Synthesis and Studies of the Inhibitory Effect of Hydroxylated Phenylpropanoids and Biphenols Derivatives on Tyrosinase and Laccase Enzymes
Abstract
1. Introduction
2. Results
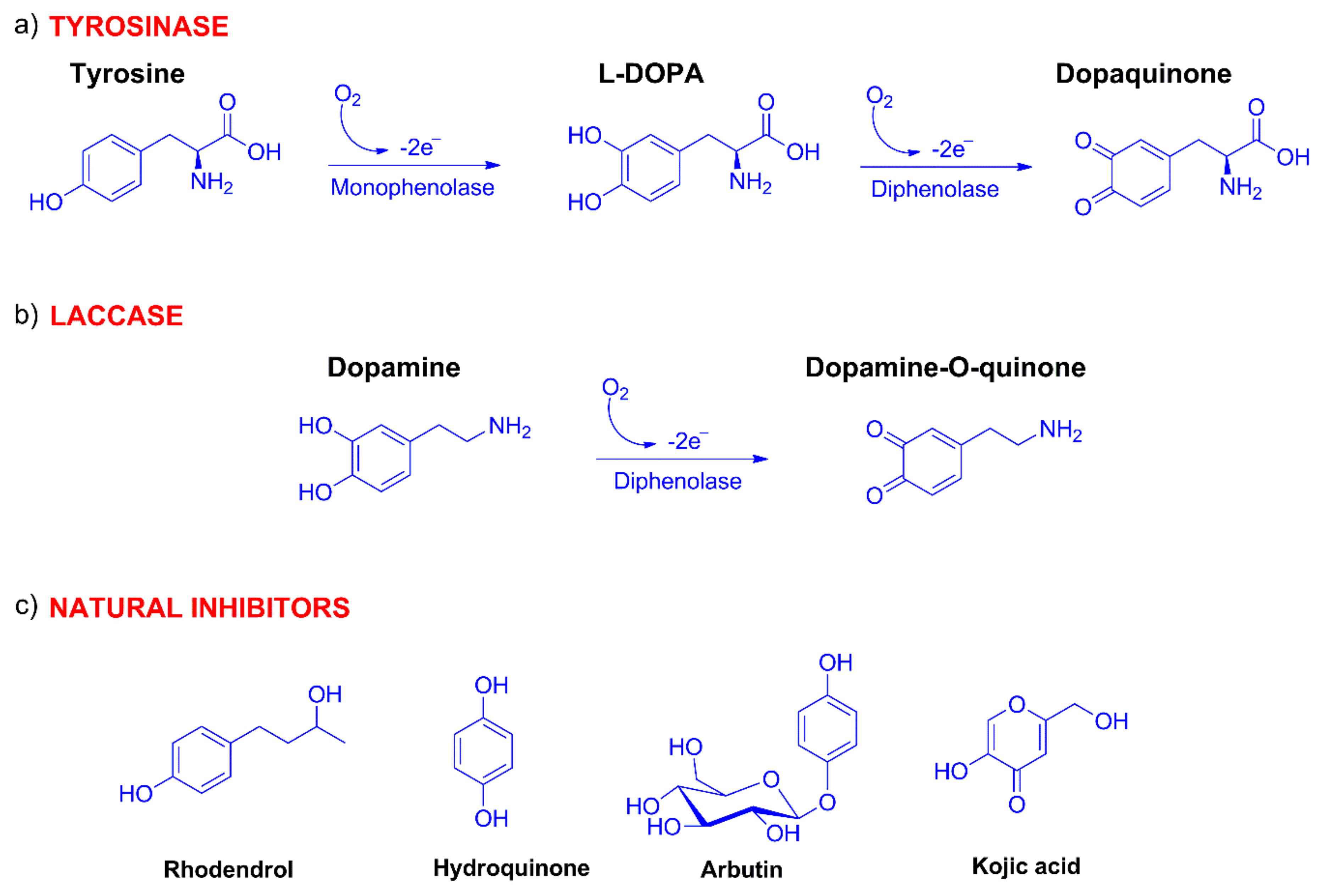
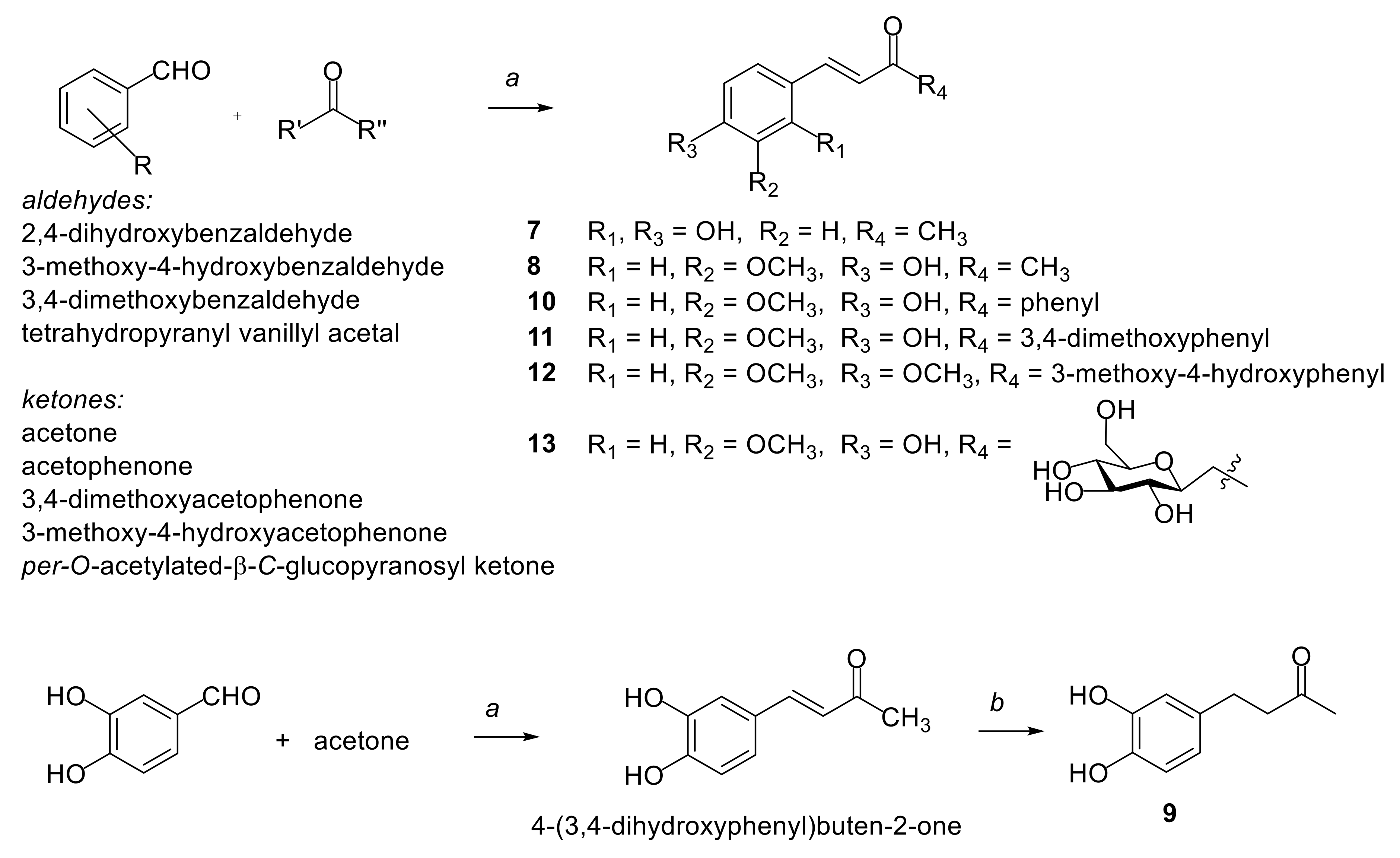
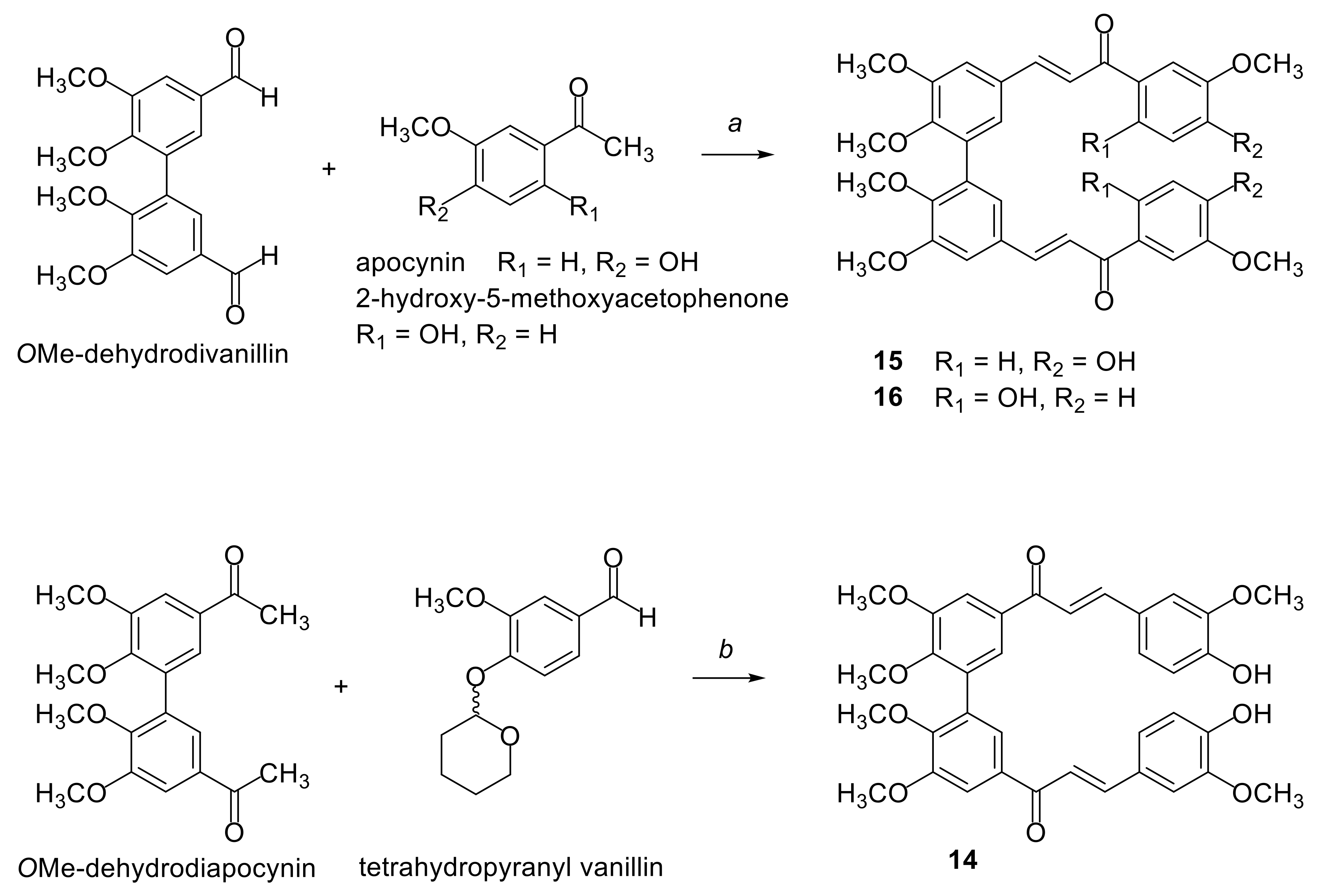
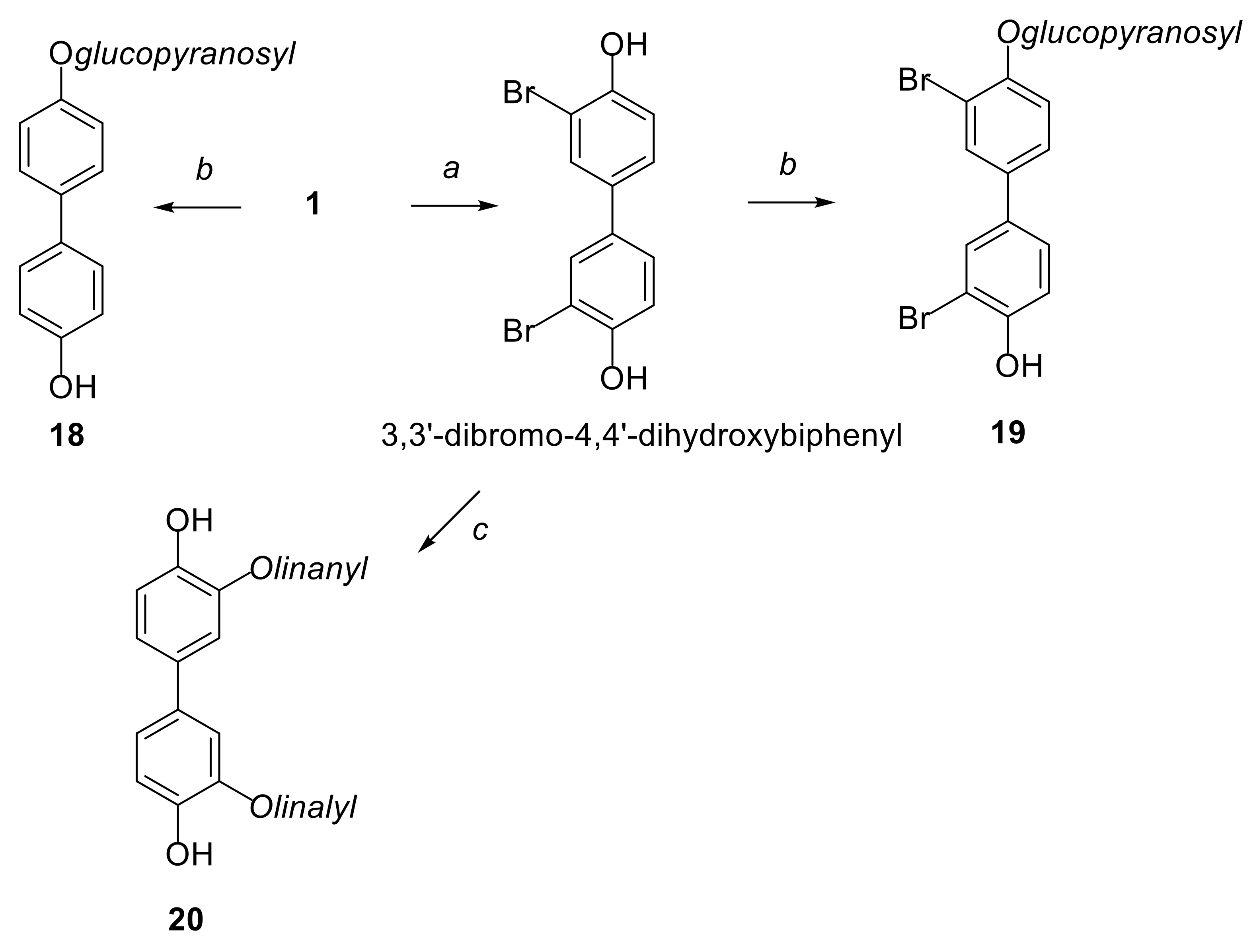
2.1. Synthesis of Compounds
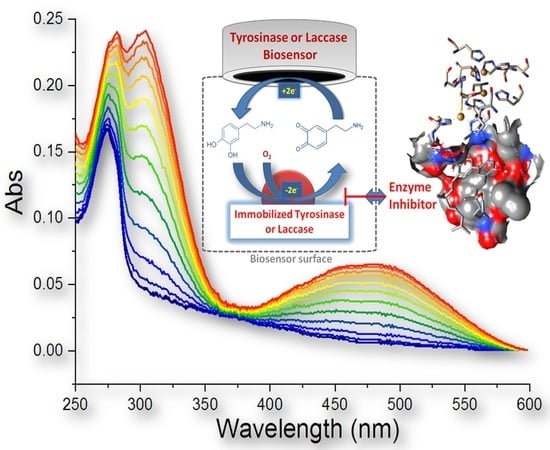
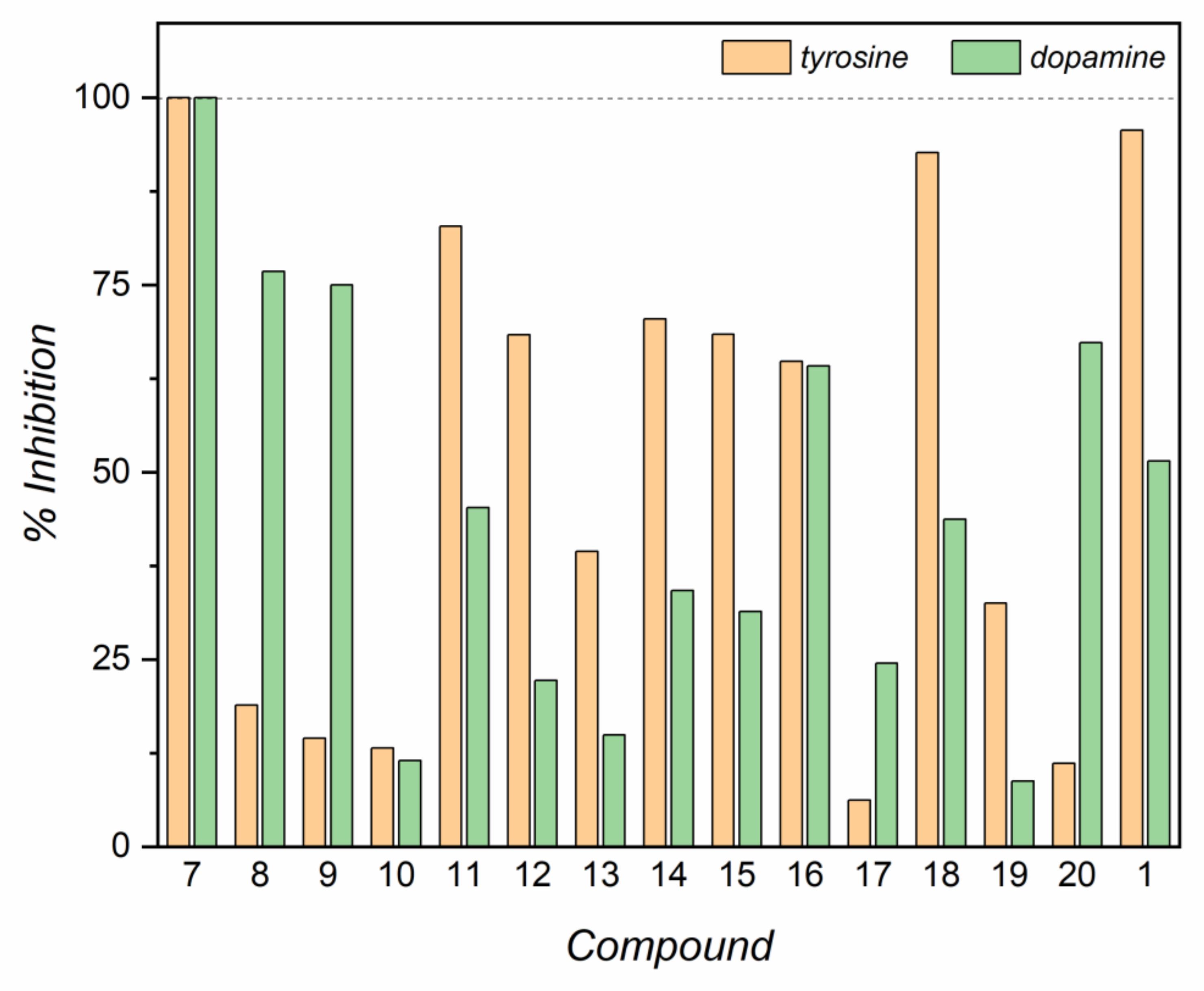
2.2. Spectrophotometric Detection of Tyrosinase Inhibitory Activity
2.3. Evaluation of IC50 and Inhibitory Activity of Compounds 7–20 with Tyrosinase and Laccase Biosensors
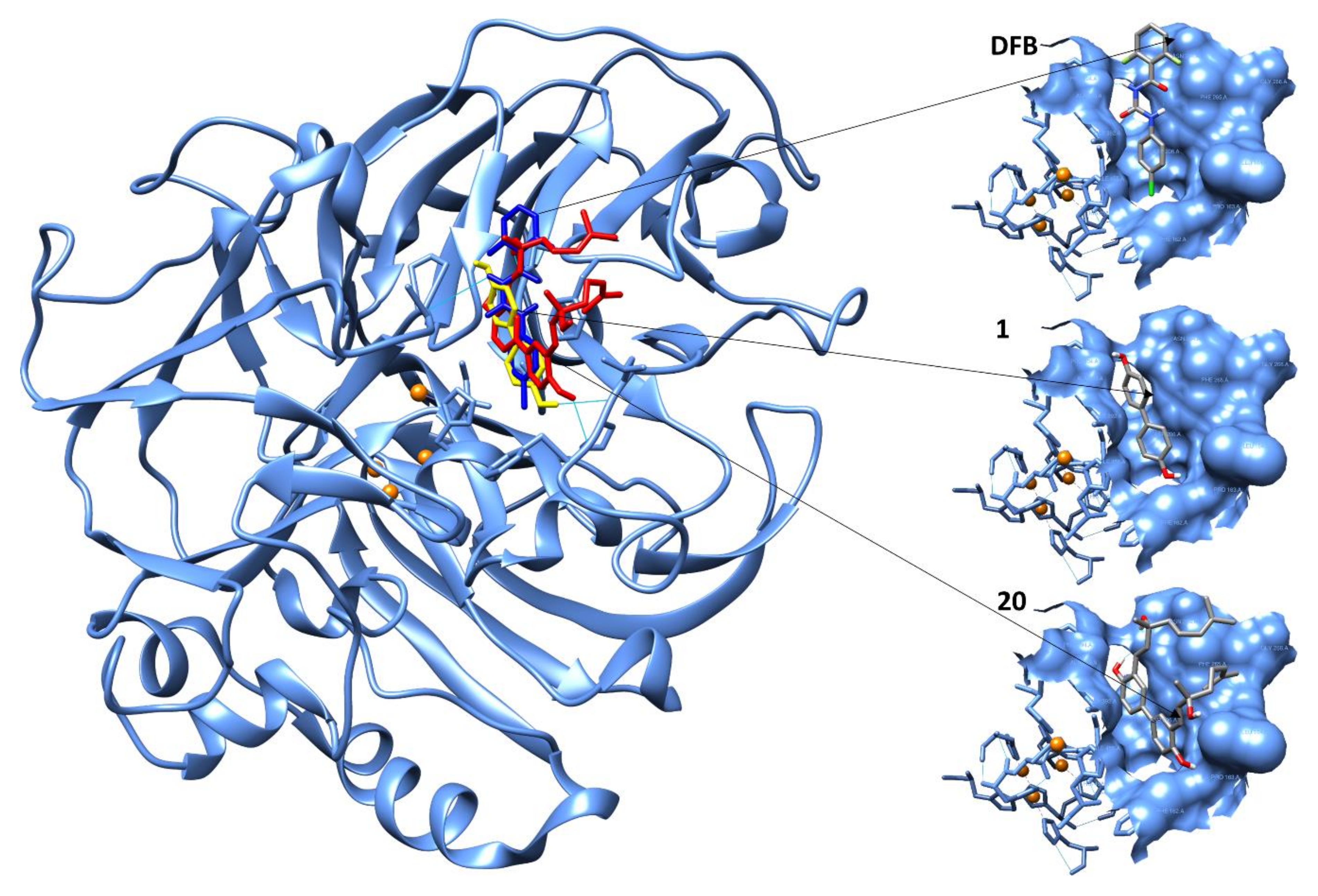
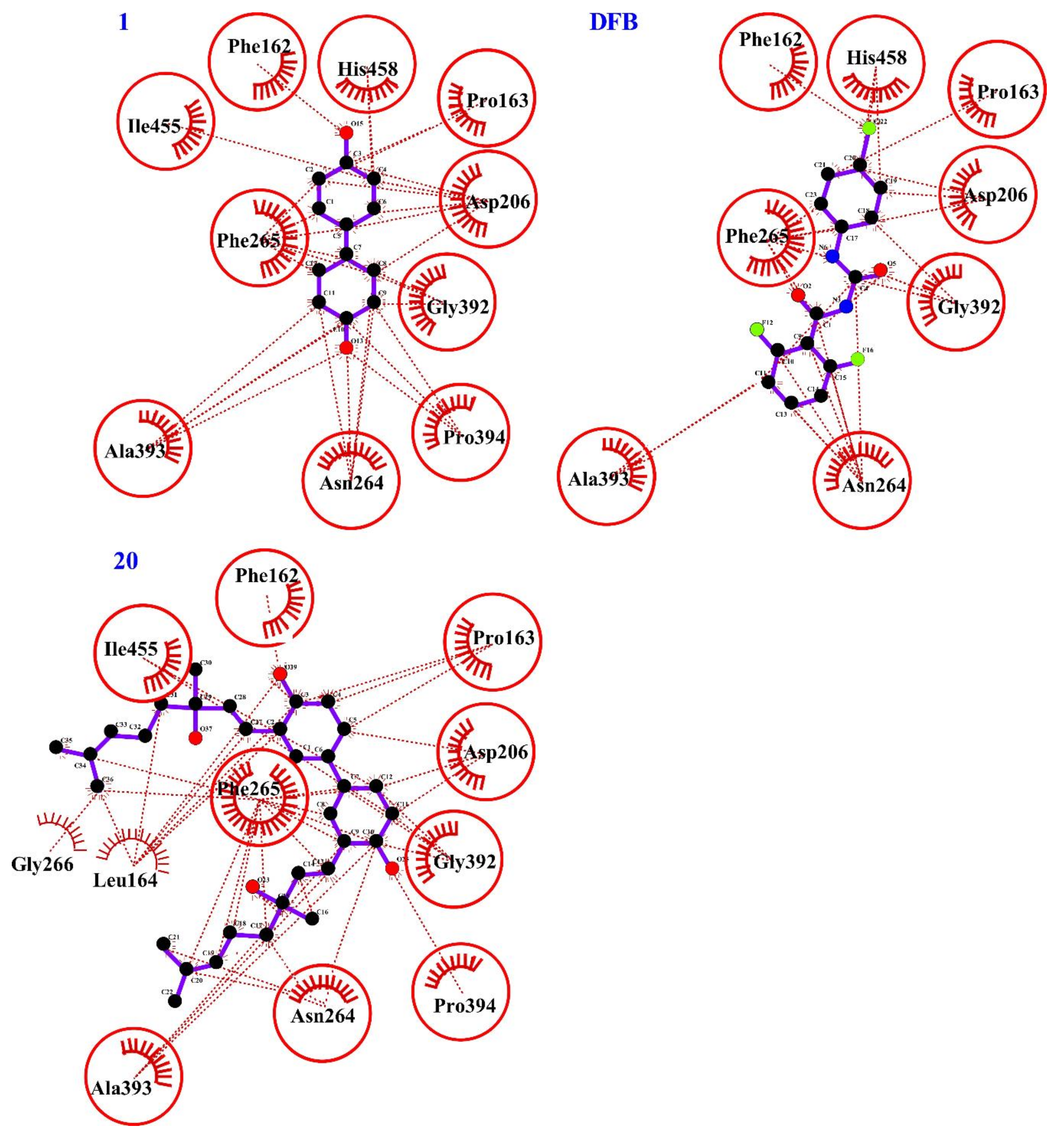
2.4. Computational Evaluation of Laccase Inhibitory Activity
2.5. Cytotoxicity of Inhibitors and Protection Against Oxidative Stress
2.6. Insecticidal Properties of Inhibitors
3. Discussion
4. Materials and Methods
4.1. Synthesis of Compounds 18 and 19 from Derivates 21 and 22
General Procedure for the Synthesis of 21 and 22
General Procedure for the Synthesis of 18 and 19
4.2. Spectrophotometric Assay
4.3. Inhibition Protocols on Tyrosinase and Laccase Biosensors
4.4. Computational Equipment and Software
4.5. Testing Insecticidal Properties on Mealworm Larvae
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Solomon, E.J.; Sundaram, U.M.; Machonkin, T.E. Multicopper oxidase and oxygenases. Chem. Rev. 1996, 96, 2563–2605. [Google Scholar] [CrossRef] [PubMed]
- Vavrocla, C.J.; Han, Q.; Mehere, P.; Ding, H.; Christensen, B.M.; Li, J. Tyrosine metabolic enzymes from insects and mammals: A comparative perspective. Insect Sci. 2014, 21, 13–19. [Google Scholar]
- Kim, Y.-J.; Uyama, H. Tyrosinase inhibitors from natural and synthetic sources: Structure, inhibition mechanism and perspective for the future. Cell. Mol. Life Sci. 2005, 62, 1707–1723. [Google Scholar] [CrossRef]
- Ullah, S.; Son, S.; Yun, H.Y.; Kim, D.H.; Chun, P.; Moon, H.R. Tyrosinase inhibitors: A patent review (2011–2015). Expert. Opin.Ther. Pat. 2016, 26, 347–362. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-C.; Huh, S.Y.; Choi, H.R.; Kim, D.-S. Biology of melanogenesis and the search for hypopigmenting agents. Dematol. Sinica 2010, 28, 53–58. [Google Scholar] [CrossRef]
- McEvily, A.J.; Iyengar, R.; Otwell, W.S. Inhibition of enzymatic browning in foods and beverages. Crit. Rev. Food Sci. Nutr. 1992, 32, 253–273. [Google Scholar] [CrossRef] [PubMed]
- Fowler, D.M.; Koulov, A.V.; Alory-Jost, C.; Marks, M.S.; Balch, W.E.; Kelly, J.W. Functional amyloid formation within mammalian tissue. PloS Biol. 2006, 4, e6. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Jin, W.; Nazir, Y.; Fercher, C.; Blaskovich, M.A.T.; Cooper, M.A.; Barnard, R.T.; Ziora, Z.M. Tyrosinase inhibitors as potential antibacterial agents. Eur. J. Med. Chem. 2020, 187, 111892. [Google Scholar] [CrossRef]
- Baldrian, P. Fungal laccases – Occurrence and properties. FEMS Microbiol. Rev. 2006, 30, 215–242. [Google Scholar] [CrossRef]
- Asano, T.; Seto, Y.; Hashimoto, K.; Kurushima, H. Mini-review an insect-specific system for terrestrializazion: Laccase-mediate cuticle formation. Insect Biochem. Mol. Biol. 2019, 108, 61–70. [Google Scholar] [CrossRef]
- Majeau, J.-A.; Brar, S.K.; Tyagi, R.D. Laccases for removal of recalcitrant and emerging pollutants. Biores. Technol. 2010, 101, 2331–2350. [Google Scholar] [CrossRef] [PubMed]
- Riva, S. Laccases: Blue enzymes for green chemistry. Trends Biotechnol. 2006, 24, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Abele, A.; Zheng, D.; Evans, J.; Sugumaran, M. Reexamination of the mechanisms of oxidative transformation of the insect cuticular sclerotizing precursor, 1,2-dehydro-N-acetyldopamine. Insect Biochem. Mol. Biol. 2010, 40, 650–659. [Google Scholar]
- Balabanidou, V.; Grigoraki, L.; Vontas, J. Insect cuticle: A critical determinant of insecticide resistance. Curr. Opin. Insect Sci. 2018, 27, 68–74. [Google Scholar] [CrossRef]
- Sendovki, M.; Kanteev, M.; Ben-Yosef, V.S.; Adir, N.; Fishman, A. First structures of an active bacterial tyrosinase reveal copper plasticity. J. Mol. Biol. 2011, 405, 227–237. [Google Scholar] [CrossRef]
- Ismaya, W.T.; Rozeboom, H.; Weijn, A.; Mes, J.J.; Fusetti, F.; Wichers, H.J.; Dijkstra, B.W. Crystal structure of Agaricus bisporus mushroom tyrosinase: Identity of the tetramer subunits and interaction with tropolone. Biochem. 2011, 50, 5477–5486. [Google Scholar] [CrossRef]
- Goldfeder, M.; Kanteev, M.; Isaschar-Ovdat, S.; Adir, N.; Fishman, A. Determination of tyrosinase substrate-binding modes reveals mechanistic differences between type-3 copper proteins. Nature Commun. 2014, 5, 4505. [Google Scholar] [CrossRef]
- Piontek, K.; Antorini, M.; Choinowski, T. Crystal structure of a laccase from the fungus Trametes versicolor at 1.90 Å resolution containing a full complement of coppers. J. Biol. Chem. 2002, 277, 37663–37669. [Google Scholar] [CrossRef]
- Lai, X.; Wichers, H.J.; Soler-López, M.; Dijkstra, B.W. Phenylthiourea binding to human tyrosinase-related protein 1. Int. J. Mol. Sci. 2020, 21, 915. [Google Scholar] [CrossRef]
- Hae, G.D.; Jo, J.M.; Kim, S.Y.; Kim, J.W. Tyrosinase inhibitors from natural source as skin-whitening agents and the application of edible insects: A mini review. Int. J. Clin. Nutr. Diet. 2019, 5, 141. [Google Scholar] [CrossRef]
- Zolghadri, S.; Bahrami, A.; Khan, M.T.H.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A comprehensive review on tyrosinase inhibitors. J. Enz. Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef]
- Smit, N.; Vicanova, J.; Pavel, S. The hunt for natural skin whitening agents. Int. Mol. Sci. 2009, 10, 5326–5349. [Google Scholar] [CrossRef]
- Chang, T.-S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef]
- Ladizinski, B.; Mistry, N.; Kundu, R.V. Widespread use of toxic skin lightening compounds: Medical and psychosocial aspects. Dermatol. Clin. 2011, 29, 111–123. [Google Scholar] [CrossRef]
- Cho, K.; Ruy, C.S.; Jeong, S.; Kim, Y. Potenial adverse effect of tyrosinase inhibitors on telosts: A review. Comp. Biochem. Physiol. Part. C 2020, 228, 108655. [Google Scholar]
- Ito, S.; Wakamatzu, K. Biochemical mechanism of rhododendrol-induced leukoderma. Int. J. Mol. Sci. 2018, 19, 552. [Google Scholar]
- Parvez, S.; Kang, M.; Chung, H.-S.; Bae, H. Naturally occurring tyrosinase inhibitors: Mechanism and applications in skin health, cosmetics and agriculture industries. Phytother. Res. 2007, 21, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Couteau, C.; Coiffard, L. Overview of skin whitening agents: Drugs and cosmetic products. Cosmetics 2016, 3, 27. [Google Scholar] [CrossRef]
- Gillbro, J.M.; Olsson, M.J. The melonogenesis and mechanisms of skin-lightening agents-existing and new approaches. Int. J. Cosmetic Sci. 2011, 33, 210–221. [Google Scholar] [CrossRef]
- Kim, Y.J.; No, J.K.; Lee, J.H.; Chung, H.Y. 4,4ʹ-Dihydroxybiphenyl as a new potent tyrosinase inhibitor. Biol. Pharm. Bull. 2005, 28, 323–327. [Google Scholar] [CrossRef]
- Ward, R.S. Lignans, neolignans and related compounds. Nat. Prod. Rep. 1999, 16, 75–96. [Google Scholar] [CrossRef]
- Hajduk, P.J.; Bures, M.; Praestagaard, J.; Fesik, S.W. Privileged molecules for protein binding identified from NMR-based screening. J. Med. Chem. 2000, 43, 3443–3447. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.; Lamb, H.K.; Brady, C.; Lefkove, B.; Bonner, M.Y.; Thompson, P.; Lovat, P.E.; Arbiser, J.L.; Hawkins, A.R.; Redfern, C.P.F. Inducing apostosis of cancer cells using small-molecule plant compounds that bind to GRP78. British J. Cancer 2013, 109, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Schuhly, W.; Hufner, A.; Pferschy-Wenzig, E.M.; Prettner, E.; Adams, M.; Bodensieck, A.; Kunert, O.; Oluwemino, A.; Haslinger, E.; Bauer, R. Design and synthesis of ten biphenyl-neolignan derivatives and their in vitro inhibitory potency against cyclooxygenase-1/2 activity and 5-lipoxygenase-mediated LTB4-formation. Bioorg. Med. Chem. 2009, 17, 4459–4465. [Google Scholar] [CrossRef]
- Bringmann, G.; Gulder, T.; Gulder, T.A.M.; Breuning, M. Atroposelective total synthesis of axially chiral biaryl natural products. Chem. Rev. 2011, 111, 563–639. [Google Scholar] [CrossRef]
- Kancheva, V.; Slavova-Kazakova, A.; Fabbri, D.; Dettori, M.A.; Delogu, G.; Janiak, M.; Amarowicz, R. Protective effects of equimolar mixture of monomer and dimer of dehydrozingerone with alfa-tocopherol and/or ascorbyl palmitate during bulk lipid autoxidation. Food Chem. 2004, 157, 263–274. [Google Scholar] [CrossRef]
- Oufensou, S.; Scherm, B.; Balmas, V.; Fabbri, D.; Dettori, M.A.; Carta, P.; Malbrán, I.; Migheli, Q.; Delogu, G. Honokiol, magnolol and its monoacetyl derivative show strong anti-fungal effect on Fusarium isolates of clinical relevance. PLoS ONE 2019, 14, e0221249. [Google Scholar] [CrossRef]
- Maioli, M.; Basoli, V.; Carta, P.; Fabbri, D.; Dettori, M.A.; Cruciani, S.; Serra, P.A.; Delogu, G. Synthesis of magnolol and honokiol derivatives and their effect against hepatocarcinoma cells. PLoS ONE 2018, 13, e0192178. [Google Scholar] [CrossRef]
- Monti, P.; Calia, G.; Marceddu, S.; Dettori, M.A.; Fabbri, D.; Jaoua, S.; O’Neill, R.; Migheli, Q.; Delogu, G.; Serra, P.A. Low electro-synthesis potentials improve permeselectivity of polymerized natural phenols in biosensor applications. Talanta 2017, 162, 151–156. [Google Scholar] [CrossRef]
- Marchiani, A.; Mammi, S.; Siligardi, G.; Hussain, R.; Tessari, I.; Bubacco, L.; Delogu, G.; Fabbri, D.; Dettori, M.A.; Sanna, D.; et al. Small molecules interacting with α-synuclein: Antiaggregating and cytoprotective properties. Amino Acids 2013, 45, 327–338. [Google Scholar] [CrossRef]
- Ruzza, P.; Serra, P.A.; Fabbri, D.; Dettori, M.A.; Rocchitta, G.; Delogu, G. Hydroxylated biphenyls as tyrosinase inhibitor: A spectrophotometric and electrochemical study. Eur. J. Med. Chem. 2017, 126, 1034–1038. [Google Scholar] [CrossRef] [PubMed]
- Boo, Y.C. p-Coumaric acid as an active ingredient in cosmetics: A review focusing on its antimelanogenic effects. Antioxidants 2019, 8, 275. [Google Scholar] [CrossRef] [PubMed]
- Kuo, P.-C.; Damu, A.G.; Cherng, C.-Y.; Jeng, J.-F.; Teng, C.-M.; Lee, E.-J.; Wu, T.-S. Isolation of a natural antioxidant, dehydrozingerone from Zingiber officinale and synthesis of its analogues for recognition of effective antioxidant and antityrosinase agents. Arch. Pharm. Res. 2005, 28, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.; Park, Y.; Lee, S.; Kang, D.; Yang, J.; Akter, J.; Chun, P.; Moon, H.R. Antioxidant, anti-tyrosinase and anti-melanogenic effects of (E)-2,3-diphenylacrylic acid derivatives. Bioorg. Med. Chem. 2019, 27, 2192–2200. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.N.; Sakeh, N.M.; Zareen, S.; Gul, S.; Lo, K.M.; Ul-Haq, Z.; Shah, S.A.A.; Ahmad, S. Design and synthesis of chalcone derivatives as potent tyrosinase inhibitors and their structural activity relationship. J. Mol. Sci. 2015, 1085, 97–103. [Google Scholar] [CrossRef]
- Sheng, Z.; Ge, S.; Xu, X.; Zhang, Y.; Wu, P.; Zhang, K.; Xu, X.; Li, C.; Zhao, D.; Tang, X. Design, synthesis and evaluation of cinnamic acid ester derivatives as mushroom tyrosinase inhibitors. Med. Chem. Comm. 2018, 9, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Chuprajob, T.; Chatchawan, C.; Chokchaisiric, R.; Chunglokd, W.; Sornkaewa, N.; Apichart Suksamrarna, A. Synthesis, cytotoxicity against human oral cancer KB cells and structure–activity relationship studies of trienone analogues of curcuminoids. Bioorg. Med. Chem. Lett. 2014, 24, 2839–2844. [Google Scholar] [CrossRef]
- Jae-Chul, J.; Soyong, J.; Yongam, L.; Dongguk, M.; Eunyoung, L.; Heyin, J.; Miyeon, O.; Seikwan, O.; Mankil, J. Efficient synthesis and neuroprotective effect of substituted 1,3-diphenyl-2-propen-1-ones. J. Med. Chem. 2008, 51, 4054–4058. [Google Scholar]
- Pathak, V.; Ahmad, I.; Kahlon, A.K.; Hasanian, M.; Sharma, S.; Srivastava, K.K.; Sarkar, J.; Shankar, K.; Gupta, A. Syntheses of 2-methoxyestradiol and eugenol template based diarylpropenes as non-steroidal anticancer agents. RSC Advances 2014, 4, 35171–35185. [Google Scholar] [CrossRef]
- Ruzza, P.; Rosato, A.; Nassi, A.; Rondina, M.; Zorzin, M.; Rossi, C.R.; Floreani, M.; Quintieri, L. Synthesis and preliminary in vitro biological evaluation of 4-[(4-hydroxyphenyl)sulfanyl] but-3-en-2-one, a 4-mercaptophenol derivative designed as a novel bifunctional antimelanoma agent. J. Med. Chem. 2009, 52, 4973–4976. [Google Scholar] [CrossRef]
- Sánchez-Ferrer, Á.; Neptuno Rodríguez-López, J.; García-Cánovas, F.; García-Carmona, F. Tyrosinase: A comprehensive review of its mechanism. BBA - Protein Struct. Mol. Enzym. 1995, 1247, 1–11. [Google Scholar] [CrossRef]
- Honisch, C.; Osto, A.; de Matos, A.D.; Vincenzi, S.; Ruzza, P. Isolation of a tyrosinase inhibitor from unripe grapes juice: A spectrophotometric study. Food Chem. 2020, 305, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera - a visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, R.A.; Swindells, M.B. LigPlot+: Multiple ligand-protein interaction diagrams for drug discovery. J. Chem. Inf. Model. 2011, 51, 2778–2786. [Google Scholar] [CrossRef]
- Pinna, A.; Ricco, R.; Migheli, R.; Rocchitta, G.; Serra, P.A.; Falcaro, P.; Malfatti, L.; Innocenzi, P. A MOF-based carrier for in situ dopamine delivery. RSC Adv. 2018, 8, 25664–25672. [Google Scholar] [CrossRef]
- Menezes, C.J.M.D.S.; Diederich, M.F. Natural dimers of coumarin, chalcones, and resveratrol and the link between structure and pharmacology. Eur. J. Med. Chem. 2019, 182, 111637. [Google Scholar] [CrossRef]
- Jia, Y.L.; Zheng, J.; Yu, F.; Cai, Y.X.; Zhan, X.L.; Wang, H.F.; Chen, Q.X. Antityrosinase kinetics and antibacterial process of caffeic acid N-nonyl ester in Chinese Olive (Canarium album) postharvest. Int. J. Biol. Macromol. 2016, 91, 486–495. [Google Scholar] [CrossRef]
- Garcia-Jimenez, A.; Teruel-Puche, J.A.; Garcia-Ruiz, P.A.; Saura-Sanmartin, A.; Berna, J.; Rodríguez-López, J.N.; Garcia-Canovas, F. Action of tyrosinase on caffeic acid and its n-nonyl ester. Catalysis and suicide inactivation. Int. J. Biol. Macromol. 2018, 107, 2650–2659. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, Z.; Zhang, J.; Moussian, B. Regionalization of surface lipids in insects. Proc. R. Soc. B 2016, 283, 20152994. [Google Scholar] [CrossRef]
- Smith, D.W.; Babb, D.A., Jr.; Snelgrove, R.V.; Townsend, P.H.; Martin, S.J. Polynaphthalene: Networks from Bisphenols. J. Amer. Chem. Soc. 1998, 120, 9078–9079. [Google Scholar] [CrossRef]
- Calia, G.; Rocchitta, G.; Migheli, R.; Puggioni, G.; Spissu, Y.; Bazzu, G.; Mazzarello, V.; Lowry, J.P.; O’Neill, R.D.; Desole, M.S.; et al. Biotelemetric monitoring of brain neurochemistry in conscious rats using microsensors and biosensors. Sensors 2009, 9, 2511–2523. [Google Scholar] [CrossRef] [PubMed]
- Bazzu, G.; Puggioni, G.G.; Dedola, S.; Calia, G.; Rocchitta, G.; Migheli, R.; Desole, M.S.; Lowry, J.P.; O’Neill, R.D.; Serra, P.A. Real-time monitoring of brain tissue oxygen using a miniaturized biotelemetric device implanted in freely moving rats. Anal. Chem. 2009, 81, 2235–2241. [Google Scholar] [CrossRef] [PubMed]
- Barberis, A.; Garbetta, A.; Cardinali, A.; Bazzu, G.; D’Antuono, I.; Rocchitta, G.; Fadda, A.; Linsalata, V.; D’Hallewin, G.; Serra, P.A.; et al. Real-time monitoring of glucose and phenols intestinal absorption through an integrated Caco-2TC7cells/biosensors telemetric device: Hypoglycemic effect of fruit phytochemicals. Biosens Bioelectron. 2017, 88, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Rocchitta, G.; Migheli, R.; Dedola, S.; Calia, G.; Desole, M.S.; Miele, E.; Lowry, J.P.; O’Neill, R.D.; Serra, P.A. Development of a distributed, fully automated, bidirectional telemetry system for amperometric microsensor and biosensor applications. Sens. Actuat. B Chem. 2007, 126, 700–709. [Google Scholar] [CrossRef]
- Serra, P.A.; Rocchitta, G.; Bazzu, G.; Manca, A.; Puggioni, G.M.; Lowry, J.P.; O’Neill, R.D. Design and construction of a low-cost single-supply embedded telemetry system for amperometric biosensor applications. Sens. Actuat. B Chem. 2007, 122, 118–126. [Google Scholar] [CrossRef]
- Gateiger, J.; Marsili, M. Iterative partial equalization of orbital electronegativity – a rapid access to atomic charges. Tetrahedron 1980, 36, 3219–3228. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. Autodock4 and AutoDockTools4: Automated docking with selective receptor flexiblity. J. Comput. Chem. 2009, 16, 2785–2791. [Google Scholar] [CrossRef]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 1988, 19, 1639–1662. [Google Scholar] [CrossRef]
- Huey, R.; Morris, G.M.; Olson, A.J.; Goodsell, D.S. A semiempirical free energy force field with charge-based desolvation. J. Comput. Chem. 2007, 28, 1145–1152. [Google Scholar] [CrossRef]
- Mohamadi, F.; Richards, N.G.; Guida, W.C.; Liskamp, R.; Lipton, M.; Caufiel, C.; Chang, G.; Hendrickson, T.; Still, W.C. Macromodel—An integrated software system for modeling organic and bioorganic molecules using molecular mechanics. J. Comput. Chem. 1990, 11, 440–467. [Google Scholar] [CrossRef]
- Chang, G.; Guida, W.C.; Still, W.C. An internal-coordinate Monte Carlo method for searching S46 conformational space. J. Am. Chem. Soc. 1989, 111, 4379–4386. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. aussian 09, Revision B, 01; Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Dennington, R.; Keith, T.; Millam, J. GaussView Version 5; Semichem Inc.: Shawnee, KS, USA, 2009. [Google Scholar]
Sample Availability: Samples of the compounds 7–20 are available from the authors. |

| Compound | IC50 | Type of Inhibition | ||
|---|---|---|---|---|
| Tyrosinase | Laccase | Tyrosinase | Laccase | |
| 7 | 26 | 27 | competitive | competitive |
| 8 | 54 | 218 | mixed | noncompetitive |
| 9 | 122 | 2238 | competitive | competitive |
| 10 | 101 | 212 | mixed | competitive |
| 11 | 38 | 62 | competitive | mixed |
| 12 | 72 | 1043 | noncompetitive | competitive |
| 13 | 20 | 23 | uncompetitive | competitive |
| 14 | 22 | 47 | mixed | mixed |
| 15 | 120 | 209 | competitive | competitive |
| 16 | 62 | 987 | competitive | competitive |
| 17 | 130 | 184 | mixed | competitive |
| 18 | 65 | 2619 | competitive | competitive |
| 19 | 24 | 130 | noncompetitive | competitive |
| 20 | 423 | 118 | competitive | competitive |
| Compound | Toxic Concentration (µM) 1 | H2O2 Damage Protection (µM) | MnCl2 Damage Protection (µM) |
|---|---|---|---|
| 7 | >10 | 1 | n.p. |
| 8 | 40 | n.p. | n.a. |
| 9 | >10 | n.p. | n.a. |
| 10 | >20 | 20 | n.p. |
| 11 | >10 | n.a. | n.a. |
| 12 | >1 | n.a. | n.a. |
| 13 | >20 | n.p. | n.a. |
| 14 | >5 | n.a. | n.a. |
| 15 | >5 | n.a. | n.a. |
| 16 | >5 | n.a. | n.a. |
| 17 | none | n.p. | n.a. |
| 18 | none | n.p. | n.a. |
| 19 | none | n.p. | n.a. |
| 20 | none | >5 | 1 |
| Compounds | Mortality% (Mean ± SD)** | Arcsine(√x) Transformation | Mean Comparison*** (Duncan Test, α = 0.05) | ||||
|---|---|---|---|---|---|---|---|
| DFB | 97.0 ± 4.47 | 1.46 ± 0.15 | a | ||||
| control | 1.0 ± 2.24 | 0.05 ± 0.10 | e | ||||
| 1 | 2.0 ± 2.74 | 0.09 ± 0.12 | e | ||||
| 2 | 4.0 ± 5.48 | 0.13 ± 0.18 | d | e | |||
| 3 | 3.0 ± 2.74 | 0.14 ± 0.12 | d | e | |||
| 4 | 3.0 ± 4.47 | 0.11 ± 0.15 | d | e | |||
| 5 | 16.0 ± 9.62 | 0.40 ± 0.13 | c | ||||
| 6 | 1.0 ± 2.24 | 0.05 ± 0.10 | e | ||||
| 7 | 5.0 ± 7.07 | 0.14 ± 0.20 | d | e | |||
| 8 | 10.0 ± 7.07 | 0.29 ± 0.17 | c | d | |||
| 9 | 4.0 ± 6.51 | 0.12 ± 0.18 | d | e | |||
| 11 | 4.0 ± 2.24 | 0.18 ± 0.10 | d | e | |||
| 12 | 0 | 0 | e | ||||
| 14 | 0 | 0 | e | ||||
| 15 | 0 | 0 | e | ||||
| 20 | 67.0 ± 14.83 | 0.97 ± 0.16 | b | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dettori, M.A.; Fabbri, D.; Dessì, A.; Dallocchio, R.; Carta, P.; Honisch, C.; Ruzza, P.; Farina, D.; Migheli, R.; Serra, P.A.; et al. Synthesis and Studies of the Inhibitory Effect of Hydroxylated Phenylpropanoids and Biphenols Derivatives on Tyrosinase and Laccase Enzymes. Molecules 2020, 25, 2709. https://doi.org/10.3390/molecules25112709
Dettori MA, Fabbri D, Dessì A, Dallocchio R, Carta P, Honisch C, Ruzza P, Farina D, Migheli R, Serra PA, et al. Synthesis and Studies of the Inhibitory Effect of Hydroxylated Phenylpropanoids and Biphenols Derivatives on Tyrosinase and Laccase Enzymes. Molecules. 2020; 25(11):2709. https://doi.org/10.3390/molecules25112709
Chicago/Turabian StyleDettori, Maria Antonietta, Davide Fabbri, Alessandro Dessì, Roberto Dallocchio, Paola Carta, Claudia Honisch, Paolo Ruzza, Donatella Farina, Rossana Migheli, Pier Andrea Serra, and et al. 2020. "Synthesis and Studies of the Inhibitory Effect of Hydroxylated Phenylpropanoids and Biphenols Derivatives on Tyrosinase and Laccase Enzymes" Molecules 25, no. 11: 2709. https://doi.org/10.3390/molecules25112709
APA StyleDettori, M. A., Fabbri, D., Dessì, A., Dallocchio, R., Carta, P., Honisch, C., Ruzza, P., Farina, D., Migheli, R., Serra, P. A., Pantaleoni, R. A., Fois, X., Rocchitta, G., & Delogu, G. (2020). Synthesis and Studies of the Inhibitory Effect of Hydroxylated Phenylpropanoids and Biphenols Derivatives on Tyrosinase and Laccase Enzymes. Molecules, 25(11), 2709. https://doi.org/10.3390/molecules25112709