The Intrinsic Virtues of EGCG, an Extremely Good Cell Guardian, on Prevention and Treatment of Diabesity Complications
Abstract
1. Introduction
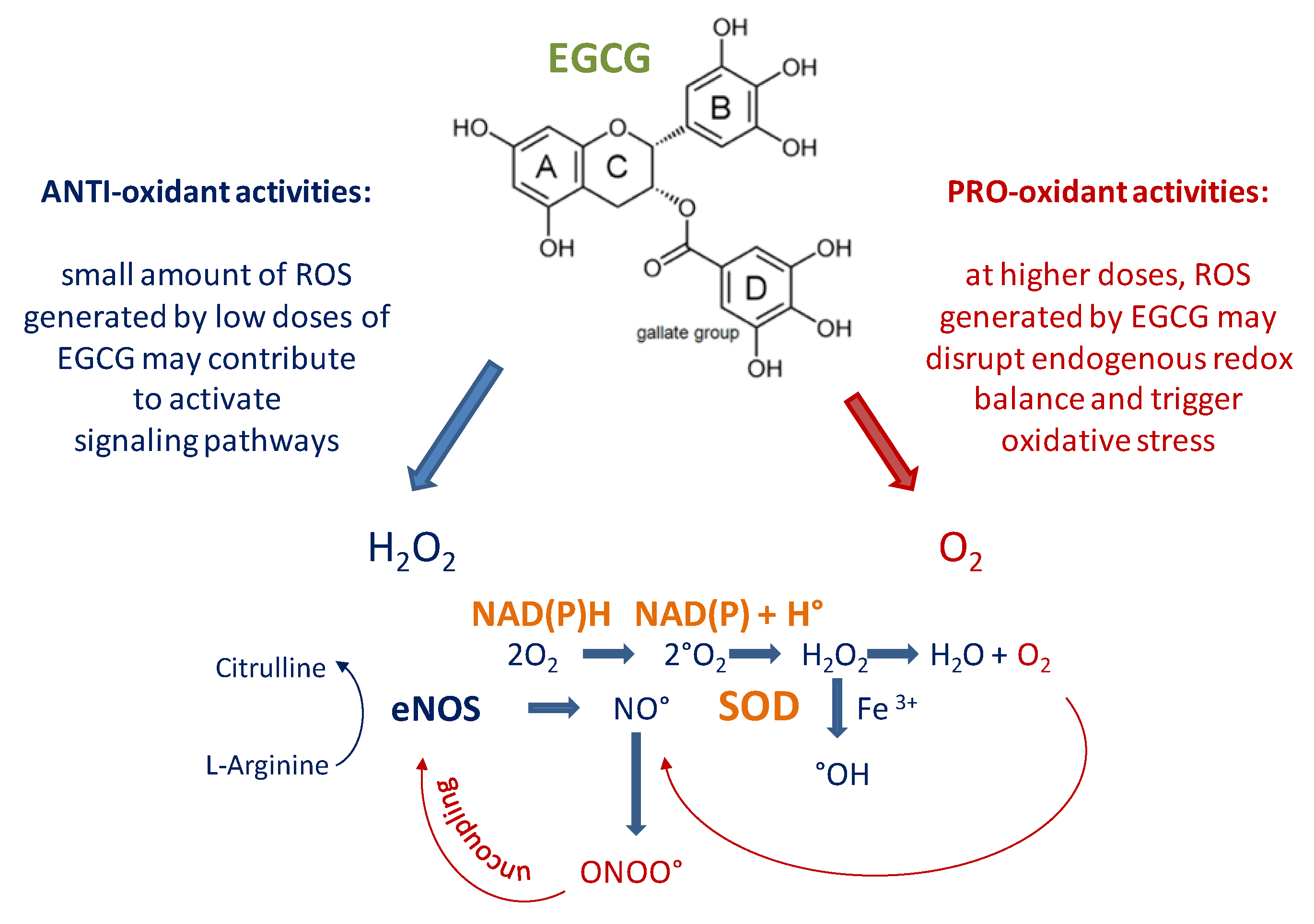
2. Structure of EGCG: Is an “Antioxidant” or “Pro-oxidant” Molecule?
3. Effects of EGCG: Evidence from Epidemiological and Intervention Studies
4. Effect of EGCG on Lipid Metabolism and Glucose Control
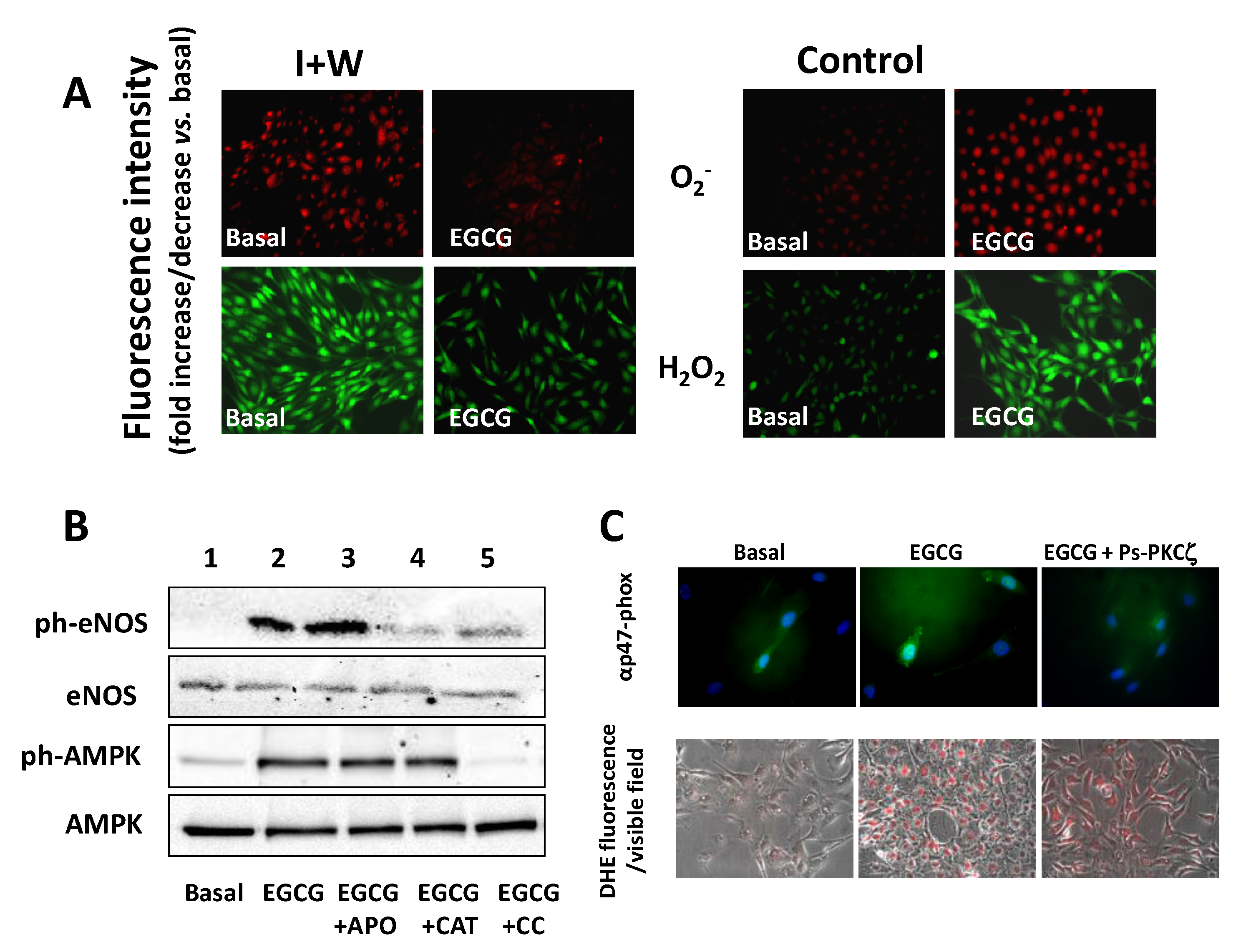
5. Effect of EGCG on Endothelial Function
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AGEs | advanced glycosylated end products |
| AMPK | 5’AMP-activated protein kinase |
| CaMKKβ | Ca/calmodulin-dependent protein kinase beta |
| DAG | diacylglycerol |
| DNMT | DNA methyltransferase |
| EGCG | epigallocatechin-3-gallate |
| eNOS | endothelial NO synthase |
| ERK | extracellular signal-regulated kinase |
| FFA | free fatty acids |
| GPx | glutathione peroxidase |
| HAT | histone acetyltransferase |
| HDAC | histone deacetylase |
| HSP27 | heat shock protein 27 |
| IRS-1 | insulin receptor substrate-1 |
| MAPK | mitogen-activated protein kinase |
| miRNA | microRNA |
| NF-kB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NLRP3 | NLR family pyrin domain containing protein 3 inflammasome |
| NO | nitric oxide |
| NOX | NADPH oxidase enzyme |
| PGC1α | peroxisome proliferator-activated receptor-γ–coactivator1α |
| PI3K | phosphatidylinositol 3-kinase |
| PKC | protein kinase C |
| ROS | radical oxygen species |
| SCD-1 | sterol-coenzyme A desaturase |
| SIRT-1 | sirtuin-1 |
| SOD | superoxide dismutase |
| T2DM | type 2 diabetes mellitus |
| TRXR | thioredoxin reductase |
| UCP2/3 | uncoupling proteins 2 and 3 |
| VEGF | vascular endothelial growth factor |
References
- Pappachan, J.M.; Viswanath, A.K. Medical Management of Diabesity: Do We Have Realistic Targets? Curr. Diabetes Rep. 2017, 17, 4. [Google Scholar] [CrossRef] [PubMed]
- Astrup, A.; Finer, N. Redefining Type 2 diabetes: ’Diabesity’ or ’Obesity Dependent Diabetes Mellitus’? Obes. Rev. 2000, 1, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Farag, Y.; Gaballa, M.R. Diabesity: An overview of a rising epidemic. Nephrol. Dial. Transplant. 2010, 26, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Brandão, B.B.; Guerra, B.A.; Mori, M.A. Shortcuts to a functional adipose tissue: The role of small non-coding RNAs. Redox Boil. 2017, 12, 82–102. [Google Scholar] [CrossRef]
- Gæde, P.; Lund-Andersen, H.; Parving, H.-H.; Pedersen, O. Effect of a Multifactorial Intervention on Mortality in Type 2 Diabetes. N. Engl. J. Med. 2008, 358, 580–591. [Google Scholar] [CrossRef]
- Kahan, S. Overweight and obesity management strategies. Am. J. Manag. Care 2016, 22, S186–S196. [Google Scholar]
- Kosiborod, M.; for The DISCOVER investigators; Gomes, M.B.; Nicolucci, A.; Pocock, S.; Rathmann, W.; Shestakova, M.V.; Watada, H.; Shimomura, I.; Chen, H.; et al. Vascular complications in patients with type 2 diabetes: Prevalence and associated factors in 38 countries (the DISCOVER study program). Cardiovasc. Diabetol. 2018, 17, 150. [Google Scholar] [CrossRef]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef]
- Mohammedi, K.; Woodward, M.; Hirakawa, Y.; Zoungas, S.; Williams, B.; Lisheng, L.; Rodgers, A.; Mancia, G.; Neal, B.; Harrap, S.; et al. Microvascular and Macrovascular Disease and Risk for Major Peripheral Arterial Disease in Patients With Type 2 Diabetes. Diabetes Care 2016, 39, 1796–1803. [Google Scholar] [CrossRef]
- Yamagishi, S.-I.; Maeda, S.; Matsui, T.; Ueda, S.; Fukami, K.; Okuda, S. Role of advanced glycation end products (AGEs) and oxidative stress in vascular complications in diabetes. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2012, 1820, 663–671. [Google Scholar] [CrossRef]
- Montagnani, M.; Chen, H.; Barr, V.A.; Quon, M. Insulin-stimulated Activation of eNOS Is Independent of Ca2+ but Requires Phosphorylation by Akt at Ser1179. J. Boil. Chem. 2001, 276, 30392–30398. [Google Scholar] [CrossRef] [PubMed]
- Montagnani, M.; Golovchenko, I.; Kim, I.; Koh, G.Y.; Goalstone, M.L.; Mundhekar, A.N.; Johansen, M.; Kucik, D.F.; Quon, M.; Draznin, B. Inhibition of Phosphatidylinositol 3-Kinase Enhances Mitogenic Actions of Insulin in Endothelial Cells. J. Boil. Chem. 2001, 277, 1794–1799. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-A.; Montagnani, M.; Koh, K.K.; Quon, M. Reciprocal Relationships Between Insulin Resistance and Endothelial Dysfunction. Circulation 2006, 113, 1888–1904. [Google Scholar] [CrossRef] [PubMed]
- Potenza, M.A.; Nacci, C.; Gagliardi, S.; Montagnani, M. Cardiovascular complications in diabetes: Lessons from animal models. Curr. Med. Chem. 2011, 18, 1806–1819. [Google Scholar] [CrossRef] [PubMed]
- Montagnani, M. Diabetic cardiomyopathy: How much does it depend on AGE? Br. J. Pharmacol. 2009, 154, 725–726. [Google Scholar] [CrossRef]
- Madamanchi, N.; Runge, M.S. Redox signaling in cardiovascular health and disease. Free Radic. Boil. Med. 2013, 61, 473–501. [Google Scholar] [CrossRef]
- Wallace, U.C. Diseases of the Mitochondrial DNA. Annu. Rev. Biochem. 1992, 61, 1175–1212. [Google Scholar] [CrossRef]
- Iacobazzi, D.; Mangialardi, G.; Gubernator, M.; Hofner, M.; Wielscher, M.; Vierlinger, K.; Reni, C.; Oikawa, A.; Spinetti, G.; Vono, R.; et al. Increased Antioxidant Defense Mechanism in Human Adventitia-Derived Progenitor Cells Is Associated with Therapeutic Benefit in Ischemia. Antioxidants Redox Signal. 2014, 21, 1591–1604. [Google Scholar] [CrossRef]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef]
- Maxwell, S.R.J.; Thomason, H.; Sandler, D.; Leguen, C.; Baxter, M.A.; Thorpe, G.H.G.; Jones, A.F.; Barnett, A.H. Antioxidant status in patients with uncomplicated insulin-dependent and non-insulin-dependent diabetes mellitus. Eur. J. Clin. Investig. 1997, 27, 484–490. [Google Scholar] [CrossRef]
- Rocic, B.; Vucic, M.; Knežević-Ćuća, J.; Radica, A.; Pavlić-Renar, I.; Profozic, V.; Metelko, Z. Total plasma antioxidants in first-degree relatives of patients with insulin-dependent diabetes. Exp. Clin. Endocrinol. Diabetes 2009, 105, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Santini, S.A.; Marra, G.; Giardina, B.; Cotroneo, P.; Mordente, A.; Martorana, G.E.; Manto, A.; Ghirlanda, G. Defective Plasma Antioxidant Defenses and Enhanced Susceptibility to Lipid Peroxidation in Uncomplicated IDDM. Diabetes 1997, 46, 1853–1858. [Google Scholar] [CrossRef] [PubMed]
- Roy, J.; Galano, J.-M.; Durand, T.; Le Guennec, J.-Y.; Lee, J.C.-Y. Physiological role of reactive oxygen species as promoters of natural defenses. FASEB J. 2017, 31, 3729–3745. [Google Scholar] [CrossRef] [PubMed]
- Schini-Kerth, V.; Etienne-Selloum, N.; Chataigneau, T.; Auger, C. Vascular Protection by Natural Product-Derived Polyphenols: In Vitro and In Vivo Evidence. Planta Med. 2011, 77, 1161–1167. [Google Scholar] [CrossRef]
- Vincent, H.K.; Innes, K.E.; Vincent, K.R. Oxidative stress and potential interventions to reduce oxidative stress in overweight and obesity. Diabetes Obes. Metab. 2007, 9, 813–839. [Google Scholar] [CrossRef]
- Corcoran, M.P.; McKay, D.L.; Blumberg, J. Flavonoid Basics: Chemistry, Sources, Mechanisms of Action, and Safety. J. Nutr. Gerontol. Geriatr. 2012, 31, 176–189. [Google Scholar] [CrossRef]
- Kishimoto, Y.; Tani, M.; Kondo, K. Pleiotropic preventive effects of dietary polyphenols in cardiovascular diseases. Eur. J. Clin. Nutr. 2013, 67, 532–535. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Andres-Lacueva, C. Polyphenols and Health: Current State and Progress. J. Agric. Food Chem. 2012, 60, 8773–8775. [Google Scholar] [CrossRef]
- Kuriyama, S.; Shimazu, T.; Ohmori, K.; Kikuchi, N.; Nakaya, N.; Nishino, Y.; Tsubono, Y.; Tsuji, I. Green Tea Consumption and Mortality Due to Cardiovascular Disease, Cancer, and All Causes in Japan. JAMA 2006, 296, 1255–1265. [Google Scholar] [CrossRef]
- Del Rio, D.; Stewart, A.J.; Mullen, W.; Burns, J.; Lean, M.E.J.; Brighenti, F.; Crozier, A. HPLC-MSnAnalysis of Phenolic Compounds and Purine Alkaloids in Green and Black Tea. J. Agric. Food Chem. 2004, 52, 2807–2815. [Google Scholar] [CrossRef]
- Basu, A.; Sanchez, K.; Leyva, M.J.; Wu, M.; Betts, N.M.; Aston, C.E.; Lyons, T.J. Green tea supplementation affects body weight, lipids, and lipid peroxidation in obese subjects with metabolic syndrome. J. Am. Coll. Nutr. 2010, 29, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Kim, J. Mechanisms underlying beneficial health effects of tea catechins to improve insulin resistance and endothelial dysfunction. Endocr. Metab. Immune Disord.—Drug Targets 2008, 8, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-A.; Formoso, G.; Li, Y.; Potenza, M.A.; Marasciulo, F.L.; Montagnani, M.; Quon, M. Epigallocatechin Gallate, a Green Tea Polyphenol, Mediates NO-dependent Vasodilation Using Signaling Pathways in Vascular Endothelium Requiring Reactive Oxygen Species and Fyn. J. Boil. Chem. 2007, 282, 13736–13745. [Google Scholar] [CrossRef] [PubMed]
- Potenza, M.A.; Marasciulo, F.L.; Tarquinio, M.; Tiravanti, E.; Colantuono, G.; Federici, A.; Kim, J.-A.; Quon, M.; Montagnani, M. EGCG, a green tea polyphenol, improves endothelial function and insulin sensitivity, reduces blood pressure, and protects against myocardial I/R injury in SHR. Am. J. Physiol. Metab. 2007, 292, E1378–E1387. [Google Scholar] [CrossRef]
- Chyu, K.-Y.; Babbidge, S.M.; Zhao, X.; Dandillaya, R.; Rietveld, A.G.; Yano, J.; Dimayuga, P.; Cercek, B.; Shah, P. Differential Effects of Green Tea–Derived Catechin on Developing Versus Established Atherosclerosis in Apolipoprotein E–Null Mice. Circulation 2004, 109, 2448–2453. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Moustaid-Moussa, N.; Chen, L.; Mo, H.; Shastri, A.; Su, R.; Bapat, P.; Kwun, I.; Shen, C.-L. Novel insights of dietary polyphenols and obesity. J. Nutr. Biochem. 2014, 25, 1–18. [Google Scholar] [CrossRef]
- Bose, M.; Lambert, J.D.; Ju, J.; Reuhl, K.R.; Shapses, S.A.; Yang, C.S. The Major Green Tea Polyphenol, (−)-Epigallocatechin-3-Gallate, Inhibits Obesity, Metabolic Syndrome, and Fatty Liver Disease in High-Fat–Fed Mice. J. Nutr. 2008, 138, 1677–1683. [Google Scholar] [CrossRef]
- Chen, N.; Bezzina, R.; Hinch, E.; Lewandowski, P.A.; Cameron-Smith, D.; Mathai, M.L.; Jois, M.; Sinclair, A.J.; Begg, D.P.; Wark, J.D.; et al. Green tea, black tea, and epigallocatechin modify body composition, improve glucose tolerance, and differentially alter metabolic gene expression in rats fed a high-fat diet. Nutr. Res. 2009, 29, 784–793. [Google Scholar] [CrossRef]
- Klaus, S.; Pültz, S.; Thöne-Reineke, C.; Wolfram, S.; Uuml, S.P. Epigallocatechin gallate attenuates diet-induced obesity in mice by decreasing energy absorption and increasing fat oxidation. Int. J. Obes. 2005, 29, 615–623. [Google Scholar] [CrossRef]
- Li, H.; Kek, H.C.; Lim, J.; Gelling, R.W.; Han, W. Green tea (−)-epigallocatechin-3-gallate counteracts daytime overeating induced by high-fat diet in mice. Mol. Nutr. Food Res. 2016, 60, 2565–2575. [Google Scholar] [CrossRef]
- Ueda, M.; Nishiumi, S.; Nagayasu, H.; Fukuda, I.; Yoshida, K.-I.; Ashida, H. Epigallocatechin gallate promotes GLUT4 translocation in skeletal muscle. Biochem. Biophys. Res. Commun. 2008, 377, 286–290. [Google Scholar] [CrossRef]
- Sae-Tan, S.; Grove, K.A.; Kennett, M.J.; Lambert, J.D. (−)-Epigallocatechin-3-gallate increases the expression of genes related to fat oxidation in the skeletal muscle of high fat-fed mice. Food Funct. 2011, 2, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Thielecke, F.; Boschmann, M. The potential role of green tea catechins in the prevention of the metabolic syndrome – A review. Phytochemistry 2009, 70, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Keske, M.A.; Ng, H.L.; Premilovac, D.; Rattigan, S.; Kim, J.-A.; Munir, K.; Yang, P.; Quon, M. Vascular and metabolic actions of the green tea polyphenol epigallocatechin gallate. Curr. Med. Chem. 2015, 22, 59–69. [Google Scholar] [CrossRef]
- Yan, J.; Zhao, Y.; Suo, S.; Liu, Y.; Zhao, B. Green tea catechins ameliorate adipose insulin resistance by improving oxidative stress. Free Radic. Boil. Med. 2012, 52, 1648–1657. [Google Scholar] [CrossRef]
- Katiyar, S.K.; Afaq, F.; Perez, A.; Mukhtar, H. Green tea polyphenol (−)-epigallocatechin-3-gallate treatment of human skin inhibits ultraviolet radiation-induced oxidative stress. Carcinogenesis 2001, 22, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Montana, V.; Jang, H.-J.; Parpura, V.; Kim, J. Epigallocatechin Gallate (EGCG) Stimulates Autophagy in Vascular Endothelial Cells. J. Boil. Chem. 2013, 288, 22693–22705. [Google Scholar] [CrossRef]
- Valenti, D.; De Bari, L.; Manente, G.A.; Rossi, L.; Mutti, L.; Moro, L.; Vacca, R.A. Negative modulation of mitochondrial oxidative phosphorylation by epigallocatechin-3 gallate leads to growth arrest and apoptosis in human malignant pleural mesothelioma cells. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2013, 1832, 2085–2096. [Google Scholar] [CrossRef]
- Valenti, D.; De Rasmo, D.; Signorile, A.; Rossi, L.; De Bari, L.; Scala, I.; Granese, B.; Papa, S.; Vacca, R.A. Epigallocatechin-3-gallate prevents oxidative phosphorylation deficit and promotes mitochondrial biogenesis in human cells from subjects with Down’s syndrome. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2013, 1832, 542–552. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, N.-D.; Zhou, F.; Shen, T.; Duan, T.; Zhou, J.; Shi, Y.; Zhu, X.-Q.; Shen, H.-M. (−)-Epigallocatechin-3-Gallate Induces Non-Apoptotic Cell Death in Human Cancer Cells via ROS-Mediated Lysosomal Membrane Permeabilization. PLoS ONE 2012, 7, e46749. [Google Scholar] [CrossRef]
- Niedowicz, D.M.; Daleke, D.L. The Role of Oxidative Stress in Diabetic Complications. Cell Biophys. 2005, 43, 289–330. [Google Scholar] [CrossRef]
- Addabbo, F.; Montagnani, M.; Goligorsky, M.S. Mitochondria and reactive oxygen species. Hypertension 2009, 53, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Sena, L.A.; Chandel, N.S. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell 2012, 48, 158–167. [Google Scholar] [CrossRef]
- Dorta, D.; Pigoso, A.A.; Mingatto, F.E.; Rodrigues, T.; Pestana, C.R.; Uyemura, S.A.; Dos Santos, N.A.G.; Curti, C. Antioxidant activity of flavonoids in isolated mitochondria. Phytotherapy Res. 2008, 22, 1213–1218. [Google Scholar] [CrossRef] [PubMed]
- Babu, P.A.; Liu, N.; Velayutham, A.B.P.; Babu, A. Green Tea Catechins and Cardiovascular Health: An Update. Curr. Med. Chem. 2008, 15, 1840–1850. [Google Scholar] [CrossRef] [PubMed]
- Casanova, E.; Salvadó, J.; Crescenti, A.; Gibert-Ramos, A. Epigallocatechin Gallate Modulates Muscle Homeostasis in Type 2 Diabetes and Obesity by Targeting Energetic and Redox Pathways: A Narrative Review. Int. J. Mol. Sci. 2019, 20, 532. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, J. Recent advances on tea polyphenols. Front. Biosci. 2012, 4, 111. [Google Scholar] [CrossRef]
- Landis-Piwowar, K.R.; Kuhn, D.J.; Wan, S.B.; Chen, D.; Chan, T.-H.; Dou, Q.P. Evaluation of proteasome-inhibitory and apoptosis-inducing potencies of novel (−)-EGCG analogs and their prodrugs. Int. J. Mol. Med. 2005, 15, 735–742. [Google Scholar] [CrossRef]
- Khandelwal, A.; Hall, J.A.; Blagg, B.S. Synthesis and Structure–Activity Relationships of EGCG Analogues, a Recently Identified Hsp90 Inhibitor. J. Org. Chem. 2013, 78, 7859–7884. [Google Scholar] [CrossRef]
- Elbling, L.; Weiss, R.-M.; Teufelhofer, O.; Uhl, M.; Knasmueller, S.; Schulte-Hermann, R.; Berger, W.; Micksche, M. Green tea extract and (−)-epigallocatechin-3-gallate, the major tea catechin, exert oxidant but lack antioxidant activities. FASEB J. 2005, 19, 1–26. [Google Scholar] [CrossRef]
- Hou, Z.; Sang, S.; You, H.; Lee, M.-J.; Hong, J.; Chin, K.-V.; Yang, C.S. Mechanism of Action of (−)-Epigallocatechin-3-Gallate: Auto-oxidation–Dependent Inactivation of Epidermal Growth Factor Receptor and Direct Effects on Growth Inhibition in Human Esophageal Cancer KYSE 150 Cells. Cancer Res. 2005, 65, 8049–8056. [Google Scholar] [CrossRef] [PubMed]
- Sakagami, H.; Arakawa, H.; Maeda, M.; Satoh, K.; Kadofuku, T.; Fukuchi, K.; Gomi, K. Production of hydrogen peroxide and methionine sulfoxide by epigallocatechin gallate and antioxidants. Anticancer. Res. 2001, 21, 2633–2641. [Google Scholar] [PubMed]
- Yang, G.-Y.; Liao, J.; Li, C.; Chung, J.; Yurkow, E.J.; Ho, C.-T. Effect of black and green tea polyphenols on c-jun phosphorylation and H2O2 production in transformed and non-transformed human bronchial cell lines: Possible mechanisms of cell growth inhibition and apoptosis induction. Carcinogenesis 2000, 21, 2035–2039. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, H.; Hasumi, K.; Woo, J.-T.; Nagai, K.; Wachi, M. Generation of hydrogen peroxide primarily contributes to the induction of Fe(II)-dependent apoptosis in Jurkat cells by (−)-epigallocatechin gallate. Carcinogenesis 2004, 25, 1567–1574. [Google Scholar] [CrossRef]
- Nakagawa, H.; Wachi, M.; Woo, J.-T.; Kato, M.; Kasai, S.; Takahashi, F.; Lee, I.-S.; Nagai, K. Fenton reaction is primarily involved in a mechanism of (−)-epigallocatechin-3-gallate to induce osteoclastic cell death. Biochem. Biophys. Res. Commun. 2002, 292, 94–101. [Google Scholar] [CrossRef]
- Tian, B.; Sun, Z.; Xu, Z.; Hua, Y. Chemiluminescence analysis of the prooxidant and antioxidant effects of epigallocatechin-3-gallate. Asia Pac. J. Clin. Nutr. 2007, 16, 153–157. [Google Scholar]
- Zhang, H.; Cao, N.; Cui, W.; Ji, M.; Qian, X.; Zhong, L. Molecular bases of thioredoxin and thioredoxin reductase-mediated prooxidant actions of (−)-epigallocatechin-3-gallate. Free. Radic. Boil. Med. 2010, 49, 2010–2018. [Google Scholar] [CrossRef]
- Manohar, M.; Fatima, I.; Saxena, R.; Chandra, V.; Sankhwar, P.L.; Dwivedi, A. (−)-Epigallocatechin-3-gallate induces apoptosis in human endometrial adenocarcinoma cells via ROS generation and p38 MAP kinase activation. J. Nutr. Biochem. 2013, 24, 940–947. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Forester, S.C.; Lambert, J.D. The role of the mitochondrial oxidative stress in the cytotoxic effects of the green tea catechin, (-)-epigallocatechin-3-gallate, in oral cells. Mol. Nutr. Food Res. 2013, 58, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Wang, X.; Lu, G.; Picinich, S.C. Cancer prevention by tea: Animal studies, molecular mechanisms and human relevance. Nat. Rev. Cancer 2009, 9, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xie, J.; Gan, R.; Wu, Z.; Luo, H.; Chen, X.; Lu, Y.; Wu, L.; Zheng, D. Synergistic inhibition of lung cancer cells by EGCG and NF-κB inhibitor BAY11-7082. J. Cancer 2019, 10, 6543–6556. [Google Scholar] [CrossRef] [PubMed]
- Mazzanti, G.; Menniti-Ippolito, F.; Moro, P.A.; Cassetti, F.; Raschetti, R.; Santuccio, C.; Mastrangelo, S. Hepatotoxicity from green tea: A review of the literature and two unpublished cases. Eur. J. Clin. Pharmacol. 2009, 65, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.D.; Kennett, M.J.; Sang, S.; Reuhl, K.R.; Ju, J.; Yang, C.S. Hepatotoxicity of high oral dose (−)-epigallocatechin-3-gallate in mice. Food Chem. Toxicol. 2010, 48, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Bitzer, Z.T.; Elias, R.J.; Vijay-Kumar, M.; Lambert, J.D. (-)-Epigallocatechin-3-gallate decreases colonic inflammation and permeability in a mouse model of colitis, but reduces macronutrient digestion and exacerbates weight loss. Mol. Nutr. Food Res. 2016, 60, 2267–2274. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, S.; Saito, K.; Miyoshi, N.; Ohishi, T.; Oishi, Y.; Miyoshi, M.; Nakamura, Y. Anti-Cancer Effects of Green Tea by Either Anti- or Pro-Oxidative Mechanisms. Asian Pac. J. Cancer Prev. 2016, 17, 1649–1654. [Google Scholar] [CrossRef] [PubMed]
- Elbling, L.; Herbacek, I.; Weiss, R.-M.; Jantschitsch, C.; Micksche, M.; Gerner, C.; Pangratz, H.; Grusch, M.; Knasmueller, S.; Berger, W. Hydrogen peroxide mediates EGCG-induced antioxidant protection in human keratinocytes. Free. Radic. Boil. Med. 2010, 49, 1444–1452. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Chen, Y.; Li, R.C. Oral Absorption and Bioavailability of Tea Catechins. Planta Med. 2000, 66, 444–447. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Chen, L.; Lee, M.J.; Balentine, D.; Kuo, M.C.; Schantz, S.P. Blood and urine levels of tea catechins after ingestion of different amounts of green tea by human volunteers. Cancer Epidemiol. Biomark. Prev. 1998, 7, 351–354. [Google Scholar]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxidants Redox Signal. 2012, 18, 1818–1892. [Google Scholar] [CrossRef]
- Lambert, J.D.; Lee, M.-J.; Diamond, L.; Ju, J.; Hong, J.; Bose, M.; Newmark, H.L.; Yang, C.S. Dose-dependent levels of epigallocatechin-3-gallate in human colon cancer cells and mouse plasma and tissues. Drug Metab. Dispos. 2005, 34, 8–11. [Google Scholar] [CrossRef]
- Lee, M.-J.; Maliakal, P.; Chen, L.; Meng, X.; Bondoc, F.Y.; Prabhu, S.; Lambert, G.; Mohr, S.; Yang, C.S. Pharmacokinetics of tea catechins after ingestion of green tea and (-)-epigallocatechin-3-gallate by humans: Formation of different metabolites and individual variability. Cancer Epidemiol. Biomark. Prev. 2002, 11, 1025–1032. [Google Scholar]
- Zinellu, A.; Sotgia, S.; Scanu, B.; Forteschi, M.; Giordo, R.; Cossu, A.; Posadino, A.M.; Carru, C.; Pintus, G. Human Serum Albumin Increases the Stability of Green Tea Catechins in Aqueous Physiological Conditions. PLoS ONE 2015, 10, e0134690. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.D.; Lee, M.-J.; Lu, H.; Meng, X.; Hong, J.J.J.; Seril, D.N.; Sturgill, M.G.; Yang, C.S. Epigallocatechin-3-gallate is absorbed but extensively glucuronidated following oral administration to mice. J. Nutr. 2003, 133, 4172–4177. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Sang, S.; Zhu, N.; Lu, H.; Sheng, S.; Lee, M.-J.; Ho, C.-T.; Yang, C.S. Identification and Characterization of Methylated and Ring-Fission Metabolites of Tea Catechins Formed in Humans, Mice, and Rats. Chem. Res. Toxicol. 2002, 15, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N.; Van Der Hooft, J.J.J.; Crozier, A. Human studies on the absorption, distribution, metabolism, and excretion of tea polyphenols. Am. J. Clin. Nutr. 2013, 98, 1619S–1630S. [Google Scholar] [CrossRef]
- Kida, K.; Suzuki, M.; Matsumoto, N.; Nanjo, F.; Hara, Y. Identification of Biliary Metabolites of (−)-Epigallocatechin Gallate in Rats. J. Agric. Food Chem. 2000, 48, 4151–4155. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Y.; Xie, Z.; Zhou, Y.; Zhang, Y.; Wan, X. The anti-obesity effects of green tea in human intervention and basic molecular studies. Eur. J. Clin. Nutr. 2014, 68, 1075–1087. [Google Scholar] [CrossRef]
- Grosso, G.; Stepaniak, U.; Micek, A.; Topór-Mądry, R.; Pikhart, H.; Szafraniec, K.; Pająk, A. Association of daily coffee and tea consumption and metabolic syndrome: Results from the Polish arm of the HAPIEE study. Eur. J. Nutr. 2014, 54, 1129–1137. [Google Scholar] [CrossRef]
- Takami, H.; Nakamoto, M.; Uemura, H.; Katsuura, S.; Yamaguchi, M.; Hiyoshi, M.; Sawachika, F.; Juta, T.; Arisawa, K. Inverse Correlation Between Coffee Consumption and Prevalence of Metabolic Syndrome: Baseline Survey of the Japan Multi-Institutional Collaborative Cohort (J-MICC) Study in Tokushima, Japan. J. Epidemiol. 2013, 23, 12–20. [Google Scholar] [CrossRef]
- Vernarelli, J.A.; Lambert, J.D. Tea consumption is inversely associated with weight status and other markers for metabolic syndrome in US adults. Eur. J. Nutr. 2012, 52, 1039–1048. [Google Scholar] [CrossRef]
- Rains, T.M.; Agarwal, S.; Maki, K.C. Antiobesity effects of green tea catechins: A mechanistic review. J. Nutr. Biochem. 2011, 22, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Grove, K.A.; Lambert, J.D. Laboratory, epidemiological, and human intervention studies show that tea (Camellia sinensis) may be useful in the prevention of obesity. J. Nutr. 2010, 140, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Nagao, T.; Komine, Y.; Soga, S.; Meguro, S.; Hase, T.; Tanaka, Y.; Tokimitsu, I. Ingestion of a tea rich in catechins leads to a reduction in body fat and malondialdehyde-modified LDL in men. Am. J. Clin. Nutr. 2005, 81, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Zhang, J.; Zhang, L.; Huang, J.; Wang, Y. Mechanisms of body weight reduction and metabolic syndrome alleviation by tea. Mol. Nutr. Food Res. 2015, 60, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, T.; Tanaka, Y.; Kamimaki, I.; Nagao, T.; Tokimitsu, I. Catechin Safely Improved Higher Levels of Fatness, Blood Pressure, and Cholesterol in Children. Obesity 2008, 16, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Most, J.; Van Can, J.G.P.; Van Dijk, J.-W.; Goossens, G.H.; Jocken, J.; Hospers, J.J.; Bendik, I.; Blaak, E.E. A 3-day EGCG-supplementation reduces interstitial lactate concentration in skeletal muscle of overweight subjects. Sci. Rep. 2015, 5, 17896. [Google Scholar] [CrossRef] [PubMed]
- Byambasukh, O.; Saris, W.H.M.; Blaak, E.E. Metabolic flexibility in the development of insulin resistance and type 2 diabetes: Effects of lifestyle. Obes. Rev. 2009, 10, 178–193. [Google Scholar] [CrossRef]
- Boschmann, M.; Thielecke, F. The effects of epigallocatechin-3-gallate on thermogenesis and fat oxidation in obese men: A pilot study. J. Am. Coll. Nutr. 2007, 26, 389S–395S. [Google Scholar] [CrossRef]
- Wolfram, S.; Raederstorff, D.; Preller, M.; Wang, Y.; Teixeira, S.R.; Riegger, C.; Weber, P. Epigallocatechin Gallate Supplementation Alleviates Diabetes in Rodents. J. Nutr. 2006, 136, 2512–2518. [Google Scholar] [CrossRef]
- Kapoor, M.P.; Sugita, M.; Fukuzawa, Y.; Okubo, T. Physiological effects of epigallocatechin-3-gallate (EGCG) on energy expenditure for prospective fat oxidation in humans: A systematic review and meta-analysis. J. Nutr. Biochem. 2017, 43, 1–10. [Google Scholar] [CrossRef]
- Hamer, M.; Witte, D.; Mosdøl, A.; Marmot, M.; Brunner, E.J. Prospective study of coffee and tea consumption in relation to risk of type 2 diabetes mellitus among men and women: The Whitehall II study. Br. J. Nutr. 2008, 100, 1046–1053. [Google Scholar] [CrossRef] [PubMed]
- Van Woudenbergh, G.J.; Kuijsten, A.; Drogan, D.; van der, A.D.; Romaguera, D.; Ardanaz, E.; Amiano, P.; Barricarte, A.; Beulens, J.W.; Boeing, H.; et al. Tea consumption and incidence of type 2 diabetes in Europe: The EPIC-InterAct case-cohort study. PLoS ONE 2012, 7, e36910. [Google Scholar] [CrossRef] [PubMed]
- Huxley, R.R.; Lee, C.M.Y.; Barzi, F.; Timmermeister, L.; Czernichow, S.; Perkovic, V.; Grobbee, D.E.; Batty, D.; Woodward, M. Coffee, Decaffeinated Coffee, and Tea Consumption in Relation to Incident Type 2 Diabetes Mellitus. Arch. Intern. Med. 2009, 169, 2053–2063. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Han, G.; Hu, Y.; Bi, Y.; Li, L.; Zhu, D.-L. Tea Consumption and Risk of Type 2 Diabetes: A Meta-Analysis of Cohort Studies. J. Gen. Intern. Med. 2009, 24, 557–562. [Google Scholar] [CrossRef]
- Zheng, X.-X.; Xu, Y.-L.; Li, S.-H.; Hui, R.; Wu, Y.; Huang, X.-H. Effects of green tea catechins with or without caffeine on glycemic control in adults: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2013, 97, 750–762. [Google Scholar] [CrossRef]
- Oba, S.; Nagata, C.; Nakamura, K.; Fujii, K.; Kawachi, T.; Takatsuka, N.; Shimizu, H. Consumption of coffee, green tea, oolong tea, black tea, chocolate snacks and the caffeine content in relation to risk of diabetes in Japanese men and women. Br. J. Nutr. 2009, 103, 453–459. [Google Scholar] [CrossRef]
- Odegaard, A.O.; Pereira, M.A.; Koh, W.-P.; Arakawa, K.; Lee, H.-P.; Yu, M.C. Coffee, tea, and incident type 2 diabetes: The Singapore Chinese Health Study. Am. J. Clin. Nutr. 2008, 88, 979–985. [Google Scholar] [CrossRef]
- Polychronopoulos, E.; Zeimbekis, A.; Kastorini, C.-M.; Papairakleous, N.; Vlachou, I.; Bountziouka, V.; Panagiotakos, D.B. Effects of black and green tea consumption on blood glucose levels in non-obese elderly men and women from Mediterranean Islands (MEDIS epidemiological study). Eur. J. Nutr. 2008, 47, 10–16. [Google Scholar] [CrossRef]
- Fukino, Y.; Shimbo, M.; Aoki, N.; Okubo, T.; Iso, H. Randomized controlled trial for an effect of green tea consumption on insulin resistance and inflammation markers. J. Nutr. Sci. Vitaminol. 2005, 51, 335–342. [Google Scholar] [CrossRef]
- Nagao, T.; Meguro, S.; Hase, T.; Otsuka, K.; Komikado, M.; Tokimitsu, I.; Yamamoto, T.; Yamamoto, K. A Catechin-rich Beverage Improves Obesity and Blood Glucose Control in Patients With Type 2 Diabetes. Obesity 2009, 17, 310–317. [Google Scholar] [CrossRef]
- Brown, A.L.; Lane, J.; Coverly, J.; Stocks, J.; Jackson, S.; Stephen, A.; Bluck, L.; Coward, A.; Hendrickx, H. Effects of dietary supplementation with the green tea polyphenol epigallocatechin-3-gallate on insulin resistance and associated metabolic risk factors: Randomized controlled trial. Br. J. Nutr. 2008, 101, 886–894. [Google Scholar] [CrossRef] [PubMed]
- Venables, M.; Hulston, C.J.; Cox, H.R.; Jeukendrup, A.E. Green tea extract ingestion, fat oxidation, and glucose tolerance in healthy humans. Am. J. Clin. Nutr. 2008, 87, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Tsuneki, H.; Ishizuka, M.; Terasawa, M.; Wu, J.-B.; Sasaoka, T.; Kimura, I. Effect of green tea on blood glucose levels and serum proteomic patterns in diabetic (db/db) mice and on glucose metabolism in healthy humans. BMC Pharmacol. 2004, 4, 18. [Google Scholar] [CrossRef] [PubMed]
- Frank, J.; George, T.; Lodge, J.K.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Minihane, A.M.; Rimbach, G. Daily Consumption of an Aqueous Green Tea Extract Supplement Does Not Impair Liver Function or Alter Cardiovascular Disease Risk Biomarkers in Healthy Men. J. Nutr. 2008, 139, 58–62. [Google Scholar] [CrossRef]
- Meng, Q.; Velalar, C.N.; Ruan, R. Regulating the Age-Related Oxidative Damage, Mitochondrial Integrity, and Antioxidative Enzyme Activity in Fischer 344 Rats by Supplementation of the Antioxidant Epigallocatechin-3-Gallate. Rejuvenation Res. 2008, 11, 649–660. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, S.; Zhang, W.; Zhao, P.; He, B.; Wu, N.; Han, P. Epigallocatechin-3-O-gallate (EGCG) attenuates FFAs-induced peripheral insulin resistance through AMPK pathway and insulin signaling pathway in vivo. Diabetes Res. Clin. Pr. 2011, 93, 205–214. [Google Scholar] [CrossRef]
- Babu, P.V.A.; Liu, D.; Gilbert, E.R. Recent advances in understanding the anti-diabetic actions of dietary flavonoids. J. Nutr. Biochem. 2013, 24, 1777–1789. [Google Scholar] [CrossRef]
- Wolfram, S.; Raederstorff, D.; Wang, Y.; Teixeira, S.R.; Elste, V.; Weber, P. TEAVIGOTM (Epigallocatechin Gallate) Supplementation Prevents Obesity in Rodents by Reducing Adipose Tissue Mass. Ann. Nutr. Metab. 2005, 49, 54–63. [Google Scholar] [CrossRef]
- Murase, T.; Nagasawa, A.; Suzuki, J.; Hase, T.; Tokimitsu, I. Beneficial effects of tea catechins on diet-induced obesity: Stimulation of lipid catabolism in the liver. Int. J. Obes. 2002, 26, 1459–1464. [Google Scholar] [CrossRef]
- Chung, S.; Yao, H.; Caito, S.; Hwang, J.-W.; Arunachalam, G.; Rahman, I. Regulation of SIRT1 in cellular functions: Role of polyphenols. Arch. Biochem. Biophys. 2010, 501, 79–90. [Google Scholar] [CrossRef]
- Moon, H.-S.; Chung, C.-S.; Lee, H.-G.; Kim, T.-G.; Choi, Y.-J.; Cho, C.-S. Inhibitory Effect of (−)-Epigallocatechin-3-Gallate on Lipid Accumulation of 3T3-L1 Cells**. Obesity 2007, 15, 2571–2582. [Google Scholar] [CrossRef] [PubMed]
- Murase, T.; Misawa, K.; Haramizu, S.; Hase, T. Catechin-induced activation of the LKB1/AMP-activated protein kinase pathway. Biochem. Pharmacol. 2009, 78, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Wang, H. Mechanistic issues concerning cancer prevention by tea catechins. Mol. Nutr. Food Res. 2011, 55, 819–831. [Google Scholar] [CrossRef] [PubMed]
- Yuliana, N.D.; Korthout, H.; Wijaya, C.H.; Kim, H.K.; Verpoorte, R. Plant-Derived Food Ingredients for Stimulation of Energy Expenditure. Crit. Rev. Food Sci. Nutr. 2013, 54, 373–388. [Google Scholar] [CrossRef] [PubMed]
- Collins, Q.F.; Liu, H.-Y.; Pi, J.; Liu, Z.; Quon, M.; Cao, W. Epigallocatechin-3-gallate (EGCG), a green tea polyphenol, suppresses hepatic gluconeogenesis through 5’-AMP-activated protein kinase. J. Boil. Chem. 2007, 282, 30143–30149. [Google Scholar] [CrossRef] [PubMed]
- Sheena, A.; Mohan, S.S.; Haridas, P.A.N.; Anilkumar, G. Elucidation of the Glucose Transport Pathway in Glucose Transporter 4 via Steered Molecular Dynamics Simulations. PLoS ONE 2011, 6, e25747. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, Q.; Liang, J.; Dai, X.; Ding, Y.; Wang, J.; Li, Y. Epigallocatechin-3-O-gallate (EGCG) protects the insulin sensitivity in rat L6 muscle cells exposed to dexamethasone condition. Phytomedicine 2010, 17, 14–18. [Google Scholar] [CrossRef]
- Jung, K.H.; Choi, H.S.; Kim, N.H.; Han, M.Y.; Chang, U.J.; Yim, S.-V.; Song, B.C.; Kim, C.-H.; Kang, S.A. Epigallocatechin Gallate Stimulates Glucose Uptake Through the Phosphatidylinositol 3-Kinase-Mediated Pathway in L6 Rat Skeletal Muscle Cells. J. Med. Food 2008, 11, 429–434. [Google Scholar] [CrossRef]
- Nishiumi, S.; Bessyo, H.; Kubo, M.; Aoki, Y.; Tanaka, A.; Yoshida, K.-I.; Ashida, H. Green and Black Tea Suppress Hyperglycemia and Insulin Resistance by Retaining the Expression of Glucose Transporter 4 in Muscle of High-Fat Diet-Fed C57BL/6J Mice. J. Agric. Food Chem. 2010, 58, 12916–12923. [Google Scholar] [CrossRef]
- Tabas, I.; García-Cardeña, G.; Owens, G.K. Recent insights into the cellular biology of atherosclerosis. J. Cell Boil. 2015, 209, 13–22. [Google Scholar] [CrossRef]
- Bonetti, P.O.; Lerman, L.O.; Lerman, L.O. Endothelial Dysfunction. Arter. Thromb. Vasc. Boil. 2003, 23, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Von Scholten, B.J.; Reinhard, H.; Hansen, P.R.; Schalkwijk, C.G.; Stehouwer, C.D.; Parving, H.-H.; Jacobsen, P.; Rossing, P. Markers of inflammation and endothelial dysfunction are associated with incident cardiovascular disease, all-cause mortality, and progression of coronary calcification in type 2 diabetic patients with microalbuminuria. J. Diabetes Its Complicat. 2016, 30, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Potenza, M.A.; Nacci, C.; De Salvia, M.; Sgarra, L.; Collino, M.; Montagnani, M. Targeting endothelial metaflammation to counteract diabesity cardiovascular risk: Current and perspective therapeutic options. Pharmacol. Res. 2017, 120, 226–241. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-M. ROS Generation by Nonphagocytic NADPH Oxidase: Potential Relevance in Diabetic Nephropathy. J. Am. Soc. Nephrol. 2003, 14, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Dal, S.; Jeandidier, N.; Schaschkow, A.; Spizzo, A.-H.; Seyfritz, E.; Sookhareea, C.; Bietiger, W.; Peronet, C.; Moreau, F.; Pinget, M.; et al. Portal or subcutaneous insulin infusion: Efficacy and impact on liver inflammation. Fundam. Clin. Pharmacol. 2015, 29, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Selemidis, S.; Sobey, C.G.; Wingler, K.; Schmidt, H.H.; Drummond, G.R. NADPH oxidases in the vasculature: Molecular features, roles in disease and pharmacological inhibition. Pharmacol. Ther. 2008, 120, 254–291. [Google Scholar] [CrossRef]
- Dal-Ros, S.; Zoll, J.; Lang, A.-L.; Auger, C.; Keller, N.; Bronner, C.; Geny, B.; Schini-Kerth, V. Chronic intake of red wine polyphenols by young rats prevents aging-induced endothelial dysfunction and decline in physical performance: Role of NADPH oxidase. Biochem. Biophys. Res. Commun. 2011, 404, 743–749. [Google Scholar] [CrossRef]
- Dal-Ros, S.; Bronner, C.; Schott, C.; Kane, M.O.; Chataigneau, M.; Schini-Kerth, V.B.; Chataigneau, T. Angiotensin II-Induced Hypertension Is Associated with a Selective Inhibition of Endothelium-Derived Hyperpolarizing Factor-Mediated Responses in the Rat Mesenteric Artery. J. Pharmacol. Exp. Ther. 2008, 328, 478–486. [Google Scholar] [CrossRef]
- Landmesser, U.; Dikalov, S.; Price, S.R.; McCann, L.; Fukai, T.; Holland, S.M.; Mitch, W.E.; Harrison, D.G. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J. Clin. Investig. 2003, 111, 1201–1209. [Google Scholar] [CrossRef]
- Siragusa, M.; Fleming, I. The eNOS signalosome and its link to endothelial dysfunction. Pflügers Arch.—Eur. J. Physiol. 2016, 468, 1125–1137. [Google Scholar] [CrossRef]
- Meza, C.A.; La Favor, J.D.; Kim, D.-H.; Hickner, R.C. Endothelial Dysfunction: Is There a Hyperglycemia-Induced Imbalance of NOX and NOS? Int. J. Mol. Sci. 2019, 20, 3775. [Google Scholar] [CrossRef] [PubMed]
- Potenza, M.A.; Addabbo, F.; Montagnani, M. Vascular actions of insulin with implications for endothelial dysfunction. Am. J. Physiol. Metab. 2009, 297, E568–E577. [Google Scholar] [CrossRef]
- Szabo, C.; Mabley, J.G.; Moeller, S.M.; Shimanovich, R.; Pacher, P.; Virág, L.; Soriano, F.G.; Van Duzer, J.H.; Williams, W.; Salzman, A.L.; et al. Part I: Pathogenetic role of peroxynitrite in the development of diabetes and diabetic vascular complications: Studies with FP15, a novel potent peroxynitrite decomposition catalyst. Mol. Med. 2002, 8, 571–580. [Google Scholar] [CrossRef]
- Moreira, J.D.; Pernomian, L.; Gomes, M.S.; Moreira, R.P.; Prado, A.F.D.; Da Silva, C.H.; De Oliveira, A.M. Enhanced nitric oxide generation from nitric oxide synthases as the cause of increased peroxynitrite formation during acute restraint stress: Effects on carotid responsiveness to angiotensinergic stimuli in type-1 diabetic rats. Eur. J. Pharmacol. 2016, 783, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, M.; Wessler, S.; Follmann, E.; Michaelis, W.; Düsterhöft, T.; Baumann, G.; Stangl, K.; Stangl, V. A Constituent of Green Tea, Epigallocatechin-3-gallate, Activates Endothelial Nitric Oxide Synthase by a Phosphatidylinositol-3-OH-kinase-, cAMP-dependent Protein Kinase-, and Akt-dependent Pathway and Leads to Endothelial-dependent Vasorelaxation. J. Boil. Chem. 2003, 279, 6190–6195. [Google Scholar] [CrossRef]
- Wu, J.; Xu, X.; Li, Y.; Kou, J.; Huang, F.; Liu, B.; Liu, K. Quercetin, luteolin and epigallocatechin gallate alleviate TXNIP and NLRP3-mediated inflammation and apoptosis with regulation of AMPK in endothelial cells. Eur. J. Pharmacol. 2014, 745, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Colombo, S.; Moncada, S. AMPKα1 regulates the antioxidant status of vascular endothelial cells. Biochem. J. 2009, 421, 163–169. [Google Scholar] [CrossRef]
- Akiyama, S.; Katsumata, S.-I.; Suzuki, K.; Nakaya, Y.; Ishimi, Y.; Uehara, M. Hypoglycemic and Hypolipidemic Effects of Hesperidin and Cyclodextrin-Clathrated Hesperetin in Goto-Kakizaki Rats with Type 2 Diabetes. Biosci. Biotechnol. Biochem. 2009, 73, 2779–2782. [Google Scholar] [CrossRef]
- Yang, D.; Liu, J.; Tian, C.; Zeng, Y.; Zheng, Y.-H.; Fang, Q.; Li, H.-H. Epigallocatechin gallate inhibits angiotensin II-induced endothelial barrier dysfunction via inhibition of the p38 MAPK/HSP27 pathway. Acta Pharmacol. Sin. 2010, 31, 1401–1406. [Google Scholar] [CrossRef]
- Menard, S.; Castronovo, V.; Tagliabue, E.; Sobel, M.E. New insights into the metastasis-associated 67 kD laminin receptor. J. Cell. Biochem. 1997, 67, 155–165. [Google Scholar] [CrossRef]
- Fujimura, Y.; Sumida, M.; Sugihara, K.; Tsukamoto, S.; Yamada, K.; Tachibana, H. Green Tea Polyphenol EGCG Sensing Motif on the 67-kDa Laminin Receptor. PLoS ONE 2012, 7, e37942. [Google Scholar] [CrossRef]
- Byun, E.-B.; Kim, W.S.; Sung, N.-Y.; Byun, E.-H. Epigallocatechin-3-Gallate Regulates Anti-Inflammatory Action Through 67-kDa Laminin Receptor-Mediated Tollip Signaling Induction in Lipopolysaccharide-Stimulated Human Intestinal Epithelial Cells. Cell. Physiol. Biochem. 2018, 46, 2072–2081. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Yang, C.; Kim, J.; MacNevin, C.J.; Hahn, K.M.; Park, N.; Ginsberg, M.H.; Kim, C. Epigallocatechin gallate has pleiotropic effects on transmembrane signaling by altering the embedding of transmembrane domains. J. Boil. Chem. 2017, 292, 9858–9864. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Ohta, T.; Igura, K.; Hara, Y.; Kaji, K. Tea catechins inhibit angiogenesis in vitro, measured by human endothelial cell growth, migration and tube formation, through inhibition of VEGF receptor binding. Cancer Lett. 2002, 180, 139–144. [Google Scholar] [CrossRef]
- Liang, Y.-C.; Lin-Shiau, S.-Y.; Chen, C.-F.; Lin, J.-K. Suppression of extracellular signals and cell proliferation through EGF receptor binding by (-)-epigallocatechin gallate in human A431 epidermoid carcinoma cells. J. Cell. Biochem. 1997, 67, 55–65. [Google Scholar] [CrossRef]
- Shimizu, M.; Deguchi, A.; Hara, Y.; Moriwaki, H.; Weinstein, I.B. EGCG inhibits activation of the insulin-like growth factor-1 receptor in human colon cancer cells. Biochem. Biophys. Res. Commun. 2005, 334, 947–953. [Google Scholar] [CrossRef]
- Pullikotil, P.; Chen, H.; Muniyappa, R.; Greenberg, C.C.; Yang, S.; Reiter, C.E.N.; Lee, J.-W.; Chung, J.H.; Quon, M.J. Epigallocatechin gallate induces expression of heme oxygenase-1 in endothelial cells via p38 MAPK and Nrf-2 that suppresses proinflammatory actions of TNF-α. J. Nutr. Biochem. 2012, 23, 1134–1145. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Hsu, M.; Hsieh, C.; Lin, J.; Lai, P.; Wung, B.-S. Upregulation of heme oxygenase-1 by Epigallocatechin-3-gallate via the phosphatidylinositol 3-kinase/Akt and ERK pathways. Life Sci. 2006, 78, 2889–2897. [Google Scholar] [CrossRef]
- Toussaint, F.; Charbel, C.; Allen, B.G.; LeDoux, J. Vascular CaMKII: Heart and brain in your arteries. Am. J. Physiol. Physiol. 2016, 311, C462–C478. [Google Scholar] [CrossRef]
- Murthy, S.; Koval, O.M.; Diaz, J.M.R.; Kumar, S.; Nuno, D.; Scott, J.A.; Allamargot, C.; Zhu, L.J.; Broadhurst, K.; Santhana, V.; et al. Endothelial CaMKII as a regulator of eNOS activity and NO-mediated vasoreactivity. PLoS ONE 2017, 12, e0186311. [Google Scholar] [CrossRef]
- Kim, H.-S.; Quon, M.; Kim, J. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Boil. 2014, 2, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Manea, S.-A. NADPH oxidase-derived reactive oxygen species: Involvement in vascular physiology and pathology. Cell Tissue Res. 2010, 342, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-T.; Ha, J.; Park, I.-J.; Lee, S.-K.; Baik, H.W.; Kim, Y.M.; Park, O.J. Apoptotic effect of EGCG in HT-29 colon cancer cells via AMPK signal pathway. Cancer Lett. 2007, 247, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Coco, C.; Sgarra, L.; Potenza, M.A.; Nacci, C.; Pasculli, B.; Barbano, R.; Parrella, P.; Montagnani, M. Can Epigenetics of Endothelial Dysfunction Represent the Key to Precision Medicine in Type 2 Diabetes Mellitus? Int. J. Mol. Sci. 2019, 20, 2949. [Google Scholar] [CrossRef]
- Prattichizzo, F.; Giuliani, A.; Ceka, A.; Rippo, M.R.; Bonfigli, A.R.; Testa, I.; Procopio, A.D.; Olivieri, F. Epigenetic mechanisms of endothelial dysfunction in type 2 diabetes. Clin. Epigenet. 2015, 7, 56. [Google Scholar] [CrossRef]
- Esser, N.; Legrand-Poels, S.; Piette, J.; Scheen, A.J.; Paquot, N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res. Clin. Pr. 2014, 105, 141–150. [Google Scholar] [CrossRef]
- Lumeng, C.N.; Saltiel, A.R. Inflammatory links between obesity and metabolic disease. J. Clin. Investig. 2011, 121, 2111–2117. [Google Scholar] [CrossRef]
- Fang, M.; Chen, D.; Yang, C.S. Dietary Polyphenols May Affect DNA Methylation. J. Nutr. 2007, 137, 223S–228S. [Google Scholar] [CrossRef]
- Fang, M.Z.; Wang, Y.; Ai, N.; Hou, Z.; Sun, Y.; Lu, H.; Welsh, W.; Yang, C.S. Tea polyphenol (-)-epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Cancer Res. 2003, 63, 7563–7570. [Google Scholar]
- Choi, K.-C.; Jung, M.G.; Lee, Y.-H.; Yoon, J.C.; Kwon, S.-H.; Kang, H.-B.; Kim, M.-J.; Cha, J.-H.; Kim, Y.J.; Jun, W.J.; et al. Epigallocatechin-3-Gallate, a Histone Acetyltransferase Inhibitor, Inhibits EBV-Induced B Lymphocyte Transformation via Suppression of RelA Acetylation. Cancer Res. 2009, 69, 583–592. [Google Scholar] [CrossRef]
- Liu, D.; Perkins, J.T.; Hennig, B. EGCG prevents PCB-126-induced endothelial cell inflammation via epigenetic modifications of NF-κB target genes in human endothelial cells. J. Nutr. Biochem. 2015, 28, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Sáez, T.; Toledo, F.; Sobrevia, L. Impaired signalling pathways mediated by extracellular vesicles in diabesity. Mol. Asp. Med. 2019, 66, 13–20. [Google Scholar] [CrossRef]
- Nesca, V.; Guay, C.; Jacovetti, C.; Menoud, V.; Peyot, M.-L.; Laybutt, D.R.; Prentki, M.; Regazzi, R. Identification of particular groups of microRNAs that positively or negatively impact on beta cell function in obese models of type 2 diabetes. Diabetol. 2013, 56, 2203–2212. [Google Scholar] [CrossRef] [PubMed]
- Bagge, A.; Clausen, T.R.; Larsen, S.; Ladefoged, M.; Rosenstierne, M.W.; Larsen, L.; Vang, O.; Nielsen, J.H.; Dalgaard, L.T. MicroRNA-29a is up-regulated in beta-cells by glucose and decreases glucose-stimulated insulin secretion. Biochem. Biophys. Res. Commun. 2012, 426, 266–272. [Google Scholar] [CrossRef]
- Collares, C.V.; Evangelista, A.F.; Xavier, D.J.; Rassi, D.M.; Arns, T.; De Freitas, M.C.F.; Foss, M.C.; Puthier, D.; Sakamoto-Hojo, E.T.; Passos, G.A.; et al. Identifying common and specific microRNAs expressed in peripheral blood mononuclear cell of type 1, type 2, and gestational diabetes mellitus patients. BMC Res. Notes 2013, 6, 491. [Google Scholar] [CrossRef] [PubMed]
- Tsang, W.P.; Kwok, T.-T. Epigallocatechin gallate up-regulation of miR-16 and induction of apoptosis in human cancer cells. J. Nutr. Biochem. 2010, 21, 140–146. [Google Scholar] [CrossRef]
- Otton, R.; Bolin, A.P.; Ferreira, L.T.; Marinovic, M.P.; Rocha, A.L.S.; Mori, M.A. Polyphenol-rich green tea extract improves adipose tissue metabolism by down-regulating miR-335 expression and mitigating insulin resistance and inflammation. J. Nutr. Biochem. 2018, 57, 170–179. [Google Scholar] [CrossRef]
- Jang, J.-Y.; Lee, J.-K.; Jeon, Y.K.; Kim, C.-W. Exosome derived from epigallocatechin gallate treated breast cancer cells suppresses tumor growth by inhibiting tumor-associated macrophage infiltration and M2 polarization. BMC Cancer 2013, 13, 421. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Potenza, M.A.; Iacobazzi, D.; Sgarra, L.; Montagnani, M. The Intrinsic Virtues of EGCG, an Extremely Good Cell Guardian, on Prevention and Treatment of Diabesity Complications. Molecules 2020, 25, 3061. https://doi.org/10.3390/molecules25133061
Potenza MA, Iacobazzi D, Sgarra L, Montagnani M. The Intrinsic Virtues of EGCG, an Extremely Good Cell Guardian, on Prevention and Treatment of Diabesity Complications. Molecules. 2020; 25(13):3061. https://doi.org/10.3390/molecules25133061
Chicago/Turabian StylePotenza, Maria Assunta, Dominga Iacobazzi, Luca Sgarra, and Monica Montagnani. 2020. "The Intrinsic Virtues of EGCG, an Extremely Good Cell Guardian, on Prevention and Treatment of Diabesity Complications" Molecules 25, no. 13: 3061. https://doi.org/10.3390/molecules25133061
APA StylePotenza, M. A., Iacobazzi, D., Sgarra, L., & Montagnani, M. (2020). The Intrinsic Virtues of EGCG, an Extremely Good Cell Guardian, on Prevention and Treatment of Diabesity Complications. Molecules, 25(13), 3061. https://doi.org/10.3390/molecules25133061







