Dendrimer-Functionalized Hybrid Materials Based on Silica as Novel Carriers of Bioactive Acids
Abstract
1. Introduction
2. Results and Discussion
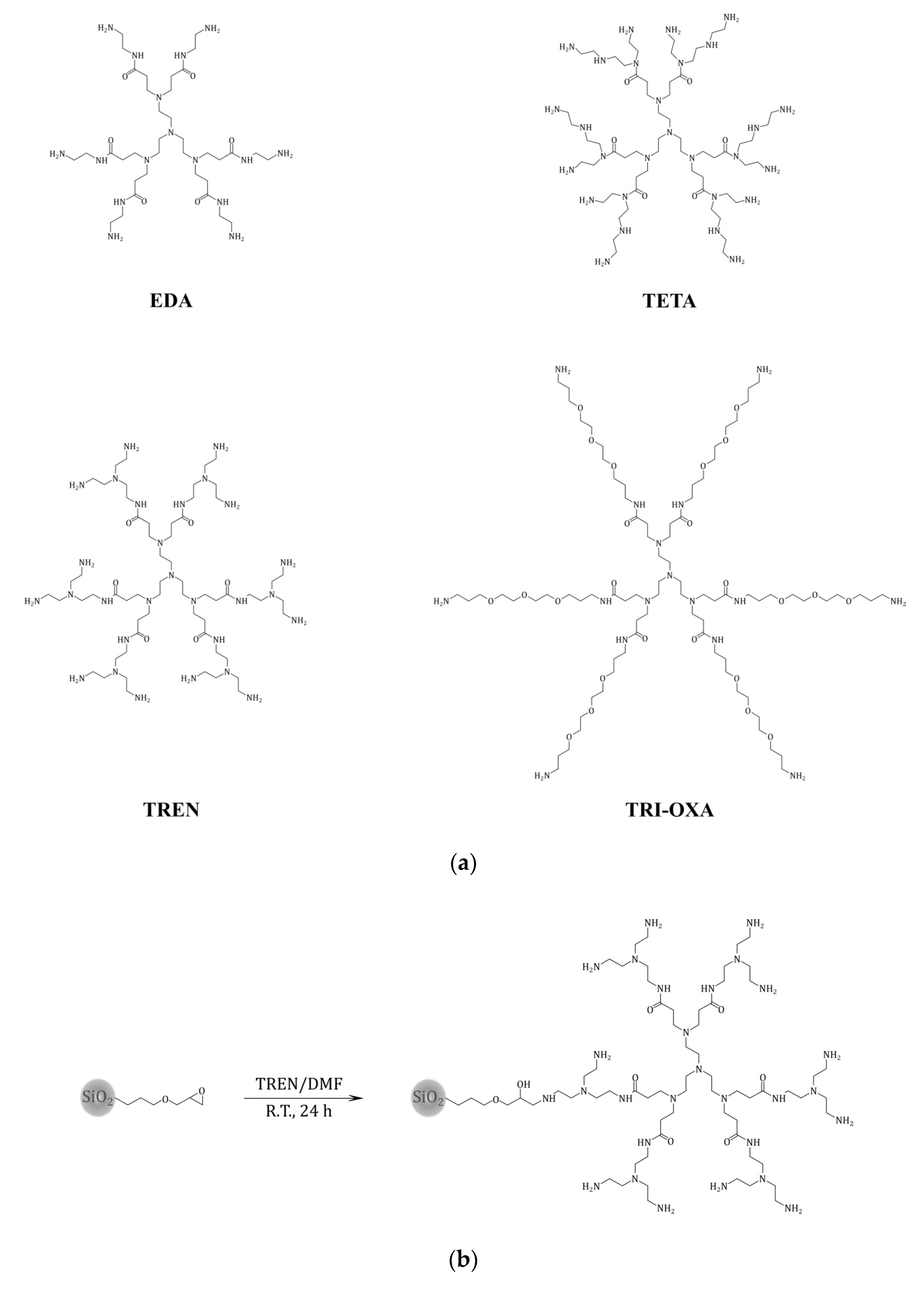
2.1. Synthesis of PAMAM Dendrimers
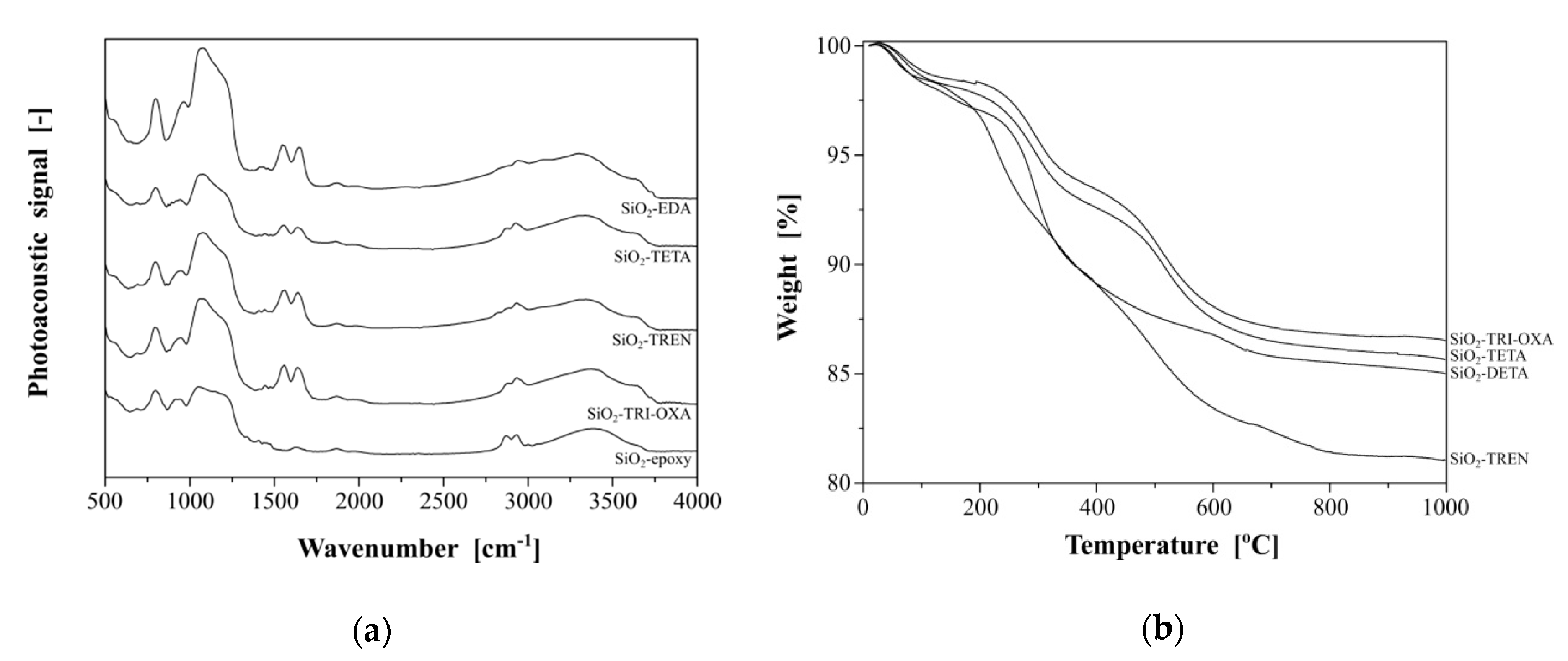
2.2. Synthesis and Characterization of the Hybrid Materials
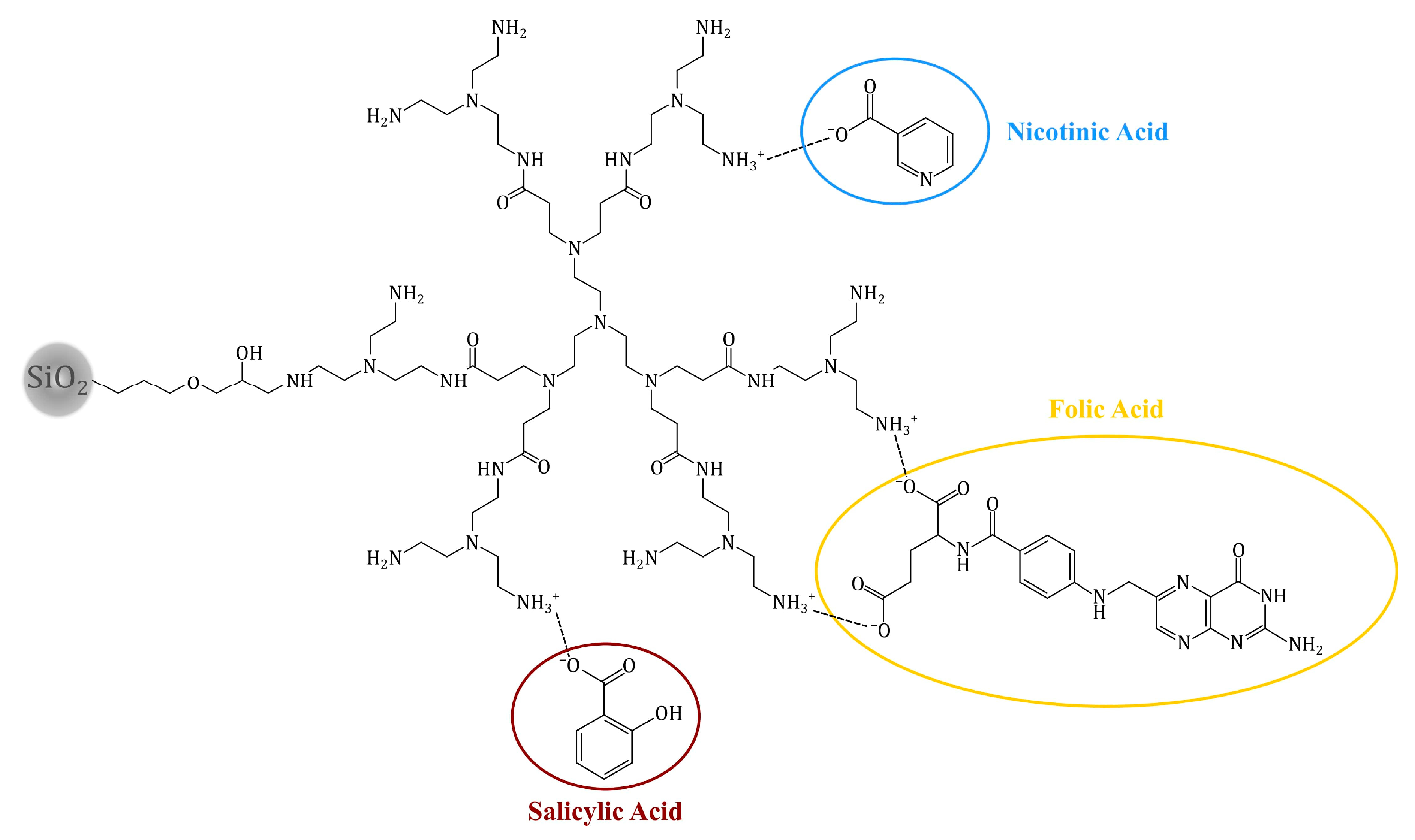
2.3. Adsorption of Bioactive Compounds on the Hybrid Materials
2.4. Thermodynamic Studies of the Drug Adsorption Processes on the Hybrid Materials
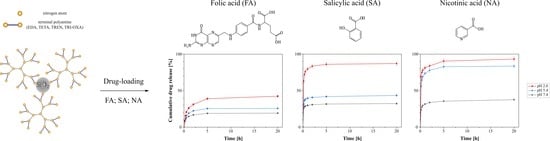
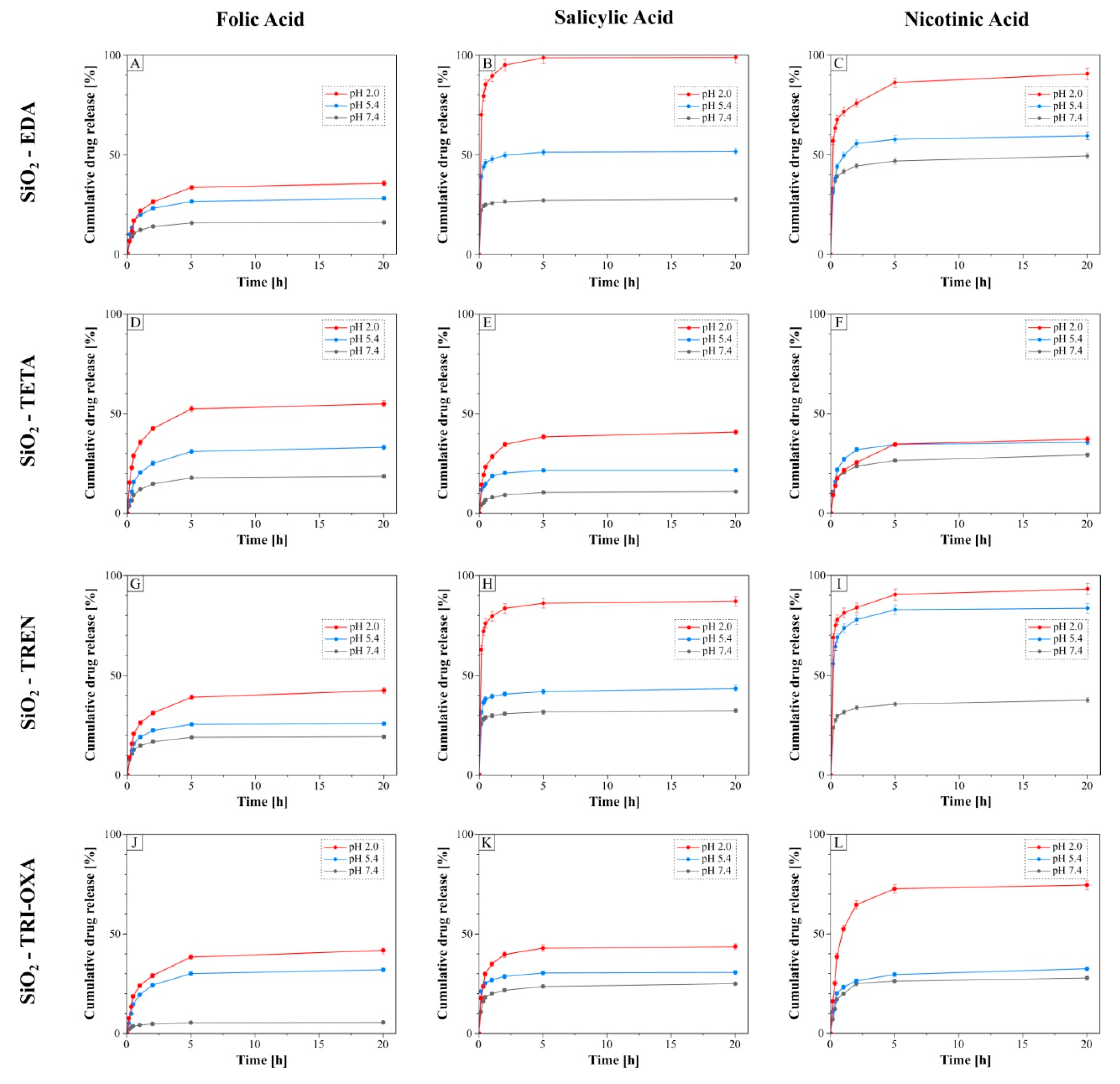
2.5. pH-Dependent Drug Release from the Drug-Loaded Hybrid Materials
3. Materials and Methods
3.1. Materials
3.2. Instruments
3.3. Synthesis of PAMAM Dendrimers
3.4. Synthesis of Hybrid Materials
3.5. Biomolecules Adsorption Isotherms
3.6. Thermodynamic Studies of Biomolecules Adsorption on the Hybrid Materials
3.7. In-Vitro Release of Bioactive Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. Dendritic macromolecules: Synthesis of starburst dendrimers. Macromolecules 1986, 19, 2466–2468. [Google Scholar] [CrossRef]
- Silva, N.P., Jr.; Menacho, F.P.; Chorilli, M. Dendrimers as potential platform in nanotechnology-based drug delivery systems. IOSE J. Pharm. 2012, 2, 23–30. [Google Scholar]
- Sadekar, S.; Ghandehari, H. Transepithelial transport and toxicity of PAMAM dendrimers: Implications for oral drug delivery. Adv. Drug. Deliv. Rev. 2012, 64, 571–588. [Google Scholar] [CrossRef]
- Chanphai, P.; Tajmir–Riahi, H.A. Characterization of folic acid-PAMAM conjugates: Drug loading efficacy and dendrimer morphology. J. Biomol. Struct. Dynamics 2018, 36, 1918–1924. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liang, H.; Liu, J.; Wang, Z. Poly (amidoamine) (PAMAM) dendrimer mediated delivery of drug and pDNA/siRNA for cancer therapy. Int. J. Pharm. 2018, 546, 215–225. [Google Scholar] [CrossRef]
- Fox, L.J.; Richardson, R.M.; Briscoe, W.H. PAMAM dendrimer-cell membrane interactions. Adv. Colloid Interf. Sci. 2018, 257, 1–18. [Google Scholar] [CrossRef]
- Ehsan, M.; Kharat, A.N.; Adeli, M. Polyamidoamine and polyglycerol; their linear, dendritic and linear-dendritic architectures as anticancer drug delivery systems. J. Mater. Chem. B 2015, 3, 3896–3921. [Google Scholar]
- Jones, C.F.; Campbell, R.A.; Brooks, A.E.; Assemi, S.; Tadjiki, S.; Thiagarajan, G.; Mulcock, C.; Weyrich, A.S.; Brooks, B.D.; Ghandehari, H.; et al. Cationic PAMAM Dendrimers Aggressively Initiate Blood Clot Formation. ACS Nano 2012, 6, 9900–9910. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; De Paoli, S.H.; Elhelu, O.K.; Farooq, S.; Simak, J. Cell membrane disintegration and extracellular vesicle release in a model of different size and charge PAMAM dendrimers effects on cultured endothelial cells. Nanotoxicology 2019, 13, 664–681. [Google Scholar] [CrossRef]
- Sweet, D.M.; Kolhatkar, R.B.; Ray, A.; Swaan, P.; Ghandehari, H. Transepithelial transport of PEGylated anionic poly (amidoamine) dendrimers: Implications for oral drug delivery. J. Control. Release 2009, 138, 78–85. [Google Scholar] [CrossRef]
- Kumar, P.D.; Kumar, P.V.; Selvam, T.P.; Sambasiva Rao, K.R.S. Prolonged Drug Delivery System of PEGylated PAMAM Dendrimers with a Anti-HIV Drug. Res. Pharm. 2013, 3, 8–17. [Google Scholar]
- Yan, C.; Gu, J.; Lv, Y.; Shi, W.; Wang, Y.; Liao, Y.; Deng, Y. Caproyl-Modified G2 PAMAM Dendrimer (G2-AC) Nanocomplexes Increases the Pulmonary Absorption of Insulin. AAPS Pharm. Sci. Tech. 2019, 20, 298–304. [Google Scholar] [CrossRef]
- Nguyen, T.T.C.; Nguyen, C.K.; Nguyen, T.H.; Tran, N.Q. Highly lipophilic pluronics-conjugated polyamidoamine dendrimer nanocarriers as potential delivery system for hydrophobic drugs. Mater. Sci. Eng. C 2017, 70, 992–999. [Google Scholar] [CrossRef] [PubMed]
- Kolhatkar, R.B.; Kitchens, K.M.; Swaan, P.W.; Ghandehari, H. Surface acetylation of polyamidoamine (PAMAM) dendrimers decreases cytotoxicity while maintaining membrane permeability. Bioconjug. Chem. 2007, 18, 2054–2060. [Google Scholar] [CrossRef]
- Pisal, D.S.; Yellepeddi, V.K.; Kumar, A.; Kaushik, R.S.; Hildreth, M.B.; Guan, X.; Palakurthi, S. Permeability of surface-modified polyamidoamine (PAMAM) dendrimers across Caco-2 cell monolayers. Int. J. Pharm. 2008, 350, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Pourianazar, N.T.; Mutlu, P.; Gunduz, U. Bioapplications of poly(amidoamine) (PAMAM) dendrimers in nanomedicine. J. Nanopart. Res. 2014, 16, 2342–2379. [Google Scholar] [CrossRef]
- Ciolkowski, M.; Petersen, J.F.; Ficker, M.; Janaszewska, A.; Christensen, J.B.; Klajnert, B.; Bryszewska, M. Surface modification of PAMAM dendrimer improves its biocompability. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 815–817. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, P.; Xie, L.; Mao, G.; Padhye, S.; Iyer, A.K. Hyaluronic acid-conjugated polyamidoamine dendrimers for targeted delivery of 3,4-difluorobenzylidene curcumin to CD44 overexpressing pancreatic cancer cells. Colloids Surf. B Biointerf. 2015, 136, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Qi, X.; Chen, Y.; Ma, N.; Zhang, Z.; Xing, J.; Zhu, X.; Li, Z.; Wu, Z. Octreotide-conjugated PAMAM for targeted delivery to somatostatin receptors over–expressed tumor cells. J. Drug Target. 2014, 22, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Pawlaczyk, M.; Kurczewska, J.; Schroeder, G. Nanomaterials Modification by Dendrimers—A review. World J. Res. Rev. 2018, 6, 14–30. [Google Scholar]
- Zhou, L.; Yuan, J.; Wei, Y. Core-shell structural iron oxide hybrid nanoparticles: From controlled synthesis to biomedical applications. J. Mater. Chm. 2010, 21, 2823–2840. [Google Scholar] [CrossRef]
- Arachchige, M.P.; Laha, S.S.; Naik, A.R.; Lewis, K.T.; Naik, R.; Jena, B.P. Functionalized nanoparticles enable tracking the rapid entry and release of doxorubicin in human pancreatic cancer cells. Micron 2017, 92, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, J.Y.; Yang, F.; Zhang, Y.J.; Yao, Q.; Cui, T.Y.; Zhao, X.; Zhang, Z.D. A new strategy for assembling multifunctional nanocomposites with iron oxide and amino-terminated PAMAM dendrimers. J. Mater. Sci. Mater. Med. 2009, 20, 2433–2440. [Google Scholar] [CrossRef] [PubMed]
- Tajabadi, M.; Khosroshahi, M.E.; Bonakdar, S. An efficient method of SPION synthesis coated with third generation PAMAM dendrimer. Colloids Surf. A Physicochem. Eng. Aspects 2013, 431, 18–26. [Google Scholar] [CrossRef]
- Zhao, H.; Gu, W.; Ye, L.; Yang, H. Biodistribution of PAMAM dendrimer conjugated magnetic nanoparticles in mice. J. Mater. Sci. Mater. Med. 2014, 25, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Khodadust, R.; Unsoy, G.; Yalcin, S.; Gunduz, G.; Gunduz, U. PAMAM dendrimer-coated iron oxide nanoparticles: Synthesis and characterization of different generations. J. Nanopart. Res. 2013, 15, 1488–1500. [Google Scholar] [CrossRef]
- Banei, M.; Salami-Kalajahi, M. A “Grafting to” Approach to Synthesize Low Cytotoxic Poly(aminoamide)-Dendrimer-grafted Fe3O4 Magnetic Nanoparticles. Adv. Polym. Technol. 2018, 37, 943–948. [Google Scholar] [CrossRef]
- Chang, Y.; Li, Y.; Meng, X.; Liu, N.; Sun, D.; Liu, H.; Wang, J. Dendrimer functionalized water soluble magnetic iron oxide conjugates as dual imaging probe for tumor targeting and drug delivery. Polym. Chem. 2013, 4, 789–794. [Google Scholar] [CrossRef]
- Xu, S.; Wu, J.; Jiang, W.; Tian, R. Synthesis and Characterization of a Poly(amido amine) Modified Magnetic Nanocarrier for Controlled Delivery of Doxorubicin. J. Nanosci. Nanotechnol. 2016, 16, 1363–1369. [Google Scholar] [CrossRef]
- Nigam, S.; Chandra, S.; Newgreen, D.F.; Bahadur, D.; Chen, Q. Poly(ethylene glycol)-Modified PAMAM–Fe3O4-Doxorubicin Triads with the Potential for Improved Therapeutic Efficacy: Generation-Dependent Increased Drug Loading and Retention at Neutral pH and Increased Release at Acidic pH. Langmuir 2014, 30, 1004–1011. [Google Scholar] [CrossRef]
- Nigam, S.; Bahadur, D. Dendrimerized Magnetic Nanoparticles as Carriers for the Anticancer Compound, Epigallocatechin Gallate. IEEE Trans. Magnet. 2016, 52, 1–5. [Google Scholar] [CrossRef]
- Nosrati, H.; Adibtabar, M.; Sharafi, A.; Danafar, H.; Kheiri, M.H. PAMAM-modified citric acid-coated magnetic nanoparticles as pH sensitive biocompatible carrier against human breast cancer cells. Drug Dev. Ind. Pharm. 2018, 44, 1377–1384. [Google Scholar] [CrossRef]
- El-Sigeny, S.M.; Abou Taleb, M.F. Synthesis, Characterization, and Application of Dendrimer Modified Magnetite Nanoparticles as Antimicrobial Agent. Life Sci. J. 2015, 12, 161–170. [Google Scholar]
- Yu, S.; Li, G.; Liu, R.; Ma, D.; Xue, W. Dendritic Fe3O4@Poly(dopamine)@PAMAM Nanocomposite as Controllable NO-Releasing Material: A Synergistic Photothermal and NO Antibacterial Study. Adv. Funct. Mater. 2018, 28, 1707440. [Google Scholar] [CrossRef]
- Cancino, J.; Paino, I.M.M.; Micocci, K.C.; Selistre de Araujo, H.S.; Zucolotto, V. In vitro nanotoxicity of single-walled carbon nanotube–dendrimer nanocomplexes against murine myoblast cells. Toxic. Letters 2013, 219, 18–25. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, T.; Jiang, H.; Li, Y.; Jiang, T.; Zhang, J.; Wang, S. Incorporation of Carvedilol into PAMAM-functionalized MWNTs as a sustained drug delivery system for enhanced dissolution and drug-loading capacity. Asian J. Pharm. Sci. 2013, 8, 278–286. [Google Scholar] [CrossRef]
- Wen, S.; Liu, H.; Cai, H.; Shen, M.; Shi, X. Targeted and pH-Responsive Delivery of Doxorubicin to Cancer Cells Using Multifunctional Dendrimer-Modified Multi-Walled Carbon Nanotubes. Adv. Healthcare Mater. 2013, 2, 1267–1276. [Google Scholar] [CrossRef]
- Kurczewska, J.; Cegłowski, M.; Messyasz, B.; Schroeder, G. Dendrimer-functionalized halloysite nanotubes for effective drug delivery. Appl. Clay Sci. 2018, 153, 134–143. [Google Scholar] [CrossRef]
- Eskandarloo, H.; Arshadi, M.; Abbaspourrad, A. Magnetic Dendritic Halloysite Nanotube for Highly Selective Recovery of Heparin Digested from Porcine Intestinal Mucosa. ACS Sustain. Chem. Eng. 2018, 6, 14561–14573. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, J.; Li, X.; Sun, Y.; Huang, S.; Li, Y.; Liu, H.; Xu, J.; Zhong, S. Multifunctional halloysite nanotubes for targeted delivery and controlled release of doxorubicin in-vitro and in-vivo studies. Nanotechnology 2017, 28, 375101. [Google Scholar] [CrossRef]
- Xu, X.; Lu, S.; Gao, C.; Wang, X.; Bai, X.; Gao, N.; Liu, M. Facile preparation of pH-sensitive and self-fluorescent mesoporous silica nanoparticles modified with PAMAM dendrimers for label-free imaging and drug delivery. Chem. Eng. J. 2015, 266, 171–178. [Google Scholar] [CrossRef]
- Lotfi, R.; Hayati, B.; Rahimi, S.; Shekarchi, A.A.; Mahmoodi, N.M.; Bagheri, A. Synthesis and characterization of PAMAM/SiO2 nanohybrid as a new promising adsorbent for pharmaceutical. Microchem. J. 2019, 146, 1150–1159. [Google Scholar] [CrossRef]
- Torres, C.C.; Campos, C.H.; Diáz, C.; Jiménez, V.A.; Vidal, F.; Guzmán, L.; Alderete, J.B. PAMAM-grafted TiO2 nanotubes as novel versatile materials for drug delivery applications. Mater. Sci. Eng. C 2016, 65, 164–171. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, L.; Jiang, Y.; Gao, W.; Wang, Y.; Yang, X.; Yang, X.; Liu, Z. Gold Nanorods with Silica Shell and PAMAM Dendrimers for Efficient Photothermal Therapy and Low Toxic Codelivery of Anticancer Drug and siRNA. Adv. Mater. Interfaces 2017, 4, 1701166. [Google Scholar] [CrossRef]
- Xiao, H.; Yan, L.; Dempsey, E.M.; Song, W.; Qi, R.; Li, W.; Huang, Y.; Jing, X.; Zhou, D.; Ding, J.; et al. Recent progress in polymer-based platinum drug delivery systems. Progress Polym. Sci. 2018, 87, 70–106. [Google Scholar] [CrossRef]
- Akin, M.; Bongartz, R.; Walter, J.G.; Demirkol, D.O.; Stahl, F.; Timur, S.; Scheper, T. PAMAM–functionalized water soluble quantum dots for cancer cell targeting. J. Mater. Chem. 2012, 22, 11529–11536. [Google Scholar] [CrossRef]
- Geraldo, D.A.; Duran–Lara, E.F.; Aguayo, D.; Cachau, R.E.; Tapia, J.; Esparza, R.; Yacaman, M.J.; Gonzalez-Nilo, F.D.; Santos, L.S. Supramolecular complexes of quantum dots and a polyamidoamine (PAMAM)–folate derivative for molecular imaging of cancer cells. Anal. Bioanal. Chem. 2011, 400, 483–492. [Google Scholar] [CrossRef]
- Kaczorowska, M.A.; Cooper, H.J. Electron Capture Dissociation, Electron Detachment Dissociation, and Collision-Induced Dissociation of Polyamidoamine (PAMAM) Dendrimer Ions with Amino, Amidoethanol, and Sodium Carboxylate Surface Groups. J. Am. Soc. Mass Spectrom. 2008, 19, 1312–1319. [Google Scholar] [CrossRef][Green Version]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies. Tables and Charts, 3rd ed.; John Wiley and Sons Ltd.: Chichester, UK, 2001; pp. 50–53, 94–108, 139–148. [Google Scholar]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and Interpretation of Adsorption Isotherms. J. Chem. 2017, 2017, 1–11. [Google Scholar] [CrossRef]
- Vahidhabanu, S.; Idowu, A.A.; Babu, R.B. Magnetic core layered double hydroxide over halloysite as a robust adsorbent for the treatment of dye-contaminated wastewater: A clean approach for industrial applications. Desalin. Water Treat. 2019, 138, 379–388. [Google Scholar] [CrossRef]
- Sanchez Zambrano, K.S.; Vilarassa–Garcia, E.; Maia, D.A.S.; Bastos-Neto, M.; Rodriguez-Castellon, E.; Azevedo, D.C.S. Adsorption microcalorimetry as a tool in the characterization of amine-grafted mesoporous silicas for CO2 capture. Adsorption 2019, 26, 165–175. [Google Scholar] [CrossRef]
- Bohrey, S.; Chourasiya, V.; Pandey, A. Polymeric nanoparticles containing diazepam: Preparation, optimization, characterization, in–vitro drug release and release kinetic study. Nano Converg. 2016, 3, 1–7. [Google Scholar] [CrossRef]
- Somanathan, T.; Krishna, V.M.; Saravanan, V.; Kumar, R.; Kumar, R. MgO Nanoparticles for Effective Uptake and Release of Doxorubicin Drug: pH Sensitive Controlled Drug Release. J. Nanosci. Nanotechnol. 2016, 16, 9421–9431. [Google Scholar] [CrossRef]
- Wua, I.Y.; Balaa, S.; Skalko–Basneta, N.; Pio di Cagno, M. Interpreting non-linear drug diffusion data: Utilizing Korsmeyer-Peppas model to study drug release from liposomes. Eur. J. Pharm. Sci. 2019, 138, 105026. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds SiO2-EDA, SiO2-TETA, SiO2-TREN and SiO2-TRI-OXA are available from the authors. |


| Biomolecule | Adsorbent | Langmuir Isotherm | Freundlich Isotherm | |||||
|---|---|---|---|---|---|---|---|---|
| qm (mg g−1) | RL (-) | R2 | χ2 | 1/n (−) | R2 | χ2 | ||
| Folic Acid | SiO2-epoxy | 12.46 ± 0.96 | 0.9966 | 0.9654 | 1.000 | 0.80 ± 0.03 | 0.9907 | 0.054 |
| SiO2-EDA | 274.72 ± 6.87 | 0.9987 | 0.9963 | 0.003 | 0.93 ± 0.02 | 0.9979 | 0.017 | |
| SiO2-TETA | 230.95 ± 18.18 | 0.9882 | 0.9977 | 0.025 | 0.96 ± 0.01 | 0.9994 | 0.029 | |
| SiO2-TREN | 236.97 ± 11.79 | 0.9984 | 0.9853 | 0.008 | 0.91 ± 0.02 | 0.9978 | 0.012 | |
| SiO2-TRI-OXA | 202.43 ± 4.34 | 0.9972 | 0.9972 | 0.727 | 0.93 ± 0.02 | 0.9982 | 0.010 | |
| Salicylic Acid | SiO2-epoxy | 1.50 ± 0.09 | 0.9549 | 0.9827 | 8.739 | 0.50 ± 0.02 | 0.9887 | 0.072 |
| SiO2-EDA | 157.48 ± 9.67 | 0.9956 | 0.9777 | 0.006 | 0.93 ± 0.02 | 0.9973 | 0.013 | |
| SiO2-TETA | 15.44 ± 1.17 | 0.9803 | 0.9667 | 0.056 | 0.70 ± 0.03 | 0.9858 | 0.046 | |
| SiO2-TREN | 42.92 ± 2.10 | 0.8741 | 0.9848 | 0.222 | 0.46 ± 0.03 | 0.9855 | 0.037 | |
| SiO2-TRI-OXA | 29.53 ± 1.69 | 0.9902 | 0.9807 | 0.104 | 0.84 ± 0.03 | 0.9914 | 0.028 | |
| Nicotinic Acid | SiO2-epoxy | 1.33 ± 0.10 | 0.9610 | 0.9641 | 8.190 | 0.54 ± 0.02 | 0.9871 | 0.030 |
| SiO2-EDA | 20.11 ± 2.25 | 0.8758 | 0.9408 | 0.673 | 0.64 ± 0.03 | 0.9907 | 0.073 | |
| SiO2-TETA | 4.86 ± 0.25 | 0.8499 | 0.9870 | 1.245 | 0.54 ± 0.03 | 0.9866 | 0.052 | |
| SiO2-TREN | 17.74 ± 1.08 | 0.7724 | 0.9816 | 0.353 | 0.42 ± 0.08 | 0.9888 | 0.032 | |
| SiO2-TRI-OXA | 7.18 ± 0.46 | 0.9508 | 0.9803 | 1.002 | 0.60 ± 0.03 | 0.9857 | 0.072 | |
| Biomolecule | Adsorbent | ΔH° (kJ mol−1) | ΔS° (J mol−1 K−1) | R2 | χ2·103 | ΔG° (kJ mol−1) | ||
|---|---|---|---|---|---|---|---|---|
| 301 K | 313 K | 328 K | ||||||
| Folic Acid | SiO2-EDA | 1.41 ± 0.12 | 43.8 ± 0.4 | 0.9855 | 0.002 | −11.78 | −12.32 | −12.96 |
| SiO2-TETA | 2.29 ± 0.02 | 51.8 ± 0.1 | 0.9998 | 0.001 | −13.31 | −13.93 | −14.71 | |
| SiO2-TREN | 1.43 ± 0.09 | 44.2 ± 0.3 | 0.9924 | 0.001 | −11.86 | −12.39 | −13.06 | |
| SiO2-TRI-OXA | 4.64 ± 0.28 | 53.3 ± 0.9 | 0.9929 | 0.007 | −11.41 | −12.03 | −12.85 | |
| Salicylic Acid | SiO2-EDA | 4.40 ± 0.13 | 47.3 ± 0.4 | 0.9982 | 0.641 | −9.84 | −10.42 | −11.12 |
| SiO2-TETA | 42.94 ± 3.02 | 147.6 ± 9.6 | 0.9901 | 4.545 | −1.58 | −3.12 | −5.56 | |
| SiO2-TREN | 6.35 ± 0.21 | 53.7 ± 0.7 | 0.9979 | 0.649 | −9.81 | −10.43 | −11.25 | |
| SiO2-TRI-OXA | 13.59 ± 0.76 | 60.0 ± 2.4 | 0.9938 | 0.157 | −4.44 | −5.22 | −6.07 | |
| Nicotinic Acid | SiO2-EDA | 4.38 ± 0.25 | 38.1 ± 0.8 | 0.9936 | 0.011 | −7.10 | −7.57 | −8.13 |
| SiO2-TETA | 9.42 ± 0.36 | 49.8 ± 1.1 | 0.9972 | 0.029 | −5.59 | −6.16 | −6.94 | |
| SiO2-TREN | 6.26 ± 0.49 | 43.2 ± 1.6 | 0.9879 | 0.046 | −6.72 | −7.28 | −7.89 | |
| SiO2-TRI-OXA | 10.45 ± 0.74 | 44.7 ± 2.4 | 0.9899 | 0.221 | −3.04 | −3.52 | −4.25 | |
| Biomolecule | Adsorbent | First-Order Model | Higuchi Model | Korsemeyer-Peppas Model | ||||
|---|---|---|---|---|---|---|---|---|
| k1 (% h−1) | R2 (χ2) | kH (% h−1/2) | R2 (χ2) | n | kK-P (% h−1) | R2 (χ2) | ||
| Folic Acid | SiO2-EDA | 0.006 ± 0.002 | 0.5519 (0.039) | 6.5 ± 1.8 | 0.7205 (10.340) | 0.34 | 17.1 ± 1.8 | 0.8311 (0.064) |
| SiO2-TETA | 0.011 ± 0.004 | 0.5748 (0.116) | 8.8 ± 2.4 | 0.7224 (10.763) | 0.26 | 31.1 ± 2.1 | 0.8743 (0.020) | |
| SiO2-TREN | 0.008 ± 0.003 | 0.5883 (0.058) | 7.3 ± 1.9 | 0.7399 (9.740) | 0.31 | 21.8 ± 2.0 | 0.8476 (0.041) | |
| SiO2-TRI-OXA | 0.008 ± 0.003 | 0.6061 (0.061) | 7.7 ± 1.9 | 0.7602 (10.207) | 0.34 | 19.6 ± 1.9 | 0.8594 (0.048) | |
| Salicylic Acid | SiO2-EDA | 0.103 ± 0.030 | 0.7071 (7.928) | 5.6 ± 2.3 | 0.5520 (3.596) | 0.07 | 86.9 ± 2.0 | 0.8118 (0.002) |
| SiO2-TETA | 0.006 ± 0.003 | 0.5238 (0.038) | 5.8 ± 1.7 | 0.6941 (6.777) | 0.22 | 25.4 ± 1.5 | 0.8735 (0.015) | |
| SiO2-TREN | 0.016 ± 0.008 | 0.4629 (0.319) | 4.5 ± 1.8 | 0.5522 (2.629) | 0.06 | 76.4 ± 1.6 | 0.8024 (0.002) | |
| SiO2-TRI-OXA | 0.006 ± 0.003 | 0.4110 (0.036) | 5.3 ± 2.0 | 0.5822 (7.903) | 0.18 | 30.2 ± 1.9 | 0.7974 (0.018) | |
| Nicotinic Acid | SiO2-EDA | 0.029 ± 0.007 | 0.7707 (0.781) | 7.6 ± 1.7 | 0.8062 (2.315) | 0.10 | 70.5 ± 1.0 | 0.9613 (0.001) |
| SiO2-TETA | 0.007 ± 0.002 | 0.6211 (0.041) | 6.5 ± 1.5 | 0.7847 (6.462) | 0.29 | 19.1 ± 1.2 | 0.9061 (0.021) | |
| SiO2-TREN | 0.028 ± 0.008 | 0.7378 (0.820) | 5.2 ± 1.3 | 0.7596 (1.318) | 0.06 | 79.8 ± 0.8 | 0.9443 (0.001) | |
| SiO2-TRI-OXA | 0.020 ± 0.009 | 0.5047 (0.418) | 12.7 ± 4.5 | 0.6164 (27.616) | 0.31 | 40.1 ± 4.4 | 0.7941 (0.049) | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawlaczyk, M.; Schroeder, G. Dendrimer-Functionalized Hybrid Materials Based on Silica as Novel Carriers of Bioactive Acids. Molecules 2020, 25, 2660. https://doi.org/10.3390/molecules25112660
Pawlaczyk M, Schroeder G. Dendrimer-Functionalized Hybrid Materials Based on Silica as Novel Carriers of Bioactive Acids. Molecules. 2020; 25(11):2660. https://doi.org/10.3390/molecules25112660
Chicago/Turabian StylePawlaczyk, Mateusz, and Grzegorz Schroeder. 2020. "Dendrimer-Functionalized Hybrid Materials Based on Silica as Novel Carriers of Bioactive Acids" Molecules 25, no. 11: 2660. https://doi.org/10.3390/molecules25112660
APA StylePawlaczyk, M., & Schroeder, G. (2020). Dendrimer-Functionalized Hybrid Materials Based on Silica as Novel Carriers of Bioactive Acids. Molecules, 25(11), 2660. https://doi.org/10.3390/molecules25112660