Extraction of Phenolic Compounds from Tabernaemontana catharinensis Leaves and Their Effect on Oxidative Stress Markers in Diabetic Rats
Abstract
:1. Introduction
2. Results and Discussion
2.1. Total Phenolic Compounds and Antioxidant Activity in Vitro
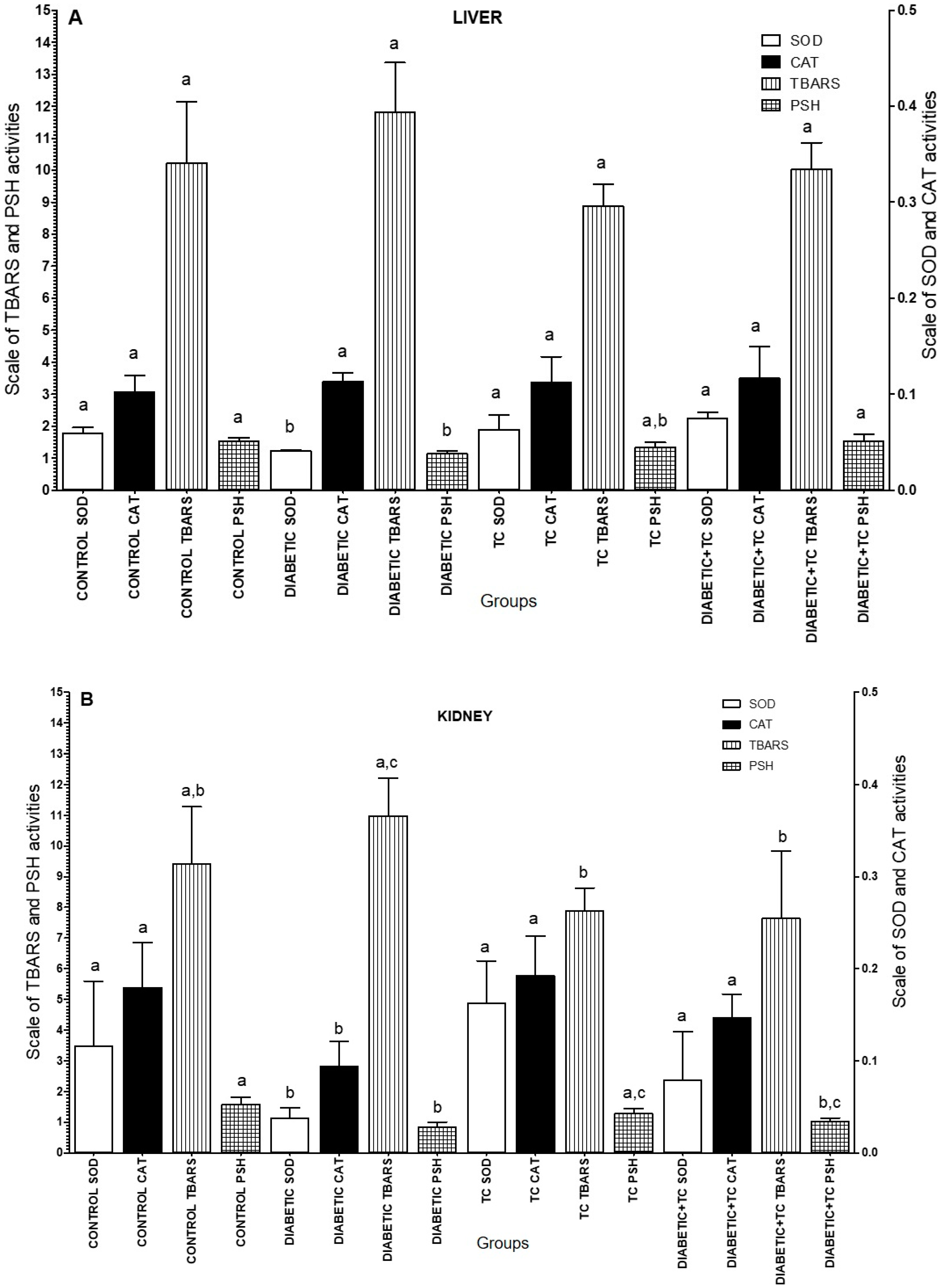
2.2. Biochemical Markers
3. Materials and Methods
3.1. Chemicals
3.2. Samples
3.3. Preparation of Extract and Experimental Design
3.4. HPLC-DAD Profile of Phenolic Compounds
3.5. Total Phenolic Compounds (TPC) and Antioxidant Activities in Vitro
3.5.1. Total Phenolic Compounds (TPC)
3.5.2. DPPH (2,2-Diphenyl-1-picryl-hydrazyl) Radical Scavenging Assay
3.5.3. Ferric Reducing Antioxidant Power (FRAP) Assay
3.5.4. ABTS (2,2-azino-bis-(3-ethylbenzothiazoline-6-sulphonic acid) Assay
3.5.5. Coupled Oxidation of β-carotene and Linoleic Acid Assay
3.6. Animals
3.7. Preparation of Extracts for Animals Diet
3.8. Experimental Design Animals and Induction of Diabetes
3.9. Biochemical Analysis
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Soares, D.C.; Pereira, C.G.; Meireles, M.A.A.; Saraiva, E.M. Leishmanicidal activity of a supercritical fluid fraction obtained from Tabernaemontana catharinensis. Parasitol. Int. 2007, 56, 35–139. [Google Scholar] [CrossRef] [PubMed]
- Raj, P.; Louis, X.L.; Thandapilly, S.J.; Movahed, A.; Zieroth, S.; Netticadan, T. Potential of resveratrol in the treatment of heart failure. Life Sci. 2014, 95, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Selmi, S.; Rtibi, K.; Grami, D.; Sebai, H.; Marzouki, L. Rosemary (Rosmarinus officinalis) essential oil components exhibit anti-hyperglycemic, anti-hyperlipidemic and antioxidant effects in experimental diabetes. Pathophysiology 2017, 24, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xi-Qiang, L.; Chun-Ping, T.; Sheng, Y. Natural products chemistry research 2010′s progress in China. Chin. J. Nat. Med. 2012, 10, 0001–0012. [Google Scholar]
- Guo-Xun, Y.; Guang-Lei, M.A.; Hao, L.I.; Ting, H.; Juan, X.; Jin-Feng, H. Advanced natural products chemistry research in China between 2015 and 2017. Chin. J. Nat. Med. 2018, 16, 0881–0906. [Google Scholar]
- Xu, C.-C.; Wang, B.; Pu, Y.-Q.; Tao, J.-S.; Zhang, T. Advances in extraction and analysis of phenolic compounds from plant materials. Chin. J. Nat. Med. 2017, 15, 0721–0731. [Google Scholar]
- Almeida, J.F.; dos Reis, A.S.; Heldt, L.F.S.; Pereira, D.; Bianchin, M.; de Moura, C.; Plata-Oviedo, M.V.; Haminiuk, C.W.I.; Ribeiro, I.S.; da Luz, C.F.P.; et al. Lyophilized bee pollen extract: A natural antioxidant source to prevent lipid oxidation in refrigerated sausages. LWT-Food Sci. Technol. 2017, 76, 299–305. [Google Scholar] [CrossRef]
- Reis, A.S.; Diedrich, C.; Moura, C.; Pereira, D.; Almeida, J.F.; Silva, L.D.; Plata-Oviedo, M.S.V.; Tavares, R.A.W.; Carpes, S.T. Physico-chemical characteristics of microencapsulated propolis by-product extract and its effect on storage stability of burger meat during storage at −15 °C. LWT-Food Sci. Technol. 2017, 76, 306–313. [Google Scholar] [CrossRef]
- Anjos, O.; Fernandes, R.; Cardoso, S.M.; Delgado, T.; Farinha, N.; Paula, V.; Estevinho, L.M.; Carpes, S.T. Bee pollen as a natural antioxidant source to prevent lipid oxidation in black pudding. LWT-Food Sci. Technol. 2019, 111, 869–875. [Google Scholar] [CrossRef] [Green Version]
- Boligon, A.A.; Piana, M.; Kubiça, T.F.; Mario, D.N.; Dalmolin, T.V.; Bonez, P.C.; Weiblen, R.; Lovato, L.; Alves, S.H.; Campos, M.M.A.; et al. HPLC analysis and antimicrobial, antimycobacterial and antiviral activities of Tabernaemontana catharinensis A. DC. J. Appl. Biomed. 2014, 13, 7–18. [Google Scholar] [CrossRef]
- Brum, E.S.; Moreira, L.R.; da Silva, A.R.H.; Boligon, A.A.; Carvalho, F.B.; Athayde, M.L.; Brandão, R.; Oliveira, S.M. Tabernaemontana catharinensis ethyl acetate fraction presents antinociceptive activity without causing toxicological effects in mice. J. Ethnopharm. 2016, 191, 115–124. [Google Scholar] [CrossRef]
- Vetere, A.; Choudhary, A.; Burns, S.M.; Wagner, B.K. Targeting the pancreatic β-cell to treat diabetes. Nat. Rev. Drug Discov. 2014, 13, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Latifi, E.; Mohammadpour, A.A.; Fathi, B.H.; Nourani, H. Antidiabetic and antihyperlipidemic effects of ethanolic Ferula assa-foetida oleo-gum-resin extract in streptozotocin-induced diabetic wistar rats. Biomed. Pharmacother. 2019, 110, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Goboza, M.; Aboua, Y.G.; Chegou, N.; Oguntibeju, O.O. Vindoline effectively ameliorated diabetes-induced hepatotoxicity by docking oxidative stress, inflammation and hypertriglyceridemia in type 2 diabetes-induced male Wistar rats. Biomed. Pharmacother. 2019, 112, 108638. [Google Scholar] [CrossRef] [PubMed]
- Chandran, R.; George, B.P.; Abrahamse, H.; Parimelazhagan, T. Therapeutic effects of Syzygium mundagam bark methanol extract on type-2 diabetic complications in rats. Biomed. Pharmacother. 2017, 95, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Silveira, D.; de Melo, A.M.M.F.; Magalhães, P.O.; Fonseca-Bazzo, Y.M. Tabernaemontana species: Promising sources of new useful drugs. Stud. Nat. Prod. Chem. 2017, 54, 227–289. [Google Scholar]
- Pereira, C.G.; Leal, P.F.; Sato, D.N.; Meireles, M.A.A. Antioxidant and antimycobacterial activities of Tabernaemontana catharinensis extracts obtained by supercritical CO2 + cosolvent. J. Med. Food 2005, 8, 533–538. [Google Scholar] [CrossRef]
- Marinho, F.F.; Simões, A.O.S.; Barcellos, T.; Moura, S. Brazilian Tabernaemontana genus: Indole alkaloids and phytochemical activities. Fitoterapia 2016, 114, 127–137. [Google Scholar] [CrossRef]
- Jain, S.; Sharma, P.; Ghule, S.; Jain, A.; Jain, N. In vivo anti-inflammatory activity of Tabernaemontana divaricata leaf extract on male albino mice. Chin. J. Nat. Med. 2013, 11, 0472–0476. [Google Scholar] [CrossRef]
- Gomes, R.C.; Neto, A.C.; Melo, V.L.; Fernandes, V.C.; Dagrava, G.; Santos, W.S.; Pereira, O.S.; Couto, L.B.; Beleboni, R.O. Antinociceptive and anti-inflammatory activities of Tabernaemontanana catharinensis. Pharm. Biol. 2009, 47, 372–376. [Google Scholar] [CrossRef] [Green Version]
- Nicola, C.; Salvador, M.; Gower, A.E.; Moura, S.; Echeverrigaray, S. Chemical constituents antioxidant and anticholinesterasic activity of Tabernaemontana catharinensis. Sci. World J. 2013, 13, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Boligon, A.A.; Freitas, R.B.; Brum, T.F.; Piana, M.; Belke, B.V.; Rocha, J.B.T.; Athayde, M.L. Phytochemical constituents and in vitro antioxidant capacity of Tabernaemontana catharinensis A. DC. Free Radic. Antioxidant 2013, 3, 77–80. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, D.M.; Araújo, J.H.B.; Francisco, M.S.; Coelho, M.A.; Franco, J.M. Evaluation of in vitro antimicrobial activity of Tabernaemontana catharinensis A. DC extract. Rev. Bras. Plantas Med. 2011, 13, 197–202. [Google Scholar] [CrossRef]
- Casagrande, M.; Zanela, J.; Pereira, D.; Lima, V.A.; Oldoni, T.L.C.; Carpes, S.T. Optimization of the extraction of antioxidant phenolic compounds from grape pomace using response surface methodology. J. Food Meas. Charact. 2018, 13, 1120–1129. [Google Scholar] [CrossRef]
- De Moura, C.; Reis, A.S.; Silva, L.D.; Lima, V.A.; Oldoni, T.L.C.; Pereira, C.; Carpes, S.T. Optimization of phenolic compounds extraction with antioxidant activity from açaí, blueberry and goji berry using response surface methodology. Emir. J. Food Agric. 2018, 30, 180–189. [Google Scholar]
- Vedana, M.I.S.; Ziemer, C.; Miguel, O.; Portella, A.C.; Candido, L.M.B. Efeito do processamento na atividade antioxidante de uva. Alim. Nutr. 2008, 19, 159–165. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [CrossRef] [Green Version]
- Bitencourt, R.G.; Cabral, F.A.; Meirelles, A.J.A. Ferulic acid solubility in supercritical carbon dioxide, ethanol and water mixtures. J. Chem. Thermodyn. 2016, 103, 285–291. [Google Scholar] [CrossRef]
- Chanioti, S.; Tzia, C. Extraction of phenolic compounds from olive pomace by using natural deep eutectic solvents and innovative extraction techniques. Innov. Food Sci. Emerg. Technol. 2018, 48, 228–239. [Google Scholar] [CrossRef]
- Stefanello, N.; Schmatz, R.; Pereira, L.B.; Rubin, M.A.; da Rocha, J.B.; Facco, G.; Pereira, M.E.; Mazzanti, C.M.; Passamonti, S.; Rodrigues, M.V.; et al. Effects of chlorogenic acid, caffeine, and coffee on behavioral and biochemical parameters of diabetic rats. Mol. Cell. Biochem. 2014, 388, 277–286. [Google Scholar] [CrossRef]
- Rekha, N.; Balaji, R.; Deecaraman, M. Effect of aqueous extract of Syzygium cumini pulp on antioxidant defense system in streptozotocin induced diabetic rats. Iran. J. Pharm.Ther. 2008, 7, 135–145. [Google Scholar]
- de Moura Barbosa, H.; Amaral, D.; do Nascimento, J.N.; Machado, D.C.; de Sousa Araújo, T.A.; de Albuquerque, U.P.; da Silva Almeida, G., Jr.; Rolim, L.A.; Lopes, N.P.; Gomes, D.A.; et al. Spondias tuberosa inner bark extract exert antidiabetic effects in streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2018, 227, 248–257. [Google Scholar] [CrossRef]
- Sagbo, I.J.; van de Venter, M.; Koekemoer, T.; Bradley, G. In vitro antidiabetic activity and mechanism of action of Brachylaena elliptica (Thunb.) DC. Evid. Based Complement. Alternat. Med. 2018, 2018, 4170372. [Google Scholar]
- Collier, A.; Wilson, R.; Bradley, H.; Thomson, J.A.; Small, M. Free Radical Activity in Type 2 Diabetes. Diabet. Med. 1990, 7, 27–30. [Google Scholar] [CrossRef]
- Gulpamuk, B.; Tekin, K.; Sonmez, K.; Inanc, M.; Neselioglu, S.; Erel, O.; Yilmazbas, P. The significance of thiol/disulfide homeostasis and ischemia-modified albumin levels to assess the oxidative stress in patients with different stages of diabetes mellitus. Scand. J. Clin. Lab. Investig. 2018, 78, 136–142. [Google Scholar] [CrossRef]
- Seyyedebrahimi, S.; Khodabandehloo, H.; Nasli Esfahani, E.; Meshkani, R. The effects of resveratrol on markers of oxidative stress in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled clinical trial. Acta Diabetol. 2018, 55, 341–353. [Google Scholar] [CrossRef]
- Sharifzadeh, M.; Ranjbar, A.; Hosseini, A.; Khanavi, M. The effect of green tea extract on oxidative stress and spatial learning in Streptozotocin-diabetic rats. Iran J Pharm Res. 2017, 16, 201–209. [Google Scholar]
- Bacanli, M.; Anlar, H.G.; Aydın, S.; Cal, T.; Ari, N.; Ündeğer Bucurgat, Ü.; Başaran, A.A.; Başaran, N. d-limonene ameliorates diabetes and its complications in streptozotocin-induced diabetic rats. Food Chem. Toxicol. 2017, 110, 434–442. [Google Scholar] [CrossRef]
- Hassan, N.S.; Emam, M.A. Protective effect of camel milk and Ginkgo biloba extract against alloxan-induced diabetes in rats. J. Diabetes Metab. 2012, 3, 231–238. [Google Scholar] [CrossRef] [Green Version]
- Han, X.X.; Jiang, Y.P.; Liu, N.; Wu, J.; Yang, J.M.; Li, Y.X.; Sun, M.; Sun, T.; Zheng, P.; Jian-Qiang, Y. Protective effects of Astragalin on spermatogenesis in streptozotocin- induced diabetes in male mice by improving antioxidant activity and inhibiting inflammation. Biomed. Pharmacother. 2019, 110, 561–570. [Google Scholar] [CrossRef]
- Hao, H.; Shao, Z.; Tang, D.; Lu, Q.; Chen, X.; Yin, X.; Wu, J.; Chen, H. Preventive effects of rutin on the development of experimental diabetic nephropathy in rats. Life Sci. 2012, 91, 959–967. [Google Scholar] [CrossRef]
- Son, H.J.; Han, S.H.; Lee, J.A.; Lee, C.S.; Seo, J.W.; Dae Yoon Chi, D.Y.; Hwang, O. 2-Acetyl-7-hydroxy-6-methoxy-1-methyl-1,2,3,4,-tetrahydroisoquinoline exhibits anti-inflammatory properties and protects the nigral dopaminergic neurons. Eur. J. Pharmacol. 2016, 771, 152–161. [Google Scholar] [CrossRef]
- Zabad, O.M.; Samra, Y.A.; Eissa, L.A. P-coumaric acid alleviates experimental diabetic nephropatty through modulation of Toll like receptor-4 in rats. Life Sci. 2019, 238, 116965. [Google Scholar] [CrossRef]
- Fujita, A.; Sasaki, H.; Doi, A.; Okamoto, K.; Matsuno, S.; Furuta, H.; Nishi, M.; Nakao, T.; Tsuno, T.; Taniguchi, H.; et al. Ferulic acid prevents pathological and functional abnormalities of the kidney in Otsuka Long-Evans Tokushima fatty diabetic rats. Diabetes Res. Clin. Pr. 2008, 79, 11–17. [Google Scholar] [CrossRef]
- Roslan, J.; Giribabu, N.; Karim, K.; Salleh, N. Quercetin ameliorates oxidative stress, inflammation and apoptosis in the heart of streptozotocin-nicotinamide-induced adult male diabetic rats. Biomed. Pharmacother. 2017, 86, 570–582. [Google Scholar] [CrossRef]
- Borriello, M.; Iannuzzi, C.; Sirangelo, I. Pinocembrin protects from AGE-Induced cytotoxicity and Inhibits non-enzymatic glycation in human insulin. Cells 2019, 8, 385. [Google Scholar] [CrossRef] [Green Version]
- Man, S.; Ma, J.; Wang, C.; Li, Y.; Gao, W.; Lu, F. Chemical composition and hypoglycaemic effect of polyphenol extracts from Litchi chinensis seeds. J. Funct. Foods 2016, 22, 313–324. [Google Scholar] [CrossRef]
- Oldoni, T.L.C.; Oliveira, S.C.; Andolfato, S.; Karling, M.; Calegari, M.A.; Sado, R.Y.; Maia, F.M.; Alencar, S.M.; Lima, V.A. Chemical characterization and optimization of the extraction process of bioactive compounds from propolis produced by selected bees. J. Braz. Chem. Soc. 2015, 26, 2054–2062. [Google Scholar]
- Pauleti, N.N.; Mello, J.; Siebert, D.A.; Micke, G.A.; de Albuquerque, C.A.C.; Alberton, M.D.; Barauna, S.C. Characterisation of phenolic compounds of the ethyl acetate fraction from Tabernaemontana catharinensis and its potential antidepressant-like effect. Nat. Prod. Res. 2018, 32, 1987–1990. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Method Enzymol. 1999, 299, 152–179. [Google Scholar]
- Re, R.; Pellegrini, N.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Ahn, M.; Kumazawa, S.; Hamasaka, T.; Bang, K.; Nakayama, T. Antioxidant activity and constituents of propolis collected in various areas of Korea. J. Agric. Food Chem. 2004, 52, 7286–7292. [Google Scholar] [CrossRef]
- Maritim, A.C.; Sanders, R.A.; Watkins, J.B. Diabetes, oxidative stress, and antioxidants: A review. J. Biochem. Mol. Toxicol. 2003, 17, 24–38. [Google Scholar] [CrossRef]
- Aebi, H.; Chiu, W.M.R. Role of catalase on antioxidative defenses. J. Struct. Biol. 1995, 2, 117–118. [Google Scholar]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem.Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Jentzsch, A.M.; Bachmann, H.; Fürst, P.; Biesalski, H.K. Improved analysis of malondialdehyde in human body fluids. Free Rad. Biol. Med. 1996, 20, 251–256. [Google Scholar] [CrossRef]
Sample Availability: Samples of the extract are available from the authors. |
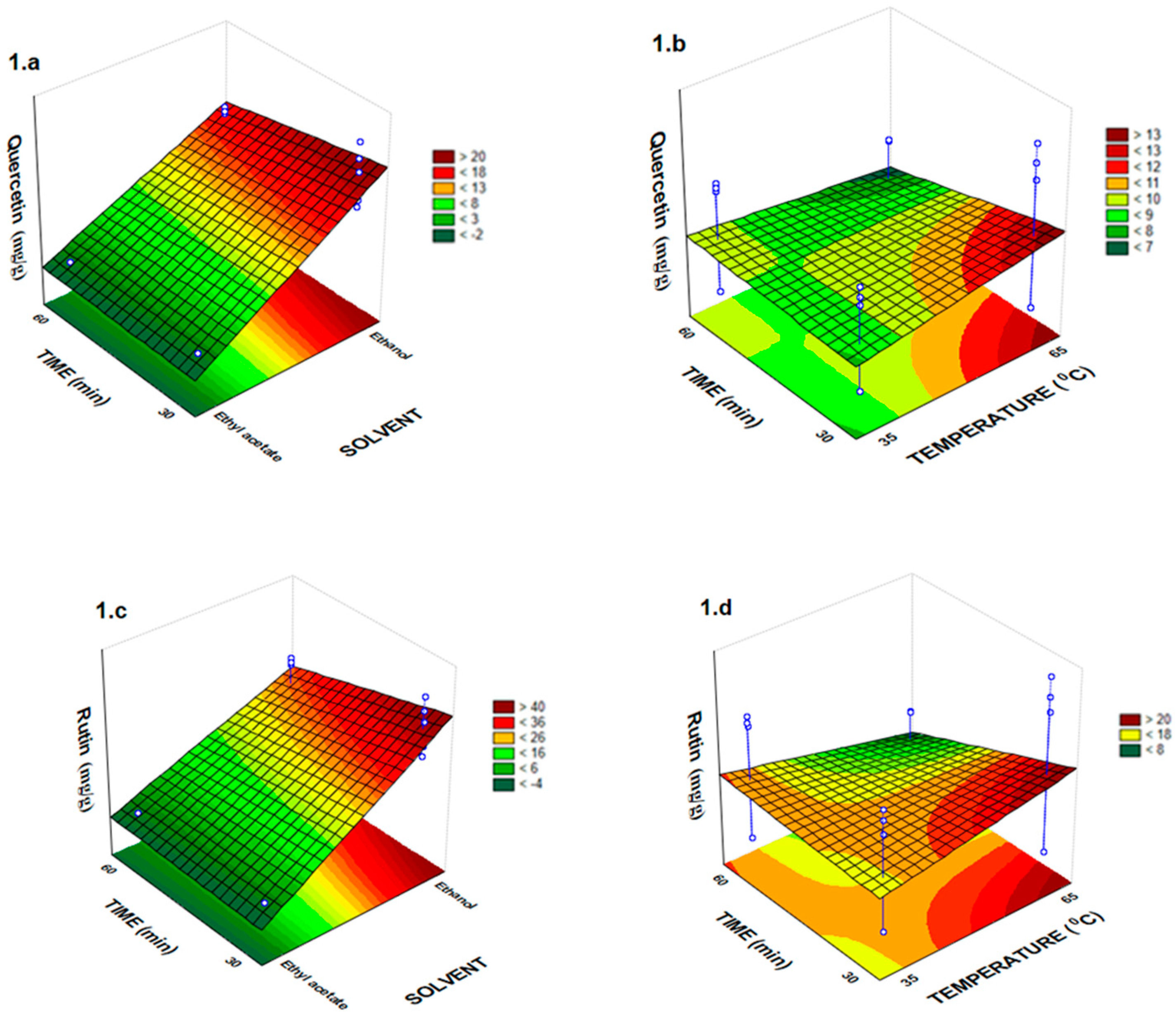
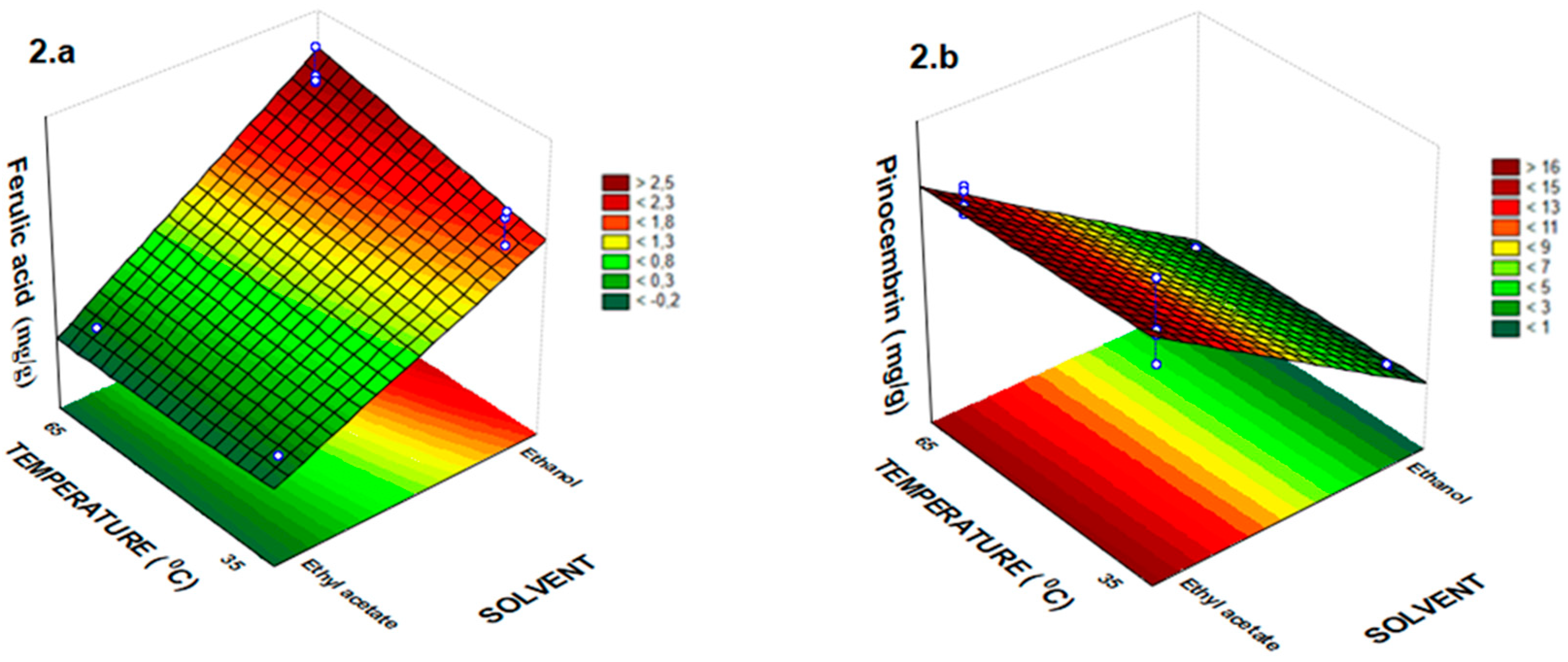
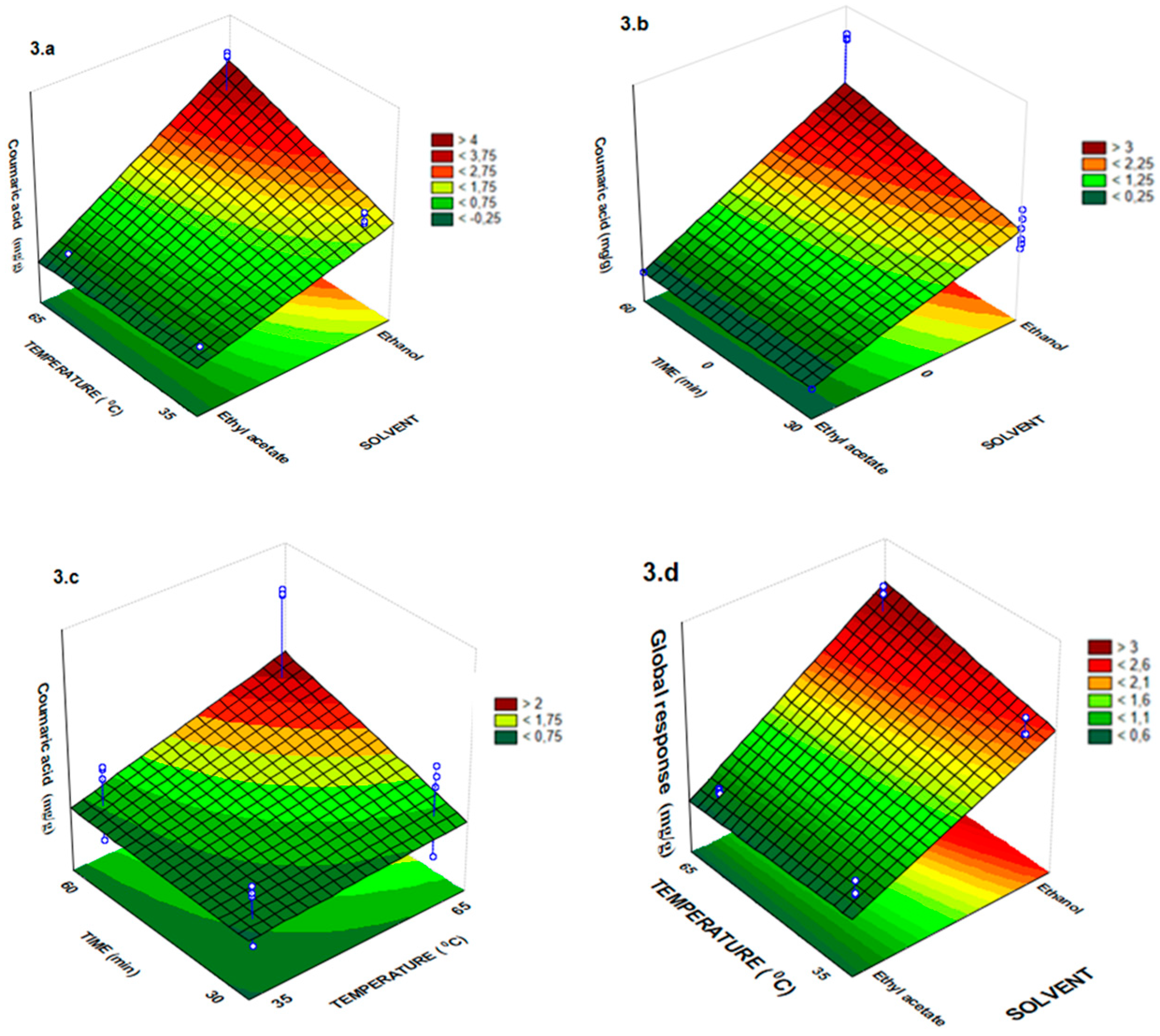
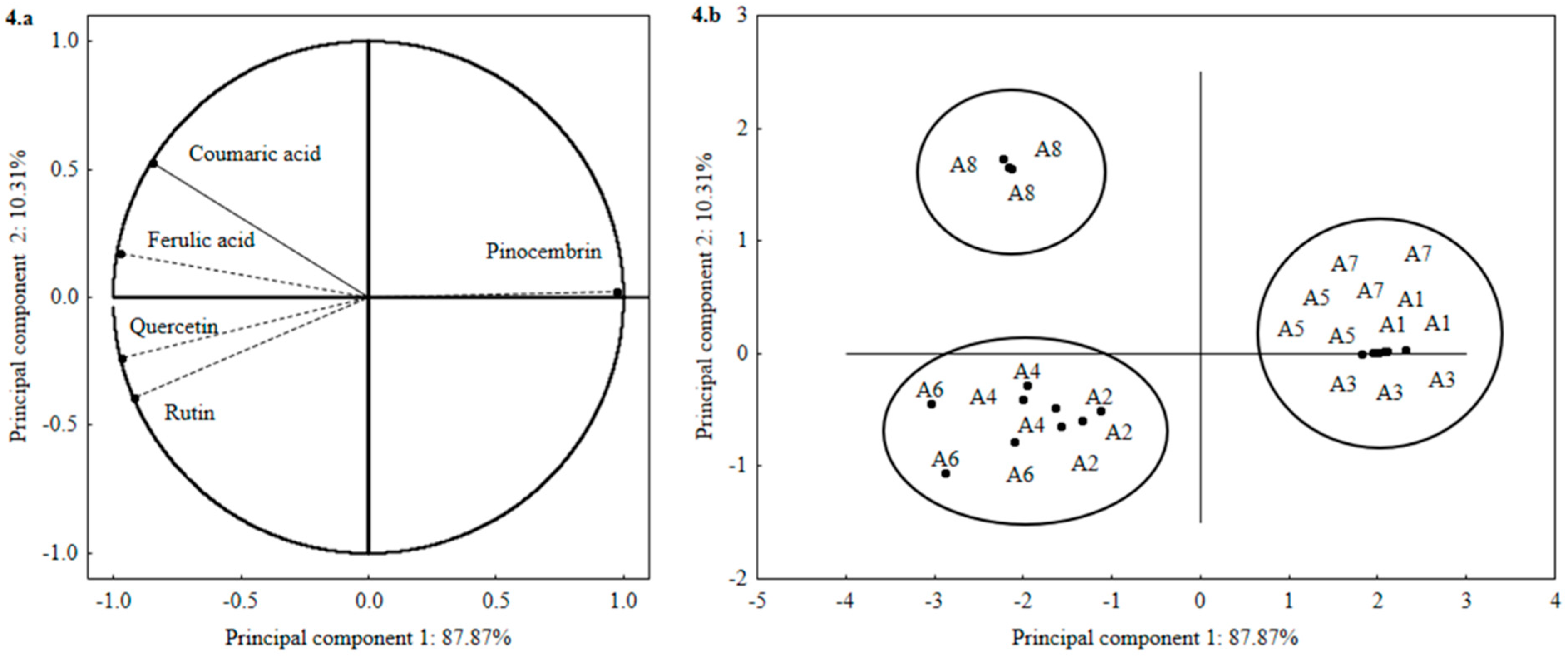
| Assay | Solvent (X1) | Time (min) X2 | Temperature (°C) X3 | Quercetin | Rutin | Ferulic Acid | Coumaric Acid | Pinocembrin |
|---|---|---|---|---|---|---|---|---|
| A1 | Ethyl Acetate (−1) | 30 (−1) | 35 (−1) | <LD | <LD | <LD | <LD | 0.15 a ± 0.01 |
| A2 | Ethanol (+1) | 30 (−1) | 35 (−1) | 0.17 c ± 0.01 | 0.35 c ± 0.04 | 0.02 b ± 0.00 | 0.01c ± 0.00 | 0.02 b ± 0.00 |
| A3 | Ethyl Acetate (−1) | 60 (+1) | 35 (−1) | <LD | <LD | <LD | <LD | 0.15 a ± 0.01 |
| A4 | Ethanol (+1) | 60 (+1) | 35 (−1) | 0.19 b ± 0.01 | 0.38 b ± 0.01 | 0.02 b ± 0.00 | 0.02 b,c ± 0.00 | 0.02 b ± 0.00 |
| A5 | Ethyl Acetate (−1) | 30 (−1) | 65 (+1) | <LD | <LD | <LD | <LD | 0.16 a ± 0.01 |
| A6 | Ethanol (+1) | 30 (−1) | 65 (+1) | 0.26 a ± 0.03 | 0.51 a ± 0.04 | 0.02 b ± 0.01 | 0.02 b ± 0.00 | 0.02 b ± 0.00 |
| A7 | Ethyl Acetate (−1) | 60 (+1) | 65 (+1) | <LD | <LD | <LD | <LD | 0.14 a ± 0.01 |
| A8 | Ethanol (+1) | 60 (+1) | 65 (+1) | 0.25 a ± 0.00 | 0.55 a ± 0.00 | 0.09 a± 0.00 | 0.05 a ± 0.00 | 0.04 b ± 0.00 |
| Source | Quercetin | Rutin | Ferulic Acid | Coumaric Acid | Pinocembrin | Global Response |
|---|---|---|---|---|---|---|
| β0 | 9.38 | 17.98 | 1.07 | 1.37 | 8.31 | 1.66 |
| β1 | 9.38 | 17.98 | 1.07 | 1.37 | −6.74 | 0.89 |
| β2 | 0.00 | 0.00 | 0.19 | 0.47 | 0.00 | 0.15 |
| β3 | −1.21 | −3.68 | 0.00 | 0.39 | 0.00 | 0.00 |
| β12 | 0.00 | 0.00 | 0.19 | 0.47 | 0.00 | 0.16 |
| β13 | −1.21 | −3.68 | 0.00 | 0.39 | 0.00 | 0.00 |
| β23 | −1.70 | −4.39 | 0.00 | 0.27 | 0.00 | −0.10 |
| R2 | 0.96 | 0.94 | 0.95 | 0.97 | 0.97 | 0.96 |
| Main effects | ||||||
| Solvent (X1) | *** | *** | *** | *** | *** | *** |
| Temperature (X2) | ns | ns | ** | *** | ns | ** |
| Time (X3) | * | ** | ns | *** | ns | ns |
| Fvalue | 261.26 | 342.78 | 149.12 | 492.79 | 757.56 | 136.63 |
| Fstatistic table | 2.70 | 2.77 | 3.10 | 2.66 | 4.30 | 2.77 |
| Fratio | 96.76 | 123.75 | 48.10 | 185.26 | 176.18 | 49.32 |
| Lack of fit (p-value) | 0.00 | 0.00 | 0.42 | 0.00 | 0.64 | 0.03 |
| Analysis | Results |
|---|---|
| TPC DPPH FRAP ABTS β-Carotene/linolenic acid | 23.34 mg EAG/g 34.26 µmol Trolox 24.13 µmol Fe2+ 12.57 µmol Trolox 78.85 (%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sari, R.; Conterno, P.; da Silva, L.D.; de Lima, V.A.; Oldoni, T.L.C.; Thomé, G.R.; Carpes, S.T. Extraction of Phenolic Compounds from Tabernaemontana catharinensis Leaves and Their Effect on Oxidative Stress Markers in Diabetic Rats. Molecules 2020, 25, 2391. https://doi.org/10.3390/molecules25102391
Sari R, Conterno P, da Silva LD, de Lima VA, Oldoni TLC, Thomé GR, Carpes ST. Extraction of Phenolic Compounds from Tabernaemontana catharinensis Leaves and Their Effect on Oxidative Stress Markers in Diabetic Rats. Molecules. 2020; 25(10):2391. https://doi.org/10.3390/molecules25102391
Chicago/Turabian StyleSari, Rafael, Paula Conterno, Leticia Dangui da Silva, Vanderlei Aparecido de Lima, Tatiane Luiza Cadorin Oldoni, Gustavo Roberto Thomé, and Solange Teresinha Carpes. 2020. "Extraction of Phenolic Compounds from Tabernaemontana catharinensis Leaves and Their Effect on Oxidative Stress Markers in Diabetic Rats" Molecules 25, no. 10: 2391. https://doi.org/10.3390/molecules25102391







