain. Chem. Eng. 2019, 7, 2530–2537. [Google Scholar] [CrossRef]
- Tarannum, A.; Muvva, C.; Mehta, A.; Rao, J.R.; Fathima, N.N. Phosphonium based ionic liquids-stabilizing or destabilizing agents for collagen? RSC Adv. 2016, 6, 4022–4033. [Google Scholar] [CrossRef]
- Wise, S.G.; Weiss, A.S. Tropoelastin. Int. J. Biochem. Cell Biol. 2009, 41, 494–497. [Google Scholar] [CrossRef]
- Csiszar, K. Lysyl oxidases: A novel multifunctional amine oxidase family. In Progress in Nucleic Acid Research and Molecular Biology; Academic Press: Cambridge, MA, USA, 2001; Volume 70, pp. 1–32. [Google Scholar]
- Waterhouse, A.; Wise, S.G.; Ng, M.K.C.; Weiss, A.S. Elastin as a Nonthrombogenic Biomaterial. Tissue Eng. Part B Rev. 2011, 17, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Daamen, W.F.; Veerkamp, J.H.; van Hest, J.C.M.; van Kuppevelt, T.H. Elastin as a biomaterial for tissue engineering. Biomaterials 2007, 28, 4378–4398. [Google Scholar] [CrossRef] [PubMed]
- Wright, E.R.; McMillan, R.A.; Cooper, A.; Apkarian, R.P.; Conticello, V.P. Thermoplastic Elastomer Hydrogels via Self-Assembly of an Elastin-Mimetic Triblock Polypeptide. Adv. Funct. Mater. 2002, 12, 149–154. [Google Scholar] [CrossRef]
- Mithieux, S.M.; Rasko, J.E.; Weiss, A.S.; Weiss, A.S. Synthetic elastin hydrogels derived from massive elastic assemblies of self-organized human protein monomers. Biomaterials 2004, 25, 4921–4927. [Google Scholar] [CrossRef]
- Herrero-Vanrell, R.; Rincon, A.C.; Alonso, M.; Reboto, V.; Molina-Martinez, I.T.; Rodriguez-Cabello, J.C. Self-assembled particles of an elastin-like polymer as vehicles for controlled drug release. J. Control. Release 2005, 102, 113–122. [Google Scholar] [CrossRef]
- Bellingham, C.M.; Lillie, M.A.; Gosline, J.M.; Wright, G.M.; Starcher, B.C.; Bailey, A.J.; Woodhouse, K.A.; Keeley, F.W. Recombinant human elastin polypeptides self-assemble into biomaterials with elastin-like properties. Biopolymers 2003, 70, 445–455. [Google Scholar] [CrossRef]
- Reguera, J.; Fahmi, A.; Moriarty, P.; Girotti, A.; Rodríguez-Cabello, J.C. Nanopore Formation by Self-Assembly of the Model Genetically Engineered Elastin-like Polymer [(VPGVG)2(VPGEG)(VPGVG)2]15. J. Am. Chem. Soc. 2004, 126, 13212–13213. [Google Scholar] [CrossRef]
- Hecht Stacie, E.; Niehoff Raymond, L.; Narasimhan, K.; Neal Charles, W.; Forshey Paul, A.; Phan Dean, V.; Brooker Anju Deepali, M.; Combs Katherine, H. Extracting Biopolymers from a Biomass Using Ionic Liquids. U.S. Patent 7,763,715, 27 July 2010. [Google Scholar]
- Zhang, Y.; Li, W.-Y.; Lan, R.; Wang, J.-Y. Quality Monitoring of Porous Zein Scaffolds: A Novel Biomaterial. Engineering 2017, 3, 130–135. [Google Scholar] [CrossRef]
- Zein: A potential biomaterial for tissue engineering: Tissue engineering. Mater. Today 2004, 7, 24. [CrossRef]
- Lee, S.; Alwahab, N.S.A.; Moazzam, Z.M. Zein-based oral drug delivery system targeting activated macrophages. Int. J. Pharm. 2013, 454, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.; Huang, Y.; Yu, H.; Lee, T.-C.; Huang, Q. Reducing the brittleness of zein films through chemical modification. J. Agric. Food Chem. 2011, 59, 56. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.; Shogren, R.L.; Stevenson, D.G.; Willett, J.L.; Bhowmik, P.K. Ionic liquids as solvents for biopolymers: Acylation of starch and zein protein. Carbohydr. Polym. 2006, 66, 546–550. [Google Scholar] [CrossRef]
- Choi, H.-M.; Kwon, I. Dissolution of Zein Using Protic Ionic Liquids: N-(2-Hydroxyethyl) Ammonium Formate and N-(2-Hydroxyethyl) Ammonium Acetate. Ind. Eng. Chem. Res. 2011, 50, 2452–2454. [Google Scholar] [CrossRef]
- Desai, H.E. Synthesis and Structural Characterization of Reflectin Proteins; North Georgia Coll and State Univ Dahlonega: Dahlonega, GA, USA, 2012. [Google Scholar]
- Tao, A.R.; Demartini, D.G.; Izumi, M.; Sweeney, A.M.; Holt, A.L.; Morse, D.E. The role of protein assembly in dynamically tunable bio-optical tissues. Biomaterials 2010, 31, 793–801. [Google Scholar] [CrossRef]
- Arulmoli, J. Scaffolds for Neural Stem Cell Tissue Engineering; UC Irvine: Irvine, CA, USA, 2016. [Google Scholar]
- Kramer, R.M.; Crookes-Goodson, W.J.; Naik, R.R. The self-organizing properties of squid reflectin protein. Nat. Mater. 2007, 6, 533–538. [Google Scholar] [CrossRef]
- Levenson, R.; Demartini, D.G.; Morse, D.E. Molecular mechanism of reflectin’s tunable biophotonic control: Opportunities and limitations for new optoelectronics. APL Mater. 2017, 5, 104801. [Google Scholar] [CrossRef]
- Levenson, R.; Bracken, C.; Sharma, C.; Santos, J.; Arata, C.; Malady, B.; Morse, D.E. Calibration between trigger and color: Neutralization of a genetically encoded coulombic switch and dynamic arrest precisely tune reflectin assembly. J. Biol. Chem. 2019, 294, 16804–16815. [Google Scholar] [CrossRef]
- Thomas, M.S.; Koshy, R.R.; Mary, S.K.; Thomas, S.; Pothan, L.A. Starch, Chitin and Chitosan Based Composites and Nanocomposites; Springer International Publishing: Cham, Swizerland, 2018. [Google Scholar]
- Liu, H.; Xie, F.; Yu, L.; Chen, L.; Li, L. Thermal processing of starch-based polymers. Prog. Polym. Sci. 2009, 34, 1348–1368. [Google Scholar] [CrossRef]
- Kadokawa, J.-I.; Murakami, M.-A.; Takegawa, A.; Kaneko, Y. Preparation of cellulose–starch composite gel and fibrous material from a mixture of the polysaccharides in ionic liquid. Carbohydr. Polym. 2009, 75, 180–183. [Google Scholar] [CrossRef]
- Carreño, N.L.V. Advances in Nanostructured Cellulose-based Biomaterials, 1st ed.; Springer International Publishing: Cham, Swizerland, 2017. [Google Scholar]
- Freire, M.G.; Teles, A.R.R.; Ferreira, R.A.S.; Carlos, L.D.; Lopes-da-Silva, J.A.; Coutinho, J.A.P. Electrospun nanosized cellulose fibers using ionic liquids at room temperature. Green Chem. 2011, 13, 3173–3180. [Google Scholar] [CrossRef]
- Xu, H.; Bronner, T.; Yamamoto, M.; Yamane, H. Regeneration of cellulose dissolved in ionic liquid using laser-heated melt-electrospinning. Carbohydr. Polym. 2018, 201, 182–188. [Google Scholar] [CrossRef]
- Hermanutz, F.; Vocht, M.P.; Panzier, N.; Buchmeiser, M.R. Processing of Cellulose Using Ionic Liquids. Macromol. Mater. Eng. 2019, 304, 1800450. [Google Scholar] [CrossRef]
- Liu, Y.; Meyer, A.S.; Nie, Y.; Zhang, S.; Thomsen, K. Low energy recycling of ionic liquids via freeze crystallization during cellulose spinning. Green Chem. 2018, 20, 493–501. [Google Scholar] [CrossRef]
- Defrates, K.; Markiewicz, T.; Callaway, K.; Xue, Y.; Stanton, J.; Salas-de La Cruz, D.; Hu, X. Structure–property relationships of Thai silk–microcrystalline cellulose biocomposite materials fabricated from ionic liquid. Int. J. Biol. Macromol. 2017, 104 Pt A, 919–928. [Google Scholar] [CrossRef]
- Kaya, M.; Mujtaba, M.; Ehrlich, H.; Salaberria, A.M.; Baran, T.; Amemiya, C.T.; Galli, R.; Akyuz, L.; Sargin, I.; Labidi, J. On chemistry of γ-chitin. Carbohydr. Polym. 2017, 176, 177–186. [Google Scholar] [CrossRef]
- Levengood, S.K.L.; Zhang, M. Chitosan-based scaffolds for bone tissue engineering. J. Mater. Chem. B 2014, 2, 3161–3184. [Google Scholar] [CrossRef]
- Barber, P.S.; Kelley, S.P.; Griggs, C.S.; Wallace, S.; Rogers, R.D. Surface modification of ionic liquid-spun chitin fibers for the extraction of uranium from seawater: Seeking the strength of chitin and the chemical functionality of chitosan. Green Chem. 2014, 16, 1828–1836. [Google Scholar] [CrossRef]
- Jaworska, M.M.; Stępniak, I.; Galiński, M.; Kasprzak, D.; Biniaś, D.; Górak, A. Modification of chitin structure with tailored ionic liquids. Carbohydr. Polym. 2018, 202, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Shigemasa, Y.; Matsuura, H.; Sashiwa, H.; Saimoto, H. Evaluation of different absorbance ratios from infrared spectroscopy for analyzing the degree of deacetylation in chitin. Int. J. Biol. Macromol. 1996, 18, 237–242. [Google Scholar] [CrossRef]
- Rehm, B.H.A.; Moradali, M.F. (Eds.) Alginates and Their Biomedical Applications, 1st ed.; Springer: Singapore, 2018. [Google Scholar]
- Moradali, M.F.; Donati, I.; Sims, I.M.; Ghods, S.; Rehm, B.H.A. Alginate Polymerization and Modification are Linked in Pseudomonas aeruginosa. MBio 2015, 6, e00453-e15. [Google Scholar] [CrossRef] [PubMed]
- Douthit, S.A.; Dlakic, M.; Ohman, D.E.; Franklin, M.J. Epimerase Active Domain of Pseudomonas aeruginosa AlgG, a Protein That Contains a Right-Handed β-Helix. J. Bacteriol. 2005, 187, 4573. [Google Scholar] [CrossRef] [PubMed]
- Mørch, Ý.A.; Donati, I.; Strand, B.L. Effect of Ca2+, Ba2+, and Sr2+ on Alginate Microbeads. Biomacromolecules 2006, 7, 1471–1480. [Google Scholar] [CrossRef]
- Ouwerx, C.; Velings, N.; Mestdagh, M.M.; Axelos, M.A.V. Physico-chemical properties and rheology of alginate gel beads formed with various divalent cations. Polym. Gels Netw. 1998, 6, 393–408. [Google Scholar] [CrossRef]
- Braccini, I.; Pérez, S. Molecular Basis of Ca2+-Induced Gelation in Alginates and Pectins: The Egg-Box Model Revisited. Biomacromolecules 2001, 2, 1089–1096. [Google Scholar] [CrossRef]
- Sikorski, P.; Mo, F.; Skjåk-Bræk, G.; Stokke, B.T. Evidence for Egg-Box-Compatible Interactions in Calcium−Alginate Gels from Fiber X-ray Diffraction. Biomacromolecules 2007, 8, 2098–2103. [Google Scholar] [CrossRef]
- Skjåk-Bræk, G.; Paoletti, S.; Gianferrara, T. Selective acetylation of mannuronic acid residues in calcium alginate gels. Carbohydr. Res. 1989, 185, 119–129. [Google Scholar] [CrossRef]
- Straatmann, A.; Windhues, T.; Borchard, W. Effects of Acetylation on Thermodynamic Properties of Seaweed Alginate in Sodium Chloride Solutions. In Analytical Ultracentrifugation VII. Progress in Colloid and Polymer Science; Lechner, M.D., Börger, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 26–30. [Google Scholar]
- Delben, F.; Cesaro, À.; Paoletti, S.; Crescenzi, V. Monomer composition and acetyl content as main determinants of the ionization behavior of alginates. Carbohydr. Res. 1982, 100, C46–C50. [Google Scholar] [CrossRef]
- Cook, W. Alginate dental impression materials: Chemistry, structure, and properties. J. Biomed. Mater. Res. 1986, 20, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Craig, R.G. Review of Dental Impression Materials. Adv. Dent. Res. 1988, 2, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Groves, A.R.; Lawrence, J.C. Alginate dressing as a donor site haemostat. Ann. R. Coll. Surg. Engl. 1986, 68, 27–28. [Google Scholar] [PubMed]
- Barnett, S.E.; Varley, S.J. The effects of calcium alginate on wound healing. Ann. R. Coll. Surg. Engl. 1987, 69, 153–155. [Google Scholar] [PubMed]
- Hrynyk, M.; Martins-Green, M.; Barron, A.E.; Neufeld, R.J. Alginate-PEG sponge architecture and role in the design of insulin release dressings. Biomacromolecules 2012, 13, 1478–1485. [Google Scholar] [CrossRef] [PubMed]
- Barbetta, A.; Barigelli, E.; Dentini, M. Porous Alginate Hydrogels: Synthetic Methods for Tailoring the Porous Texture. Biomacromolecules 2009, 10, 2328–2337. [Google Scholar] [CrossRef]
- Andersen, T.; Melvik, J.E.; Gåserød, O.; Alsberg, E.; Christensen, B.E. Ionically Gelled Alginate Foams: Physical Properties Controlled by Operational and Macromolecular Parameters. Biomacromolecules 2012, 13, 3703–3710. [Google Scholar] [CrossRef]
- Shin, S.-J.; Park, J.-Y.; Lee, J.-Y.; Park, H.; Park, Y.-D.; Lee, K.-B.; Whang, C.-M.; Lee, S.-H. “On the Fly” Continuous Generation of Alginate Fibers Using a Microfluidic Device. Langmuir 2007, 23, 9104–9108. [Google Scholar] [CrossRef]
- Daemi, H.; Barikani, M.; Barmar, M. Highly stretchable nanoalginate based polyurethane elastomers. Carbohydr. Polym. 2013, 95, 630–636. [Google Scholar] [CrossRef]
- Senuma, Y.; Lowe, C.; Zweifel, Y.; Hilborn, J.G.; Marison, I. Alginate hydrogel microspheres and microcapsules prepared by spinning disk atomization. Biotechnol. Bioeng. 2000, 67, 616–622. [Google Scholar] [CrossRef]
- Bodmeier, R.; Chen, H.; Paeratakul, O. A Novel Approach to the Oral Delivery of Micro- or Nanoparticles. Pharm. Res. 1989, 6, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, B.; Sarfaraz, Z.; Muhammad, N.; Ahmad, P.; Iqbal, J.; Khan, Z.U.H.; Gonfa, G.; Iqbal, F.; Jamal, A.; Rahim, A. Ionic liquid as a potential solvent for preparation of collagen-alginate-hydroxyapatite beads as bone filler. J. Biomater. Sci. Polym. Ed. 2018, 29, 1168–1184. [Google Scholar] [CrossRef] [PubMed]
- Rufato, K.B.; Almeida, V.C.; Kipper, M.J.; Rubira, A.F.; Martins, A.F.; Muniz, E.C. Polysaccharide-based adsorbents prepared in ionic liquid with high performance for removing Pb(II) from aqueous systems. Carbohydr. Polym. 2019, 215, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.M.; Hohman, M.M.; Brenner, M.P.; Rutledge, G.C. Experimental characterization of electrospinning: The electrically forced jet and instabilities. Polymer 2001, 42, 09955–09967. [Google Scholar] [CrossRef]
- Lyons, J.; Li, C.; Ko, F. Melt-electrospinning part I: Processing parameters and geometric properties. Polymer 2004, 45, 7597–7603. [Google Scholar] [CrossRef]
- Chronakis, I.S. Novel nanocomposites and nanoceramics based on polymer nanofibers using electrospinning process—A review. J. Mater. Process. Technol. 2005, 167, 283–293. [Google Scholar] [CrossRef]
- Green, M.D.; Long, T.E. Designing Imidazole-Based Ionic Liquids and Ionic Liquid Monomers for Emerging Technologies. Polym.Rev. 2009, 49, 291–314. [Google Scholar] [CrossRef]
- Xu, J.-M.; Wu, W.-B.; Qian, C.; Liu, B.-K.; Lin, X.-F. A novel and highly efficient protocol for Markovnikov’s addition using ionic liquid as catalytic green solvent. Tetrahedron Lett. 2006, 47, 1555–1558. [Google Scholar] [CrossRef]
- Kong, C.S.; Yoo, W.S.; Jo, N.G.; Kim, H.S. Electrospinning Mechanism for Producing Nanoscale Polymer Fibers. J. Macromol. Sci. Part B 2010, 49, 122–131. [Google Scholar] [CrossRef]
- Kulpinski, P. Cellulose nanofibers prepared by the N-methylmorpholine-N-oxide method. J. Appl. Polym. Sci. 2005, 98, 1855–1859. [Google Scholar] [CrossRef]
- Rosenau, T.; Potthast, A.; Adorjan, I.; Hofinger, A.; Sixta, H.; Firgo, H.; Kosma, P. Cellulose solutions in N-methylmorpholine-N-oxide (NMMO)—Degradation processes and stabilizers. Cellulose 2002, 9, 283–291. [Google Scholar] [CrossRef]
- de Vasconcelos, C.L.; Bezerril, P.M.; Pereira, M.R.; Ginani, M.F.; Fonseca, J.L.C. Viscosity–temperature behavior of chitin solutions using lithium chloride/DMA as solvent. Carbohydr. Res. 2011, 346, 614–618. [Google Scholar] [CrossRef]
- Kurland, N.E.; Dey, T.; Wang, C.; Kundu, S.C.; Yadavalli, V.K. Silk Protein Lithography as a Route to Fabricate Sericin Microarchitectures. Adv. Mater. 2014, 26, 4431–4437. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.K.; Kurland, N.E.; Wang, C.; Kundu, S.C.; Yadavalli, V.K. Biopatterning of Silk Proteins for Soft Micro-optics. ACS Appl. Mater. Interfaces 2015, 7, 8809–8816. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.K.; Farghaly, A.A.; Wang, C.; Collinson, M.M.; Kundu, S.C.; Yadavalli, V.K. Conducting polymer-silk biocomposites for flexible and biodegradable electrochemical sensors. Biosens. Bioelectron. 2016, 81, 294–302. [Google Scholar] [CrossRef]
- Yao, C.; Li, X.; Song, T.; Li, Y.; Pu, Y. Biodegradable nanofibrous membrane of zein/silk fibroin by electrospinning. Polym. Int. 2009, 58, 396–402. [Google Scholar] [CrossRef]
- Wang, F.; Yu, H.-y.; Gu, Z.-G.; Si, L.; Liu, Q.-C.; Hu, X. Impact of calcium chloride concentration on structure and thermal property of Thai silk fibroin films. J. Ther. Anal. Calorim. 2017, 130, 851–859. [Google Scholar] [CrossRef]
- Gubitosi, M.; Duarte, H.; Gentile, L.; Olsson, U.; Medronho, B. On cellulose dissolution and aggregation in aqueous tetrabutylammonium hydroxide. Biomacromolecules 2016, 17, 2873–2881. [Google Scholar] [CrossRef]
- Behrens, M.A.; Holdaway, J.A.; Nosrati, P.; Olsson, U. On the dissolution state of cellulose in aqueous tetrabutylammonium hydroxide solutions. RSC Adv. 2016, 6, 30199–30204. [Google Scholar] [CrossRef]
- Medronho, B.; Filipe, A.; Napso, S.; Khalfin, R.L.; Pereira, R.F.P.; de Zea Bermudez, V.; Romano, A.; Cohen, Y. Silk Fibroin Dissolution in Tetrabutylammonium Hydroxide Aqueous Solution. Biomacromolecules 2019, 20, 4107–4116. [Google Scholar] [CrossRef]
- Sang, Q.; Williams, G.R.; Wu, H.; Liu, K.; Li, H.; Zhu, L.-M. Electrospun gelatin/sodium bicarbonate and poly(lactide-co-ε-caprolactone)/sodium bicarbonate nanofibers as drug delivery systems. Mater. Sci. Eng. C 2017, 81, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Anton, F. Process and Apparatus for Preparing Artificial Threads. U.S. Patent 1,975,504, 1930. [Google Scholar]
- Abdul Khalil, H.P.S.; Davoudpour, Y.; Bhat, A.H.; Rosamah, E.; Tahir, P.M. Electrospun Cellulose Composite Nanofibers. In Handbook of Polymer Nanocomposites. Processing, Performance and Application: Volume C: Polymer Nanocomposites of Cellulose Nanoparticles; Pandey, J.K., Takagi, H., Nakagaito, A.N., Kim, H.-J., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 191–227. [Google Scholar]
- Baji, A.; Mai, Y.-W.; Wong, S.-C.; Abtahi, M.; Chen, P. Electrospinning of polymer nanofibers: Effects on oriented morphology, structures and tensile properties. Compos. Sci. Technol. 2010, 70, 703–718. [Google Scholar] [CrossRef]
- Gupta, P.; Wilkes, G.L. Some investigations on the fiber formation by utilizing a side-by-side bicomponent electrospinning approach. Polymer 2003, 44, 6353–6359. [Google Scholar] [CrossRef]
- Kenawy, E.-R.; Abdel-Hay, F.I.; El-Newehy, M.H.; Wnek, G.E. Processing of polymer nanofibers through electrospinning as drug delivery systems. Mater. Chem. Phys. 2009, 113, 296–302. [Google Scholar] [CrossRef]
- Hu, X.; Liu, S.; Zhou, G.; Huang, Y.; Xie, Z.; Jing, X. Electrospinning of polymeric nanofibers for drug delivery applications. J. Control. Release 2014, 185, 12–21. [Google Scholar] [CrossRef]
- Shamshina, J.L.; Zavgorodnya, O.; Bonner, J.R.; Gurau, G.; Di Nardo, T.; Rogers, R.D. “Practical” Electrospinning of Biopolymers in Ionic Liquids. ChemSusChem 2017, 10, 106–111. [Google Scholar] [CrossRef]
- Pelipenko, J.; Kristl, J.; Janković, B.; Baumgartner, S.; Kocbek, P. The impact of relative humidity during electrospinning on the morphology and mechanical properties of nanofibers. Int. J. Pharm. 2013, 456, 125–134. [Google Scholar] [CrossRef]
- Cramariuc, B.; Cramariuc, R.; Scarlet, R.; Manea, L.R.; Lupu, I.G.; Cramariuc, O. Fiber diameter in electrospinning process. J. Electrost. 2013, 71, 189–198. [Google Scholar] [CrossRef]
- Bae, H.-S.; Haider, A.; Selim, K.M.K.; Kang, D.-Y.; Kim, E.-J.; Kang, I.-K. Fabrication of highly porous PMMA electrospun fibers and their application in the removal of phenol and iodine. J. Polym. Res. 2013, 20, 158. [Google Scholar] [CrossRef]
- Yudin, V.E.; Dobrovolskaya, I.P.; Neelov, I.M.; Dresvyanina, E.N.; Popryadukhin, P.V.; Ivan’kova, E.M.; Elokhovskii, V.Y.; Kasatkin, I.A.; Okrugin, B.M.; Morganti, P. Wet spinning of fibers made of chitosan and chitin nanofibrils. Carbohydr. Polym. 2014, 108, 176–182. [Google Scholar] [CrossRef]
- Lucia, L.; Ayoub, A. Polysaccharide-Based Fibers and Composites: Chemical and Engineering Fundamentals and Industrial Applications; Springer International Publishing: Cham, Swizerland, 2017. [Google Scholar]
- DeFrates, K.G.; Moore, R.; Borgesi, J.; Lin, G.; Mulderig, T.; Beachley, V.; Hu, X. Protein-Based Fiber Materials in Medicine: A Review. Nanomaterials 2018, 8, 457. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Baek, D.; Ki, C.; Park, Y.H. Preparation and characterization of wet spun silk fibroin/poly(vinyl alcohol) blend filaments. Int. J. Biol. Macromol. 2007, 41, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Nayak, R.; Padhye, R.; Kyratzis, I.L.; Truong, Y.B.; Arnold, L. Recent advances in nanofibre fabrication techniques. Text. Res. J. 2011, 82, 129–147. [Google Scholar] [CrossRef]
- Grimmelsmann, N.; Grothe, T.; Homburg, S.V.; Ehrmann, A. Electrospinning and Stabilization of Chitosan Nanofiber Mats; Institute of Physics Publishing: Bristol, UK, 2017; Volume 254. [Google Scholar]
- Stanton, J.; Xue, Y.; Waters, J.C.; Lewis, A.; Cowan, D.; Hu, X.; la Cruz, D.S.-d. Structure–property relationships of blended polysaccharide and protein biomaterials in ionic liquid. Cellulose 2017, 24, 1775–1789. [Google Scholar] [CrossRef]
- Love, S.A.; Popov, E.; Rybacki, K.; Hu, X.; Salas-de la Cruz, D. Facile treatment to fine-tune cellulose crystals in cellulose-silk biocomposites through hydrogen peroxide. Int. J. Biol. Macromol. 2020, 147, 569–575. [Google Scholar] [CrossRef]
- Pereira, R.F.P.; Brito-Pereira, R.; Goncalves, R.; Silva, M.P.; Costa, C.M.; Silva, M.M.; de Zea Bermudez, V.; Lanceros-Mendez, S. Silk Fibroin Separators: A Step Toward Lithium-Ion Batteries with Enhanced Sustainability. ACS Appl. Mater. Interfaces 2018, 10, 5385–5394. [Google Scholar] [CrossRef] [PubMed]
- Haverhals, L.M.; Sulpizio, H.M.; Fayos, Z.A.; Trulove, M.A.; Reichert, W.M.; Foley, M.P.; De Long, H.C.; Trulove, P.C. Process variables that control natural fiber welding: Time, temperature, and amount of ionic liquid. Cellulose 2012, 19, 13–22. [Google Scholar] [CrossRef]
- Ebner, G.; Schiehser, S.; Potthast, A.; Rosenau, T. Side reaction of cellulose with common 1-alkyl-3-methylimidazolium-based ionic liquids. Tetrahedron Lett. 2008, 49, 7322–7324. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, J.; Zhang, H.; He, J.; Ren, Q.; Guo, M. Homogeneous Acetylation of Cellulose in a New Ionic Liquid. Biomacromolecules 2004, 5, 266–268. [Google Scholar] [CrossRef]
- Salas-de La Cruz, D.; Green, M.D.; Ye, Y.; Elabd, Y.A.; Long, T.E.; Winey, K.I. Correlating backbone-to-backbone distance to ionic conductivity in amorphous polymerized ionic liquids. J. Polym. Sci. B Polym. Phys. 2012, 50, 338–346. [Google Scholar] [CrossRef]
- Klein, R.J.; Zhang, S.; Dou, S.; Jones, B.H.; Colby, R.H.; Runt, J. Modeling electrode polarization in dielectric spectroscopy: Ion mobility and mobile ion concentration of single-ion polymer electrolytes. J. Chem. Phys. 2006, 124, 144903. [Google Scholar] [CrossRef] [PubMed]
- Salas-de La Cruz, D. Morphology and Ionic Conductivity of Polymerized Ionic Liquids; Winey, K.I., Ed.; ProQuest Dissertations Publishing: Ann Arbor, MI, USA, 2011. [Google Scholar]
- Lovrić, M. Solid state electrochemistry (1995) Peter G. Bruce (ed). J. Solid State Electrochem. 1997, 1, 116. [Google Scholar] [CrossRef]
- Chen, H.; Choi, J.-H.; Salas-de la Cruz, D.; Winey, K.I.; Elabd, Y.A. Polymerized Ionic Liquids: The Effect of Random Copolymer Composition on Ion Conduction. Macromolecules 2009, 42, 4809–4816. [Google Scholar] [CrossRef]
- Shin, E.J.; Choi, S.M.; Singh, D.; Zo, S.M.; Lee, Y.H.; Kim, J.H.; Han, S.S. Fabrication of cellulose-based scaffold with microarchitecture using a leaching technique for biomedical applications. Cellulose 2014, 21, 3515–3525. [Google Scholar] [CrossRef]
- Li, L.; Meng, L.; Zhang, X.; Fu, C.; Lu, Q. The ionic liquid-associated synthesis of a cellulose/SWCNT complex and its remarkable biocompatibility. J. Mater. Chem. 2009, 19, 3612–3617. [Google Scholar] [CrossRef]
- Liu, Y.; Nguyen, A.; Allen, A.; Zoldan, J.; Huang, Y.; Chen, J.Y. Regenerated cellulose micro-nano fiber matrices for transdermal drug release. Mater. Sci. Eng. C 2017, 74, 485–492. [Google Scholar] [CrossRef]
- Ma, H.; Hsiao, B.S.; Chu, B. Thin-film nanofibrous composite membranes containing cellulose or chitin barrier layers fabricated by ionic liquids. Polymer 2011, 52, 2594–2599. [Google Scholar] [CrossRef]
- Heinze, T.; Liebert, T. Unconventional methods in cellulose functionalization. Prog. Polym. Sci. 2001, 26, 1689–1762. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Ono, T. Separation and characterization of cellulose fibers from cypress wood treated with ionic liquid prior to laccase treatment. Bioresour. Technol. 2013, 127, 132–137. [Google Scholar] [CrossRef]
- Haerens, K.; Van Deuren, S.; Matthijs, E.; Van der Bruggen, B. Challenges for recycling ionic liquids by using pressure driven membrane processes. Green Chem. 2010, 12, 2182–2188. [Google Scholar] [CrossRef]
- Yu, X.; Bao, X.; Zhou, C.; Zhang, L.; Yagoub, A.E.-G.A.; Yang, H.; Ma, H. Ultrasound-ionic liquid enhanced enzymatic and acid hydrolysis of biomass cellulose. Ultrason. Sonochem. 2018, 41, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Dias, J.C.; Lopes, A.C.; Magalhães, B.; Botelho, G.; Silva, M.M.; Esperança, J.M.S.S.; Lanceros-Mendez, S. High performance electromechanical actuators based on ionic liquid/poly(vinylidene fluoride). Polym. Test. 2015, 48, 199–205. [Google Scholar] [CrossRef]
- Kim, O.; Shin, T.J.; Park, M.J. Fast low-voltage electroactive actuators using nanostructured polymer electrolytes. Nat. Commun. 2013, 4, 2208. [Google Scholar] [CrossRef] [PubMed]


| Ionic Liquid | Abbreviation(s) | Chemical Structure |
|---|---|---|
| 1-Allyl-3-methylimidazolium chloride | AMIMCl | 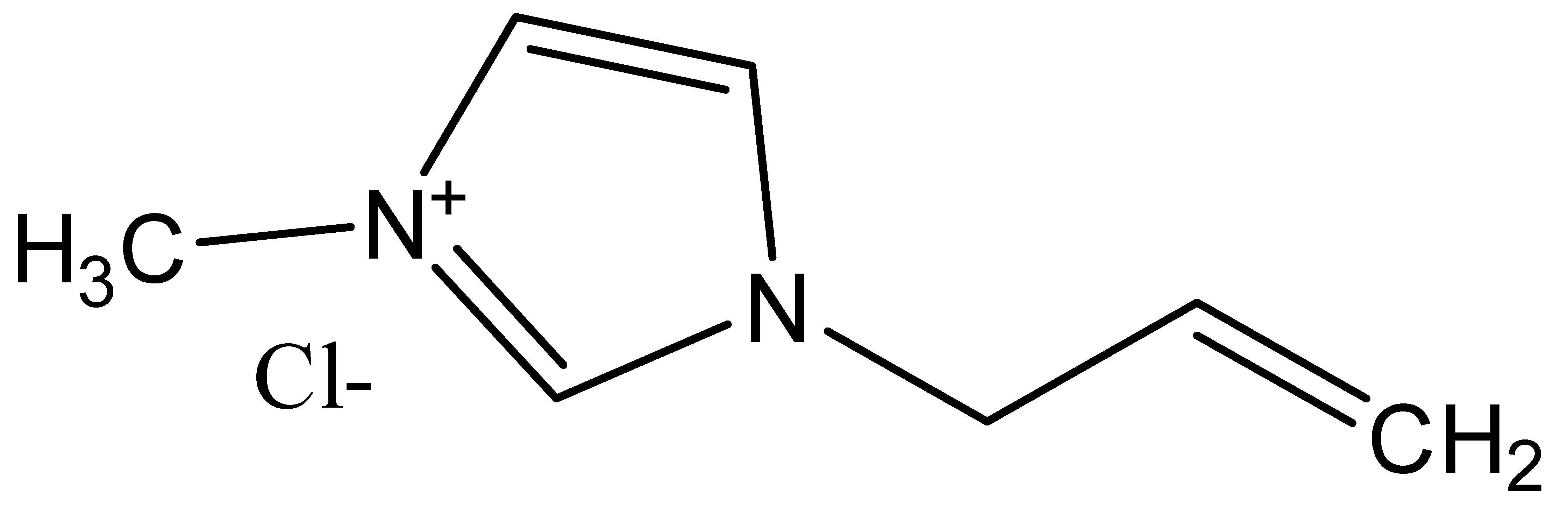 |
| 1-Ethyl-3-methylimidazolium chloride | [C2MIM][Cl] or EMIMCl | 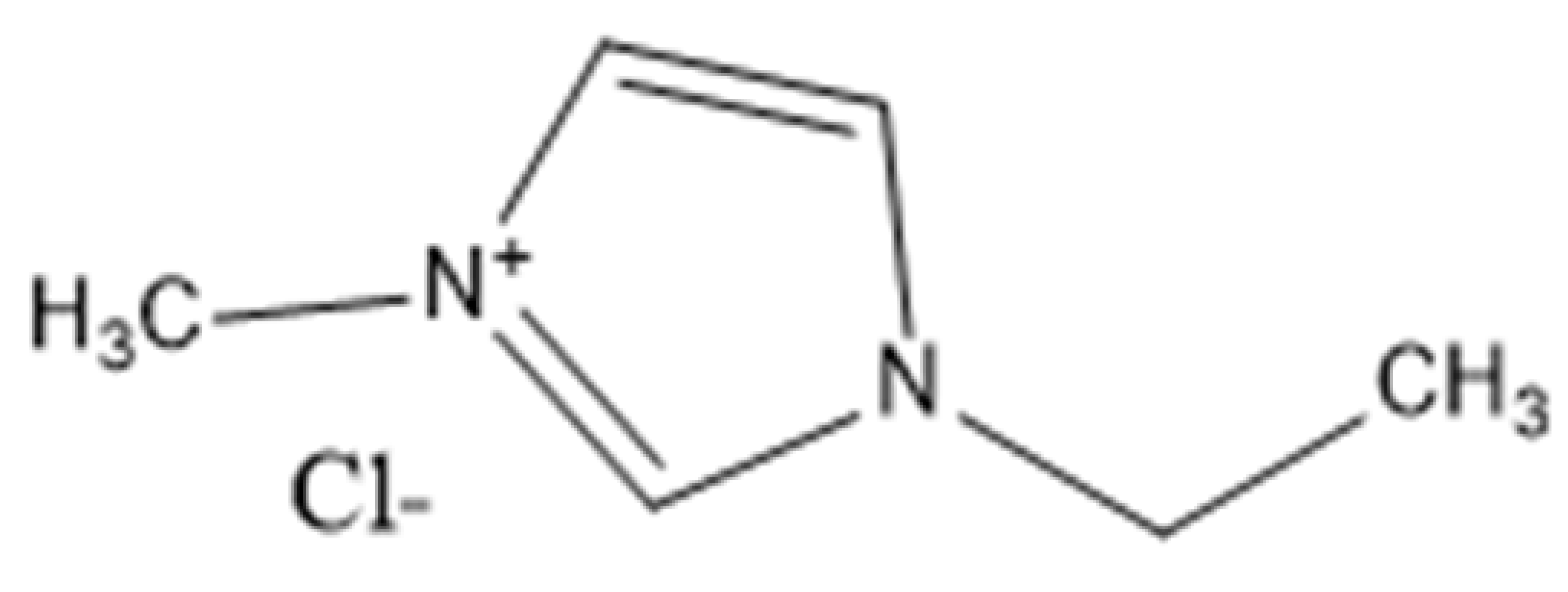 |
| 1-Butyl-3-methylimidazolium chloride | [C4C1IM]Cl or BMIMCl | 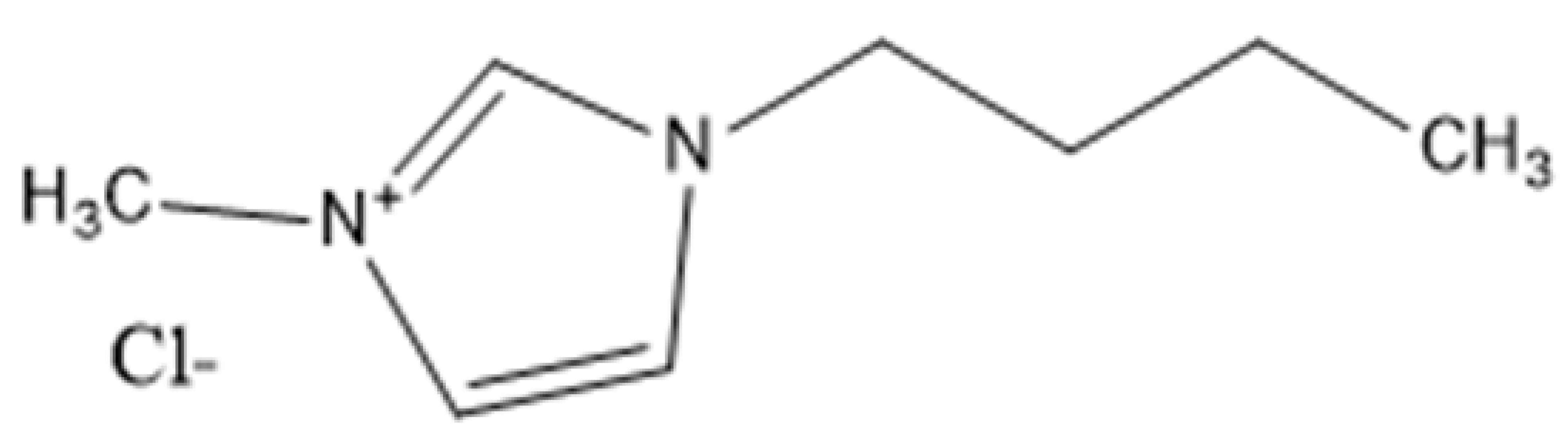 |
| 1-Allyl-3-methylimidazolium acetate | AMIMAc | 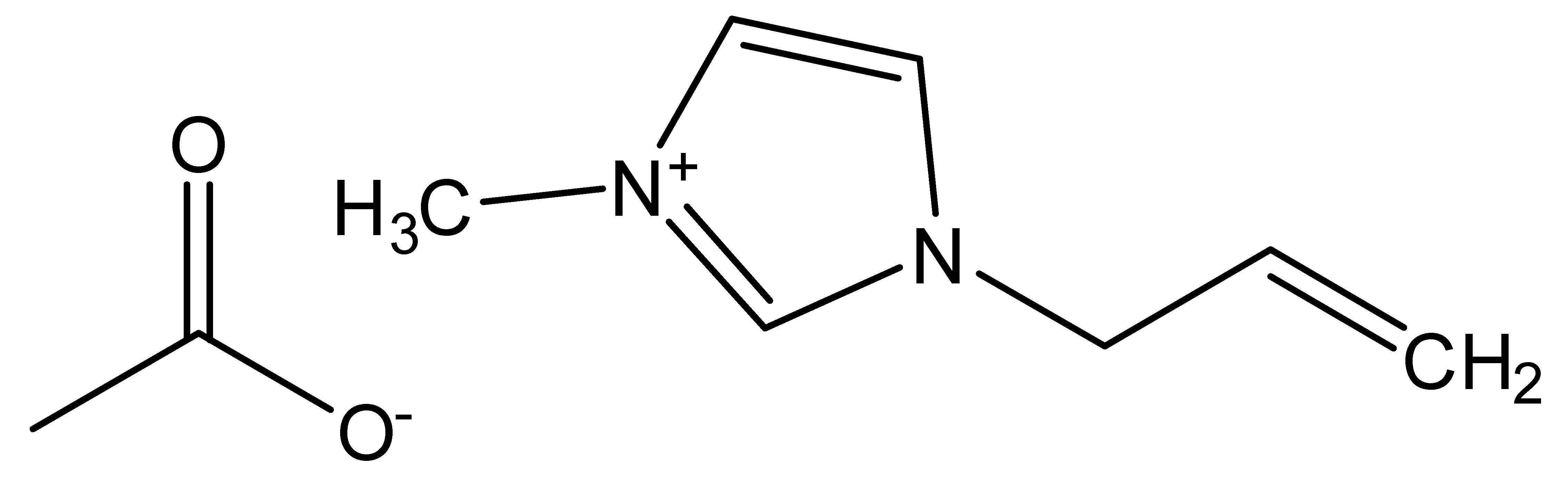 |
| 1-Ethyl-3-methylimidazolium acetate | [C2MIM][CH3CO2] or [C2MIM][OAc] or EMIMAc | 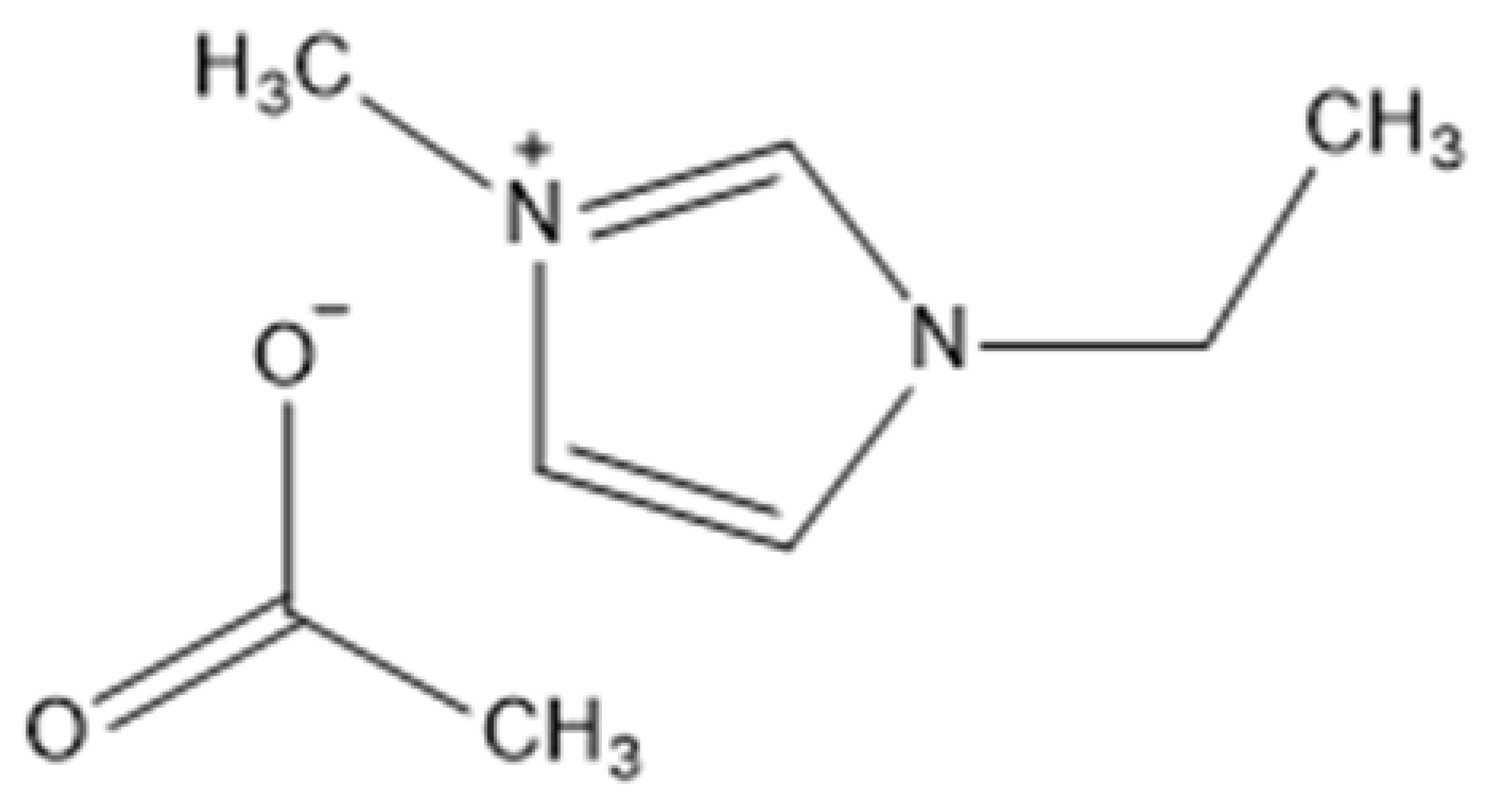 |
| 1-Butyl-3-methylimidazolium acetate | BMIMAc | 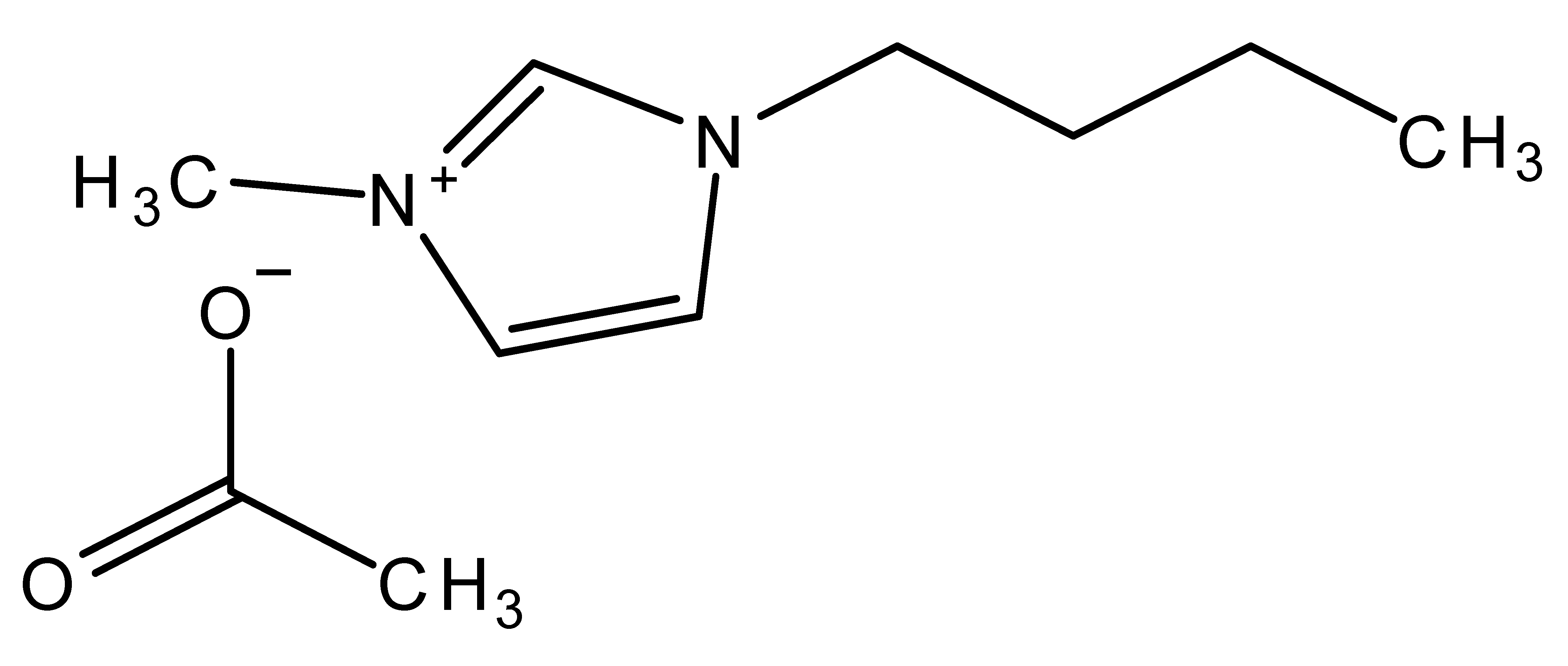 |
| 1-Butyl-3-methylimidazolium dicyanamide | [BMIM][DCA] | 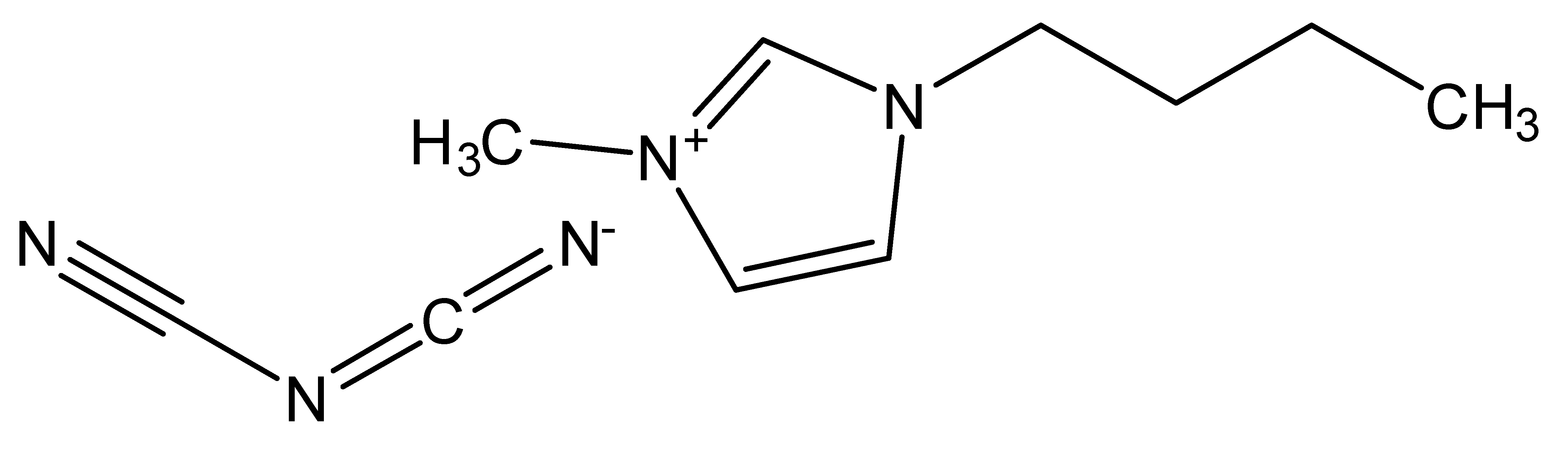 |
| 1-Butyl-3-methylimidazolium bromide | BMIMBr |  |
| 1-Butyl-3-methylimidazolium methanesulfonate | BMIMMeSO3 | 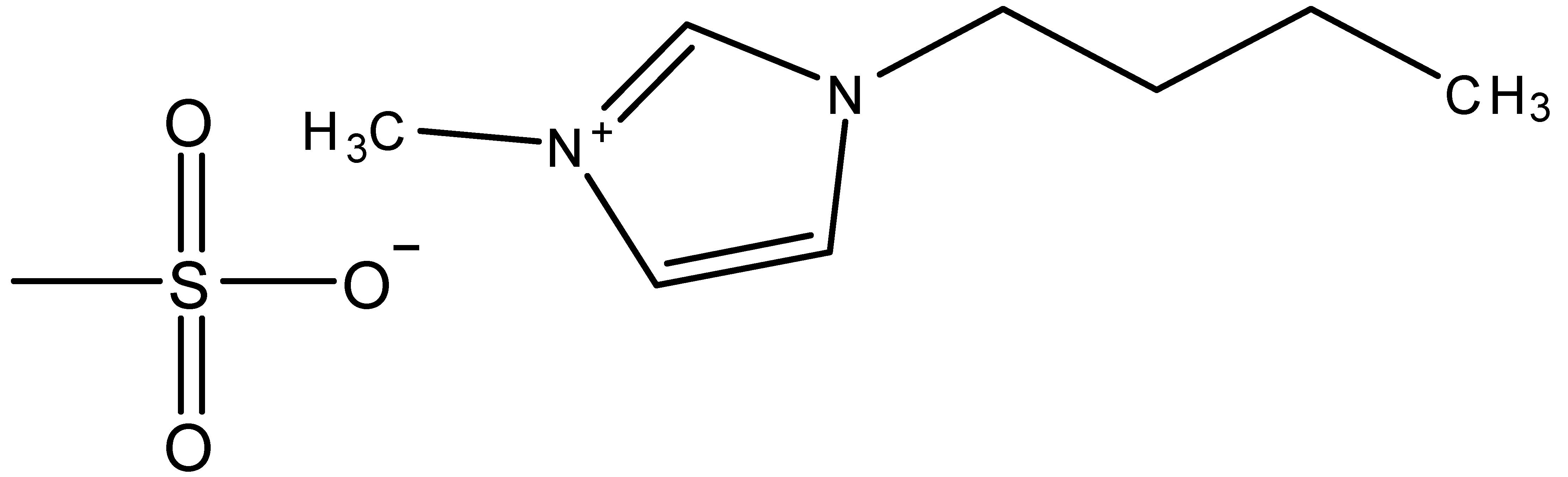 |
| 1-Ethyl-3-methylimidazolium diethyl phosphate | EMIMDep |  |
| 2-Hydroxyethylammonium formate | [NH3(CH2CH2OH)][OFo] | 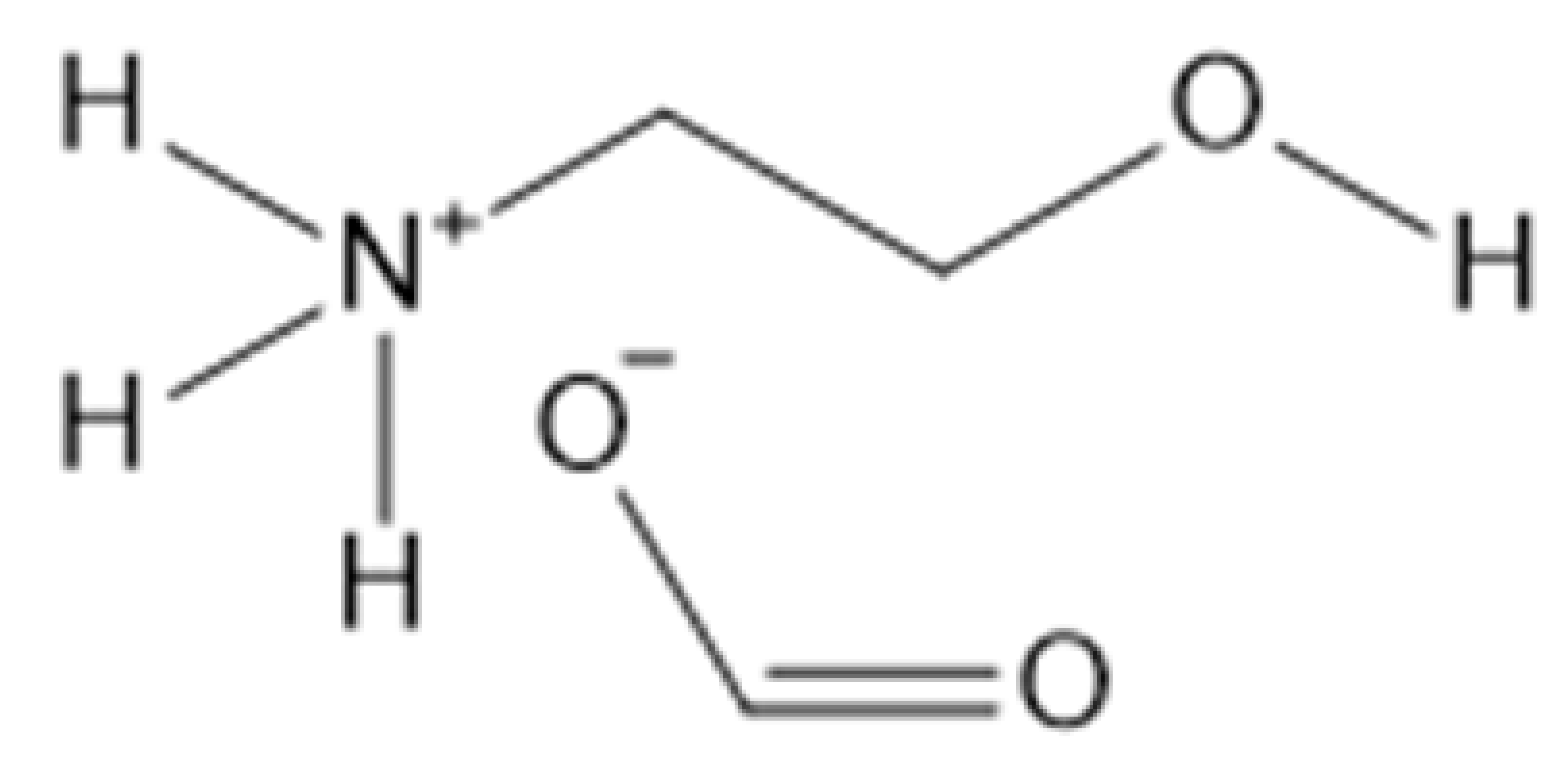 |
| 2-Hydroxyethylammonium acetate | [NH3(CH2CH2OH)][OAc] |  |
| 1-Ethyl-3-methylimidazolium hexafluorophosphate | [C2MIM][PF6] | 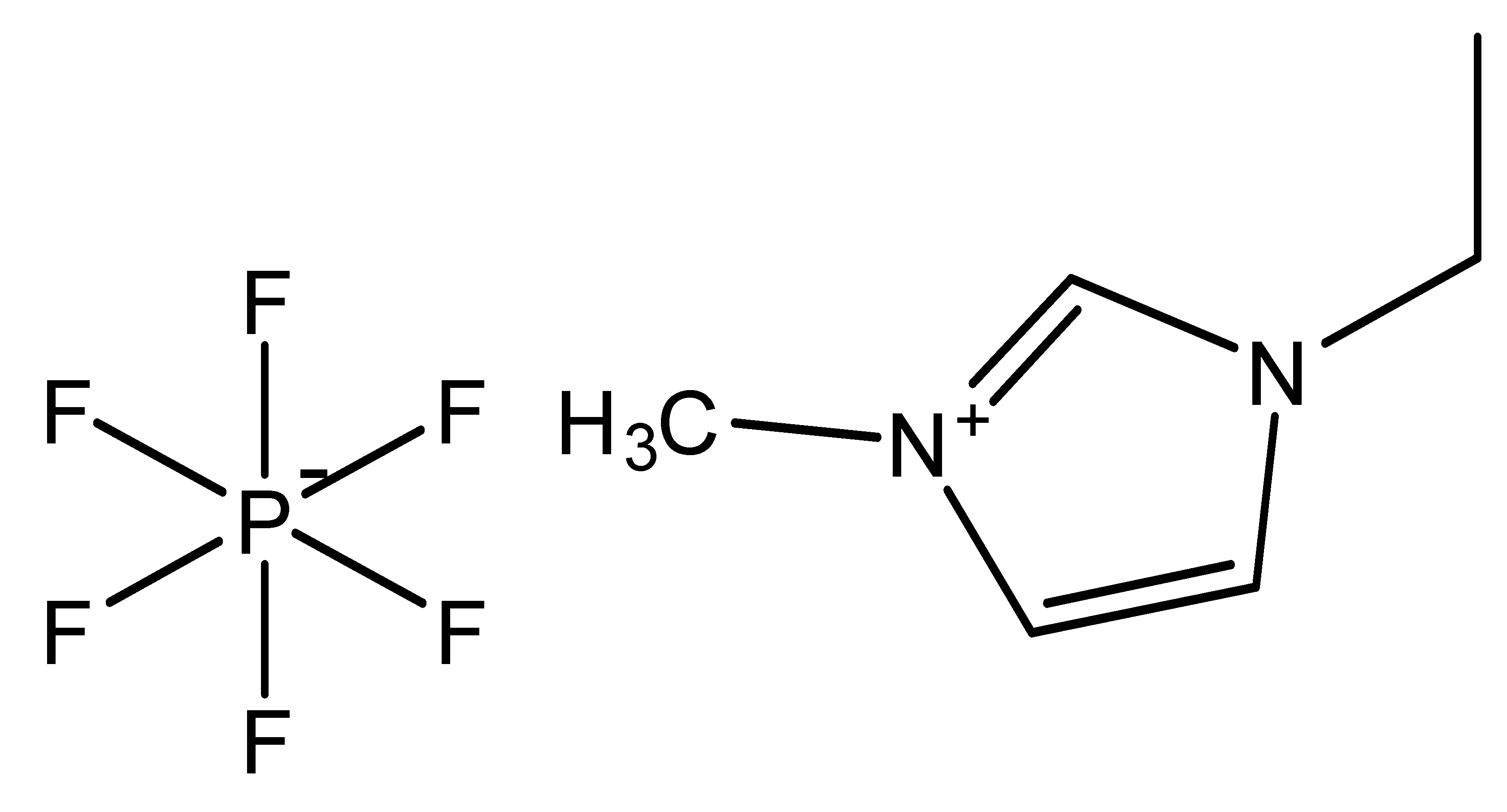 |
| 1-Hexyl-3-methylimidazolium hexafluorophosphate | [C6MIM][PF6] | 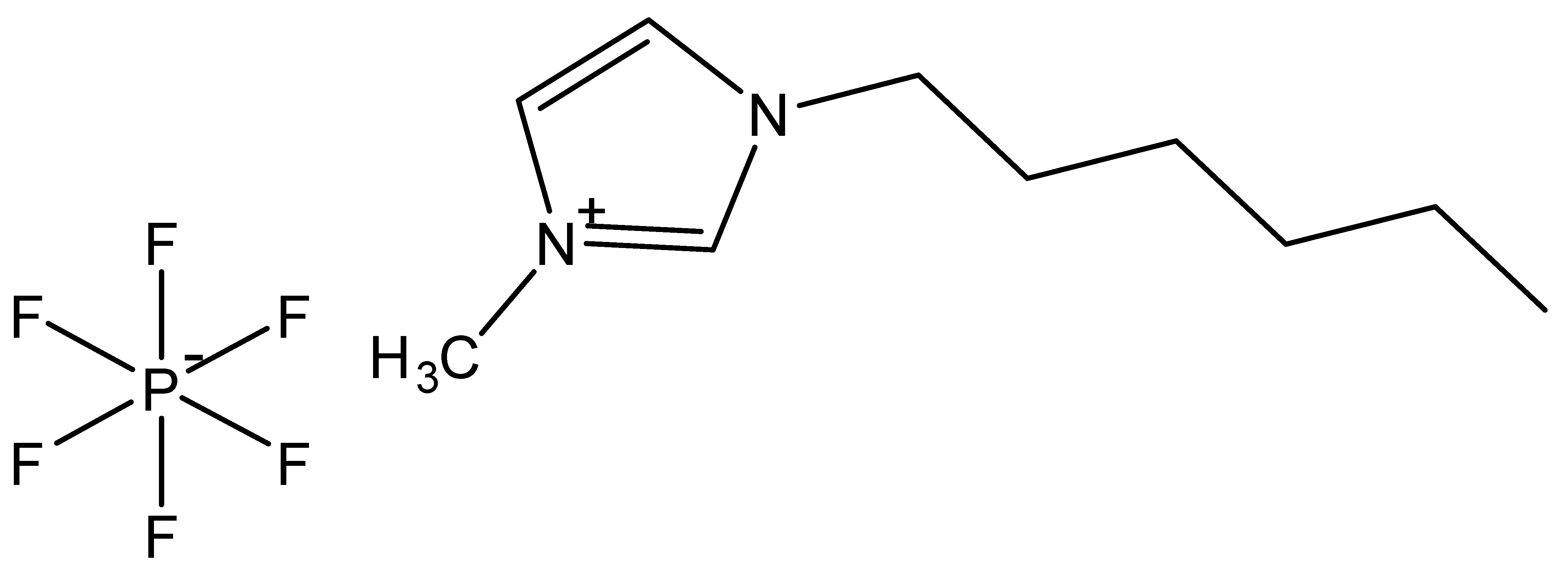 |
| 1-Ethyl-3-methylimidazolium trifluoromethanesulfonate | [C2MIM][Tf] |  |
| 1-Butyl-1-methylpyrrolidinium bis(trifluoromethylsulfonyl)imide | [BMP][NTf2] |  |
| Triethylsulfonium bis(trifluoromethylsulfonyl)imide | [TES][NTf2] | 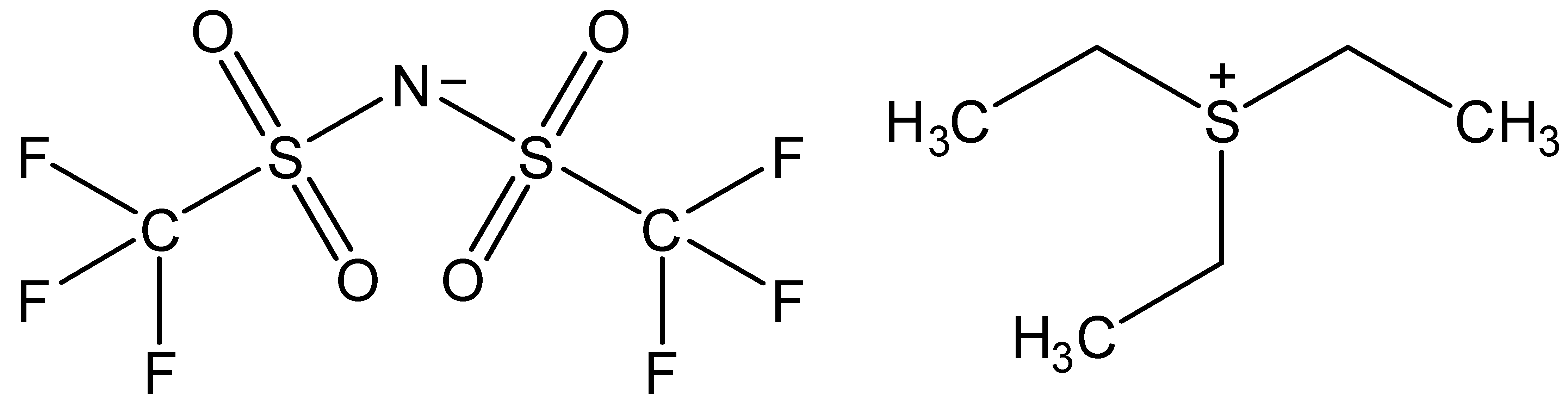 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gough, C.R.; Rivera-Galletti, A.; Cowan, D.A.; Salas-de la Cruz, D.; Hu, X. Protein and Polysaccharide-Based Fiber Materials Generated from Ionic Liquids: A Review. Molecules 2020, 25, 3362. https://doi.org/10.3390/molecules25153362
Gough CR, Rivera-Galletti A, Cowan DA, Salas-de la Cruz D, Hu X. Protein and Polysaccharide-Based Fiber Materials Generated from Ionic Liquids: A Review. Molecules. 2020; 25(15):3362. https://doi.org/10.3390/molecules25153362
Chicago/Turabian StyleGough, Christopher R., Ashley Rivera-Galletti, Darrel A. Cowan, David Salas-de la Cruz, and Xiao Hu. 2020. "Protein and Polysaccharide-Based Fiber Materials Generated from Ionic Liquids: A Review" Molecules 25, no. 15: 3362. https://doi.org/10.3390/molecules25153362
APA StyleGough, C. R., Rivera-Galletti, A., Cowan, D. A., Salas-de la Cruz, D., & Hu, X. (2020). Protein and Polysaccharide-Based Fiber Materials Generated from Ionic Liquids: A Review. Molecules, 25(15), 3362. https://doi.org/10.3390/molecules25153362










