One-Pot Multicomponent Synthesis and Bioevaluation of Tetrahydroquinoline Derivatives as Potential Antioxidants, α-Amylase Enzyme Inhibitors, Anti-Cancerous and Anti-Inflammatory Agents
Abstract
1. Introduction
2. Results and Discussions
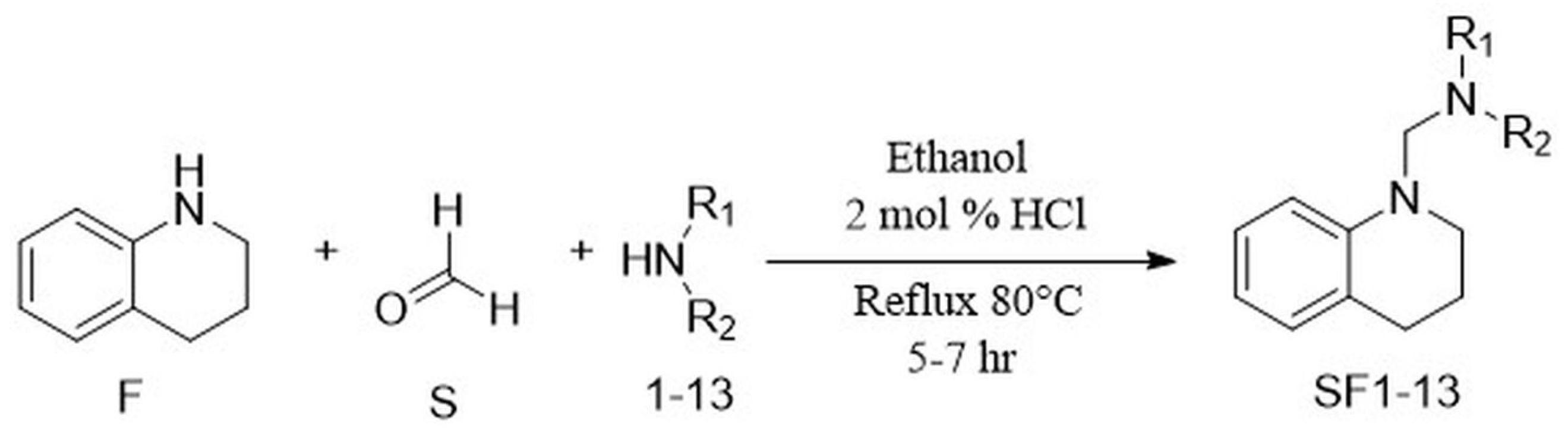
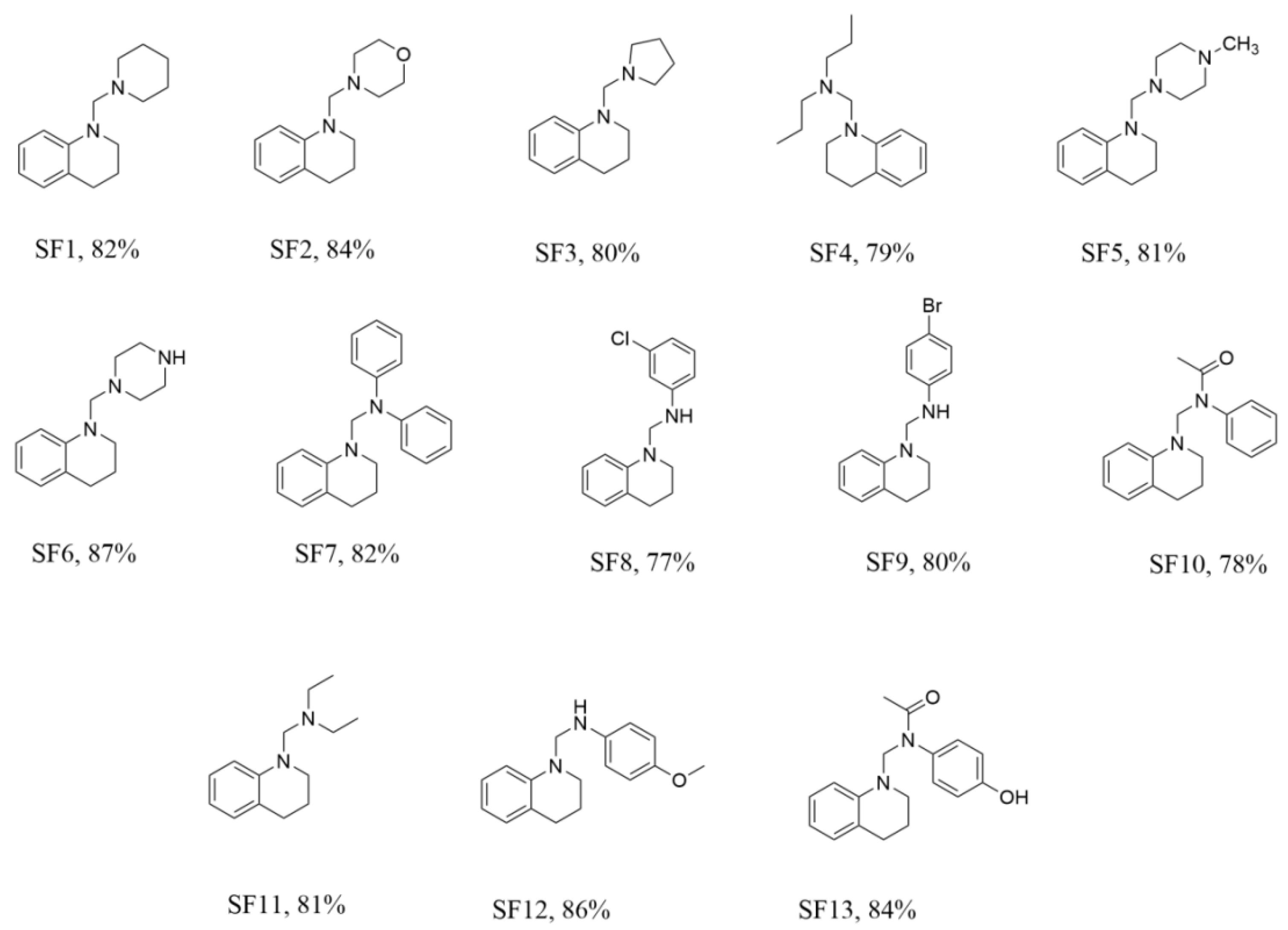
2.1. Chemistry
2.2. ADME Predictions
2.3. Bioevaluation
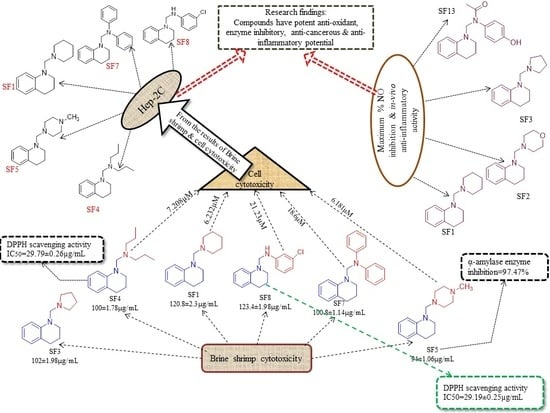
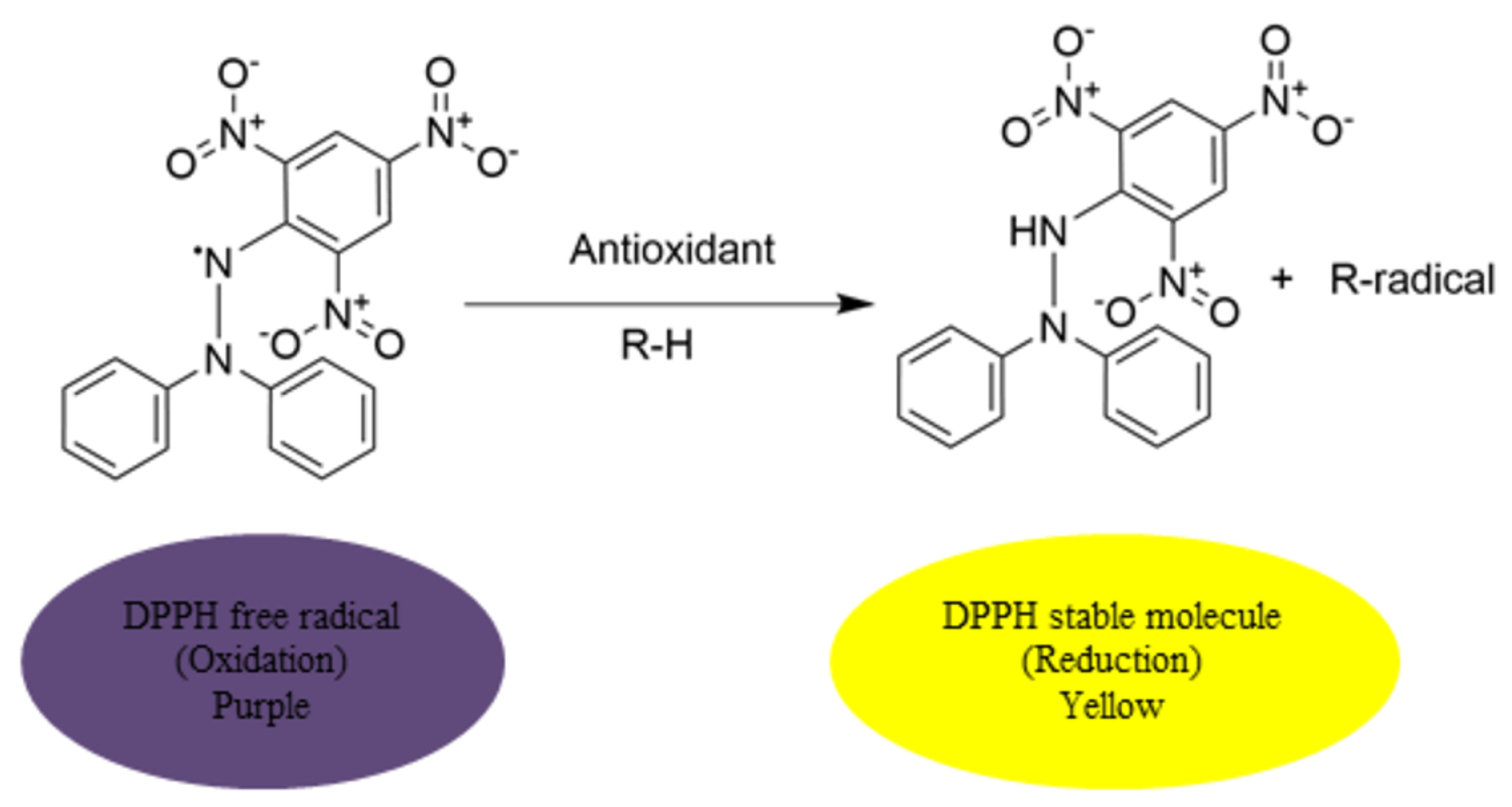
2.3.1. Antioxidant Assays
DPPH Assay
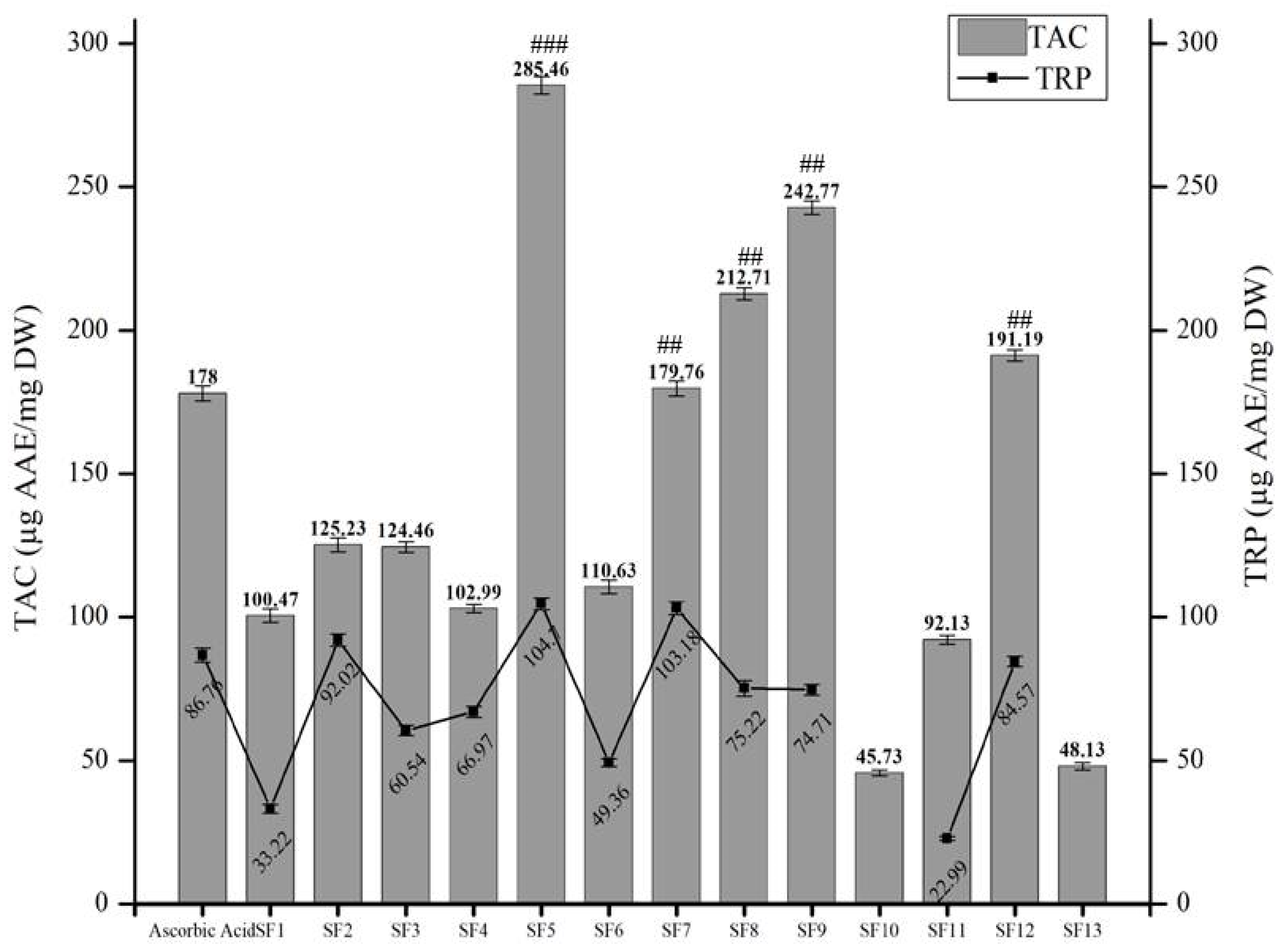
Phosphomolybdenum-Based Total Antioxidant Capacity (TAC) and Total Reducing Power (TRP)
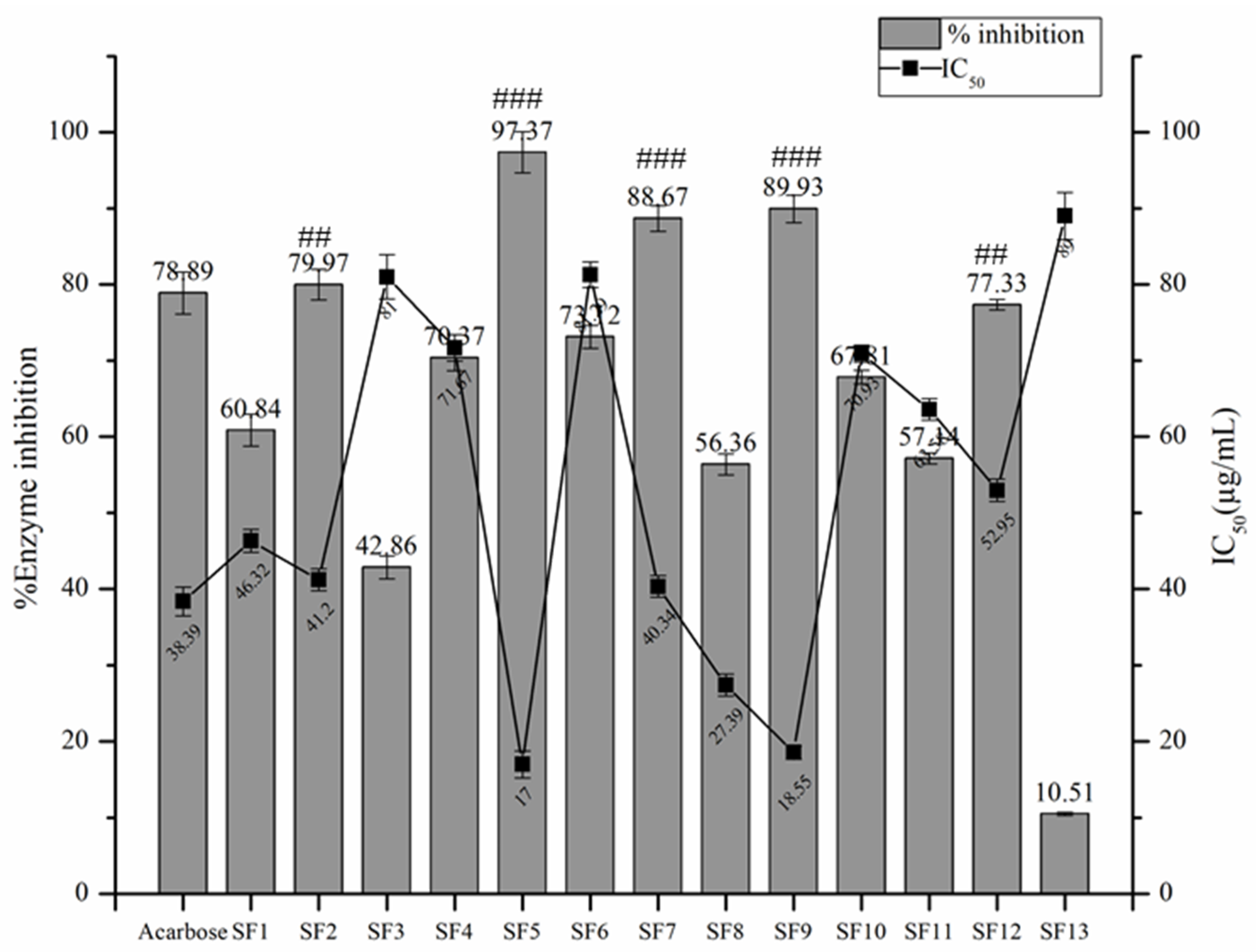
2.3.2. α- Amylase Inhibitory Assay
2.3.3. Evaluation of Cytotoxic Potential
Brine Shrimp Cytotoxicity Assay
Cytotoxicity Against Raw Macrophages and Cancer Cell Lines (Hep-2C)
MTT Assay Against Hep-2C Cells
2.3.4. Anti-Inflammatory Activity
Nitric Oxide Scavenging Assay (NO)
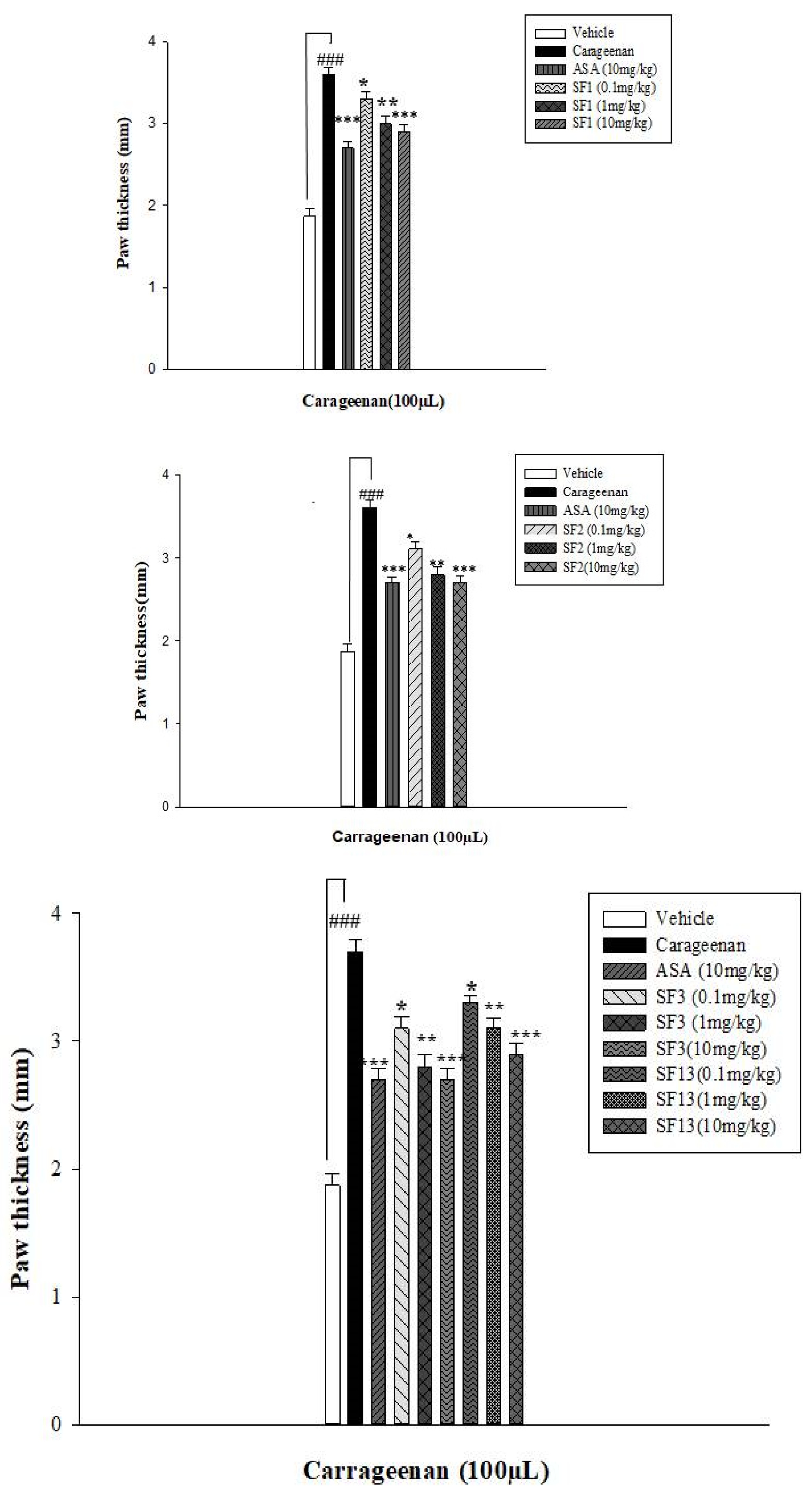
In Vivo Anti-Inflammatory Activity
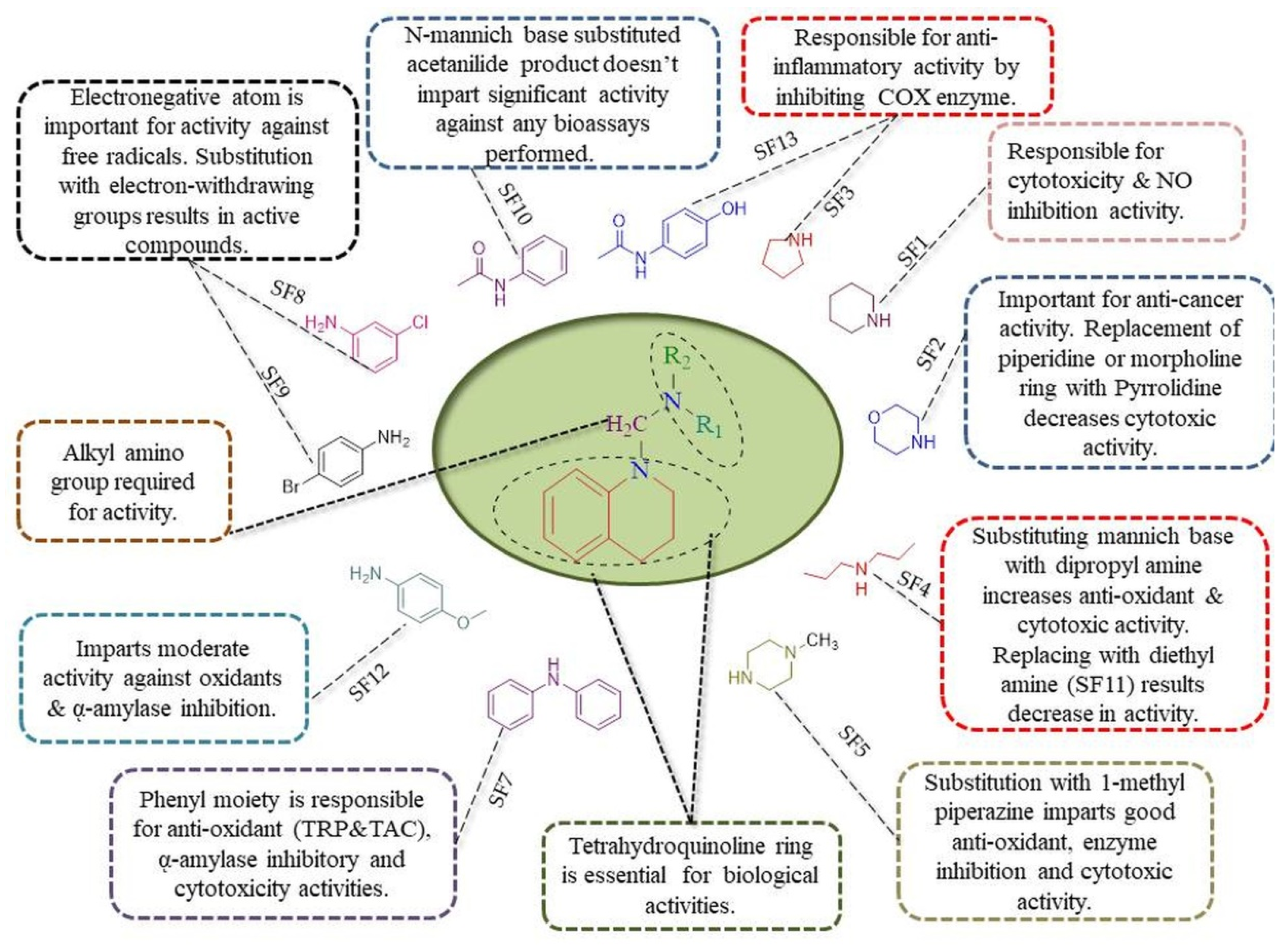
2.3.5. Structure-Activity Relationship (SAR)
3. Materials and Methods
3.1. General Procedure for the Synthesis of N-Mannich Bases of Tetrahydroquinoline
3.2. ADME Predictions
3.3. Bioevaluation
3.3.1. Antioxidant Assay
DPPH Assay
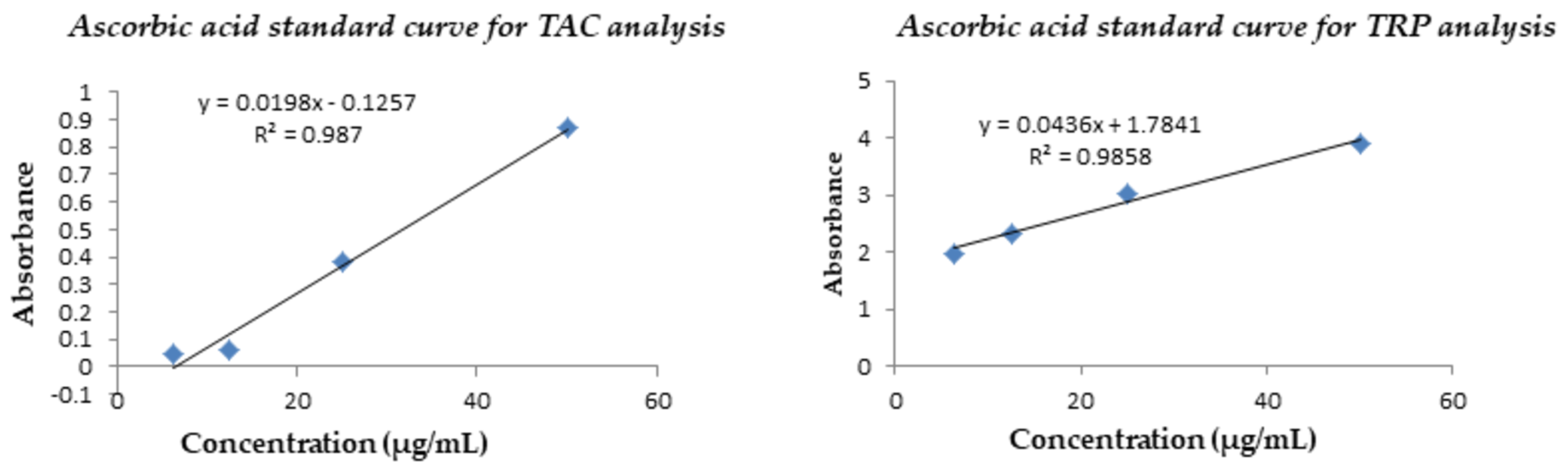
Determination of Total Antioxidant Capacity (TAC)
Total Reducing Power (TRP) Determination
3.3.2. α- Amylase Enzyme Inhibitory Assay
3.3.3. Cytotoxicity Evaluation
Brine Shrimp Cytotoxicity Assay
Cytotoxicity Against Raw Macrophages
MTT Assay Against Hep-2C Cells
3.3.4. Nitric Oxide Assay
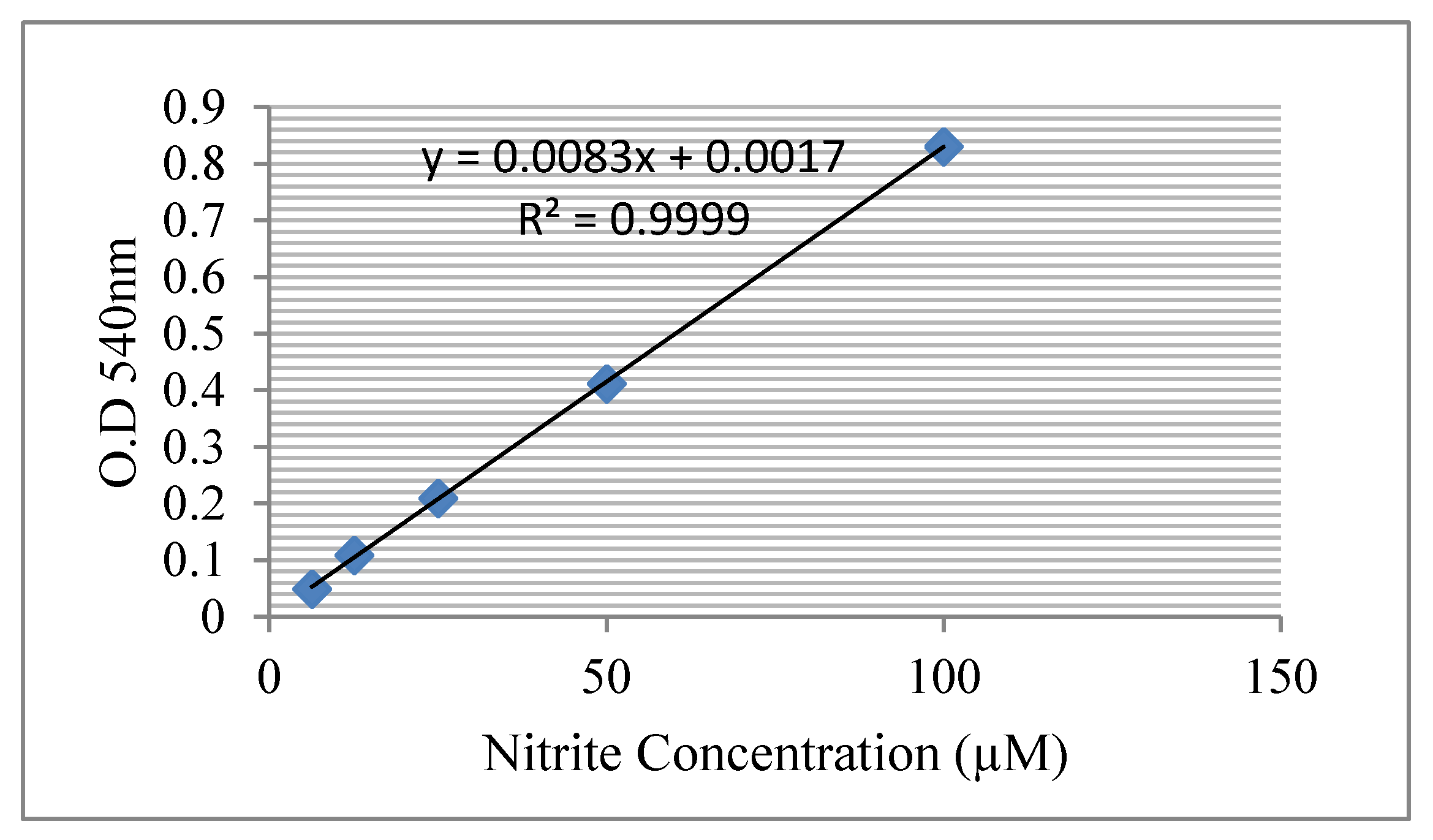
Isolation of Peritoneal Macrophages and Measurement of Nitrite Production
In Vivo Anti-Inflammatory Activity
3.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| THQ | Tetrahydroquinoline |
| ASA | Acetylsalicylic acid |
| FRSA | Free radical scavenging activity |
| TAC | Total antioxidant capacity |
| TRP | Total reducing power |
| NO | Nitric oxide |
| SEM | Standard error of the mean |
| SD | Standard deviation |
| DMSO | Dimethylsulfoxide |
| KBr | Potassium bromide |
| DM | Diabetes mellitus |
| ROS | Reactive oxygen species |
| COX-I/II | Cyclooxygenase I/II |
| MCR | Multicomponent reaction |
| CDCl3 | Deuterated chloroform |
| ADME | Absorption, distribution, metabolism, excretion |
| AAE | Ascorbic acid equivalent |
| LD | Lethal dose |
| TLC | Thin-layer chromatography |
| DCM | Dichloromethane |
| SAR | Structure–activity relationship |
| RO5 | Rule of five (Lipinski’s five pints) |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide |
| DPPH | 2, 2-Diphenyl-1-picrylhydrazine |
References
- Patwardhan, B.; Vaidya, A.D.; Chorghade, M.; Joshi, S.P. Reverse pharmacology and systems approaches for drug discovery and development. Curr. Bioact. Compd. 2008, 4, 201–212. [Google Scholar] [CrossRef]
- Campos, K.R.; Coleman, P.J.; Alvarez, J.C.; Dreher, S.D.; Garbaccio, R.M.; Terrett, N.K.; Tillyer, R.D.; Truppo, M.D.; Parmee, E.R. The importance of synthetic chemistry in the pharmaceutical industry. Science 2019, 363, eaat0805. [Google Scholar] [CrossRef]
- Afsah, E.M.; Fadda, A.A.; Hammouda, M.M. Synthesis and antioxidant activity of 2-indolinone bis (Mannich bases) and related compounds. Mon. Chem. Chem. Mon. 2016, 147, 2009–2016. [Google Scholar] [CrossRef]
- Kattappagari, K.K.; Teja, C.R.; Kommalapati, R.K.; Poosarla, C.; Gontu, S.R.; Reddy, B.V.R. Role of antioxidants in facilitating the body functions: A review. J. Orofac. Sci. 2015, 7, 71. [Google Scholar] [CrossRef]
- Garcia, E.J.; Oldoni, T.L.C.; Alencar, S.M.d.; Reis, A.; Loguercio, A.D.; Grande, R.H.M. Antioxidant activity by DPPH assay of potential solutions to be applied on bleached teeth. Braz. Dent. J. 2012, 23, 22–27. [Google Scholar] [CrossRef]
- Demirci, S.; Demirbaş, N. Anticancer activities of novel Mannich bases against prostate cancer cells. Med. Chem. Res. 2019, 28, 1945–1958. [Google Scholar] [CrossRef]
- Aschner, P. Current concepts of diabetes mellitus. Int. Ophthalmol. Clin. 1998, 38, 1–10. [Google Scholar] [CrossRef]
- Sõukand, R.; Pieroni, A.; Biró, M.; Dénes, A.; Dogan, Y.; Hajdari, A.; Kalle, R.; Reade, B.; Mustafa, B.; Nedelcheva, A. An ethnobotanical perspective on traditional fermented plant foods and beverages in Eastern Europe. J. Ethnopharmacol. 2015, 170, 284–296. [Google Scholar] [CrossRef]
- Etxeberria, U.; de la Garza, A.L.; Campión, J.; Martinez, J.A.; Milagro, F.I. Antidiabetic effects of natural plant extracts via inhibition of carbohydrate hydrolysis enzymes with emphasis on pancreatic alpha amylase. Expert Opin. Ther. Targets 2012, 16, 269–297. [Google Scholar] [CrossRef]
- Balan, K.; Ratha, P.; Prakash, G.; Viswanathamurthi, P.; Adisakwattana, S.; Palvannan, T. Evaluation of invitro α-amylase and α-glucosidase inhibitory potential of N2O2 schiff base Zn complex. Arab. J. Chem. 2017, 10, 732–738. [Google Scholar] [CrossRef]
- Arora, R.K.; Kaur, N.; Bansal, Y.; Bansal, G. Novel coumarin–benzimidazole derivatives as antioxidants and safer anti-inflammatory agents. Acta Pharm. Sin. B 2014, 4, 368–375. [Google Scholar] [CrossRef]
- Madar, J.M.; Shastri, L.A.; Shastri, S.L.; Holiyachi, M.; Naik, N.; Kulkarni, R.; Shaikh, F.; Sungar, V. Design, synthesis, characterization, and biological evaluation of pyrido [1, 2-a] pyrimidinone coumarins as promising anti-inflammatory agents. Synth. Commun. 2018, 48, 375–386. [Google Scholar] [CrossRef]
- Yin, L.-L.; Zhang, W.-Y.; Li, M.-H.; Shen, J.-K.; Zhu, X.-Z. CC05, a novel anti-inflammatory compound, exerts its effect by inhibition of cyclooxygenase-2 activity. Eur. J. Pharmacol. 2005, 520, 172–178. [Google Scholar] [CrossRef]
- Zhang, X.; Cao, J.; Zhong, L. Hydroxytyrosol inhibits pro-inflammatory cytokines, iNOS, and COX-2 expression in human monocytic cells. Naunyn-Schmiedebergs Arch. Pharmacol. 2009, 379, 581. [Google Scholar] [CrossRef]
- Hseu, Y.-C.; Wu, F.-Y.; Wu, J.-J.; Chen, J.-Y.; Chang, W.-H.; Lu, F.-J.; Lai, Y.-C.; Yang, H.-L. Anti-inflammatory potential of Antrodia camphorata through inhibition of iNOS, COX-2 and cytokines via the NF-κB pathway. Int. Immunopharmacol. 2005, 5, 1914–1925. [Google Scholar] [CrossRef]
- Sharma, J.; Al-Omran, A.; Parvathy, S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef]
- Bogdan, C. Nitric oxide and the immune response. Nat. Immunol. 2001, 2, 907–916. [Google Scholar] [CrossRef]
- Aktan, F. iNOS-mediated nitric oxide production and its regulation. Life Sci. 2004, 75, 639–653. [Google Scholar] [CrossRef]
- Insuasty, D.; Castillo, J.; Becerra, D.; Rojas, H.; Abonia, R. Synthesis of Biologically Active Molecules through Multicomponent Reactions. Molecules 2020, 25, 505. [Google Scholar] [CrossRef]
- Cioc, R.C.; Ruijter, E.; Orru, R.V. Multicomponent reactions: Advanced tools for sustainable organic synthesis. Green Chem. 2014, 16, 2958–2975. [Google Scholar] [CrossRef]
- Fields, E.K. The synthesis of esters of substituted amino phosphonic acids1a. J. Am. Chem. Soc. 1952, 74, 1528–1531. [Google Scholar] [CrossRef]
- Lal, J.; Gupta, S.K.; Thavaselvam, D.; Agarwal, D.D. Synthesis and pharmacological activity evaluation of curcumin derivatives. Chin. Chem. Lett. 2016, 27, 1067–1072. [Google Scholar] [CrossRef]
- Dayal, N.; Mikek, C.G.; Hernandez, D.; Naclerio, G.A.; Chu, E.F.Y.; Carter-Cooper, B.A.; Lapidus, R.G.; Sintim, H.O. Potently inhibiting cancer cell migration with novel 3H-pyrazolo [4, 3-f] quinoline boronic acid ROCK inhibitors. Eur. J. Med. Chem. 2019, 180, 449–456. [Google Scholar] [CrossRef]
- Tenti, G.; Parada, E.; León, R.; Egea, J.; Martínez-Revelles, S.; Briones, A.M.; Sridharan, V.; López, M.G.; Ramos, M.T.; Menéndez, J.C. New 5-unsubstituted dihydropyridines with improved CaV1. 3 selectivity as potential neuroprotective agents against ischemic injury. J. Med. Chem. 2014, 57, 4313–4323. [Google Scholar] [CrossRef]
- Pourshojaei, Y.; Abiri, A.; Eskandari, R.; Dourandish, F.; Eskandari, K.; Asadipo, A. Synthesis, biological evaluation, and computational studies of novel fused six-membered O-containing heterocycles as potential acetylcholinesterase inhibitors. Comput. Biol. Chem. 2019, 80, 249–258. [Google Scholar] [CrossRef]
- Lakshmi, N.V.; Thirumurugan, P.; Noorulla, K.; Perumal, P.T. InCl3 mediated one-pot multicomponent synthesis, anti-microbial, antioxidant and anticancer evaluation of 3-pyranyl indole derivatives. Bioorg. Med. Chem. Lett. 2010, 20, 5054–5061. [Google Scholar] [CrossRef]
- Murlykina, M.V.; Sakhno, Y.I.; Desenko, S.M.; Konovalova, I.S.; Shishkin, O.V.; Sysoiev, D.A.; Kornet, M.N.; Chebanov, V.A. Features of switchable multicomponent heterocyclizations of salicylic aldehydes and 5-aminopyrazoles with pyruvic acids and antimicrobial activity of the reaction products. Tetrahedron 2013, 69, 9261–9269. [Google Scholar] [CrossRef]
- Darandale, S.N.; Pansare, D.N.; Mulla, N.A.; Shinde, D.B. Green synthesis of tetrahydropyrimidine analogues and evaluation of their antimicrobial activity. Bioorg. Med. Chem. Lett. 2013, 23, 2632–2635. [Google Scholar] [CrossRef]
- Aouali, M.; Mhalla, D.; Allouche, F.; El Kaim, L.; Tounsi, S.; Trigui, M.; Chabchoub, F. Synthesis, antimicrobial and antioxidant activities of imidazotriazoles and new multicomponent reaction toward 5-amino-1-phenyl [1, 2, 4] triazole derivatives. Med. Chem. Res. 2015, 24, 2732–2741. [Google Scholar] [CrossRef]
- Kenchappa, R.; Bodke, Y.D.; Chandrashekar, A.; Telkar, S.; Manjunatha, K.; Sindhe, M.A. Synthesis of some 2, 6-bis (1-coumarin-2-yl)-4-(4-substituted phenyl) pyridine derivatives as potent biological agents. Arab. J. Chem. 2017, 10, S1336–S1344. [Google Scholar] [CrossRef]
- Ezzatzadeh, E.; Hossaini, Z.; Varasteh Moradi, A.; Salimifard, M.; Afshari-Sharif Abad, S. Copper iodide and ZnO nanoparticles catalyzed multicomponent synthesis of 1, 3-cyclopentadiene: Study of antioxidant activity. Can. J. Chem. 2019, 97, 270–276. [Google Scholar] [CrossRef]
- Kouznetsov, V.V.; Puentes, C.O.; Bohorquez, A.R.; Zacchino, S.A.; Sortino, M.; Gupta, M.; Vazquez, Y.; Bahsas, A.; Amaro-Luis, J. A straightforward synthetic approach to antitumoral pyridinyl substituted 7H-indeno [2, 1-c] quinoline derivatives via three-component imino Diels-Alder reaction. Lett. Org. Chem. 2006, 3, 300–304. [Google Scholar] [CrossRef]
- Roopan, S.M.; Khan, F.N.; Jin, J.S.; Kumar, R.S. An efficient one pot–three component cyclocondensation in the synthesis of 2-(2-chloroquinolin-3-yl)-2, 3-dihydroquinazolin-4 (1H)-ones: Potential antitumor agents. Res. Chem. Intermed. 2011, 37, 919. [Google Scholar] [CrossRef]
- Nagaraju, B.; Kovvuri, J.; Babu, K.S.; Adiyala, P.R.; Nayak, V.L.; Alarifi, A.; Kamal, A. A facile one pot CC and CN bond formation for the synthesis of spiro-benzodiazepines and their cytotoxicity. Tetrahedron 2017, 73, 6969–6976. [Google Scholar] [CrossRef]
- Islam, M.S.; Ghawas, H.M.; El-Senduny, F.F.; Al-Majid, A.M.; Elshaier, Y.A.; Badria, F.A.; Barakat, A. Synthesis of new thiazolo-pyrrolidine–(spirooxindole) tethered to 3-acylindole as anticancer agents. Bioorg. Chem. 2019, 82, 423–430. [Google Scholar] [CrossRef]
- Wu, L.; Liu, Y.; Li, Y. Synthesis of Spirooxindole-O-Naphthoquinone-Tetrazolo [1, 5-a] Pyrimidine Hybrids as Potential Anticancer Agents. Molecules 2018, 23, 2330. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-L.; Feng, T.-T.; Jiang, W.-D.; Yang, C.; Tian, M.-Y.; Jiang, Y.; Lin, B.; Zhao, Z.; Zhou, Y. Molecular hybridization-guided 1, 3-dipolar cycloaddition reaction enabled pyrimidine-fused spiropyrrolidine oxindoles synthesis as potential anticancer agents. Tetrahedron Lett. 2016, 57, 4411–4416. [Google Scholar] [CrossRef]
- Sudhapriya, N.; Perumal, P.; Balachandran, C.; Ignacimuthu, S.; Sangeetha, M.; Doble, M. Synthesis of new class of spirocarbocycle derivatives by multicomponent domino reaction and their evaluation for antimicrobial, anticancer activity and molecular docking studies. Eur. J. Med. Chem. 2014, 83, 190–207. [Google Scholar] [CrossRef]
- Liao, S.-R.; Du, L.-J.; Qin, X.-C.; Xu, L.; Wang, J.-F.; Zhou, X.-F.; Tu, Z.-C.; Li, J.; Liu, Y.-H. Site selective synthesis of cytotoxic 1, 3, 6-trisubstituted 3, 6-diunsaturated (3Z, 6Z)-2, 5-diketopiperazines via a one-pot multicomponent method. Tetrahedron 2016, 72, 1051–1057. [Google Scholar] [CrossRef]
- Abadi, A.H.; Hegazy, G.H.; El-Zaher, A.A. Synthesis of novel 4-substituted-7-trifluoromethylquinoline derivatives with nitric oxide releasing properties and their evaluation as analgesic and anti-inflammatory agents. Bioorg. Med. Chem. 2005, 13, 5759–5765. [Google Scholar] [CrossRef]
- Liu, J.; Ming, B.; Gong, G.-H.; Wang, D.; Bao, G.-L.; Yu, L.-J. Current research on anti-breast cancer synthetic compounds. RSC Adv. 2018, 8, 4386–4416. [Google Scholar] [CrossRef]
- Husain, A.; Maaz, M.; Ahmad Ansari, K.; Ahmad, A.; Rashid, M. Synthesis and microbiological evaluation of mannich bases derived from 4, 6-diacetylresorcinol. J. Chil. Chem. Soc. 2010, 55, 332–334. [Google Scholar] [CrossRef][Green Version]
- Keri, R.S.; Patil, S.A. Quinoline: A promising antitubercular target. Biomed. Pharmacother. 2014, 68, 1161–1175. [Google Scholar] [CrossRef] [PubMed]
- Eswaran, S.; Adhikari, A.V.; Shetty, N.S. Synthesis and antimicrobial activities of novel quinoline derivatives carrying 1, 2, 4-triazole moiety. Eur. J. Med. Chem. 2009, 44, 4637–4647. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.-C.; Jiménez, E.; Portilla, J.; Insuasty, B.; Quiroga, J.; Moreno-Fuquen, R.; Kennedy, A.R.; Abonia, R. Application of a catalyst-free Domino Mannich/Friedel-Crafts alkylation reaction for the synthesis of novel tetrahydroquinolines of potential antitumor activity. Tetrahedron 2018, 74, 932–947. [Google Scholar] [CrossRef]
- Nandagokula, C.; Poojary, B.; Vittal, S.; Shenoy, S.; Shetty, P.; Tangavelu, A. Synthesis, characterization, and biological evaluation of some N-aryl hydrazones and their 2, 3-disubstituted-4-thiazolidinone derivatives. Med. Chem. Res. 2013, 22, 253–266. [Google Scholar] [CrossRef]
- Asolkar, R.N.; Schroeder, D.; Heckmann, R.; Lang, S.; Wagner-Doebler, I.; Laatsch, H. Helquinoline, a new tetrahydroquinoline antibiotic from Janibacter limosus Hel 1. J. Antibiot. 2004, 57, 17–23. [Google Scholar] [CrossRef]
- Gudmundsson, K.S.; Boggs, S.D.; Catalano, J.G.; Svolto, A.; Spaltenstein, A.; Thomson, M.; Wheelan, P.; Jenkinson, S. Imidazopyridine-5, 6, 7, 8-tetrahydro-8-quinolinamine derivatives with potent activity against HIV-1. Bioorg. Med. Chem. Lett. 2009, 19, 6399–6403. [Google Scholar] [CrossRef] [PubMed]
- Fotie, J.; Kaiser, M.; Delfin, D.A.; Manley, J.; Reid, C.S.; Paris, J.-M.; Wenzler, T.; Maes, L.; Mahasenan, K.V.; Li, C. Antitrypanosomal activity of 1, 2-dihydroquinolin-6-ols and their ester derivatives. J. Med. Chem. 2010, 53, 966–982. [Google Scholar] [CrossRef]
- Lukevics, E.; Segal, I.; Zablotskaya, A.; Germane, S. Synthesis and neurotropic activity of novel quinoline derivatives. Molecules 1997, 2, 180–185. [Google Scholar] [CrossRef]
- Faber, K.; StÚckler, H.; Kappe, T. Non-steroidal antiinflammatory agents. 1. Synthesis of 4-hydroxy-2-oxo-1, 2-dihydroquinolin-3-yl alkanoic acids by the wittig reaction of quinisatines. J. Heterocycl. Chem. 1984, 21, 1177–1181. [Google Scholar] [CrossRef]
- Jarvest, R.L.; Armstrong, S.A.; Berge, J.M.; Brown, P.; Elder, J.S.; Brown, M.J.; Copley, R.C.; Forrest, A.K.; Hamprecht, D.W.; O’Hanlon, P.J. Definition of the heterocyclic pharmacophore of bacterial methionyl tRNA synthetase inhibitors: Potent antibacterially active non-quinolone analogues. Bioorg. Med. Chem. Lett. 2004, 14, 3937–3941. [Google Scholar] [CrossRef]
- Bendale, P.; Olepu, S.; Suryadevara, P.K.; Bulbule, V.; Rivas, K.; Nallan, L.; Smart, B.; Yokoyama, K.; Ankala, S.; Pendyala, P.R. Second generation tetrahydroquinoline-based protein farnesyltransferase inhibitors as antimalarials. J. Med. Chem. 2007, 50, 4585–4605. [Google Scholar] [CrossRef]
- Gholap, A.R.; Toti, K.S.; Shirazi, F.; Kumari, R.; Bhat, M.K.; Deshpande, M.V.; Srinivasan, K.V. Synthesis and evaluation of antifungal properties of a series of the novel 2-amino-5-oxo-4-phenyl-5, 6, 7, 8-tetrahydroquinoline-3-carbonitrile and its analogues. Bioorg. Med. Chem. 2007, 15, 6705–6715. [Google Scholar] [CrossRef]
- Liou, J.-P.; Wu, Z.-Y.; Kuo, C.-C.; Chang, C.-Y.; Lu, P.-Y.; Chen, C.-M.; Hsieh, H.-P.; Chang, J.-Y. Discovery of 4-amino and 4-hydroxy-1-aroylindoles as potent tubulin polymerization inhibitors. J. Med. Chem. 2008, 51, 4351–4355. [Google Scholar] [CrossRef] [PubMed]
- Tramontini, M. Advances in the chemistry of Mannich bases. Synthesis 1973, 1973, 703–775. [Google Scholar] [CrossRef]
- Malinakova, H.C. Multicomponent Reaction Sequences Using Palladacyclic Complexes. In Palladacycles; Elsevier: Amsterdam, The Netherlands, 2019; pp. 263–295. [Google Scholar]
- Paprocki, D.; Madej, A.; Koszelewski, D.; Brodzka, A.; Ostaszewski, R. Multicomponent reactions accelerated by aqueous micelles. Front. Chem. 2018, 6, 502. [Google Scholar] [CrossRef] [PubMed]
- Malinakova, H.C. Recent advances in the discovery and design of multicomponent reactions for the generation of small-molecule libraries. Rep. Org. Chem. 2015, 5, 75–90. [Google Scholar] [CrossRef][Green Version]
- Weber, L. The application of multi-component reactions in drug discovery. Curr. Med. Chem. 2002, 9, 2085–2093. [Google Scholar] [CrossRef] [PubMed]
- Mannich, C.; Krösche, W. Ueber ein kondensationsprodukt aus formaldehyd, ammoniak und antipyrin. Arch. Pharm. 1912, 250, 647–667. [Google Scholar] [CrossRef]
- Blicke, F. The M annich Reaction. Org. React. 2004, 1, 303–341. [Google Scholar]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Benet, L.Z.; Hosey, C.M.; Ursu, O.; Oprea, T.I. BDDCS, the rule of 5 and drugability. Adv. Drug Deliv. Rev. 2016, 101, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A. Drug-like properties and the causes of poor solubility and poor permeability. J. Pharmacol. Toxicol. Methods 2000, 44, 235–249. [Google Scholar] [CrossRef]
- Rahman, L.; Shinwari, Z.K.; Iqrar, I.; Rahman, L.; Tanveer, F. An assessment on the role of endophytic microbes in the therapeutic potential of Fagonia indica. Ann. Clin. Microbiol. Antimicrob. 2017, 16, 53. [Google Scholar] [CrossRef]
- Seelolla, G.; Cheera, P.; Ponneri, V. Synthesis, antimicrobial and antioxidant activities of novel series of cinnamamide derivatives having morpholine moiety. Med. Chem. 2014, 4, 778–783. [Google Scholar] [CrossRef]
- Katbab, A.; Scott, G. Mechanisms of antioxidant action: The involvement of nitroxyl radicals in the antifatigue action of secondary amines. Eur. Polym. J. 1981, 17, 559–565. [Google Scholar] [CrossRef]
- Mistry, B.; Patel, R.V.; Keum, Y.-S.; Kim, D.H. Synthesis of N-Mannich bases of berberine linking piperazine moieties revealing anticancer and antioxidant effects. Saudi J. Biol. Sci. 2017, 24, 36–44. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric Quantitation of Antioxidant Capacity through the Formation of a Phosphomolybdenum Complex: Specific Application to the Determination of Vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Kanzler, C.; Haase, P.T.; Schestkowa, H.; Kroh, L.W. Antioxidant properties of heterocyclic intermediates of the Maillard reaction and structurally related compounds. J. Agric. Food Chem. 2016, 64, 7829–7837. [Google Scholar] [CrossRef]
- Singh, N.; Rajini, P. Free radical scavenging activity of an aqueous extract of potato peel. Food Chem. 2004, 85, 611–616. [Google Scholar] [CrossRef]
- Semon, W.L. Antioxidant. U.S. Patent No. 2,166,223, 18 July 1939. [Google Scholar]
- Shahidi, F.; Janitha, P.; Wanasundara, P. Phenolic antioxidants. Crit. Rev. Food Sci. Nutr. 1992, 32, 67–103. [Google Scholar] [CrossRef]
- El-Gohary, N.; Shaaban, M. Antimicrobial and antiquorum-sensing studies. Part 2: Synthesis, antimicrobial, antiquorum-sensing and cytotoxic activities of new series of fused [1, 3, 4] thiadiazole and [1, 3] benzothiazole derivatives. Med. Chem. Res. 2014, 23, 287–299. [Google Scholar] [CrossRef]
- Solis, P.N.; Wright, C.W.; Anderson, M.M.; Gupta, M.P.; Phillipson, J.D. A microwell cytotoxicity assay using Artemia salina (brine shrimp). Planta Med. 1993, 59, 250–252. [Google Scholar] [CrossRef]
- Birndorf, H.C.; D’Alessio, J.; Bagshaw, J.C. DNA-dependent RNA-polymerases from Artemia embryos: Characterization of polymerases I and II from nauplius larvae. Dev. Biol. 1975, 45, 34–43. [Google Scholar] [CrossRef]
- Sanchez, C.; Gupta, M.; Vasquez, M.; Montenegro, G. Bioessay with brine Artemia to predict antibacterial and pharmacologic activity. Rev. Med. Panama 1993, 18, 62–69. [Google Scholar]
- Das, S.; da Silva, C.J.; Silva, M.d.M.; Dantas, M.D.d.A.; de Fátima, Â.; Ruiz, A.L.T.G.; da Silva, C.M.; de Carvalho, J.E.; Santos, J.C.; Figueiredo, I.M. Highly functionalized piperidines: Free radical scavenging, anticancer activity, DNA interaction and correlation with biological activity. J. Adv. Res. 2018, 9, 51–61. [Google Scholar] [CrossRef]
- Abdo, N.Y.M.; Kamel, M.M. Synthesis and anticancer evaluation of 1, 3, 4-oxadiazoles, 1, 3, 4-thiadiazoles, 1, 2, 4-triazoles and Mannich bases. Chem. Pharm. Bull. 2015, 63, 369–376. [Google Scholar] [CrossRef]
- Wiji Prasetyaningrum, P.; Bahtiar, A.; Hayun, H. Synthesis and cytotoxicity evaluation of novel asymmetrical mono-carbonyl analogs of curcumin (AMACs) against Vero, HeLa, and MCF7 Cell Lines. Sci. Pharm. 2018, 86, 25. [Google Scholar] [CrossRef]
- Kumar, G.S.; Prasad, Y.R.; Mallikarjuna, B.; Chandrashekar, S. Synthesis and pharmacological evaluation of clubbed isopropylthiazole derived triazolothiadiazoles, triazolothiadiazines and mannich bases as potential antimicrobial and antitubercular agents. Eur. J. Med. Chem. 2010, 45, 5120–5129. [Google Scholar] [CrossRef] [PubMed]
- Dimmock, J.R.; Vashishtha, S.C.; Quail, J.W.; Pugazhenthi, U.; Zimpel, Z.; Sudom, A.M.; Allen, T.M.; Kao, G.Y.; Balzarini, J.; De Clercq, E. 4-(β-Arylvinyl)-3-(β-arylvinylketo)-1-ethyl-4-piperidinols and Related Compounds: A Novel Class of Cytotoxic and Anticancer Agents. J. Med. Chem. 1998, 41, 4012–4020. [Google Scholar] [CrossRef]
- Rao, U.M.; Ahmad, B.A.; Mohd, K.S. In vitro nitric oxide scavenging and anti-inflammatory activities of different solvent extracts of various parts of Musa paradisiaca. Malays. J. Anal. Sci. 2016, 20, 1191–1202. [Google Scholar] [CrossRef]
- Koksal, M.; Ozkan-Dagliyan, I.; Ozyazici, T.; Kadioglu, B.; Sipahi, H.; Bozkurt, A.; Bilge, S.S. Some Novel Mannich Bases of 5-(3, 4-Dichlorophenyl)-1, 3, 4-oxadiazole-2 (3H)-one and Their Anti-Inflammatory Activity. Arch. Pharm. 2017, 350, 1700153. [Google Scholar] [CrossRef] [PubMed]
- Ames, B.N.; Shigenaga, M.K.; Hagen, T.M. Oxidants, antioxidants, and the degenerative diseases of aging. Proc. Natl. Acad. Sci. USA 1993, 90, 7915–7922. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.-F.; Sun, J.; Wu, X.; Liu, R.H. Antioxidant and antiproliferative activities of common vegetables. J. Agric. Food Chem. 2002, 50, 6910–6916. [Google Scholar] [CrossRef] [PubMed]
- Simmons, D.L.; Wagner, D.; Westover, K. Nonsteroidal anti-inflammatory drugs, acetaminophen, cyclooxygenase 2, and fever. Clin. Infect. Dis. 2000, 31, S211–S218. [Google Scholar] [CrossRef]
- SwissADME Software. Available online: http://swissadme.ch/index.php (accessed on 8 January 2020).
- Tai, Z.; Cai, L.; Dai, L.; Dong, L.; Wang, M.; Yang, Y.; Cao, Q.; Ding, Z. Antioxidant activity and chemical constituents of edible flower of Sophora viciifolia. Food Chem. 2011, 126, 1648–1654. [Google Scholar] [CrossRef]
- Aguilar Urbano, M.; Pineda Priego, M.; Prieto, P. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E1. Anal. Biochem. 2013, 269, 337–341. [Google Scholar]
- Khan, K.; Fatima, H.; Taqi, M.M.; Zia, M.; Mirza, B. Phytochemical and in vitro biological evaluation of Artemisia scoparia Waldst. & Kit for enhanced extraction of commercially significant bioactive compounds. J. Appl. Res. Med. Aromat. Plants 2015, 2, 77–86. [Google Scholar] [CrossRef]
- Kim, J.-S.; Kwon, C.-S.; SoN, K.H. Inhibition of alpha-glucosidase and amylase by luteolin, a flavonoid. Biosci. Biotechnol. Biochem. 2000, 64, 2458–2461. [Google Scholar] [CrossRef] [PubMed]
- Meyer, B.; Ferrigni, N.; Putnam, J.; Jacobsen, L.; Nichols, D.J.; McLaughlin, J.L. Brine shrimp: A convenient general bioassay for active plant constituents. Planta Med. 1982, 45, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Shin, E.M.; Choi, R.J.; Jung, Y.H.; Kim, J.; Tosun, A.; Kim, Y.S. Suppression of LPS-induced inflammatory and NF-κB responses by anomalin in RAW 264.7 macrophages. J. Cell. Biochem. 2011, 112, 2179–2188. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Mao, J.; Huang, Y.; Xu, W.; Duan, Y.; Zhang, J. Synthesis and biological evaluation of novel benzofuroxan-based pyrrolidine hydroxamates as matrix metalloproteinase inhibitors with nitric oxide releasing activity. Bioorg. Med. Chem. 2018, 26, 4363–4374. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.S.; Noh, E.J.; Zhao, H.L.; Jung, S.H.; Kang, S.S.; Kim, Y.S. Inhibition of inducible nitric oxide synthase and cyclooxygenase II by Platycodon grandiflorum saponins via suppression of nuclear factor-κB activation in RAW 264.7 cells. Life Sci. 2005, 76, 2315–2328. [Google Scholar] [CrossRef] [PubMed]
- Zeeshan, S.; Naveed, M.; Khan, A.; Atiq, A.; Arif, M.; Ahmed, M.N.; Kim, Y.S.; Khan, S. N-Pyrazoloyl and N-thiopheneacetyl hydrazone of isatin exhibited potent anti-inflammatory and anti-nociceptive properties through suppression of NF-κB, MAPK and oxidative stress signaling in animal models of inflammation. Inflamm. Res. 2019, 68, 613–632. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |




| Comp. No | MW 1 (< 500) | HBA 2 (< 10) | HBD 3 (< 5) | ilog (Po/w) 4 (< 5) | Mlog(Po/w) 5 (< 5) | Lipinski Violation |
|---|---|---|---|---|---|---|
| SF1 | 230 | 1 | 0 | 3.02 | 3.21 | No |
| SF2 | 232.32 | 2 | 0 | 2.69 | 2.07 | No |
| SF3 | 216.32 | 1 | 0 | 2.78 | 2.95 | No |
| SF4 | 246.39 | 1 | 0 | 3.41 | 3.46 | No |
| SF5 | 245.36 | 2 | 0 | 2.94 | 2.33 | No |
| SF6 | 231.34 | 2 | 1 | 2.67 | 2.07 | No |
| SF7 | 314.42 | 0 | 0 | 3.46 | 4.89 | No |
| SF8 | 272.77 | 0 | 1 | 3.08 | 3.98 | No |
| SF9 | 317.22 | 0 | 1 | 3.17 | 4.10 | No |
| SF10 | 280.36 | 1 | 0 | 2.82 | 3.30 | No |
| SF11 | 218.34 | 1 | 0 | 3.11 | 2.95 | No |
| SF12 | 268.35 | 1 | 1 | 3.21 | 3.10 | No |
| SF13 | 296.36 | 2 | 1 | 2.61 | 2.81 | No |
| Compound | % DPPH Scavenging Activity at Different Concentrations | IC50 (µg/mL) a | |||
|---|---|---|---|---|---|
| 200 µg/mL | 66.66 µg/mL | 22.22 µg/mL | 7.4 µg/mL | ||
| SF1 | 62.45 ± 0.12 | 50.08 ± 0.18 | 27.76 ± 0.11 | 11.91 ± 0.32 | 44.22 ± 0.25 |
| SF2 | 71.85 ± 0.27 | 58.39 ± 0.27 | 36.61 ± 0.22 | 17.44 ± 0.26 | 42.55 ± 0.36 |
| SF3 | 27.19 ± 0.31 | 18.32 ± 0.91 | 9.85 ± 0.18 | 3.76 ± 0.125 | 52.75 ± 0.28 |
| SF4 | 58.58 ± 0.33 | 53.65 ± 0.14 | 41.63 ± 0.28 | 18.12 ± 0.20 | 29.79 ± 0.26 |
| SF5 | 45.24 ± 0.28 | 28.77 ± 0.14 | 17.39 ± 0.16 | 7.76 ± 0.23 | 56.63 ± 0.32 |
| SF6 | 66.61 ± 0.13 | 50.32 ± 0.18 | 37.75 ± 0.31 | 8.81 ± 0.21 | 35.89 ± 0.33 |
| SF7 | 64.48 ± 0.18 | 50.48 ± 0.054 | 26.76 ± 0.17 | 9.28 ± 0.13 | 44.4 ± 0.29 |
| SF8 | 62.35 ± 0.14 | 52.12 ± 0.36 | 50.17 ± 0.32 | 27.98 ± 0.24 | 29.19 ± 0.25 |
| SF9 | 67.46 ± 0.14 | 50.28 ± 0.22 | 19.35 ± 0.11 | 7.64 ± 0.26 | 50.76 ± 0.37 |
| SF10 | 49.73 ± 0.19 | 40.77 ± 0.31 | 31.89 ± 0.21 | 23.45 ± 0.28 | 47.81 ± 0.27 |
| SF11 | 43.93 ± 0.28 | 30.9 ± 0.12 | 15.54 ± 0.09 | 7.76 ± 0.18 | 53.63 ± 0.13 |
| SF12 | 64.20 ± 0.31 | 54.10 ± 0.17 | 31.80 ± 0.42 | 11.24 ± 0.11 | 39.33 ± 0.28 |
| SF13 | 19.34 ± 0.34 | 11.78 ± 0.15 s | 4.44 ± 0.18 | 1.98 ± 0.20 | 60.78 ± 0.36 |
| Blank | 0 | 0 | 0 | 0 | -- |
| Ascorbic Acid | 89.01 ± 0.18 | 76.63 ± 0.19 | 52.12 ± 0.31 | 31.35 ± 0.23 | 41.38 ± 0.34 |
| Quercetin | 81.43 ± 0.21 | 69.81 ± 0.17 | 58.31 ± 0.28 | 42.34 ± 0.36 | 41.64 ± 1.01 |
| Compounds | LD50 (µg/mL) ± SEM 1 |
|---|---|
| SF1 | 120.8 ± 2.3 |
| SF2 | 141.4 ± 2.1 |
| SF3 | 102 ± 1.98 |
| SF4 | 100 ± 1.78 |
| SF5 | 94 ± 1.06 |
| SF6 | 121.2 ± 1.09 |
| SF7 | 100.8 ± 1.14 |
| SF8 | 123.4 ± 1.87 |
| SF9 | 139 ± 0.87 |
| SF10 | 196.1 ± 1.12 |
| SF11 | 200 ± 2.11 |
| SF12 | 128 ± 1.87 |
| SF13 | 204 ± 1.67 |
| Doxorubicin | 124 ± 1.54 |
| Compound | % Cell Viability at Different Concentrations | IC50 (µM) a | |||
|---|---|---|---|---|---|
| 100 µM | 50 µM | 10 µM | 1 µM | ||
| SF1 | 44.66 ± 0.5 | 47 ± 0.84 | 48 ± 1.34 | 58 ± 0.76 | 6.23 ± 0.01 *** |
| SF2 | 50 ± 1.52 | 60 ± 0.91 | 70 ± 0.91 | 76 ± 0.46 | 26.80 ± 0.30 |
| SF3 | 41 ± 1.07 | 72 ± 1.34 | 78 ± 0.74 | 83 ± 0.63 | 29.13 ± 0.50 |
| SF4 | 39 ± 0.88 | 42 ± 0.87 | 53 ± 0.63 | 79 ± 0.92 | 7.20 ± 0.05 *** |
| SF5 | 60 ± 1.20 | 67 ± 0.84 | 74 ± 0.42 | 98 ± 1.01 | 6.18 ± 0.05 *** |
| SF6 | 60 ± 1.21 | 65 ± 0.76 | 87 ± 0.82 | 91 ± 0.99 | 23.37 ± 0.17 |
| SF7 | 58 ± 0.83 | 84 ± 0.77 | 95 ± 0.96 | 96 ± 0.89 | 18.64 ± 0.13 ** |
| SF8 | 52 ± 1.00 | 68 ± 0.79 | 83 ± 0.78 | 97 ± 1.04 | 21.23 ± 0.33 ** |
| SF9 | 82 ± 0.96 | 84 ± 1.21 | 91 ± 0.91 | 98 ± 0.87 | 14.24 ± 0.26 ** |
| SF10 | 76 ± 1.20 | 80 ± 1.24 | 95 ± 0.93 | 100 ± 0.88 | 24.82 ± 0.269 |
| SF11 | 41 ± 0.83 | 62 ± 1.20 | 82 ± 0.84 | 95 ± 0.91 | 26.36 ± 0.31 |
| SF12 | 41 ± 0.86 | 57 ± 0.89 | 84 ± 0.87 | 87 ± 0.82 | 35.10 ± 0.50 |
| SF13 | 60 ± 1.24 | 73 ± 1.01 | 82 ± 0.66 | 85 ± 0.93 | 41.43 ± 0.69 |
| Control | 100 | 100 | 100 | 100 | 0 |
| Doxorubicin | 17 ± 0.83 | 33 ± 1.01 | 40 ± 0.87 | 51 ± 1.34 | 17.08 ± 0.37 *** |
| Compound | IC50 (µM) 1 (Mean ± SEM) Hep-2C Cells | ||
|---|---|---|---|
| 24 h | 48 h | 72 h | |
| SF1 | 27.98 ± 1.07 | 21.82 ± 1.1 | 14.82 ± 1.07 ** |
| SF4 | 23.94 ± 1.07 | 21.15 ± 1.2 | 15.53 ± 1.03 ** |
| SF5 | 21.3 ± 1.09 | 18.02 ± 1.05 | 16.78 ± 1.07 |
| SF7 | 26.68 ± 1.10 | 21.57 ± 1.05 | 16.81 ± 1.03 |
| SF8 | 20.23 ± 1.07 | 15.73 ± 1.08 | 11.90 ± 1.04 ** |
| THQ | 41.27 ± 1.4 | 38.54 ± 1.23 | 31.98 ± 1.12 |
| Cisplatin | 19.12 ± 1.06 | 17.47 ± 1.07 | 14.63 ± 1.01 ** |
| Compound | Concentration (µM) and % NO Production (Mean ± SEM) 1 | |||
|---|---|---|---|---|
| 100 | 50 | 10 | 1 | |
| Piroxicam | 13.351 ± 0.54 | 16.457 ± 0.41 | 20.710 ± 0.24 | 25.35 ± 0.45 |
| SF1 | 17.313 ± 0.35 | 23.687 ± 0.41 | 26.0406 ± 0.43 | 27.77 ± 0.46 *** |
| SF2 | 15.345 ± 0.29 | 22.091 ± 0.33 | 27.093 ± 0.36 | 32.23 ± 0.34 *** |
| SF3 | 19.67 ± 0.37 | 23.810 ± 0.39 | 29.571 ± 0.42 | 33.17 ± 0.47 ** |
| SF4 | 20.226 ± 0.28 | 32.045 ± 0.31 | 39.172 ± 0.38 | 43.03 ± 0.41 |
| SF5 | 21.924 ± 0.38 | 28.644 ± 0.44 | 43.036 ± 0.45 | 51.43 ± 0.48 |
| SF6 | 52.437 ± 0.49 | 61.110 ± 0.54 | 72.333 ± 0.59 | 77.30 ± 0.62 |
| SF7 | 17.455 ± 0.45 | 21.382 ± 0.48 | 63.122 ± 0.61 | 69.71 ± 0.65 |
| SF8 | 15.031 ± 0.33 | 24.111 ± 0.41 | 29.351 ± 0.44 | 39.40 ± 0.48 |
| SF9 | 49.073 ± 0.45 | 55.601 ± 0.51 | 58.541 ± 0.55 | 66.97 ± 0.61 |
| SF10 | 33.842 ± 0.33 | 42.487 ± 0.54 | 58.512 ± 0.59 | 62.40 ± 0.65 |
| SF11 | 25.701 ± 0.44 | 33.701 ± 0.53 | 39.799 ± 0.61 | 42.97 ± 0.66 |
| SF12 | 23.203 ± 0.55 | 26.522 ± 0.578 | 32.310 ± 0.59 | 49.70 ± 0.61 |
| SF13 | 14.494 ± 0.44 | 15.572 ± 0.47 | 22.153 ± 0.52 | 25.10 ± 0.59 *** |
| Compounds | Time After Carrageenan Injection 1 | |||
|---|---|---|---|---|
| 0 h | 2 h | 4 h | 6 h | |
| Control | 2.04 ± 0.07 | 2.05 ± 0.06 | 2.07 ± 0.04 | 2.10 ± 0.02 |
| Carrageenan (100 µL) | 2.11 ± 0.07 | 2.57 ± 0.08 ### | 2.75 ± 0.07 ### | 2.86 ± 0.05 ### |
| Acetyl salicylic acid (10 mg/kg) | 2.14 ± 0.08 | 2.26 ± 0.05 *** | 2.32 ± 0.04 *** | 2.39 ± 0.02 *** |
| SF1 (10 mg/kg) | 2.06 ± 0.07 | 2.13 ± 0.03 *** | 2.18 ± 0.06 *** | 2.25 ± 0.05 *** |
| SF2 (10 mg/kg) | 2.05 ± 0.05 | 2.11 ± 0.06 *** | 2.17 ± 0.03 *** | 2.23 ± 0.07 *** |
| SF3 (10 mg/kg) | 2.05 ± 0.06 | 2.16 ± 0.05 *** | 2.22 ± 0.02 *** | 2.28 ± 0.07 *** |
| SF13 (10 mg/kg) | 2.04 ± 0.07 | 2.18 ± 0.03 *** | 2.26 ± 0.06 *** | 2.31 ± 0.07 *** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farooq, S.; Mazhar, A.; Ghouri, A.; Ihsan-Ul-Haq; Ullah, N. One-Pot Multicomponent Synthesis and Bioevaluation of Tetrahydroquinoline Derivatives as Potential Antioxidants, α-Amylase Enzyme Inhibitors, Anti-Cancerous and Anti-Inflammatory Agents. Molecules 2020, 25, 2710. https://doi.org/10.3390/molecules25112710
Farooq S, Mazhar A, Ghouri A, Ihsan-Ul-Haq, Ullah N. One-Pot Multicomponent Synthesis and Bioevaluation of Tetrahydroquinoline Derivatives as Potential Antioxidants, α-Amylase Enzyme Inhibitors, Anti-Cancerous and Anti-Inflammatory Agents. Molecules. 2020; 25(11):2710. https://doi.org/10.3390/molecules25112710
Chicago/Turabian StyleFarooq, Samra, Aqsa Mazhar, Areej Ghouri, Ihsan-Ul-Haq, and Naseem Ullah. 2020. "One-Pot Multicomponent Synthesis and Bioevaluation of Tetrahydroquinoline Derivatives as Potential Antioxidants, α-Amylase Enzyme Inhibitors, Anti-Cancerous and Anti-Inflammatory Agents" Molecules 25, no. 11: 2710. https://doi.org/10.3390/molecules25112710
APA StyleFarooq, S., Mazhar, A., Ghouri, A., Ihsan-Ul-Haq, & Ullah, N. (2020). One-Pot Multicomponent Synthesis and Bioevaluation of Tetrahydroquinoline Derivatives as Potential Antioxidants, α-Amylase Enzyme Inhibitors, Anti-Cancerous and Anti-Inflammatory Agents. Molecules, 25(11), 2710. https://doi.org/10.3390/molecules25112710