Production and Potential Applications of Bioconversion of Chitin and Protein-Containing Fishery Byproducts into Prodigiosin: A Review
Abstract
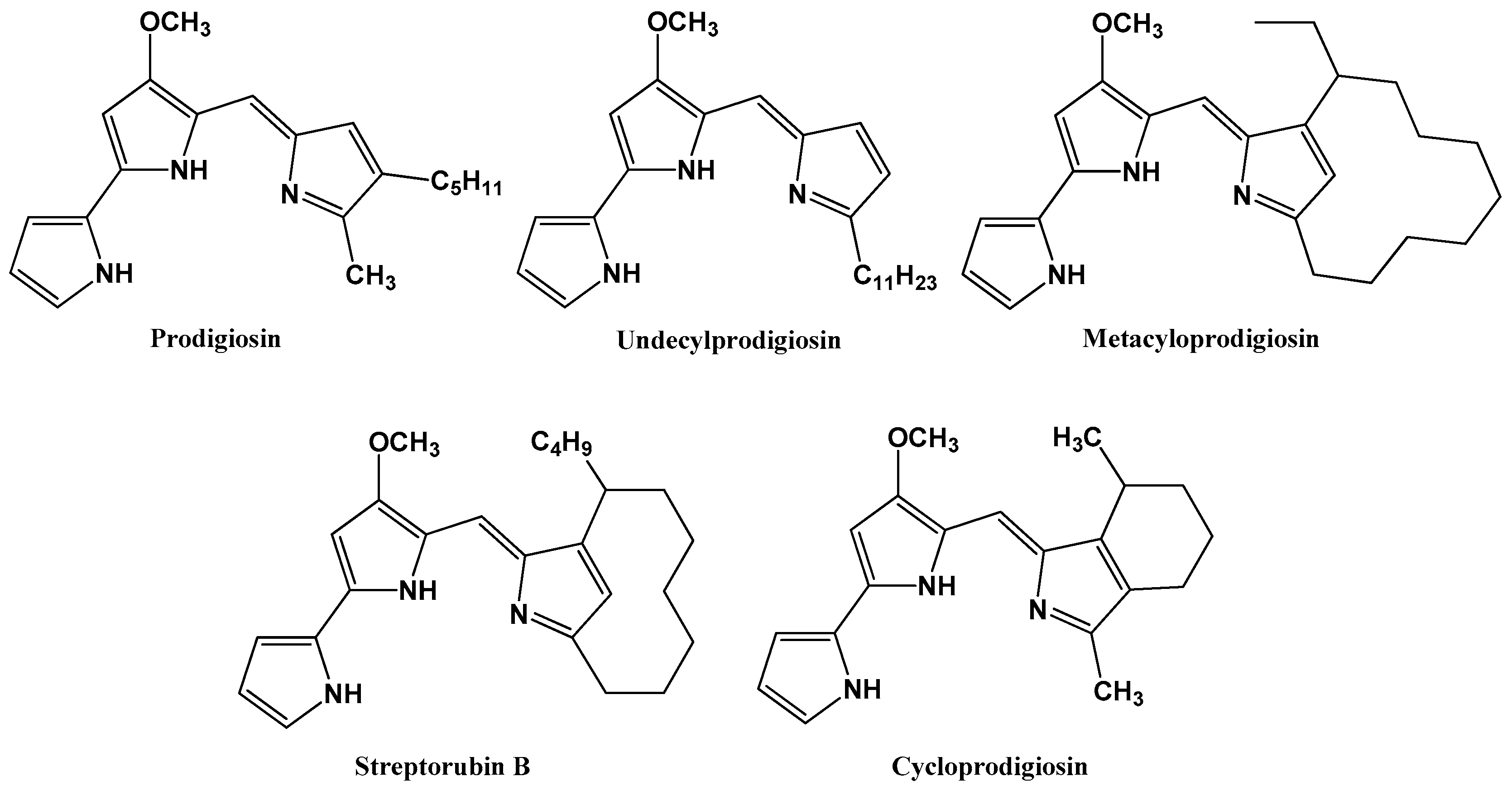
1. Introduction
2. Production of PGs
3. Bioactivity and Application of PG
3.1. Antimicrobial Activity
3.2. Antiparasitic Activity
3.3. Insecticidal Activity
3.4. Anti-Cancer Activity
3.5. Algicidal Activity
3.6. Dyes
4. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Tran, N.V.N.; Yu, J.; Nguyen, T.P.; Wang, S.L. Coagulation of chitin production wastewater from shrimp scraps with by-product chitosan and chemical coagulants. Polymers 2020, 12, 607. [Google Scholar] [CrossRef] [PubMed]
- Ha, N.M.C.; Wang, S.L.; Nguyen, A.D. Preparation of NPK nanofertilizer based on chitosan nanoparticles and its effect on biophysical characteristics and growth of coffee in green house. Res. Chem. Intermed. 2019, 45, 51–63. [Google Scholar] [CrossRef]
- Shaala, L.A.; Asfour, H.Z.; Youssef, D.T.A.; Zółtowska-Aksamitowska, S.; Wysokowski, M.; Tsurkan, M.; Galli, R.; Meissner, H.; Petrenko, I.; Tabachnick, K.; et al. New source of 3D chitin scaffolds: The Red Sea demosponge Pseudoceratina arabica (Pseudoceratinidae, Verongiida). Mar. Drugs 2019, 17, 92. [Google Scholar] [CrossRef] [PubMed]
- Casadidio, C.; Peregrina, D.V.; Gigliobianco, M.R.; Deng, S.; Censi, R.; Di Martino, P. Chitin and chitosans: Characteristics, eco-friendly processes, and applications in cosmetic science. Mar. Drugs 2019, 17, 369. [Google Scholar] [CrossRef] [PubMed]
- Doan, C.T.; Tran, T.N.; Nguyen, V.B.; Vo, T.P.K.; Nguyen, A.D.; Wang, S.L. Chitin extraction from shrimp waste by liquid fermentation using an alkaline protease-producing strain, Brevibacillus parabrevis. Int. J. Biol. Macromol. 2019, 131, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Doan, C.T.; Tran, T.N.; Nguyen, V.B.; Nguyen, A.D.; Wang, S.L. Production of a thermostable chitosanase from shrimp heads via Peanibacillus mucilaginosus TKU032 conversion and its application in the preparation of bioactive chitosan oligosaccharides. Mar. Drugs 2019, 17, 217. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, M.; Abou-Elmagd, W.S.I.; Suloma, A.; El-Shabaka, H.A.; Khalil, M.T.; El-Rahman, F.A.A. Optimization and physicochemical characterization of chitosan and chitosan nanoparticles extracted from the crayfish Procambarus clarkii wastes. J. Shellfish Res. 2019, 38, 385. [Google Scholar] [CrossRef]
- Kaczmarek, M.B.; Struszczyk-Swita, K.; Li, X.; Szczęsna-Antczak, M.; Daroch, M. Enzymatic modifications of chitin, chitosan, and chitooligosaccharides. Front. Bioeng. Biotechnol. 2019, 7, 243. [Google Scholar] [CrossRef]
- Wang, C.H.; Doan, C.T.; Nguyen, V.B.; Nguyen, A.D.; Wang, S.L. Reclamation of fishery processing waste: A mini-review. Molecules 2019, 24, 2234. [Google Scholar] [CrossRef]
- Santos-Moriano, P.; Fernandez-Arrojo, L.; Mengibar, M.; Belmonte-Reche, E.; Peñalver, P.; Acosta, F.; Ballesteros, A.; Morales, J.; Kidibule, P.; Fernandez-Lobato, M.; et al. Enzymatic production of fully deacetylated chitooligosaccharides and their neuroprotective and anti-inflammatory properties. Biocatal. Biotransform. 2018, 1, 36. [Google Scholar] [CrossRef]
- Pighinelli, W.L.; Broquá, J.; Zanin, B.G.; Zanin, B.G.; Flach, A.M.; Mallmann, C.; Taborda, F.G.D.; Machado, L.E.L.; Alves, S.M.L.; Silva, M.M.; et al. Methods of chitin production a short review. Am. J. Biomed. Sci. Res. 2019, 3, AJBSR.MS.ID.000682. [Google Scholar] [CrossRef]
- Oh, Y.S.; Shieh, I.L.; Tzeng, Y.M.; Wang, S.L. Protease produced by Pseudomonas aeruginosa K-187 and its application in the deproteinization of shrimp and crab shell wastes. Enzyme Microb. Technol. 2000, 27, 3–10. [Google Scholar] [CrossRef]
- Yang, J.K.; Shih, I.L.; Tzeng, Y.M.; Wang, S.L. Production and purification of protease from a Bacillus subtilis that can deproteinize crustacean wastes. Enzyme Microb. Technol. 2010, 26, 406–413. [Google Scholar] [CrossRef]
- Wang, S.L.; Chio, S.H. Deproteinization of shrimp and crab shell with the protease of Pseudomonas aeruginosa K-187. Enzyme Microb. Technol. 1998, 22, 629–633. [Google Scholar] [CrossRef]
- Wang, S.L.; Liang, T.W.; Yen, Y.H. Bioconversion of chitin-containing wastes for the production of enzymes and bioactive materials (review paper). Carbohydr. Polym. 2011, 84, 732–742. [Google Scholar] [CrossRef]
- Wang, S.L. Microbial reclamation of squid pen. Biocatal. Agric. Biotechnol. 2012, 1, 177–180. [Google Scholar] [CrossRef]
- Abu-Tahon, M.; Isaac, G.S. Anticancer and antifungal efficiencies of purified chitinase produced from Trichoderma viride under submerged fermentation. J. Gen. Appl. Microbiol. 2020, 66, 32–40. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Z.; Liu, Y.; Cao, Y.; He, S.; Huo, F.; Qin, C.; Yao, B.; Ringø, E. High-yield production of a chitinase from Aeromonas veronii B565 as a potential feed supplement for warm-water aquaculture. Appl. Microbiol. Biotechnol. 2014, 98, 1651–1662. [Google Scholar] [CrossRef]
- Doan, C.T.; Tran, T.N.; Nguyen, M.T.; Nguyen, V.B.; Nguyen, A.D.; Wang, S.L. Anti-α-glucosidase activity by a protease from Bacillus licheniformis. Molecules 2019, 24, 691. [Google Scholar] [CrossRef]
- Doan, C.T.; Tran, T.N.; Wen, I.H.; Nguyen, V.B.; Nguyen, A.D.; Wang, S.L. Conversion of shrimp head waste for production of a thermotolerant, detergent-stable, alkaline protease by Paenibacillus sp. Catalysts 2019, 9, 798. [Google Scholar] [CrossRef]
- Huynh, N.T.; Suyotha, W.; Yano, S.; Konno, H.; Cheirsilp, B.; Wakayama, M. Low-cost production of chitosanolytic enzymes from Lentzea sp. strain OUR-I1 for the production of antimicrobial substances against food-borne pathogens. Int. Food Res. J. 2019, 26, 1293–1304. [Google Scholar]
- Tran, T.N.; Doan, C.T.; Nguyen, M.T.; Nguyen, V.B.; Vo, T.P.K.; Nguyen, A.D.; Wang, S.L. An exochitinase with N-acetyl-β-glucosaminidase activity from shrimp heads conversion by Streptomyces speibonae and its application in hydrolyzing β-chitin powder to produce N-acetyl-D-glucosamine. Polymers 2019, 11, 1600. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.N.; Doan, C.T.; Nguyen, V.B.; Nguyen, A.D.; Wang, S.L. The isolation of chitinase from Streptomyces thermocarboxydus and its application in the preparation of chitin oligomers. Res. Chem. Intermed. 2019, 45, 727–742. [Google Scholar] [CrossRef]
- Doan, C.T.; Tran, T.N.; Nguyen, V.B.; Nguyen, A.D.; Wang, S.L. Reclamation of marine chitinous materials for chitosanase production via microbial conversion by Paenibacillus macerans. Mar. Drugs 2018, 16, 429. [Google Scholar] [CrossRef] [PubMed]
- Doan, C.T.; Tran, T.N.; Nguyen, V.B.; Nguyen, A.D.; Wang, S.L. Conversion of squid pens to chitosanases and proteases via Paenibacillus sp. TKU042. Mar. Drugs 2018, 16, 83. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.L.; Liang, T.W. Microbial reclamation of squid pens and shrimp shells. Res. Chem. Intermed. 2017, 43, 3445–3462. [Google Scholar] [CrossRef]
- Liang, T.W.; Chen, W.T.; Lin, Z.H.; Kuo, Y.H.; Nguyen, A.D.; Pan, P.S.; Wang, S.L. An amphiprotic novel chitosanase from Bacillus mycoides and its application in the production of chitooligomers with their antioxidant and anti-inflammatory evaluation. Int. J. Mol. Sci. 2016, 17, 1302. [Google Scholar] [CrossRef]
- Liang, T.W.; Lo, B.C.; Wang, S.L. Chitinolytic bacteria-assisted conversion of squid pen and its effect on dyes and pigments adsorption. Mar. Drugs 2015, 13, 4576–4593. [Google Scholar] [CrossRef]
- Nguyen, A.D.; Huang, C.C.; Liang, T.W.; Nguyen, V.B.; Pan, P.S.; Wang, S.L. Production and purification of a fungal chitosanase and chitooligomers from Penicillium janthinellum D4 and discovery of the enzyme activators. Carbohydr. Polym. 2014, 108, 331–337. [Google Scholar] [CrossRef]
- Wang, C.L.; Su, J.W.; Liang, T.W.; Nguyen, A.D.; Wang, S.L. Production, purification and characterization of a chitosanase from Bacillus cereus. Res. Chem. Intermed. 2014, 40, 2237–2248. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, W. Induction and optimization of chitosanase production by Aspergillus fumigatus YT-1 using response surface methodology. Chem. Biochem. Eng. Q. 2013, 27, 335–345. [Google Scholar]
- Brzezinska, M.S.; Walczak, M.; Lalke-Porczyk, E.; Donderski, W. Utilization of shrimp-shell waste as a substrate for the activity of chitinases produced by microorganisms. Pol. J. Environm. Stud. 2010, 19, 177–182. [Google Scholar]
- Wang, S.L.; Hsu, W.H.; Liang, T.W. Conversion of squid pen by Pseudomonas aeruginosa K187 fermentation for the production of N-acetyl chitooligosaccharides and biofertilizers. Carbohydr. Res. 2010, 345, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.L.; Chang, T.J.; Liang, T.W. Conversion and degradation of shellfish wastes by Serratia sp. TKU016 fermentation for the production of enzymes and bioactive materials. Biodegradation 2010, 21, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.T.; Chen, M.L.; Wang, S.L. An antifungal chitinase produced by Bacillus subtilis using chitin waste as a carbon source. World J. Microbiol. Biotechnol. 2010, 26, 945–950. [Google Scholar] [CrossRef]
- Liang, T.W.; Kuo, Y.H.; Wu, P.C.; Wang, C.L.; Dzung, N.A.; Wang, S.L. Purification and characterization of a chitosanase and a protease by conversion of shrimp shell wastes fermented by Serratia marcescens subsp. sakuensis TKU019. J. Chin. Chem. Soc. 2010, 57, 857–863. [Google Scholar] [CrossRef]
- Wang, S.L.; Peng, J.H.; Liang, T.W.; Liu, K.C. Purification and characterization of a chitosanase from Serratia marcescens TKU011. Carbohydr. Res. 2008, 343, 1316–1323. [Google Scholar] [CrossRef]
- Wang, S.L.; Yeh, P.Y. Purification and characterization of a chitosanases from a nattokinase producing strain Bacillus subtilis TKU007. Process Biochem. 2008, 43, 132–138. [Google Scholar] [CrossRef]
- Ruiz-Sànchez, A.; Cruz-amarillo, R.; Salcedo-Hernandez, R.; Barboza-Corona, J.E. Chitinases from Serratia marcescens Nima. Biotechnol. Lett. 2005, 27, 649–653. [Google Scholar] [CrossRef]
- Wang, S.L.; Huang, T.Y.; Wang, C.Y.; Liang, T.W.; Yen, Y.H.; Sakata, Y. Bioconversion of squid pen by Lactobacillus paracasei subsp. paracasei TKU010 for the production of proteases and lettuce growth enhancing biofertilizers. Bioresour. Technol. 2008, 99, 5436–5443. [Google Scholar] [CrossRef]
- Liang, T.W.; Yen, Y.H.; Lin, J.J.; Wang, S.L. Purification and characterization of a protease extracellularly produced by Monascus purpureus CCRC31499 in a shrimp and crab shell powder medium. Enzyme Microb. Technol. 2006, 38, 74–80. [Google Scholar] [CrossRef]
- Wang, S.L.; Lin, T.Y.; Yen, Y.H.; Liao, H.F.; Chen, Y.J. Bioconversion of shellfish chitin wastes for the production of Bacillus subtilis W-118 chitinase. Carbohydr. Res. 2006, 341, 2507–2515. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.T.; Chen, J.S.; Wang, S.L. An antifungal chitinase produced by Bacillus cereus with shrimp and crab shell powder as a carbon source. Curr. Microbiol. 2003, 47, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.L.; Hsiao, W.J.; Chang, W.T. Purification and characterization of an antimicrobial chitinase extracellularly produced by Monascus purpureus CCRC31499 in a shrimp and crab shell powder medium. J. Agric. Food Chem. 2002, 50, 2249–2255. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.L.; Yen, Y.H.; Tsiao, W.J.; Chang, W.T.; Wang, C.L. Production of antimicrobial compounds by Monascus purpureus CCRC31499 using shrimp and crab shell powder as a carbon source. Enzyme Microb. Technol. 2002, 31, 337–344. [Google Scholar] [CrossRef]
- Wang, S.L.; Shih, I.L.; Liang, T.W.; Wang, C.H. Purification and characterization of two antifungal chitinases extracellularly produced by Bacillus amyloliquefaciens V656 in a shrimp and crab shell powder medium. J. Agric. Food Chem. 2002, 50, 2241–2248. [Google Scholar] [CrossRef]
- Wang, S.L.; Shih, I.L.; Wang, C.H.; Chang, W.T.; Tu, Y.G.; Ro, J.J.; Wang, C.L. Production of antifungal compounds from chitin by Bacillus subtilis. Enzyme Microb. Technol. 2002, 31, 321–328. [Google Scholar]
- Wang, S.L.; Yieh, T.C.; Shieh, I.L. Purification and characterization of a new antifungal compound produced by Pseudomonas aeruginosa K-187 in a shrimp and crab shell powder medium. Enzyme Microb. Technol. 1999, 25, 439–446. [Google Scholar] [CrossRef]
- Wang, S.L.; Yieh, T.C.; Shih, I.L. Production of antifungal compounds by Pseudomonas aeruginosa K-187 using shrimp and crab shell powder as a carbon source. Enzyme Microb. Technol. 1999, 25, 142–148. [Google Scholar] [CrossRef]
- Wang, S.L.; Chang, W.T. Purification and characterization of two bifunctional chitinases/lysozymes extracellularly produced by Pseudomonas aeruginosa K-187 in a shrimp and crab shell powder medium. Appl. Environ. Microbiol. 1997, 63, 380–386. [Google Scholar] [CrossRef]
- Liang, T.W.; Jen, S.N.; Nguyen, A.D.; Wang, S.L. Application of chitinous materials in production and purification of a poly(L-lactic acid) depolymerase from Pseudomonas tamsuii TKU015. Polymers 2016, 8, 98. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.L.; Wu, Y.Y.; Liang, T.W. Purification and biochemical characterization of a nattokinase by conversion of shrimp shell with Bacillus subtilis TKU007. New Biotechnol. 2011, 28, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.W.; Lee, Y.C.; Wang, S.L. Tyrosinase inhbitory activity of supernatant and semi-purified extracts from squid pen fermeted with Burkholderia cepacia TKU025. Res. Chem. Intermed. 2015, 41, 6105–6116. [Google Scholar] [CrossRef]
- Hsu, C.H.; Nguyen, A.D.; Chen, Y.W.; Wang, S.L. Tyrosinase inhibitors and insecticidal materials produced by Burkholderia cepacia using squid pen as the sole carbon and nitrogen source. Res. Chem. Intermed. 2014, 40, 2249–2258. [Google Scholar] [CrossRef]
- Liang, T.W.; Tseng, S.C.; Wang, S.L. Production and characterization of antioxidant properties of exopolysaccharides from Paenibacillus mucilaginosus TKU032. Mar. Drugs 2016, 14, 40. [Google Scholar] [CrossRef]
- Liang, T.W.; Wang, S.L. Recent advances in exopolysaccharides from Paenibacillus spp.: Production, isolation, structure, and bioactivities-review. Mar. Drugs 2015, 13, 1847–1863. [Google Scholar] [CrossRef]
- Wang, S.L.; Li, H.T.; Zhang, L.J.; Lin, Z.H.; Kuo, Y.H. Conversion of squid pen to homogentisic acid via Paenibacillus sp. TKU036 and the antioxidant and anti-inflammatory activities of homogentisic acid. Mar. Drugs 2016, 14, 183. [Google Scholar] [CrossRef]
- Nguyen, V.B.; Nguyen, T.H.; Doan, C.T.; Tran, T.N.; Nguyen, A.D.; Kuo, Y.H.; Wang, S.L. Production and bioactivity-guided isolation of antioxidants with α-glucosidase inhibitory and anti-NO properties from marine chitinous materials. Molecules 2018, 23, 1124. [Google Scholar] [CrossRef]
- Nguyen, V.B.; Chen, S.P.; Nguyen, T.H.; Nguyen, M.T.; Tran, T.T.T.; Doan, C.T.; Tran, T.G.; Nguyen, A.D.; Kuo, Y.H.; Wang, S.L. Novel efficient bioprocessing of marine chitins into active anticancer prodigiosin. Mar. Drugs 2020, 18, 15. [Google Scholar] [CrossRef]
- Liang, T.W.; Chen, S.Y.; Chen, C.H.; Yen, Y.H.; Wang, S.L. Enhancement of prodigiosin production by Serratia marcescens TKU011 and its insecticidal activity relative to food colorants. J. Food Sci. 2013, 78, 1743–1751. [Google Scholar] [CrossRef]
- Wang, S.L.; Wang, C.Y.; Yen, Y.H.; Liang, T.W.; Chen, S.Y.; Chen, C.H. Enhanced production of insecticidal prodigiosin from Serratia marcescens TKU011 in media containing squid pen. Process Biochem. 2012, 47, 1684–1690. [Google Scholar] [CrossRef]
- Wang, S.L.; Chen, S.Y.; Yen, Y.H.; Liang, T.W. Utilization of chitinous materials in pigment adsorption. Food Chem. 2012, 135, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.B.; Wang, S.L. Production of potent antidiabetic compounds from shrimp head powder via Paenibacillus conversion. Process Biochem. 2019, 76, 18–24. [Google Scholar] [CrossRef]
- Nguyen, V.B.; Wang, S.L. New novel α-glucosidase inhibitors produced by microbial conversion. Process Biochem. 2018, 65, 228–232. [Google Scholar] [CrossRef]
- Nguyen, V.B.; Wang, S.L. Reclamation of marine chitinous materials for the production of α-glucosidase inhibitors via microbial conversion. Mar. Drugs 2017, 15, 350. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.B.; Nguyen, A.D.; Wang, S.L. Utilization of fishery processing α-glucosidase inhibitors production by Paenibacillus sp. Mar. Drugs 2017, 15, 274. [Google Scholar] [CrossRef]
- Nguyen, V.B.; Nguyen, A.D.; Kuo, Y.H.; Wang, S.L. Biosynthesis of α-glucosidase inhibitors by a newly isolated bacterium, Paenibacillus sp. TKU042 and its effect on reducing plasma glucose in mouse model. Int. J. Mol. Sci. 2017, 18, 700. [Google Scholar] [CrossRef]
- Liang, T.W.; Chen, Y.J.; Yen, Y.H.; Wang, S.L. The antitumor activity of the hydrolysates of chitinous materials hydrolyzed by crude enzyme from Bacillus amyloliquefaciens V656. Process Biochem. 2007, 42, 527–534. [Google Scholar] [CrossRef]
- Kuo, Y.H.; Liang, T.W.; Liu, K.C.; Hsu, Y.W.; Hsu, H.C.; Wang, S.L. Isolation and identification of a novel antioxidant with antitumour activity from Serratia ureilytica using squid pen as fermentation substrate. Mar. Biotechnol. 2011, 13, 451–461. [Google Scholar] [CrossRef]
- Rashid, H.A.; Jung, H.Y.; Kim, J.K. Enhanced reutilization value of shrimp-shell waste via fed-batch biodegradation with higher production of reducing sugar, antioxidant, and DNA protective compounds. Fish. Aquat. Sci. 2018, 21, 33. [Google Scholar] [CrossRef]
- Wang, S.L.; Liu, K.C.; Liang, T.W.; Kuo, Y.H.; Wang, C.Y. In vitro antioxidant activity of liquor and semi-purified fractions from fermented squid pen biowaste by Serratia ureilytica TKU013. Food Chem. 2010, 119, 1380–1385. [Google Scholar] [CrossRef]
- Wang, S.L.; Li, J.Y.; Liang, T.W.; Hsieh, J.L.; Tseng, W.N. Conversion of shrimp shell by using Serratia sp. TKU017 fermentation for the production of enzymes and antioxidants. J. Microbiol. Biotechnol. 2010, 20, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.W.; Chen, C.H.; Wang, S.L. Production of insecticidal materials from Pseudomonas tamsuii. Res. Chem. Intermed. 2015, 41, 7965–7971. [Google Scholar] [CrossRef]
- Liang, T.W.; Huang, C.T.; Nguyen, A.D.; Wang, S.L. Squid pen chitin oligomers as pigment adsorbents. Mar. Drugs 2015, 13, 681–696. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, C.; Vinithkumar, N.V.; Kirubagaran, R.; Venil, C.K.; Dufossé, L. Multifaceted applications of microbial pigments: Current knowledge, challenges and future directions for public health implications. Microorganisms 2019, 7, 186. [Google Scholar] [CrossRef] [PubMed]
- Mumtaz, R.; Bashir, S.; Numan, M.; Shinwari, Z.K.; Ali, M. Pigments from soil bacteria and their therapeutic properties: A mini review. Curr. Microbiol. 2019, 76, 783–790. [Google Scholar] [CrossRef]
- Soliev, A.B.; Hosokawa, K.; Enomoto, K. Bioactive pigments from marine bacteria: Applications and physiological roles. Evid.-Based Complement. Altern. Med. 2011, 2011, 670349–670356. [Google Scholar] [CrossRef]
- Chang, C.C.; Chen, W.C.; Ho, T.F.; Wu, H.S.; Wei, Y.H. Development of natural anti-tumor drugs by microorganism. J. Biosci. Bioeng. 2011, 111, 501–511. [Google Scholar] [CrossRef]
- Akilandeswari, P.; Pradeep, B.V. Exploration of industrially important pigments from soil fungi. Appl. Microbiol. Biotechnol. 2016, 100, 1631–1643. [Google Scholar] [CrossRef]
- Numan, M.; Bashir, S.; Mumtaz, R.; Tayyab, S.; Rehman, N.U.; Khan, A.L.; Shinwari, Z.K.; Al-Harrasi, A. Therapeutic applications of bacterial pigments: A review of current status and future opportunities. 3 Biotech 2018, 8, 207. [Google Scholar] [CrossRef]
- Parani, K.; Saha, B.K. Optimization of prodigiosin production from a strain of Serratia marcescens SR1 and screening for antifungal activity. J. Biol. Control 2008, 22, 73–79. [Google Scholar]
- Picha, P.; Kale, D.; Dave, I.; Pardeshi, S. Comparative studies on prodigiosin production by Serratia marcescens using various crude fatty acid sources-its characterization and applications. Int. J. Curr. Microbiol. Appl. Sci. 2015, 2, 254–267. [Google Scholar]
- Lapenda, J.C.; Silva, P.A.; Vicalvi, M.C.; Sena, K.X.F.R.; Nascimento, S.C. Antimicrobial activity of prodigiosin isolated from Serratia marcescens UFPEDA 398. World J. Microbiol. Biotechnol. 2015, 31, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, D.; Nazari, T.F.; Kassim, J.; Lim, S.H. Prodigiosin-an antibacterial red pigment produced by Serratia marcescens IBRL USM 84 associated with a marine sponge Xestopongia testudinaria. J. Appl. Pharm. Sci. 2014, 4, 001–006. [Google Scholar] [CrossRef]
- Someya, N.; Nakajima, M.; Watanabe, K.; Hibi, T.; Akutsu, K. Potential of Serratia marcescens strain B2 for biological control of rice sheath blight. Biocontrol Sci. Technol. 2005, 15, 105–109. [Google Scholar] [CrossRef]
- Someya, N.; Nakajima, M.; Hirayae, K.; Hibi, T.; Akutsu, K. Synergistic antifungal activity of chitinolytic enzymes and prodigiosin produced by biocontrol bacterium, Serratia marcescens strain B2 against gray mold pathogen, Botrytis cinerea. J. Gen. Plant Pathol. 2001, 67, 312–317. [Google Scholar] [CrossRef]
- Nguyen, V.B.; Nguyen, D.N.; Wang, S.L. Microbial reclamation of chitin and protein-containing marine by-products for the production of prodigiosin and the evaluation of its bioactivities. Polymers 2020, 12, 1328. [Google Scholar]
- Gutiérrez-Román, M.I.; Holguin-Melendez, F.; Dunn, F.M.; Guillén-Navarro, K.; Huerta, G. Antifungal activity of Serratia marcescens CFFSUR-B2 purified chitinolytic enzymes and prodigiosin against Mycosphaerella fijiensis, causal agent of black Sigatoka in banana (Musa spp.). BioControl 2015, 60, 565–572. [Google Scholar] [CrossRef]
- Purkayastha, G.D.; Mangar, P.; Saha, A.; Saha, D. Evaluation of the biocontrol efficacy of a Serratia marcescens strain indigenous to tea rhizosphere for the management of root rot disease in tea. PLoS ONE 2018, 13, e0191761. [Google Scholar] [CrossRef]
- Gutiérrez-Román, M.I.; Holguín-Meléndez, F.; Bello-Mendoza, R.; GuillénNavarro, K.; Dunn, M.F.; Huerta-Palacios, G. Production of prodigiosin and chitinases by tropical Serratia marcescens strains with potential to control plant pathogens. World J. Microbiol. Biotechnol. 2012, 28, 145–153. [Google Scholar] [CrossRef]
- El-Bondkly, A.M.A.; El-Gendy, M.M.A.; Bassyouni, R.H. Overproduction and biological activity of prodigiosin-like pigments from recombinant fusant of endophytic marine Streptomyces species. Antonie van Leeuwenhoek 2012, 102, 719–734. [Google Scholar] [CrossRef] [PubMed]
- Arivizhivendhan, K.V.; Mahesh, M.; Boopathy, R.; Swarnalatha, S.; Regina Mary, R.; Sekaran, G. Antioxidant and antimicrobial activity of bioactive prodigiosin produces from Serratia marcescens using agricultural waste as a substrate. J. Food Sci. Technol. 2018, 55, 2661–2670. [Google Scholar] [CrossRef] [PubMed]
- Arivizhivendhan, K.V.; Mahesh, M.; Regina Mary, R.; Sekaran, G. Bioactive prodigiosin isolated from Serratia marcescens using solid state fermenter and its bactericidal activity compared with conventional antibiotics. J. Microb. Biochem. Technol. 2015, 7, 305–312. [Google Scholar]
- Herráez, R.; Murm, A.; Merlos, A.; Viñas, M.; Vinuesa, T. Using prodigiosin against some gram-positive and gram-negative bacteria and Trypanosoma cruzi. J. Venom Anim. Toxins Incl. Trop. Dis. 2019, 25, e20190001. [Google Scholar] [CrossRef]
- Woodhams, D.C.; LaBumbard, B.C.; Barnhart, K.L.; Becker, M.H.; Bletz, M.C.; Escobar, L.A.; Flechas, S.V.; Forman, M.E.; Iannetta, A.A.; Joyce, M.D.; et al. Prodigiosin, violacein, and volatile organic compounds produced by widespread cutaneous bacteria of amphibians can inhibit two Batrachochytrium fungal pathogens. Microb. Ecol. 2018, 75, 1049–1062. [Google Scholar] [CrossRef]
- Suryawanshi, R.K.; Patil, C.D.; Borase, H.P.; Narkhede, C.P.; Stevenson, A.; Hallsworth, J.E.; Patil, S.V. Towards an understanding of bacterial metabolites prodigiosin and violacein and their potential for use in commercial sunscreens. Int. J. Cosmet. Sci. 2015, 37, 98–107. [Google Scholar] [CrossRef]
- Sruthy, P.B.; Anjana, J.C.; Rathinamala, J.; Jayashree, S. The role of red pigment prodigiosin from bacteria of earthworm gut as an anticancer agent. J. Microbiol. Biotechnol. Food Sci. 2017, 4, 246–251. [Google Scholar]
- Hsieh, H.Y.; Shieh, J.J.; Chen, C.J.; Pan, M.Y.; Yang, S.Y.; Lin, S.C.; Chang, J.S.; Lee, A.Y.; Chang, C.C. Prodigiosin down-regulats SKP2 to induce p27(KIP1) stabilization and antiproliferation in human lung adenocarcinoma cells. Br. J. Pharmacol. 2012, 166, 2095–2108. [Google Scholar] [CrossRef]
- Nakashima, T.; Kurachi, M.; Kato, Y.; Yamaguchi, K.; Oda, T. Characterization of bacterium isolated from the sediment at coastal area of Omura Bay in Japan and several biological activities of pigment produced by this isolate. Microbiol. Immunol. 2005, 49, 407–415. [Google Scholar] [CrossRef]
- Setiyono, E.; Adhiwibawa, M.A.S.; Indrawati, R.; Prihastyanti, M.N.T.; Shioi, Y.; Brotosudarmo, T.H.P. An Indonesian marine bacterium, Pseudoalteromonas rubra, produces antimicrobial prodiginine pigments. ACS Omega 2020, 5, 4626–4635. [Google Scholar] [CrossRef] [PubMed]
- Alihosseini, F.; Ju, K.S.; Lango, J.; Hammock, B.D.; Gang, G. Antibacterial colorants: Characterization of prodiginines and their applicationson textile. Biotechnol. Prog. 2008, 24, 742–747. [Google Scholar] [CrossRef] [PubMed]
- Rahul, S.; Chandrashekhar, P.; Hemant, B.; Bipinchandra, S.; Mouray, E.; Grellier, P.; Satish, P. In vitro antiparasitic activity of microbial pigments and their combination with phytosynthesized metal nanoparticles. Parasitol. Int. 2015, 64, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Rahul, S.; Chandrashekhar, P.; Hemant, B.; Chandrakant, N.; Laxmikant, S.; Satish, P. Nematicidal activity of microbial pigment from Serratia marcescens. Nat. Prod. Res. 2014, 28, 1399–1404. [Google Scholar] [CrossRef] [PubMed]
- Castro, A. Antimalarial activity of prodigiosin. Nature 1967, 213, 903–904. [Google Scholar] [PubMed]
- Isaka, M.; Jaturapat, A.; Kramyu, J.; Tanticharoen, M.; Thebtaranonth, Y. Potent in vitro antimalarial activity of metacycloprodigiosin isolated from Streptomyces spectabilis BCC 4785. Antimicrob. Agents Chemother. 2002, 46, 1112–1113. [Google Scholar]
- Gerber, N.N. A new prodiginne (prodigiosin-like) pigment from Streptomyces. Antimalarial activity of several prodiginnes. J. Antibiot. (Tokyo) 1975, 28, 194–199. [Google Scholar] [CrossRef][Green Version]
- Marchal, E.; Smithen, D.A.; Uddin, M.I.; Robertson, A.W.; Jakeman, D.L.; Mollard, V.; Goodman, C.D.; MacDougall, K.S.; McFarland, S.A.; McFadden, G.I.; et al. Synthesis and antimalarial activity of prodigiosenes. Org. Biomol. Chem. 2014, 12, 4132–4142. [Google Scholar]
- Papireddy, K.; Smilkstein, M.; Kelly, J.X.; Shweta, S.; Salem, S.M.; Alhamadsheh, M.; Haynes, S.W.; Challis, G.L.; Reynolds, K.A. Antimalarial activity of natural and synthetic prodiginines. J. Med. Chem. 2011, 54, 5296–5306. [Google Scholar] [CrossRef]
- Lazaro, J.E.; Nitcheu, J.; Predicala, R.Z.; Mangalindan, G.C.; Nesslany, F.; Marzin, D.; Concepcion, G.P.; Diquet, B. Heptyl prodigiosin, a bacterial metabolite, is antimalarial in vivo and non-mutagenic in vitro. J. Nat. Toxins 2002, 11, 367–377. [Google Scholar]
- Patil, C.D.; Patil, S.V.; Salunke, B.K.; Salunkhe, R.B. Prodigiosin produced by Serratia marcescens NMCC46 as a mosquito larvicidal agent against Aedes aegypti and Anopheles stephensi. Parasitol. Res. 2011, 109, 1179–1187. [Google Scholar]
- Suryawanshi, R.K.; Patil, C.D.; Borase, H.P.; Narkhede, C.P.; Salunke, B.K.; Patil, S.V. Mosquito larvicidal and pupaecidal potential of prodigiosin from Serratia marcescens and understanding its mechanism of action. Pestic. Biochem. Physiol. 2015, 123, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Jin, Z.X.; Wan, Y.J. Apoptosis of human lung adenocarcinoma A549 cells induced by prodigiosin analogue obtained from an entomopathogenic bacterium Serratia marcescens. Appl. Microbiol. Biotechnol. 2010, 88, 1269–1275. [Google Scholar] [CrossRef] [PubMed]
- Asano, S.; Ogiwara, K.; Nakagawa, Y.; Suzuki, K.; Hori, H.; Watanabe, T. Prodigiosin produced by Serratia marcescens enhances the insecticidal activity of Bacillus thuringiensis deltaendotoxin (Cry 1C) against common cutworm, Spodoptera litura. J. Pestic. Sci. 1999, 24, 381–385. [Google Scholar] [CrossRef][Green Version]
- Campàs, C.; Dalmau, M.; Montaner, B.; Barragan, M.; Bellosillo, B.; Colomer, D.; Pons, G.; Perez-Tomas, R.; Gil, J. Prodigiosin induces apoptosis of B and T cells from B-cell chronic lymphocytic leukemia. Leukemia 2003, 17, 746–750. [Google Scholar] [CrossRef]
- Liu, R.; Cui, C.B.; Duan, L.; Gu, Q.Q.; Zhu, W.M. Potent in vitro anticancer activity of metacycloprodigiosin and undecylprodigiosin from a sponge-derived actinomycete Saccharopolyspora sp. nov. Arch. Pharm. Res. 2005, 28, 1341–1344. [Google Scholar] [CrossRef]
- Chiu, W.J.; Lin, S.R.; Chen, Y.H.; Tssai, M.J.; Leong, M.K.; Weng, C.F. Prodigiosin-emerged PI3K/Beclin-1-independent pathway elicits autophagic cell death in doxorubicin-sensitive and-resistant lung cancer. J. Clin. Med. 2018, 7, 321. [Google Scholar] [CrossRef]
- Cheng, M.F.; Lin, C.S.; Chen, Y.H.; Sung, P.J.; Lin, S.R.; Tong, Y.W.; Weng, C.F. Inhibitory growth of oral squamous cell carcinoma cancer via bacterial prodigiosin. Mar. Drugs 2017, 15, 224. [Google Scholar] [CrossRef]
- Lin, S.R.; Weng, C.F. PG-priming enhances doxorubicin influx to trigger necrotic and autophagic cell death in oral squamous cell carcinoma. J. Clin. Med. 2018, 7, 325. [Google Scholar] [CrossRef] [PubMed]
- Llagostera, E.; Soto-Cerrato, V.; Joshi, R.; Montaner, B.; Gimenez-Bonafe, P.; Perez-Tomas, R. High cytotoxic sensitivity of the human small cell lung doxorubicin-resistant carcinoma (GLC4/ADR) cell line to prodigiosin through apoptosis activation. Anticancer Drugs 2005, 16, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Llagostera, E.; Soto-Cerrato, V.; Montaner, B.; Perez-Tomas, R. Prodigiosin induces apoptosis by acting on mitochondria in human lung cancer cells. Ann. N. Y. Acad. Sci. 2003, 1010, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Monge, M.; Vilaseca, M.; Soto-Cerrato, V.; Montaner, B.; Giralt, E.; Perez-Tomas, R. Proteomic analysis of prodigiosin-induced apoptosis in a breast cancer mitoxantrone-resistant (MCF-7 MR) cell line. Investig. New Drugs 2007, 25, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Soto-Cerrato, V.; Vinals, F.; Lambert, J.R.; Kelly, J.A.; Perez-Tomas, R. Prodigiosin induces the proapoptotic gene NAG-1 via glycogen synthase kinase-3β activity in human breast cancer cells. Mol. Cancer Ther. 2007, 6, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Soto-Cerrato, V.; Llagostera, E.; Montaner, B.; Scheffer, G.L.; Perez-Tomas, R. Mitochondria-mediated apoptosis operating irrespective of multidrug resistance in breast cancer cells by the anticancer agent prodigiosin. Biochem. Pharmacol. 2004, 68, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, B.; Zhou, L.; Yu, S.; Su, Z.; Song, J.; Sun, Q.; Sha, O.; Wang, X.; Jiang, W.; et al. Prodigiosin inhibits Wnt/β-catenin signaling and exerts anticancer activity in breast cancer cells. Proc. Natl. Acad. Sci. USA 2016, 113, 13150–13155. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.Y.; Shen, Y.C.; Lu, C.H.; Yang, S.Y.; Ho, T.F.; Peng, Y.T.; Chang, C.C. Prodigiosin activates endoplasmic reticulum stress cell death pathway in human breast carcinoma cell lines. Toxicol. Appl. Pharmacol. 2012, 265, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.H.; Lin, S.C.; Yang, S.Y.; Pan, M.Y.; Lin, Y.W.; Hsu, C.Y.; Wei, Y.H.; Chang, J.S.; Chang, C.C. Prodigiosin-induced cytotoxicity involves RAD51 down-regulation through the JNK and p38 MAPK pathways in human breast arcinoma cell lines. Toxicol. Lett. 2012, 212, 83–89. [Google Scholar] [CrossRef]
- Ho, T.F.; Peng, Y.T.; Chuang, S.M.; Lin, S.C.; Feng, B.L.; Lu, C.H.; Yu, W.J.; Chang, J.S.; Chang, C.C. Prodigiosin down-regulates survivin to facilitate paclitaxel sensitization in human breast carcinoma cell lines. Toxicol. Appl. Pharmacol. 2009, 235, 253–260. [Google Scholar] [CrossRef]
- Hassankhani, R.; Sam, M.R.; Esmaeilou, M.; Ahangar, P. Prodigiosin isolated from cell wall of Serratia marcescens alters expression of apoptosis-related genes and increases apoptosis in colorectal cancer cells. Med. Oncol. 2015, 32, 366. [Google Scholar] [CrossRef]
- Dalili, D.; Fouladdel, S.; Rastkari, N.; Samadi, N.; Ahmadkhaniha, R.; Ardavan, A.; Azizi, E. Prodigiosin, the red pigment of Serratia marcescens, shows cytotoxic effects and apoptosis induction in HT-29 and T47D cancer cell lines. Nat. Prod. Res. 2012, 26, 2078–2083. [Google Scholar]
- Montaner, B.; Perez-Tomas, R. Prodigiosin-induced apoptosis in human colon cancer cells. Life Sci. 2001, 68, 2025–2036. [Google Scholar] [CrossRef]
- Prabhu, V.V.; Hong, B.; Allen, J.E.; Zhang, S.; Lulla, A.R.; Dicker, D.T.; El-Deiry, W.S. Small molecule prodigiosin restores p53 tumor suppressor activity in chemoresistant colorectal cancer stem cells via c-Jun-mediated DNp73 inhibition and p73 activation. Cancer Res. 2016, 76, 1989–1999. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Avila, W.; Abal, M.; Robine, S.; Perez-Tomas, R. Non-apoptotic concentrations of prodigiosin (H+/Cl− symporter) inhibit the acidification of lysosomes and induce cell cycle blockage in colon cancer cells. Life Sci. 2005, 78, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Ruiz, C.; Montaner, B.; Pérez-Tomás, R. Prodigiosin induces cell death and morphological changes indicative of apoptosis in gastric cancer cell line HGT-1. Histol. Histopathol. 2001, 16, 415–421. [Google Scholar] [PubMed]
- Sam, M.R.; Pourpak, R.S. Regulation of p53 and survivin by prodigiosin compound derived from Serratia marcescens contribute to caspase-3-dependent apoptosis in acute lymphoblastic leukemia cells. Hum. Exp. Toxicol. 2018, 37, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Yenkejeh, R.A.; Sam, M.R.; Esmaeillou, M. Targeting survivin with prodigiosin isolated from cell wall of Serratia marcescens induces apoptosis in hepatocellular carcinoma cells. Hum. Exp. Toxicol. 2017, 36, 402–411. [Google Scholar] [CrossRef]
- Lund, K.A.R.; Figliola, C.; Kajetanowicz, A.K.; Thompson, A. Synthesis and anticancer activity of prodigiosenes bearing C-ring esters and amides. RSC Adv. 2017, 7, 18617. [Google Scholar] [CrossRef]
- Kavitha, R.; Aiswariya, S.; Chandana, R.M. Anticancer activity of red pigment from Serratia marcescens in human cervix carcinoma. Int. J. Pharm. Res. 2010, 2, 784–787. [Google Scholar]
- Hong, B.; Prabhu, V.V.; Zhang, S.; van den Heuvel, A.P.; Dicker, D.T.; Kopelovich, L.; El-Deiry, W.S. Prodigiosin rescues deficient p53 signaling and antitumor effects via upregulating p73 and disrupting its interaction with mutant p53. Cancer Res. 2014, 74, 1153–1165. [Google Scholar] [CrossRef]
- Pérez-Tomás, R.; Viñas, M. New insights on the antitumoral properties of prodiginines. Curr. Med. Chem. 2010, 17, 2222–2231. [Google Scholar] [CrossRef]
- Raj, D.N.; Dhanasekaran, D.; Thajuddin, N.; Panneerselvam, A. Production of prodigiosin from Serratia marcescens and its cytotoxicity activity. J. Pharm. Res. 2009, 2, 590–593. [Google Scholar]
- Montaner, B.; Pérez-Tomás, R. The prodigiosins: A new family of anticancer drugs. Curr. Cancer Drug Targets 2003, 3, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Montaner, B.; Navarro, S.; Pique, M.; Vilaseca, M.; Martinell, M.; Giralt, E.; Gil, J.; Pérez-Tomás, R. Prodigiosin from the supernatant of Serratia marcescens induce apoptosis in haematopoietic cancer cell line. Br. J. Pharmacol. 2000, 131, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, J.; Wang, X.; Kong, D.; Du, W.; Li, H.; Hse, C.Y.; Shupe, T.; Zhou, D.; Zhao, K. Biological potential and mechanism of prodigiosin from Serratia marcescens subsp. lawsoniana in human choriocarcinoma and prostate cancer cell lines. Int. J. Mol. Sci. 2018, 19, 3465. [Google Scholar] [CrossRef] [PubMed]
- Elahian, F.; Moghimi, B.; Dinmohammadi, F.; Ghamghami, M.; Hamidi, M.; Mirzaei, S.A. The anticancer agent prodigiosin is not a multidrug resistance protein substrate. DNA Cell Biol. 2013, 32, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Muthukumar, A.; Pradeep, P.; Thigale, I.; Mohanasrinivasan, V.; Jemimah, N.S.; Devi, C.S. Exploring the bioactive potential of Serriatia marcescens VITAPI (Acc: 1933637) isolated from soil. Front. Biol. 2016, 11, 476–480. [Google Scholar] [CrossRef]
- Abdelfattah, A.S.; Elmallah, M.I.Y.; Ebrahim, H.Y.; Almeer, R.S.; Eltanamy, R.M.A.; Moneim, A.E.A. Prodigiosins from a marine sponge-associated actinomycete attenuate HCl/ethanol-induced gastric lesion via antioxidant and anti-inflammatory mechanisms. PLoS ONE 2019, 14, e0216737. [Google Scholar] [CrossRef] [PubMed]
- Williamson, N.R.; Fineran, P.C.; Gristwood, T.; Chawrai, S.R.; Leeper, F.J.; Salmond, G.P. Anticancer and immunosuppressive properties of bacterial prodiginines. Future Microbiol. 2007, 2, 605–618. [Google Scholar] [CrossRef]
- Kim, D.; Kim, J.F.; Yim, J.H.; Kwon, S.K.; Lee, C.H. Red to red- the marine bacterium Hahella chejuensis and its product prodigiosin for mitigation of harmful algal blooms. J. Microbiol. Biotechnol. 2008, 18, 1621–1629. [Google Scholar]
- Nakashima, T.; Miyazaki, Y.; Matsuyama, Y.; Muraoka, W.; Yamaguchi, K.; Oda, T. Producing mechanism of an algicidal compound against red tide phytoplankton in a marine bacterium gamma-proteobacterium. Appl. Microbiol. Biotechnol. 2006, 73, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wei, H.Y.; Li, X.Q.; Li, Y.H.; Li, X.B.; Yin, L.H.; Pu, Y.P. Isolation and characterization of an algicidal bacterium indigenous to Lake Taihu with a red pigment able to lyse Microcystis aeruginosa. Biomed. Environ. Sci. 2013, 26, 148–154. [Google Scholar]
- Gong, J.; Liu, J.; Tan, X.; Li, Z.; Li, Q.; Zhang, J. Bio-preparation and regulation of pyrrole structure nano-pigment based on biomimetic membrane. Nanomaterial 2019, 9, 114. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, K.; Dalsaniya, P.; Pathak, H. Optimization of prodigiosin-type biochrome production and effect of mordants on textile dyeing to improve dye fastness. Fiber Polym. 2015, 16, 802–808. [Google Scholar] [CrossRef]
- Krishana, J.G.; Jacob, A.; Kurian, P.; Elyas, K.; Muthusamy, C. Marine bacterial prodigiosin as dye for rubber latex, polymethyl methacrylate sheets and paper. Afr. J. Biotechnol. 2013, 12, 2266–2269. [Google Scholar]
- Siva, R.; Subha, K.; Bhakta, D.; Ghosh, A.R.; Babu, S. Characterization and ehanced production of prodigiosin from the spoiled coconut. Appl. Biochem. Biotechnol. 2012, 166, 187–196. [Google Scholar] [CrossRef]
- Mehta, M.; Shah, G. Extraction of pigent from Serratia marcescens and its application in candle industry. Adv. Appl. Res. 2015, 7, 1–4. [Google Scholar] [CrossRef]
- Yip, C.H.; Yarkoni, O.; Ajioka, J.W.; Wan, K.L.; Nathan, S. Recent advancements in high-level synthesis of the promising clinical drug, prodigiosin. Appl. Microbiol. Biotechnol. 2019, 103, 1667–1680. [Google Scholar] [CrossRef]
- Hu, D.X.; Withall, D.M.; Challis, G.L.; Thomson, R.J. Structure, chemical synthesis, and biosynthesis of prodiginine natural products. Chem. Rev. 2016, 116, 7818–7853. [Google Scholar] [CrossRef]
- Nisha, N.; Kumar, K.; Kumar, V. Prodigiosin alkaloids: Recent advancements in total synthesis and their biological potential. RSC Adv. 2015, 5, 10899–10920. [Google Scholar] [CrossRef]
- Stankovic, N.; Senerovic, L.; Ilic-Tomic, T.; Vasiljevic, B.; Nikodinovic-Runic, J. Properties and appications of undecylprodigiosin and other bacterial prodigiosins. Appl. Microbiol. Biotechnol. 2014, 98, 3841–3858. [Google Scholar] [CrossRef]
- Williamson, N.R.; Fineran, P.C.; Leeper, F.J.; Salmond, G.P.C. The biosynthesis and regulation of bacterial prodiginines. Nat. Rev. 2006, 4, 887–899. [Google Scholar] [CrossRef]
- Andreyeva, I.N.; Ogorodnikova, T.I. Pigmentation of Serratia marcescens and special properties of prodigiosin. Microbiology 2015, 84, 28–33. [Google Scholar] [CrossRef]
- Khanafari, A.; Assadi, M.M.; Fakhr, F.A. Review of prodigiosin, pigmentation in Serratia marcescens. J. Biol. Sci. 2006, 6, 1–13. [Google Scholar] [CrossRef]
- Hubbard, R.; Rimington, C. The biosynthesis of prodigiosin, the tripyrrylmethane pigment from Bacillus prodigiosus (Serratia marcescens). Biochem. J. 1950, 46, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, H.H.; Mckeon, J.E.; Smith, L.; Forgione, P. Prodigiosin structure and partial synthesis. J. Am. Chem. Soc. 1960, 82, 506–507. [Google Scholar] [CrossRef]
- Rapoport, H.; Holden, K.G. The synthesis of prodigiosin. J. Am. Chem. Soc. 1962, 82, 5510–5511. [Google Scholar] [CrossRef]
- Araújo, H.W.C.; Andrade, R.F.S.; Montero-Rodríguez, D.; Rubio-Ribeaux, D.; Alves da Silva, C.A.; Campos-Takaki, G.M. Sustainable biosurfactant produced by Serratia marcescens UCP 1549 and its suitability for agricultural and marine bioremediation applications. Microb. Cell Factories 2019, 18, 2. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, K. Progress in microbial production of prodigiosin. J. Biol. Med. Sci. 2018, 2, 109. [Google Scholar]
- Venil, C.K.; Lakshmanaperumalsamy, P. An insightful overview on microbial pigment, prodigiosin. Electron. J. Biol. 2009, 5, 49–61. [Google Scholar]
- Kurbanoglu, E.B.; Ozdal, M.; Ozdal, O.G.; Algur, O.F. Enhanced production of prodigiosin by Serratia marcescens MO-1 using ram horn peptone. Braz. J. Microbiol. 2015, 46, 631–637. [Google Scholar] [CrossRef]
- Suryawanshi, R.K.; Patil, C.D.; Borase, H.P.; Salinke, B.K.; Patil, S.V. Studies on production and biological potential of prodigiosin by Serratia marcescens. Appl. Biochem. Biotechnol. 2014, 173, 1209–1221. [Google Scholar] [CrossRef]
- Chen, W.C.; Yu, W.J.; Chang, C.C.; Chang, J.S.; Huang, S.H.; Chang, C.H.; Chen, S.Y.; Chien, C.C.; Yao, C.L.; Chen, W.M.; et al. Enhancing production of prodigiosin from Serratia marcescens C3 by statistical experimental design and porous carrier addition strategy. Biochem. Eng. J. 2013, 78, 93–100. [Google Scholar] [CrossRef]
- Kamble, K.D.; Hiwarale, V.D. Prodigiosin production from Serratia marcescens strains obtained from farm soil. Int. J. Environm. Sci. 2012, 3, 631–638. [Google Scholar]
- Araújo, H.W.C.; Fukushima, K.; Takaki, G.M.C. Prodigiosin production by Serratia marcescens UCP1549 using renewable-resources as a low cost substrate. Molecules 2010, 15, 6931–6940. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.; Bentley, R. Seeing red: The story of prodigiosin. Adv. Appl. Microbiol. 2000, 47, 1–32. [Google Scholar] [PubMed]
- Wei, Y.H.; Chen, W.C. Enhanced production of prodigiosin-like pigment from Serratia marcescens SM△R by medium improvement and oil-supplement strategies. J. Biosci. Bioeng. 2005, 99, 616–622. [Google Scholar] [CrossRef]
- Wei, Y.H.; Yu, W.J.; Chen, W.C. Enhanced undecylprodigiosin production from Serratia marcescens SS-1 by medium formulation and amino-acid supplementation. J. Biosci. Bioeng. 2005, 100, 466–471. [Google Scholar] [CrossRef]
- Shaikh, Z. Biosynthesis of prodigiosin and its applications. IOSR J. Pharm. Biol. Sci. 2016, 11, 1–28. [Google Scholar]
- Gerber, N.N.; Gauthier, M.J. New prodigiosin-like pigment from Alteromonas rubra. Appl. Environ. Microbiol. 1979, 37, 1176–1179. [Google Scholar] [CrossRef]
- Schloss, P.D.; Allen, H.K.; Klimowicz, A.K.; Mlot, C.; Gross, J.A.; Savengsuksa, S.; McEllin, J.; Clardy, J.; Ruess, R.W.; Handelsman, J. Psychrotrophic strain of Janthinobacterium lividum from a cold Alaskan soil produces prodigiosin. DNA Cell Biol. 2010, 29, 533–541. [Google Scholar] [CrossRef]
- Darshan, N.; Manonmani, H.K. Prodigiosin and its potential applications. J. Food Sci. Technol. 2015, 52, 5393–5407. [Google Scholar] [CrossRef]
- Gerber, N.N.; Stahly, D.P. Prodiginine (prodigiosin-like) pigments from Streptoverticillium rubrireticuli, an organism that causes pink staining of polyvinyl chloride. Appl. Microbiol. 1975, 30, 807–810. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhu, H.; Zheng, G.; Jiang, W.; Lu, Y. Metabolic engineering of Streptomyces coelicolor for enhanced prodigiosins (RED) production. Sci. China Life Sci. 2017, 60, 948–957. [Google Scholar] [CrossRef] [PubMed]
- Alihosseini, F.; Lango, J.; Ju, K.S.; Hammock, B.D.; Sun, G. Mutation of bacterium Vibrio gazogenes for selective preparation of colorants. Biotechnol. Prog. 2010, 26, 352–360. [Google Scholar] [PubMed]
- D’Aoust, J.Y.; Gerber, N.N. Isolation and purification of prodigiosin from Vibrio psychroerythrus. J. Bacteriol. 1974, 118, 756–757. [Google Scholar] [CrossRef]
- Gandhi, N.M.; Patell, J.R.; Gandhi, J.; De Souza, N.J.; Kohl, H. Prodigiosin metabolites of a marine Pseudomonas species. Mar. Biol. 1976, 34, 223–227. [Google Scholar] [CrossRef]
- Sakai-Kawada, F.E.; Ip, C.G.; Hagiwara, K.A.; Awaya, J.D. Biosynthesis and bioactivity of prodiginine analogs in marine bacteria, Pseudoalteromonas: A mini review. Front. Microbiol. 2019, 10, 1715. [Google Scholar] [CrossRef]
- Perry, J.J. Prodigiosin in an actinomycete. Nature 1961, 1, 77–78. [Google Scholar] [CrossRef]
- Domröse, A.; Klein, A.S.; Hage-Hülsmann, J.; Thies, S.; Svensson, V.; Classen, T.; Pietruszka, J.; Jaeger, K.E.; Drepper, T.; Loeschcke, A. Efficient recombinant production of prodigiosin in Pseudomonas putida. Front. Microbiol. 2015, 6, 972. [Google Scholar] [CrossRef]
- Klein, A.S.; Domröse, A.; Bongen, P.; Brass, H.U.C.; Classen, T.; Loeschcke, A.; Drepper, T.; Laraia, L.; Sievers, S.; Jaeger, K.E.; et al. New prodigiosin derivatives obtained by mutasynthesis in Pseudomonas putida. ACS Synth. Biol. 2017, 6, 1757–1765. [Google Scholar] [CrossRef]
- Cang, S.; Sanada, M.; Johdo, O.; Ohta, S.; Nagamatsu, Y.; Yoshimoto, A. High production of prodigiosin by Serratia marcescens grown on ethanol. Biotechnol. Lett. 2000, 22, 1761–1765. [Google Scholar] [CrossRef]
- Gerber, N.N.; Lechevalier, M.P. Prodiginine (prodigiosin-like) pigments from Streptomyces and other aerobic Actinomycetes. Can. J. Microbiol. 1976, 22, 658–667. [Google Scholar] [CrossRef] [PubMed]
- Loriia, Z.h.K.; Briukner, B.; Egorov, N.S. Correlation between the synthesis of extracellular proteases and the synthesis of the red pigment prodigiosin in Serratia marcescens. Mikrobiology 1977, 46, 647–650. [Google Scholar]
- Loriia, Zh.K.; Briukner, B.; Egorov, N.S. Effect of amino acids on the synthesis of extracellular protease in Serratia marcescens. Mikrobiology 1977, 46, 41–45. [Google Scholar]
- Loriia, Zh.K.; Briukner, B.; Ezorov, N.S. The effect of glucose on induced synthesis of exocellular protease of Serratia marcescens. Mikrobiology 1977, 46, 926–930. [Google Scholar]
- Cruz Camarillo, R.; Albores Medina, A. Obtainment of extracellular alkaline protease from Serratia marcescens for commercial purposes. Rev. Latinoam. Microbiol. 1972, 14, 211–219. [Google Scholar] [PubMed]
- Alves, T.S.; Salgado, J.P.; Andrade, R.F.S.; Montero-Rodríguez, D.; Ferreira, W.B.; Almeida, M.M.; Campos-Takaki, G.M.; Araújo, H.W.C. Production and evaluation of biosurfactant by Serratia marcescens UCP 1549 using industrial wastes. Br. Biotechnol. J. 2014, 4, 708–719. [Google Scholar] [CrossRef]
- Wei, Y.H.; Lai, H.C.; Chen, S.Y.; Yeh, M.S.; Chang, J.S. Characterization of biosurfactant production by Serratia marcescens SS-1 and its isogenic strain SMδR defective in spnR, a quorum sensing LuxR familiy protein. Biotechnol. Lett. 2004, 26, 799–802. [Google Scholar] [CrossRef]
- Santos, D.K.; Rufino, R.D.; Luna, J.M.; Santos, V.A.; Sarubbo, L.A. Biosurfactants: Multifunctional biomolecules of the 21st century. Int. J. Mol. Sci. 2016, 17, 401. [Google Scholar] [CrossRef]
- Solé, M.; Rius, N.; Francia, A.; Lorén, J.G. The effect of pH on prodigiosin production by non-proliferating cells of Serratia marcescens. Lett. Appl. Microbiol. 1994, 19, 341–344. [Google Scholar] [CrossRef]
- Rjazantseva, I.N.; Andreeva, I.N.; Ogorodnikova, T.I. Effect of various growth conditions on pigmentation of Serratia marcescens. Microbios 1994, 79, 155–161. [Google Scholar]
- Giri, A.V.; Anandkumar, N.; Muthukumaran, G.; Pennathur, G. A novel medium for the enhanced cell growth and production of prodigiosin from Serratia marcescens isolated from soil. BMC Microbiol. 2004, 4, 11. [Google Scholar] [CrossRef] [PubMed]
- Elkenawy, N.M.; Yassin, A.S.; Elhifnawy, H.N.; Amin, M.A. Optimization of prodigiosin production by Serratia marcescens using crude glycerol and enhancing production using gamma radiation. Biotechnol. Rep. (Amst.) 2017, 14, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Williamson, N.R.; Simonsen, H.T.; Ahmed, R.A.; Goldet, G.; Slater, H.; Woodley, L.; Leeper, F.J.; Salmond, G.P. Biosynthesis of the red antibiotic, prodigiosin, in Serratia: Identification of a novel 2-methyl-3-n-amyl-pyrrole (MAP) assembly pathway, definition of the terminal condensing enzyme, and implications for undecylprodigiosin biosynthesis in Streptomyces. Mol. Microbiol. 2005, 56, 971–989. [Google Scholar] [CrossRef] [PubMed]
- Couturier, M.; Bhalara, H.D.; Chawrai, S.R.; Monson, R.; Williamson, N.R.; Salmond, G.P.C.; Leeper, F.J. Substrate flexibility of the flavin-dependent dihydropyrrole oxidases PigB and HapB involved in antibiotic prodigiosin biosynthesis. ChemBioChem 2020, 21, 523–530. [Google Scholar] [CrossRef]
- Lou, X.; Ran, T.; Han, N.; Gao, Y.; He, J.; Tang, L.; Xu, D.; Wang, W. Crystal structure of the catalytic domain of PigE: A transaminase involved in the biosynthesis of 2-methyl-3-n-amyl-pyrrole (MAP) from Serratia sp. FS14. Biochem. Biophys. Res. Commun. 2014, 447, 178–183. [Google Scholar] [CrossRef]
- Song, M.J.; Bae, J.; Lee, D.S.; Kim, C.H.; Kim, J.S.; Kim, S.W.; Hong, S.I. Purification and characterization of prodigiosin produced by integrated bioreactor from Serratia sp. KH-95. J. Biosci. Bioeng. 2006, 101, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Juang, R.S.; Yeh, C.L. Adsorptive recovery and purification of prodigiosin from methanol/water solutions of Serratia marcescens fermentation broth. Biotechnol. Bioproc. Eng. 2014, 19, 159–168. [Google Scholar] [CrossRef]
- Wang, X.; Tao, J.; Wei, D.; Shen, Y.; Tong, W. Development of an adsorption procedure for the direct separation and purification of prodigiosin from culture broth. Biotechnol. Appl. Biochem. 2004, 40, 277–280. [Google Scholar]
- Khanam, B.; Chandra, R. Comparative analysis of prodigiosin isolated from endophyte Serratia marcescens. Lett. Appl. Microbiol. 2018, 66, 194–201. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Sun, S.; Zhu, C.; Xu, W.; Park, Y.; Zhou, H. Mutant breeding of Serratia marcescens strain for enhancing prodigiosin production and application to textiles. Prepar. Biochem. Biotechnol. 2013, 43, 271–284. [Google Scholar] [CrossRef]
- Vaidyanathan, J.; Bhathena-Langdana, Z.; Adivarekar, R.V.; Nerurkar, M. Production, partial characterization, and use of a red biochrome produced by Serratia sakuensis subsp. nov. strain KRED for dyeing natural fibers. Appl. Biochem. Biotechnol. 2012, 166, 321–335. [Google Scholar] [CrossRef]
- Lin, C.; Jia, X.; Fang, Y.; Chen, L.; Zhang, H.; Lin, R.; Chen, J. Enhanced production of prodigiosin by Serratia marcescens FZSF02 in the form of pigment pellets. Electron. J. Biotechnol. 2019, 40, 58–64. [Google Scholar] [CrossRef]
- Hazarika, D.J.; Gautom, T.; Parveen, A.; Goswami, G.; Barooah, M.; Modi, M.K.; Boro, R.C. Mechanism of interaction of an endofungal bacterium Serratia marcescens D1 with its host and non-host fungi. PLoS ONE 2020, 15, e0224051. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wang, Y.Y.; Qi, X.; Gu, Q.; Geng, M.; Li, J. Undecylprodigiosin induced apoptosis in P388 cancer cells is associated with its binding to ribosome. PLoS ONE 2013, 8, e65381. [Google Scholar] [CrossRef] [PubMed]
| Culture Medium | PG | Cultivation | Reference |
|---|---|---|---|
| (C/N Source) | Productivity (mg/L) | Temp/pH/Time | |
| Chitin/casein | 4620 | 30 °C 1 Day/25 °C 2 Day | [59] |
| Squid pens | 2480 | [61] | |
| Shrimp shells | 190 | [61] | |
| Shrimp heads | 30 | [61] | |
| Crab shells | 110 | [61] | |
| Ram horn peptone/mannitol | 277.74 | 48 h | [170] |
| Peanut seed broth | 47,000 | - | [91] |
| Peanut seed broth | 3875 | 28 °C/-/36 h | [201] |
| Peanut seed broth | 1595 | 28 °C after 48 h | [82] |
| Peanut oil | 2890 | [201] | |
| Peanut powder/olive oil/beef extract | 13,622 | [212] | |
| Sunflower seed broth/maltose | 1556 | [82] | |
| Sunflower seed broth/glucose | 1525 | [82] | |
| Sesame seed broth | 16,680 | 28 °C/-/36 h | [201] |
| Sesame oil | 1006 | [201] | |
| Coconut oil | 1420 | [201] | |
| Copra seed | 1940 | [201] | |
| Casein-enriched broth/vegetable oil | 765.1 | 30 °C/7/84 h | [81] |
| Ethanol/cottonseed meal | 3000 | 28 °C/6.8/72 h | [190] |
| Casein | 4280 | [206] | |
| Cassava wastewater/mannitol | 49,500 | 28 °C/7/48 h | [174] |
| Yeast extract | 690 | [176] | |
| Yeast extract | 380.2 | [212] | |
| Yeast/tryptone | 152 | 30 °C/72 h | [172] |
| Nutrient broth/glycerol | 25 °C/72 h | [169] | |
| Tryptone | 353 | [212] | |
| Soya peptone | 1174 | [212] | |
| Peptone/maltose | 656 | 28 °C/72 h | [143] |
| Sweet potato powder/casein | 4800 | [171] | |
| Soybean powder | 0 | [212] | |
| Corn steep liquor | 0 | [212] | |
| Modified Luria–Bertani broth/sunflower oil | 790 | [175] | |
| 3-[N-morpholino]-Ethanesulfonic acid | 475 | [199] |
| Strain | Bioactivity/Application | Reference |
|---|---|---|
| (Antimicrobial activity) | ||
| S. marcescens UFPEDA398 | antibacterial | [83] |
| S. marcescens IBRL USM 84 | antibacterial | [84] |
| S. marcescens | antibacterial | [94] |
| S. marcescens | antibacterial | [92,93] |
| S. marcescens | antibacterial | [97] |
| S. marcescens | anti-chytrid fungi | [95] |
| S. marcescens PP1 | anti-fungal pathogens, antibacterial | [82] |
| S. marcescens CFFSUR-B2 | anti-fungal pathogens | [88] |
| S. marcescens ETR17 | anti-fungal pathogens | [89] |
| S. marcescens | antibacterial | [96] |
| S. marcescens SR1 | anti-fungal pathogens | [81] |
| S. marcescens B2 | anti-fungal pathogens | [85,86] |
| Streptomyces fusant NRCF69 | antidermatophytic | [91] |
| Strain MS-02-063 | antibacterial | [99] |
| Pseudoalteromonas rubra | antibacterial | [100] |
| Vibrio sp. KSJ45 | antibacterial | [101] |
| (Anti-parasitic activity) | ||
| S. marcescens 2170 | anti-parasitic euglenoids | [94] |
| S. marcescens | anti-parasitic euglenoids | [102] |
| S. marcescens | anti-nematode | [103] |
| S. marcescens | anti-malaria | [104] |
| Streptomyces spectabilis BCC 4785 | anti-malaria | [105] |
| Streptomyces sp. | anti-malaria | [106] |
| Prodigiosene | anti-malaria | [107] |
| Prodiginine | anti-malaria | [108] |
| Heptyl prodigiosin | anti-malaria | [109] |
| (Insecticidal activity) | ||
| S. marcescens TKU011 | fruit fly larvicide | [60,61] |
| S. marcescens NMCC46 | mosquito larvicide | [110] |
| S. marcescens NMCC75 | mosquito larvicide | [111] |
| S. marcescens SCQ1 | acute septicemia of silkworm | [112] |
| S. marcescens ATCC274 | anti-cutworm | [113] |
| (Anti-cancer activity) | ||
| S. marcescens TKU011 S. marcescens TNU01 | anti-cancer anti-cancer | [59] [87] |
| S. marcescens | anti-cancer | [138] |
| S. marcescens | anti-cancer | [147] |
| S. marcescens SCQ1 | anti-lung cancer | [112] |
| Vibrio sp. C1-TDSG02-1 | anti-lung cancer | [116] |
| S. marcescens | anti-lung cancer | [119,120] |
| S. marcescens | anti-oral cancer | [117] |
| Vibrio sp. C1-TDSG02-1 | anti-oral cancer | [118] |
| S. marcescens | anti-breast cancer | [97] |
| S. marcescens | anti-breast cancer | [121] |
| S. marcescens | anti-breast cancer | [124] |
| S. marcescens | anti-breast cancer | [125] |
| S. marcescens | anti-breast cancer | [126] |
| S. marcescens | anti-breast cancer | [127] |
| S. marcescens | anti-breast cancer | [122,123] |
| S. marcescens | anti-colorectal cancer | [128] |
| S. marcescens | anti-colorectal cancer | [129] |
| S. marcescens | anti-colorectal cancer | [130] |
| S. marcescens | anti-colorectal cancer | [131] |
| S. marcescens | anti-colorectal cancer | [132] |
| S. marcescens | anti-gastric cancer | [133] |
| S. marcescens | anti-leukemia | [114] |
| S. marcescens | anti-leukemia | [134] |
| Saccharopolyspora sp. | anti-leukemia | [214] |
| S. marcescens | anti-hepatocellular cancer | [135] |
| Prodigiosene | anti-cancer | [136] |
| Prodigiosin | anti-cancer | [141] |
| S. marcescens | anti-cervix carcinoma | [137] |
| S. marcescens MTCC97 | anti-cervix carcinoma | [140] |
| S. marcescens 2170 | anti-hematopoietic cancer | [142] |
| S. marcescens HDZK-BYSB107 | anti-choriocarcinoma | [143] |
| S. marcescens HDZK-BYSB107 | anti-prostate cancer | [143] |
| Streptomyces fusant NRCF69 | anti-colon cancer | [91] |
| S. fusant NRCF69 | anti-liver cancer | [91] |
| S. fusant NRCF69 | anti-breast cancer | [91] |
| (Anti-oxidation/Anti-inflammatory activity) | ||
| S. marcescens VITAPI | anti-oxidation | [145] |
| S. marcescens VITAPI | anti-inflammatory | [145] |
| S. marcescens | immunosuppressive | [147] |
| (Algicidal activity) | ||
| Hahella chejuensis KCTC 239 | algicide | [148] |
| strain MS-02-063 (γ-proteobacterium) | algicide | [149] |
| (Dyes) | ||
| S. marcescens ATCC8100 | textile | [151] |
| Serratia sp. KH-1 | textile | [152] |
| S. marcescens | candle | [163] |
| S. marcescens | sunscreen | [96] |
| Serratia rubidaea | textile | [154,177] |
| Vibrio sp. KSJ45 | textile | [101] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.-L.; Nguyen, V.B.; Doan, C.T.; Tran, T.N.; Nguyen, M.T.; Nguyen, A.D. Production and Potential Applications of Bioconversion of Chitin and Protein-Containing Fishery Byproducts into Prodigiosin: A Review. Molecules 2020, 25, 2744. https://doi.org/10.3390/molecules25122744
Wang S-L, Nguyen VB, Doan CT, Tran TN, Nguyen MT, Nguyen AD. Production and Potential Applications of Bioconversion of Chitin and Protein-Containing Fishery Byproducts into Prodigiosin: A Review. Molecules. 2020; 25(12):2744. https://doi.org/10.3390/molecules25122744
Chicago/Turabian StyleWang, San-Lang, Van Bon Nguyen, Chien Thang Doan, Thi Ngoc Tran, Minh Trung Nguyen, and Anh Dzung Nguyen. 2020. "Production and Potential Applications of Bioconversion of Chitin and Protein-Containing Fishery Byproducts into Prodigiosin: A Review" Molecules 25, no. 12: 2744. https://doi.org/10.3390/molecules25122744
APA StyleWang, S.-L., Nguyen, V. B., Doan, C. T., Tran, T. N., Nguyen, M. T., & Nguyen, A. D. (2020). Production and Potential Applications of Bioconversion of Chitin and Protein-Containing Fishery Byproducts into Prodigiosin: A Review. Molecules, 25(12), 2744. https://doi.org/10.3390/molecules25122744










