Production of Lactic Acid from Carob, Banana and Sugarcane Lignocellulose Biomass
Abstract
1. Introduction
2. Results and Discussion
2.1. Lignocellulose Biomass Analysis
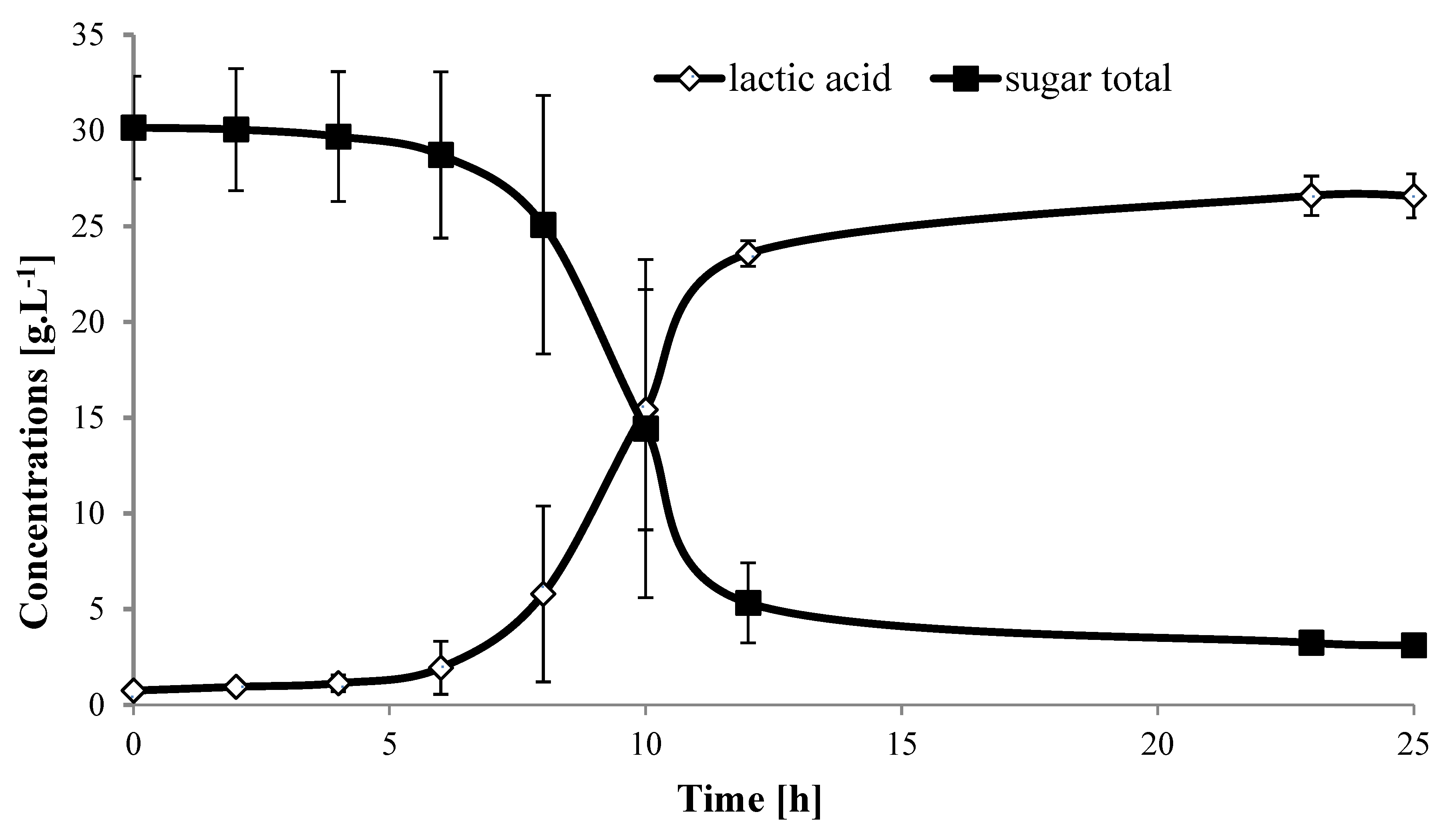
2.2. Fermentation of Banana Peduncles
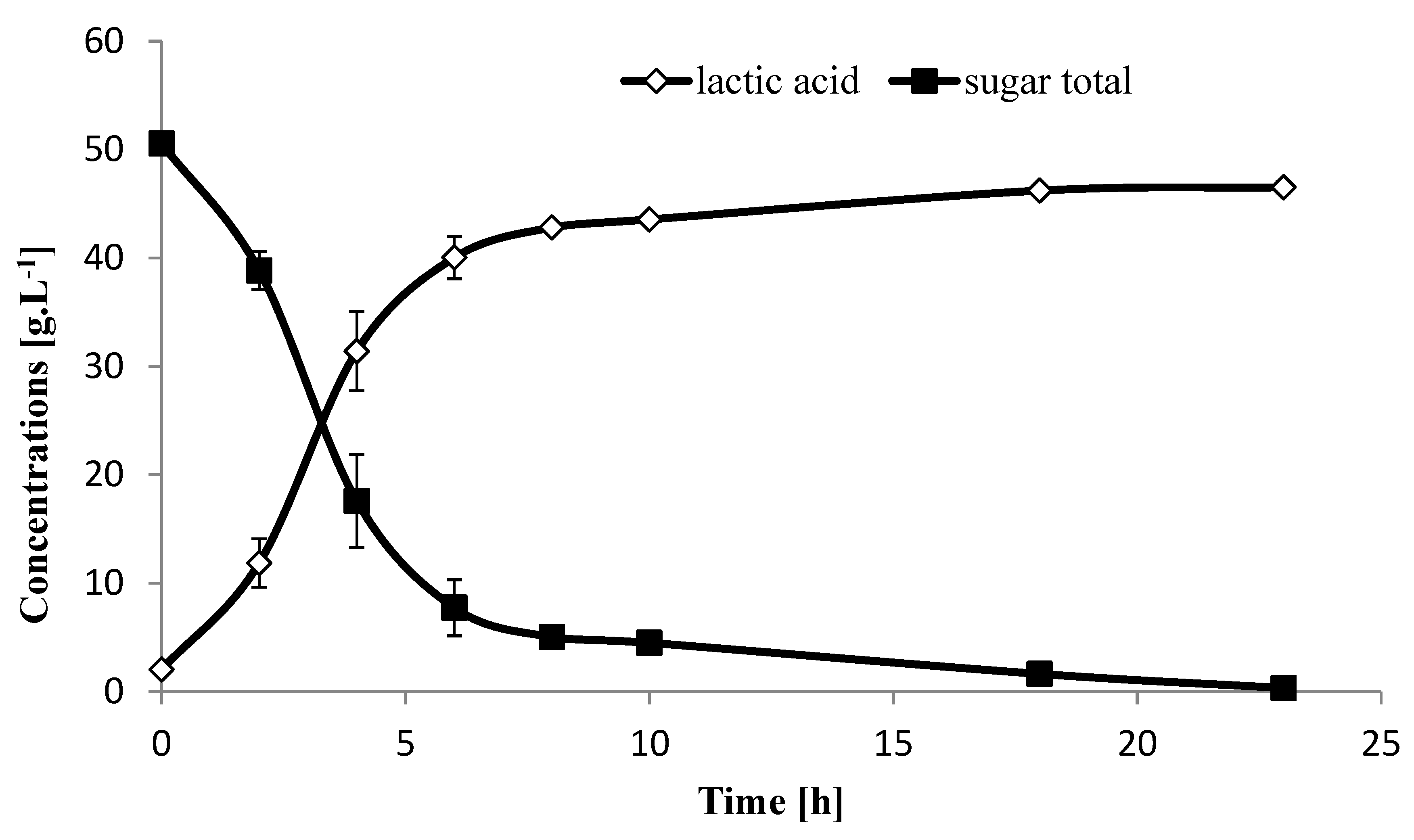
2.3. Fermentation of Sugarcane Biomass
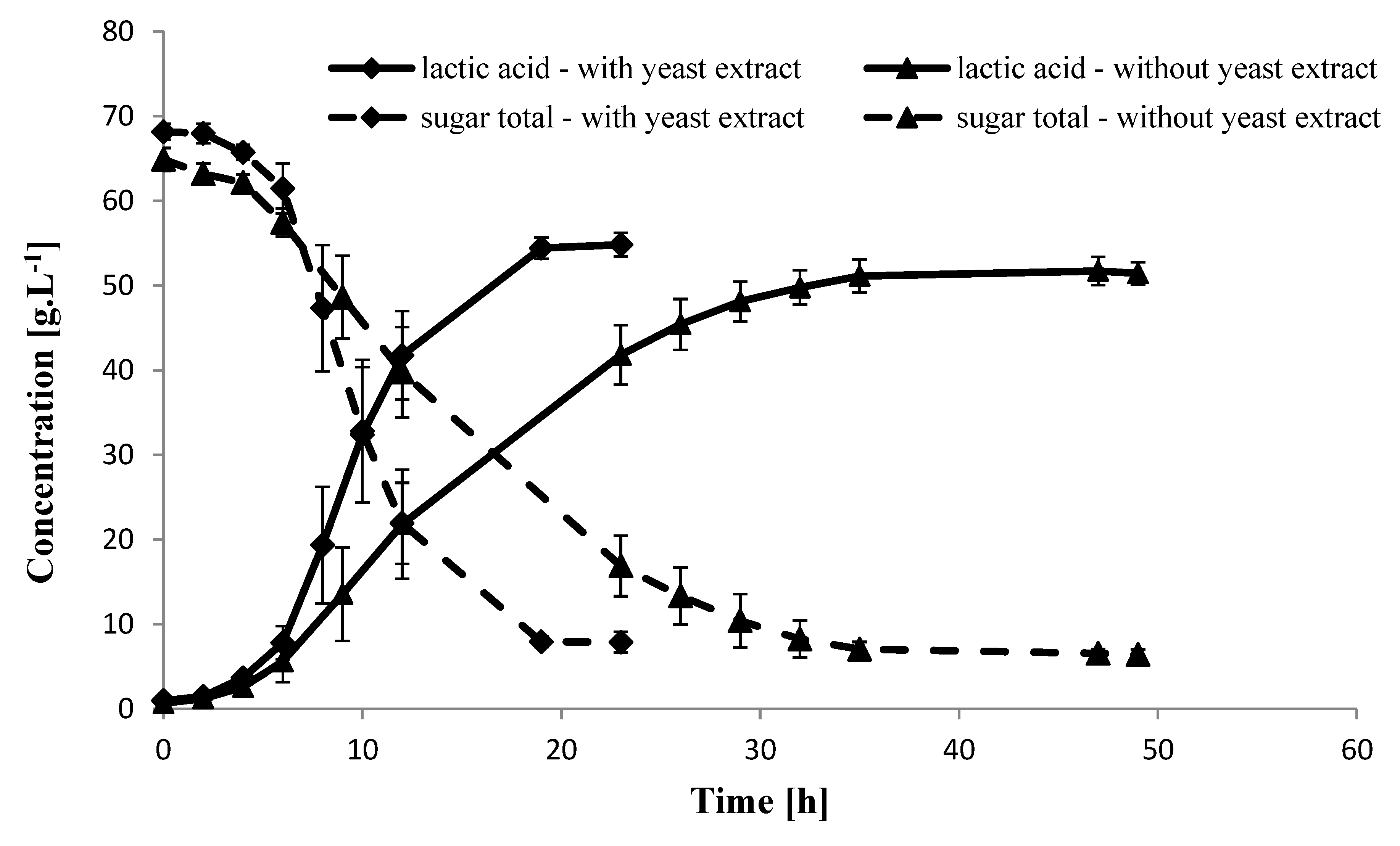
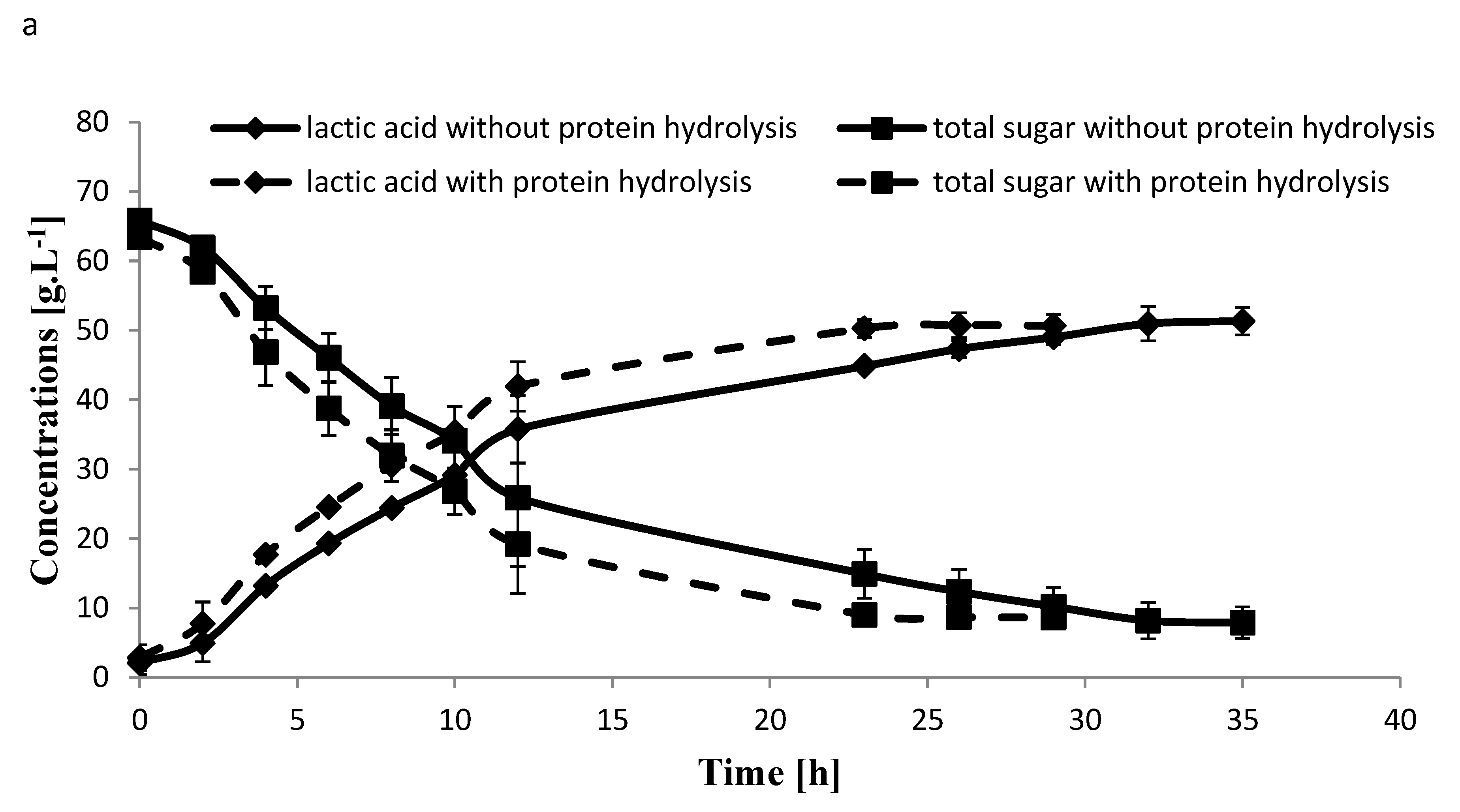
2.4. Fermentation of Carob
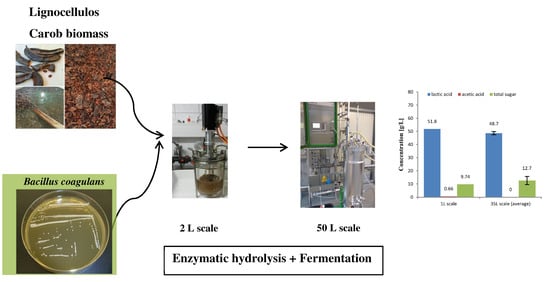
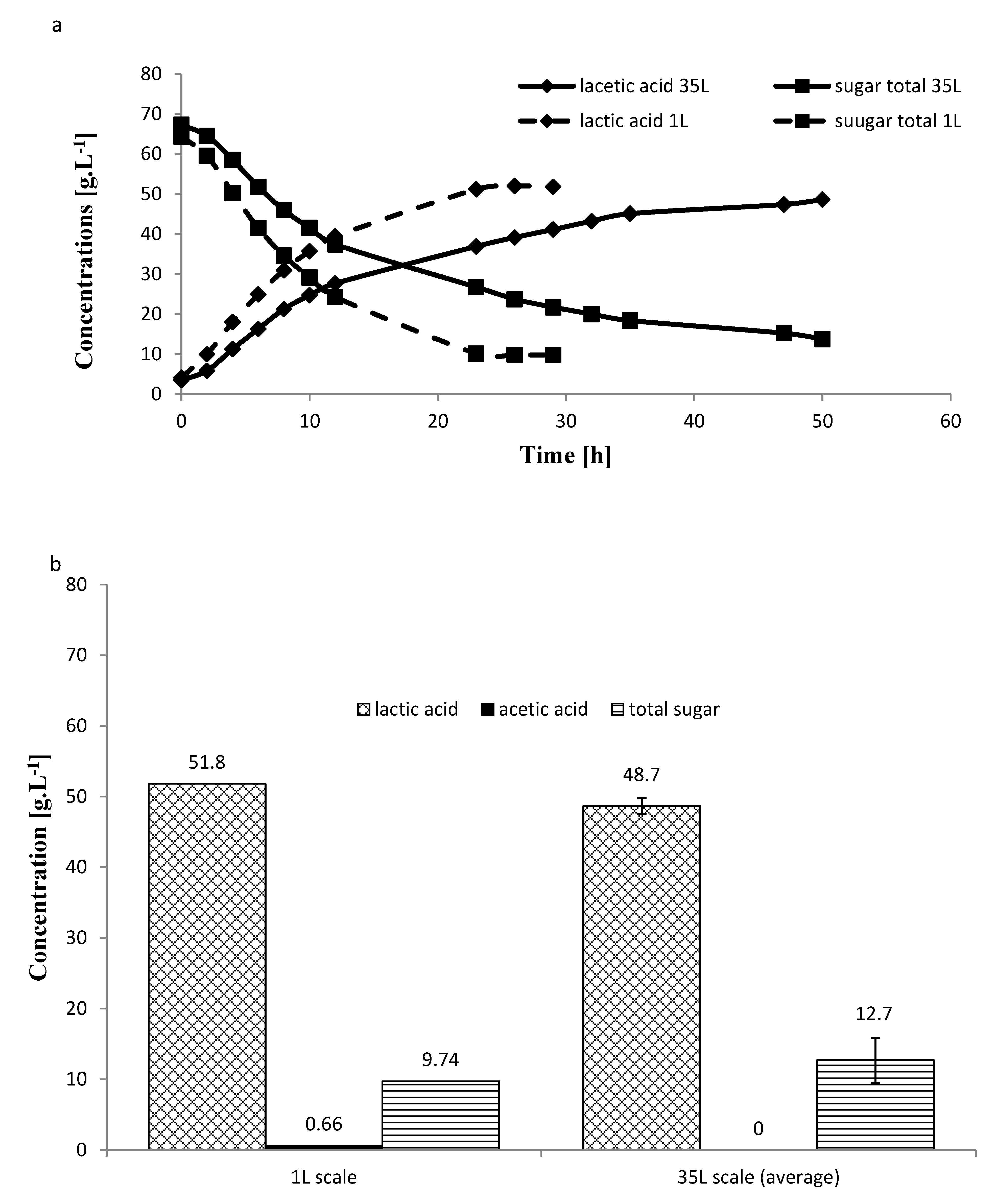
2.5. Larger Scale Fermentation for Carob
3. Materials and Methods
3.1. Feedstock and Biomass Analysis
3.2. Lignocellulose Enzymatic Hydrolysis
3.3. Fermentation
3.4. Analytical Methods
3.5. Larger Scale Fermentation for Carob
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abu Tayeh, H.; Levy-Shalev, O.; Azaizeh, H.; Dosoretz, C.G. Subcritical hydrothermal pretreatment of olive mill solid waste for biofuel production. Bioresour. Technol. 2016, 199, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Van Dyk, J.; Pletschke, B.I. A review of lignocellulose bioconversion using enzymatic hydrolysis and synergistic cooperation between enzymes—Factors affecting enzymes, conversion and synergy. Biotechnol. Adv. 2012, 30, 1458–1480. [Google Scholar] [CrossRef] [PubMed]
- Wischral, D.; Arias, J.M.; Modesto, L.F.; Passos, D.D.F.; Junior, N.P. Lactic acid production from sugarcane bagasse hydrolysates by Lactobacillus pentosus: Integrating xylose and glucose fermentation. Biotechnol. Prog. 2018, 35, e2718. [Google Scholar] [CrossRef] [PubMed]
- Mora-Villalobos, J.A.; Montero-Zamora, J.; Barboza, N.; Rojas-Garbanzo, C.; Usaga, J.; Redondo-Solano, M.; Schroedter, L.; Olszewska-Widdrat, A.; López-Gómez, J.P. Multi-Product Lactic Acid Bacteria Fermentations: A Review. Fermentation 2020, 6, 23. [Google Scholar] [CrossRef]
- Alves de Oliveira, R.; Komesu, A.; Vaz Rossell, C.E.; Maciel Filho, R. Challenges and opportunities in lactic acid bioprocess design- From economic to production aspects. Bio. Eng. J. 2018, 133, 219–239. [Google Scholar] [CrossRef]
- Hu, Y.; Kwan, T.H.; Daoud, W.A.; Lin, C.S.K. Continuous ultrasonic-mediated solvent extraction of lactic acid from fermentation broths. J. Clean. Prod. 2017, 145, 142–150. [Google Scholar] [CrossRef]
- López-Gómez, J.P.; Alexandri, M.; Schneider, R.; Venus, J. A review on the current developments in continuous lactic acid fermentations and case studies utilising inexpensive raw materials. Process. Biochem. 2018, 79, 1–10. [Google Scholar] [CrossRef]
- Panesar, P.S.; Kaur, S. Bioutilisation of agro-industrial waste for lactic acid production. Int. J. Food Sci. Technol. 2015, 50, 2143–2151. [Google Scholar] [CrossRef]
- Djukić-Vuković, A.; MladenoviĆ, D.; Ivanovic, J.; Pejin, J.; Mojović, L. Towards sustainability of lactic acid and poly-lactic acid polymers production. Renew. Sustain. Energy Rev. 2019, 108, 238–252. [Google Scholar] [CrossRef]
- Peinemann, J.C.; Caruso, A.; Demichelis, F.; Pleissner, D. Techno-economic assessment of non-sterile batch and continuous production of lactic acid from food waste. Bioresour. Technol. 2019, 289, 121631. [Google Scholar] [CrossRef]
- Liu, J.; Cai, Y.; Liu, L.; Zhu, J.; Li, H.; Zhan, R.; Xiao, N.; Zhao, S.; He, M.; Hu, G.; et al. Enhanced lactic acid production by Bacillus coagulans through simultaneous saccharification, biodetoxification, and fermentation. Biofuels Bioprod. Biorefining 2020, 14, 533–543. [Google Scholar] [CrossRef]
- Demichelis, F.; Pleissner, D.; Fiore, S.; Mariano, S.; Gutierrez, M.N.; Schneider, R.; Venus, J. Investigation of food waste valorization through sequential lactic acid fermentative production and anaerobic digestion of fermentation residues. Bioresour. Technol. 2017, 241, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Romo-Buchelly, J.; Rodríguez-Torres, M.; Orozco-Sánchez, F. Biotechnological valorization of agro industrial and household wastes for lactic acid production. Rev. Colomb. Biotecnol. 2019, 21, 113–127. [Google Scholar] [CrossRef]
- Das, M.; Kundu, D.; Rastogi, A.; Singh, J.; Banerjee, R. Biotechnological Exploitation of Poly-Lactide Produced from Cost Effective Lactic Acid. In Principles and Applications of Fermentation Technology; Wiley: Hoboken, NJ, USA, 2018; Volume 18, pp. 401–416. [Google Scholar]
- Abdel-Rahman, M.A.; Tan, J.; Tashiro, Y.; Zendo, T.; Sakai, K.; Sonomoto, K. Non-carbon loss long-term continuous lactic acid production from mixed sugars using thermophilic Enterococcus faecium QU 50. Biotechnol. Bioeng. 2020, 117, 1673–1683. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, M.A.; Tashiro, Y.; Zendo, T.; Sakai, K.; Sonomoto, K. Highly efficient l-lactic acid production from xylose in cell recycle continuous fermentation using Enterococcus mundtii QU 25. RSC Adv. 2016, 6, 17659–17668. [Google Scholar] [CrossRef]
- Ben Othmen, K.; Elfalleh, W.; Lachiheb, B.; Haddad, M. Evolution of phytochemical and antioxidant activity of Tunisian carob (Ceratonia siliqua L.) pods during maturation. EuroBiotech J. 2019, 3, 135–142. [Google Scholar] [CrossRef]
- Durazzo, A.; Turfani, V.; Narducci, V.; Azzini, E.; Maiani, G.; Carcea, M. Nutritional characterisation and bioactive components of commercial carobs flours. Food Chem. 2014, 153, 109–113. [Google Scholar] [CrossRef]
- Roseiro, L.B.; Tavares, C.S.; Roseiro, J.; Rauter, A.P.; Roseiro, M.L.D.B.W. Antioxidants from aqueous decoction of carob pods biomass (Ceretonia siliqua L.): Optimisation using response surface methodology and phenolic profile by capillary electrophoresis. Ind. Crop. Prod. 2013, 44, 119–126. [Google Scholar] [CrossRef]
- Sęczyk, Ł.; Świeca, M.; Gawlik-Dziki, U. Effect of carob (Ceratonia siliqua L.) flour on the antioxidant potential, nutritional quality, and sensory characteristics of fortified durum wheat pasta. Food Chem. 2016, 194, 637–642. [Google Scholar] [CrossRef]
- Moreira, T.C.; Da Silva, Á.T.; Fagundes, C.; Ferreira, S.M.R.; Cândido, L.M.B.; Passos, M.; Magnani, M. Elaboration of yogurt with reduced level of lactose added of carob (Ceratonia siliqua L.). LWT 2017, 76, 326–329. [Google Scholar] [CrossRef]
- Markis, D.P.; Kefalas, P. Carob pods (Ceratonia siliqua L.) as a source of polyphenolic antioxydants. Food Technol. Biotechnol. 2004, 42, 105–108. [Google Scholar]
- El Batal, H.; Hasib, A.; Dehbi, F.; Zaki, N.; Ouatmane, A.; Boulli, A. Assessment of nutritional composition of Carob pulp (Ceratonia siliqua L.) collected from various locations in Morocco. J. Mater. Environ. Sci. 2016, 7, 3278–3285. [Google Scholar]
- Karkacier, M.; Artik, N. Physical properties, chemical composition and extraction conditions of carob (Ceratonia siliqua L.). Chemistry 1995, 20, 131–136. [Google Scholar]
- Haddarah, A.; Ismail, A.; Bassal, A.; Hamieh, T.; Ioannou, I.; Ghoul, M. Morphological and chemical variability of Lebanese carob varieties. Eur. Sci. J. 2013, 9, 353–369. [Google Scholar] [CrossRef]
- Said, O.; Khalil, K.; Fulder, S.; Azaizeh, H. Ethnopharmacological survey of medicinal herbs in Israel, the Golan Heights and the West Bank region. J. Ethnopharmacol. 2002, 83, 251–265. [Google Scholar] [CrossRef]
- Sandolo, C.; Coviello, T.; Matricardi, P.; Alhaique, F. Characterization of polysaccharide hydrogels for modified drug delivery. Eur. Biophys. J. 2007, 36, 693–700. [Google Scholar] [CrossRef]
- Bahry, H.; Pons, A.; Abdallah, R.; Pierre, G.; Delattre, C.; Fayad, N.; Taha, S.; Vial, C. Valorization of carob waste: Definition of a second-generation bioethanol production process. Bioresour. Technol. 2017, 235, 25–34. [Google Scholar] [CrossRef]
- Faostat, F.; FAO. Agriculture Organization of the United Nations. 2015. Available online: http://faostat3.fao.org/faostat-gateway/go/to/download/Q/QC/S (accessed on 10 September 2014).
- Mohapatra, D.; Mishra, S.; Sutar, N. Banana and its by-product utilization: An overview. J. Sci. Ind. Res. 2010, 69, 323–329. [Google Scholar]
- Pazmiño-Hernandez, M.; Moreira, C.; Pullammanappallil, P. Feasibility assessment of waste banana peduncle as feedstock for biofuel production. Biofuels 2017, 69, 1–12. [Google Scholar] [CrossRef]
- Fagbemigun, T.K.; Fagbemi, O.D.; Buhari, F.; Mgbachiuzo, E.; Igwe, C.C. Fibre Characteristics and Strength Properties of Nigerian Pineapple Leaf (Ananas cosmosus), Banana Peduncle and Banana Leaf (Musa sapientum)—Potential Green Resources for Pulp and Paper Production. J. Sci. Res. Rep. 2016, 12, 1–13. [Google Scholar] [CrossRef]
- Tavanlar, M.; Ramirez, T.; Sapin, A. Banana Peduncle: To Waste or not To Waste? 2012. Available online: http://agris.fao.org/agris-search/search.do?record.ID=PH2014000240 (accessed on 9 February 2017).
- Bueno, C.M.; Sedano, S.A.; Beltran, E.D. Chemical, cooking and sensory characteristics of burger patties with different levels of banana peduncle powder. Philip. J. Vet. Anim. Sci. 2012, 38, 45–52. [Google Scholar]
- Belmakki, M.; Hansen, A.; Bartali, H. Lactic acid production from un-matured banana peel and flesh through simultaneous saccharification and fermentation. Rev. Mar. Sci. Agron. Vét. 2016, 4, 78–85. [Google Scholar]
- Idrees, M.; Adnan, A.; Malik, F.; Qureshi, F.A. Enzymatic saccharification and lactic acid production from banana pseudo-stem through optimized pretreatment at lowest catalyst concentration. EXCLI J. 2013, 12, 269–281. [Google Scholar] [PubMed]
- Méndez, J.; Passos, D.D.F.; Wischral, D.; Modesto, L.F.; Pereira, N. Second-generation ethanol production by separate hydrolysis and fermentation from sugarcane bagasse with cellulose hydrolysis using a customized enzyme cocktail. Biofuels 2019, 1–7. [Google Scholar] [CrossRef]
- Betancur, G.J.V.; Junior, N.P. Sugar cane bagasse as feedstock for second generation ethanol production. Part I: Diluted acid pretreatment optimization. Electron. J. Biotechnol. 2010, 13, 10–11. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Martin, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef]
- Maitan-Alfenas, G.P.; Visser, E.M.; Guimarães, V.M. Enzymatic hydrolysis of lignocellulosic biomass: Converting food waste in valuable products. Curr. Opin. Food Sci. 2015, 1, 44–49. [Google Scholar] [CrossRef]
- Neu, A.-K.; Pleissner, D.; Mehlmann, K.; Schneider, R.; Quintero, G.I.P.; Venus, J. Fermentative utilization of coffee mucilage using Bacillus coagulans and investigation of down-stream processing of fermentation broth for optically pure l(+)-lactic acid production. Bioresour. Technol. 2016, 211, 398–405. [Google Scholar] [CrossRef]
- Ouis, N.; Hariri, A. Improving of lactic acid production by Lactobacillus plantarum from carob pods syrup. Wulfenia 2018, 27, 12–25. [Google Scholar]
- Turhan, I.; Bialka, K.L.; Demirci, A.; Karhan, M. Enhanced Lactic Acid Production from Carob Extract by Lactobacillus casei Using Invertase Pretreatment. Food Biotechnol. 2010, 24, 364–374. [Google Scholar] [CrossRef]
- Ahring, B.K.; Traverso, J.J.; Murali, N.; Srinivas, K. Continuous fermentation of clarified corn stover hydrolysate for the production of lactic acid at high yield and productivity. Biochem. Eng. J. 2016, 109, 162–169. [Google Scholar] [CrossRef]
- Ajala, E.O.; Olonade, Y.O.; Ajala, M.; Akinpelu, G.S. Lactic Acid Production from Lignocellulose—A Review of Major Challenges and Selected Solutions. ChemBioEng Rev. 2020, 7, 38–49. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Structure Carbohydrates and Lignin in Biomass. Available online: https://www.nrel.gov/docs/gen/fy13/42618.pdf (accessed on 22 March 2018).
- Abu Tayeh, H.N.; Azaizeh, H.; Gerchman, Y. Circular economy in olive oil production—Olive mill solid waste to ethanol and heavy metal sorbent using microwave pretreatment. J. Waste Manag. 2020, 113, 321–328. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |

| DM 105 °C [%] | Proteins [%DM] | Sugars [%DM] | Cellulose [%DM] | Hemicellulose [%DM] | Lignin [%DM] | |
|---|---|---|---|---|---|---|
| Banana | 93.2 | 8.2 | 0.1 | 35.8 | 20.7 | 6.16 |
| Sugarcane | 91.1 | 12.5 | 11.3 | 27.9 | 25.6 | 2.79 |
| Carob | 85.8 | 9.3 | 27.7 | 19.0 | 0.35 | 28.4 |
| Glucose (g·L−1) | Disaccharides (g·L−1) | Xylose (g·L−1) | Arabinose (g·L−1) | Total Sugars (g·L−1) | |
|---|---|---|---|---|---|
| Banana | 24.9 ± 1.6 | 3.0 ± 0.4 | 4.3 ± 0.0 | 0.4 ± 0.0 | 32.6 + 2.1 |
| Sugarcane | 30.6 ± 0.7 | 3.3 ± 0.0 | 19.4 ± 0.4 | 1.3 ± 0.1 | 54.6 + 1.2 |
| Glucose (g·L−1) | Disaccharides (g·L−1) | Fructose (g·L−1) | Total Sugars (g·L−1) | |
|---|---|---|---|---|
| With Protein hydrolysis | 36.6 ± 0.3a | 3.0 ± 1.1a | 27.2 ± 0.2a | 66.8 + 1.6a |
| Without Protein hydrolysis | 38.8 ± 0.1b | 3.0 ± 1.5a | 28.5 ± 0.0b | 70.3 + 1.6a |
| Glucose (g·L−1) | Disaccharides (g·L−1) | Fructose (g·L−1) | Total Sugars (g·L−1) | |
|---|---|---|---|---|
| One liter fermenter | 36.8 | 3.8 | 27.3 | 67.9 |
| 35 L pilot scale | 38.8 | 3.8 | 28.0 | 70.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azaizeh, H.; Abu Tayeh, H.N.; Schneider, R.; Klongklaew, A.; Venus, J. Production of Lactic Acid from Carob, Banana and Sugarcane Lignocellulose Biomass. Molecules 2020, 25, 2956. https://doi.org/10.3390/molecules25132956
Azaizeh H, Abu Tayeh HN, Schneider R, Klongklaew A, Venus J. Production of Lactic Acid from Carob, Banana and Sugarcane Lignocellulose Biomass. Molecules. 2020; 25(13):2956. https://doi.org/10.3390/molecules25132956
Chicago/Turabian StyleAzaizeh, Hassan, Hiba N. Abu Tayeh, Roland Schneider, Augchararat Klongklaew, and Joachim Venus. 2020. "Production of Lactic Acid from Carob, Banana and Sugarcane Lignocellulose Biomass" Molecules 25, no. 13: 2956. https://doi.org/10.3390/molecules25132956
APA StyleAzaizeh, H., Abu Tayeh, H. N., Schneider, R., Klongklaew, A., & Venus, J. (2020). Production of Lactic Acid from Carob, Banana and Sugarcane Lignocellulose Biomass. Molecules, 25(13), 2956. https://doi.org/10.3390/molecules25132956