Antifungal Activity of Volatile Organic Compounds Produced by Bacillus methylotrophicus and Bacillus thuringiensis against Five Common Spoilage Fungi on Loquats
Abstract
1. Introduction
2. Results
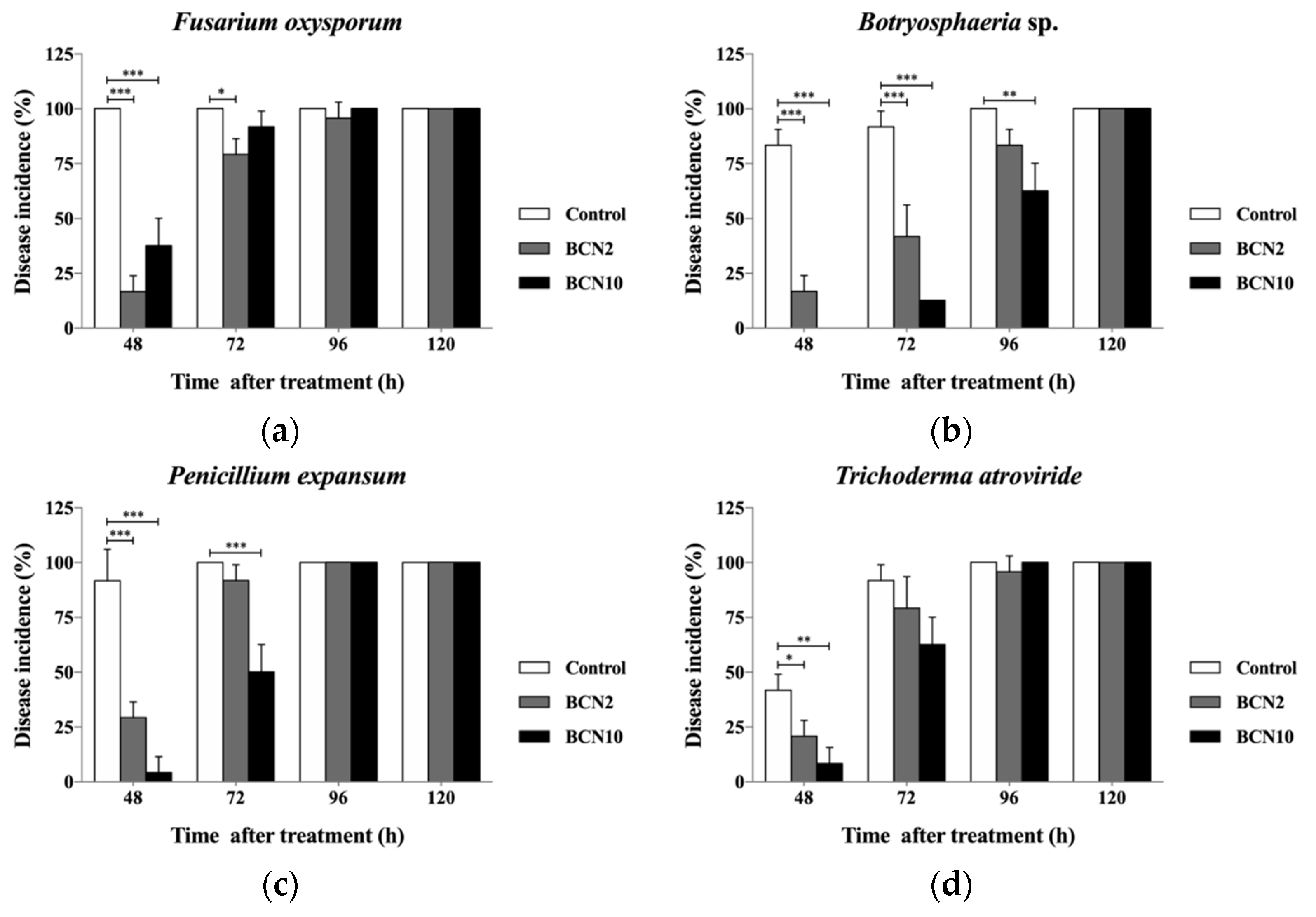
2.1. Antagonistic Activities of VOCs Produced by BCN2 and BCN10 against Five Molds on Double Petri-Dishes
2.2. Antagonistic Activities of VOCs Produced by BCN2 and BCN10 on Loquat Fruit
2.3. Gas Component Analysis of VOCs from BCN2 and BCN10
2.4. Decay Control of VOCs Produced by BCN2 and BCN10 in Natural Postharvest Storage of Loquat Fruits
3. Discussion
4. Materials and Methods
4.1. Mircoorganisms Culture Media
4.2. In Vitro Antagonistic Activities of VOCs from Two Bacillus Strains
4.3. In Vivo Antagonistic Activities of VOCs
4.4. HS-SPME GC-MS Analysis of VOCs
4.5. Effectiveness of VOCs Produced by BCN2 and BCN10 in Natural Storage of Loquats
4.6. Data Analysis
5. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mari, M.; Martini, C.; Guidarelli, M.; Neri, F. Postharvest Biocontrol of Monilinia Laxa, Monilinia Fructicola and Monilinia Fructigena on Stone Fruit by Two Aureobasidium Pullulans Strains. Biol. Control 2012, 60, 132–140. [Google Scholar] [CrossRef]
- Mari, M.; Martini, C.; Spadoni, A.; Roussi, W.; Bertolini, P. Biocontrol of Apple Postharvest Decay by Aureobasidium Pullulans. Postharvest Biol. Technol. 2012, 73, 56–62. [Google Scholar] [CrossRef]
- Lahlali, R.; Serrhini, M.N.; Jijakli, M.H. Efficacy Assessment of Candida Oleophila (Strain O) and Pichia Anomala (Strain K) against Major Post-Harvest Diseases of Citrus Fruits in Morocco. Commun. Agric. Appl. Biol. Sci. 2004, 69, 601–609. [Google Scholar] [PubMed]
- Sharma, R.R.; Singh, D.; Singh, R. Biological Control of Postharvest Diseases of Fruits and Vegetables by Microbial Antagonists: A Review. Biol. Control 2009, 50, 205–221. [Google Scholar] [CrossRef]
- Bencheqroun, S.K.; Bajji, M.; Labhilili, M.; Sebastien, M. In Vitro and In Situ Study of Postharvest Apple Blue Mold Biocontrol by Aureobasidium Pullulans: Evidence for the Involvement of Competition for Nutrients. Postharvest Biol. Technol. 2007, 46, 128–135. [Google Scholar] [CrossRef]
- Bull, C.T.; Wadsworth, M.L.; Sorensen, K.N. Syringomycin E Produced by Biological Control Agents Controls Green Mold on Lemons. Biol. Control 1998, 12, 89–95. [Google Scholar] [CrossRef]
- El-Ghaouth, A.; Wilson, C.L.; Wisniewski, M. Ultrastructural and Cytochemical Aspects of the Biological Control of Botrytis Cinerea by Candida Saitoana in Apple Fruit. Phytopathology 1998, 88, 282–291. [Google Scholar] [CrossRef]
- Ryu, C.M.; Farag, M.A.; Hu, C.H.; Reddy, M.S.; Wei, H.X.; Paré, P.W.; Kloepper, J.W. Bacterial Volatiles Promote Growth in Arabidopsis. Proc. Natl. Acad. Sci. USA 2003, 100, 4927–4932. [Google Scholar] [CrossRef]
- Gu, Y.-Q.; Mo, M.-H.; Zhou, J.-P.; Zou, C.-S.; Zhang, K.-Q. Evaluation and Identification of Potential Organic Nematicidal Volatiles from Soil Bacteria. Soil Biol. Biochem. 2007, 39, 2567–2575. [Google Scholar] [CrossRef]
- Farag, M.A.; Ryu, C.M.; Sumner, L.W.; Pare, P. Gc–Ms Spme Profiling of Rhizobacterial Volatiles Reveals Prospective Inducers of Growth Promotion and Induced Systemic Resistance in Plants. Phytochemistry 2006, 67, 2262–2268. [Google Scholar] [CrossRef]
- Fialho, M.B.; Duarte de Moraes, M.H.; Tremocorti, A.R.; Pascholati, S.F. Potential of Antimicrobial Volatile Organic Compounds to Control Sclerotinia Sclerotiorum in Bean Seeds. Pesqui. Agropecu. Bras. 2011, 46, 137–142. [Google Scholar] [CrossRef]
- Buzzini, P.; Martini, A.; Cappelli, F.; Pagnoni, U.M.; Davoli, P. A Study on Volatile Organic Compounds (VOCS) Produced by Tropical Ascomycetous Yeasts. Antonie Leeuwenhoek 2003, 84, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Minerdi, D.; Bossi, S.; Gullino, M.L.; Garibaldi, A. Volatile Organic Compounds: A Potential Direct Long-Distance Mechanism for Antagonistic Action of Fusarium Oxysporum Strain Msa 35. Environ. Microbiol. 2009, 11, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Raza, W.; Shen, Q.; Huang, Q. Antifungal Activity of Bacillus Amyloliquefaciens Njn-6 Volatile Compounds against Fusarium Oxysporum f. sp. Cubense. Appl. Environ. Microbiol. 2012, 78, 5942–5944. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-L.; Cheng, Y.-H.; Ding, F.-B.; Liu, Y.-Z.; Liu, C.-H.; Chen, Z.-Y. Inhibition Effect of 2 Bacillus Subtilis on Botryosphaeria Berengeriana f. Sp. Piricola In Vitro. Fruit Sci. 2010, 27, 823–827. [Google Scholar]
- Zheng, M.; Shi, J.; Shi, J.; Wang, Q.; Li, Y. Antimicrobial Effects of Volatiles Produced by Two Antagonistic Bacillus Strains on the Anthracnose Pathogen in Postharvest Mangos. Biol. Control 2013, 65, 200–206. [Google Scholar] [CrossRef]
- Sungpueak, R.; Sompong, M.; Thumanu, K.; Tantanuch, W.; Athinuwat, D.; Prathuangwong, S.; Buensanteai, N. Element Analysis of Cassava Leaves Induced Resistance by Bacillus Subtilis Casu007 against Colletotrichum Gloeosporioides f.sp. Manihotis Using Micro-Beam Synchrotron X-Ray Fluorescence(μ-Sxrf). C. In Proceedings of the ICPP 2013 10th International Congress of Plant Pathology, Beijing, China, 25–30 August 2013; Volume 1. Available online: http://cstm.cnki.net/stmt/TitleBrowse/KnowledgeNet/ZGVS201308001A0S?db=STMI8319. (accessed on 21 July 2020).
- Lee, H.-J.; Park, K.-C.; Lee, S.-H.; Bang, K.-H.; Park, H.-W.; Hyun, D.-Y.; Kang, S.-W.; Cha, S.-W.; Chung, I.-M. Screening of Antifungal Bacillus Spp. against Alternaria Blight Pathogen (Alternaria Panax) and Anthracnose Pathogen (Colletotrichum Gloeosporioides) of Ginseng. Korean J. Med. Crop. Sci. 2012, 20, 339–344. [Google Scholar] [CrossRef]
- Andersen, R.A.; Hamilton-Kem, T.R.; Hidelbrand, D.F.; McCraken, C.T., Jr.; Collins, R.W.; Fleming, P.D. Structure-Antifungal Activity Relationships among Volatile C6 and C9 Aliphatic Aldehydes, Ketones, and Alcohols. J. Agric. Food Chem. 1994, 42, 1563–1568. [Google Scholar] [CrossRef]
- Tyagi, A.K.; Bukvicki, D.; Gottardi, D.; Tabanelli, G.; Montanari, C.; Malik, A.; Guerzoni, M.E. Eucalyptus Essential Oil as a Natural Food Preservative: In Vivo and In Vitro Antiyeast Potential. BioMed. Res. Int. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Camele, I.; Altieri, L.; De Martino, L.; De Feo, V.; Mancini, E.; Rana, G.L. In Vitro Control of Post-Harvest Fruit Rot Fungi by Some Plant Essential Oil Components. Int. J. Mol. Sci. 2012, 13, 2290–2300. [Google Scholar] [CrossRef]
- Rais, A.; Jabeen, Z.; Shair, F.; Hafeez, F.Y.; Hassan, M.N. Bacillus spp. A Bio-Control Agent Enhances the Activity of Antioxidant Defense Enzymes in Rice against Pyricularia Oryzae. PLoS ONE 2017, 12, e0187412. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, X.; Liu, S.; Zhang, K.; Cai, Z.; Chen, X.; Zhang, Y.; Liu, J.; Wang, A. The Endochitinase of Clonostachysrosea Expression in Bacillus Amyloliquefaciens Enhances the Botrytis Cinerea Resistance of Tomato. Int. J. Mol. Sci. 2018, 19, 2221. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zheng, Q.; Xu, B.; Liu, J. Identification of the Fungal Pathogens of Postharvest Disease on Peach Fruits and the Control Mechanisms of Bacillus Subtilis Jk-14. Toxins 2019, 11, 322. [Google Scholar] [CrossRef]
- Raza, W.; Ling, N.; Yang, L.; Huang, Q.; Shen, Q. Response of Tomato Wilt Pathogen Ralstonia Solanacearum to the Volatile Organic Compounds Produced by a Biocontrol Strain Bacillus Amyloliquefaciens Sqr-9. Sci. Rep. 2016, 6, 24856. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.-H.; Liao, M.-J.; Wang, H.-K.; Zheng, M.-Z.; Xu, J.-J.; Gao, J.-H. Bacillus Velezensis, a Potential and Efficient Biocontrol Agent in Control of Pepper Gray Mold Caused by Botrytis Cinereal. Biol. Control 2018, 126, 147–157. [Google Scholar] [CrossRef]
- Wang, L.; Dou, G.; Guo, H.; Zhang, Q.; Qin, X.; Yu, W.; Jiang, C.; Xiao, H. Volatile Organic Compounds of Hanseniaspora Uvarum Increase Strawberry Fruit Flavor and Defense during Cold Storage. Food Sci. Nutr. 2019, 7, 2625–2635. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.B.; Monteiro, S.; Freitas, R.; Santos, C.N.; Chen, Z.; Borges, A.F. The Role of Plant Defence Proteins in Fungal Pathogenesis. Mol. Plant Pathol. 2007, 8, 677–700. [Google Scholar] [CrossRef]
- Ryals, J.A.; Neuenschwander, U.H.; Willits, M.G.; Molina, A.; Hunt, M.D. Systemic Acquired Resistance. Plant Cell 1996, 8, 1809–1819. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, W.; Bi, Y.; Luo, Y. Postharvest BTH Treatment Induces Resistance of Peach (Prunus Persica l. Cv. Jiubao) Fruit to Infection by Penicillium Expansum and Enhances Activity of Fruit Defense Mechanisms. Postharvest Biol. Technol. 2005, 35, 263–269. [Google Scholar] [CrossRef]
- Wang, K.; Jin, P.; Cao, S.; Rui, H.; Zheng, Y. Biological Control of Green Mould Decay in Postharvest Chinese Bayberries by Pichia ns. J. Phytopathol. 2011, 159, 417–423. [Google Scholar]
- Cao, S.-F.; Zheng, Y.-H.; Tang, S.-S.; Jin, P.; Wang, K.-T. Biological Control of Post-Harvest Anthracnose Rot of Loquat Fruit by Pichia Membranefaciens. J. Hortic. Sci. Biotechnol. 2008, 83, 816–820. [Google Scholar] [CrossRef]
- Chan, Z.; Tian, S. Interaction of Antagonistic Yeasts against Postharvest Pathogens of Apple Fruit and Possible Mode of Action. Postharvest Biol. Technol. 2005, 36, 215–223. [Google Scholar] [CrossRef]
- Wang, X.; Xu, F.; Wang, J.; Jin, P.; Zheng, Y. Bacillus Cereus AR156 Induces Resistance against Rhizopus Rot through Priming of Defense Responses in Peach Fruit. Food Chem. 2013, 136, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Randon, J.; Maret, L.; Ferronato, C. Gas Chromatography-Mass Spectroscopy Optimization by Computer Simulation, Application to the Analysis of 93 Volatile Organic Compounds in Workplace Ambient Air. Anal. Chim. Acta 2014, 812, 258–264. [Google Scholar] [CrossRef]
- Kubica, D.; Kaczmarczyk, M.; Kaszuba, A. Determination of Volatile Organic Compounds in Materials from Polystyrene Intended for Contact with Food: Comparison of HS-GS/MS and Spme-Gc/Ms Techniques. Roczniki Państwowego Zakładu Higieny 2018, 69, 235–242. [Google Scholar] [PubMed]
- Rahnamaie, T.R.; Goh, H.H.; Noor, N.M. Methyl Jasmonate-Induced Compositional Changes of Volatile Organic Compounds in Polygonum Minus Leaves. J. Plant Physiol. 2019, 240, 152994. [Google Scholar] [CrossRef]
- Arrebola, E.; Sivakumar, D.; Korsten, L. Effect of Volatile Compounds Produced by Bacillus Strains on Postharvest Decay in Citrus. Biol. Control 2010, 53, 122–128. [Google Scholar] [CrossRef]
- Chaves-López, C.; Serio, A.; Gianotti, A.; Sacchetti, G.; Ndagijimana, M.; Ciccarone, C.; Stellarini, A.; Corsetti, A.; Paparella, A. Diversity of Food-Borne Bacillus Volatile Compounds and Influence on Fungal Growth. J. Appl. Micorbiol. 2015, 119, 487–499. [Google Scholar] [CrossRef]
- Maruzzella, J.C. Antimicrobial Substances from Ferns. Nature 1961, 191, 518. [Google Scholar] [CrossRef]
- Liu, W.-W.; Mu, W.; Zhu, B.-Y.; Du, Y.-C.; Liu, F. Antagonistic Activities of Volatiles from Four Strains of Bacillus spp. and Paenibacillus spp. Against Soil-Borne Plant Pathogens. Agric. Sci. China 2008, 7, 1104–1114. [Google Scholar] [CrossRef]
- Urbanek, A.; Szadziewski, R.; Stepnowski, P.; Boros-Majewska, J.; Gabriel, I.; Dawgul, M.; Kamysz, W.; Sosnowska, D.; Gołębiowski, M. Composition and Antimicrobial Activity of Fatty Acids Detected in the Hygroscopic Secretion Collected from the Secretory Setae of Larvae of the Biting Midge Forcipomyia Nigra (Diptera: Ceratopogonidae). J. Insect Physiol. 2012, 58, 1265–1276. [Google Scholar] [CrossRef]
- Ingram, L.O.; Buttke, T.M. Effects of Alcohols on Microorganisms. Adv. Microb. Physiol. 1984, 25, 253–300. [Google Scholar] [PubMed]
- Ando, H.; Hatanaka, K.; Ohata, I.; Yamashita-Kitaguchi, Y.; Kurata, A.; Kishimoto, N. Antifungal Activities of Volatile Substances Generated by Yeast Isolated from Iranian Commercial Cheese. Food Control 2012, 26, 472–478. [Google Scholar] [CrossRef]
- Zhang, X.; Li, B.; Wang, Y.; Guo, Q.; Lu, X.; Li, S.; Ma, P. Lipopeptides, a Novel Protein, and Volatile Compounds Contribute to the Antifungal Activity of the Biocontrol Agent Bacillus Atrophaeus CAB-1. Appl. Microbiol. Biotechnol. 2013, 97, 9525–9534. [Google Scholar] [CrossRef] [PubMed]
- Fernando, W.G.D.; Ramarathnam, R.; Krishnamoorthy, A.S.; Savchuk, S.C. Identification and Use of Potential Bacterial Organic Antifungal Volatiles in Biocontrol. Soil Biol. Biochem. 2005, 37, 955–964. [Google Scholar] [CrossRef]
- Rouissi, W.; Ugolini, L.; Martini, C.; Lazzeri, L.; Mari, M. Control of Postharvest Fungal Pathogens by Antifungal Compounds from Penicillium Expansum. J. Food Prot. 2013, 76, 1879–1886. [Google Scholar] [CrossRef]
- Lesaffre, E.; Molenberghs, G. Multivariate Probit Analysis: A Neglected Procedure in Medical Statistics. Stat. Med. 1991, 10, 1391–1403. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not applicable from the authors. |



| Pathogens | Fusarium oxysporum | Botryosphaeria sp. | Penicillium expansum | Trichoderma atroviride | Colletotrichum gloeosporioides |
|---|---|---|---|---|---|
| Bacillus | |||||
| BCN2 | ++ | ++ | --- | --- | +++ |
| BCN10 | --- | +++ | ++ | ++ | + |
| BCN2+BCN10 | ++ | +++ | ++ | ++ | +++ |
| Bacillus methylotrophicus BCN2 | Bacillus thuringiensis BCN10 | |||||
|---|---|---|---|---|---|---|
| RI a | Conc b | RI a | Conc b | |||
| Alcohols | 434 | Butan-1-ol | 0.17 ± 0.02 | 430 | Butan-1-ol | 0.52 ± 0.04 |
| 506 | 3-Methylbutan-1-ol | 0.27 ± 0.10 | 513 | 3-Methylbutan-1-ol | 0.56 ± 0.08 | |
| 671 | Hexan-2-ol | 0.45 ± 0.06 | 750 | Phenylmethanol | 0.05 ± 0.00 | |
| 864 | Phenylethanol | 2.05 ± 0.12 | 862 | Phenylethanol | 1.00 ± 0.08 | |
| Phenols | 927 | 2,3,6-Trimethylphenol | 0.37 ± 0.05 | |||
| 1256 | Benzothiazole-5,7-ol | 0.19 ± 0.11 | ||||
| Ketones | 412 | Butan-2-one | 0.07 ± 0.03 | 412 | Butan-2-one | 0.25 ± 0.05 |
| 465 | 3-Hydroxybutan-2-one | 1.43 ± 0.07 | 459 | 3-Hydroxybutan-2-one | 1.80 ± 0.07 | |
| 918 | Nonan-2-one | 0.71 ± 0.12 | 403 | Butane-2,3-dione | 3.13 ± 1.08 | |
| 1076 | Decan-2-one | 0.41 ± 0.05 | 859 | Acetophenone | 0.95 ± 0.03 | |
| 1198 | Undecan-2-one | 0.22 ± 0.11 | 870 | 6-Methylheptan-2-one | 0.06 ± 0.01 | |
| 1280 | Dodecan-2-one | 2.06 ± 0.98 | ||||
| 1333 | Tridecan-2-one | 0.23 ± 0.08 | ||||
| 1598 | Pentadecan-2-one | 0.19 ± 0.11 | ||||
| Hydrocarbons | 419 | 2-Methylpropane | 0.39 ± 0.07 | 417 | 2-Methylpropane | 3.13 ± 0.08 |
| 530 | Penta-1,3-diene | 0.44 ± 0.06 | 533 | Penta-1,3-diene | 1.17 ± 0.00 | |
| 751 | Toluene | 1.21 ± 0.54 | 757 | 2,4-Diaminotoluene | 0.45 ± 0.19 | |
| 840 | Ethylbenzene | 0.98 ± 0.23 | 868 | Oct-1-ene | 0.70 ± 0.04 | |
| 941 | Propylbenzene | 1.48 ± 0.08 | 1113 | 2-Methylnaphthalene | 3.05 ± 0.51 | |
| 923 | Isopropylbenzene | 2.11 ± 0.53 | 1265 | Dodecane | 0.79 ± 0.07 | |
| 1407 | Tetradecane | 0.15 ± 0.06 | 1412 | Tetradecane | 0.34 ± 0.04 | |
| 1541 | Pentadecane | 5.98 ± 0.30 | 1971 | Eicosane | 0.16 ± 0.03 | |
| Aldehydes | 811 | Benzaldehyde | 4.01 ± 0.17 | |||
| Esters | 423 | Ethyl acetate | 0.78 ± 0.19 | |||
| Acids | 249 | Acetic acid | 1.54 ± 0.01 | 241 | Acetic acid | 0.75 ± 0.42 |
| 477 | 2-Methylpropanoic acid | 0.06 ± 0.02 | ||||
| Other compounds | 221 | Carbon disulphide | 0.34 ± 0.09 | 230 | Carbon disulphide | 0.17 ± 0.01 |
| 614 | 2,6-Dimethylpyrazine | 0.06 ± 0.01 | 745 | Benzothiazole | 0.29 ± 0.04 | |
| 855 | 2-Ethyl-3,5-dimethylpyrazine | 0.67 ± 0.15 | 762 | 4-Methyl-1,3-benzenediamine | 1.36 ± 0.03 | |
| 873 | Tetramethylpyrazine | 2.02 ± 0.07 | 881 | 2-(Methylthio)benzothiazole | 0.45 ± 0.07 | |
| 807 | 2-Methoxyphenyl oxime | 0.23 ± 0.18 | ||||
| 845 | 3-Ethyl-2,5-dimethylpyrazine | 0.89 ± 0.01 | ||||
| 976 | 2-Pentylfuran | 3.29 ± 0.16 | ||||
| 957 | 2,3,5-Trimethyl-6-ethylpyrazine | 0.09 ± 0.04 | ||||
| 965 | 2,3-Diethyl-5-methylpyrazine | 0.77 ± 0.43 | ||||
| 1132 | 2,5-Dimethyl-3-(3-methylbutyl)pyrazine | 0.24 ± 0.05 | ||||
| 1147 | 2,3-Dimethyl-5-isopentylpyrazine | 0.51 ± 0.14 | ||||
| Disease Incidence | 3 d | 5 d | 10 d | 15 d |
|---|---|---|---|---|
| Control | 12.50% | 20.83% | 54.17% | 100.00% |
| BCN2 | 0.00% | 4.17% | 20.83% | 87.50% |
| BCN10 | 0.00% | 5.77% | 22.34% | 89.08% |
| BCN2+BCN10 | 0.00% | 3.56% | 20.19% | 86.95% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, C.-N.; Ye, W.-Q.; Zhu, Y.-Y.; Zhou, W.-W. Antifungal Activity of Volatile Organic Compounds Produced by Bacillus methylotrophicus and Bacillus thuringiensis against Five Common Spoilage Fungi on Loquats. Molecules 2020, 25, 3360. https://doi.org/10.3390/molecules25153360
He C-N, Ye W-Q, Zhu Y-Y, Zhou W-W. Antifungal Activity of Volatile Organic Compounds Produced by Bacillus methylotrophicus and Bacillus thuringiensis against Five Common Spoilage Fungi on Loquats. Molecules. 2020; 25(15):3360. https://doi.org/10.3390/molecules25153360
Chicago/Turabian StyleHe, Chao-Nan, Wan-Qiong Ye, Ying-Ying Zhu, and Wen-Wen Zhou. 2020. "Antifungal Activity of Volatile Organic Compounds Produced by Bacillus methylotrophicus and Bacillus thuringiensis against Five Common Spoilage Fungi on Loquats" Molecules 25, no. 15: 3360. https://doi.org/10.3390/molecules25153360
APA StyleHe, C.-N., Ye, W.-Q., Zhu, Y.-Y., & Zhou, W.-W. (2020). Antifungal Activity of Volatile Organic Compounds Produced by Bacillus methylotrophicus and Bacillus thuringiensis against Five Common Spoilage Fungi on Loquats. Molecules, 25(15), 3360. https://doi.org/10.3390/molecules25153360






