Comparing the Thermal and Electrochemical Stabilities of Two Structurally Similar Ionic Liquids
Abstract
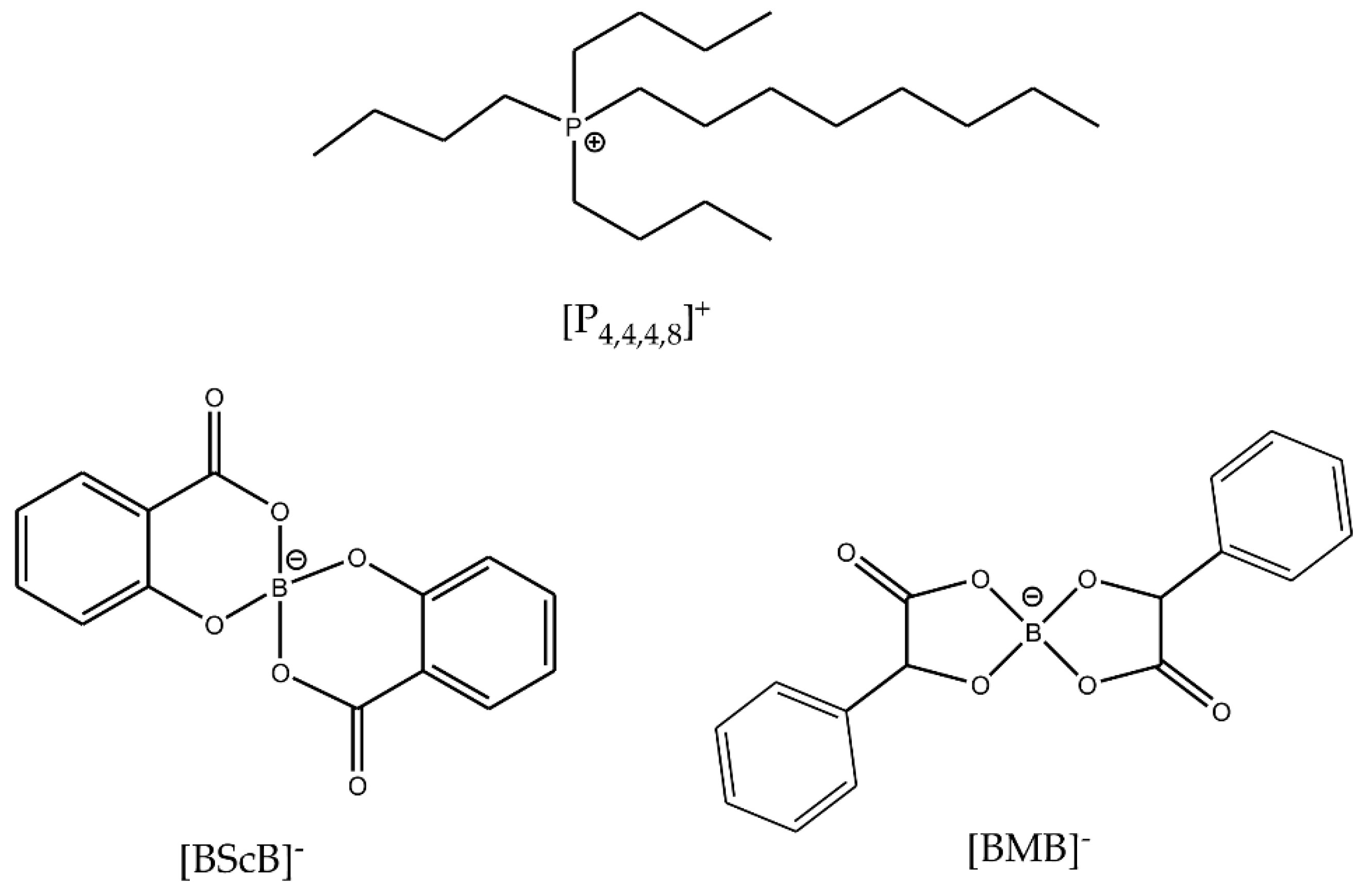
1. Introduction
2. Results and Discussion
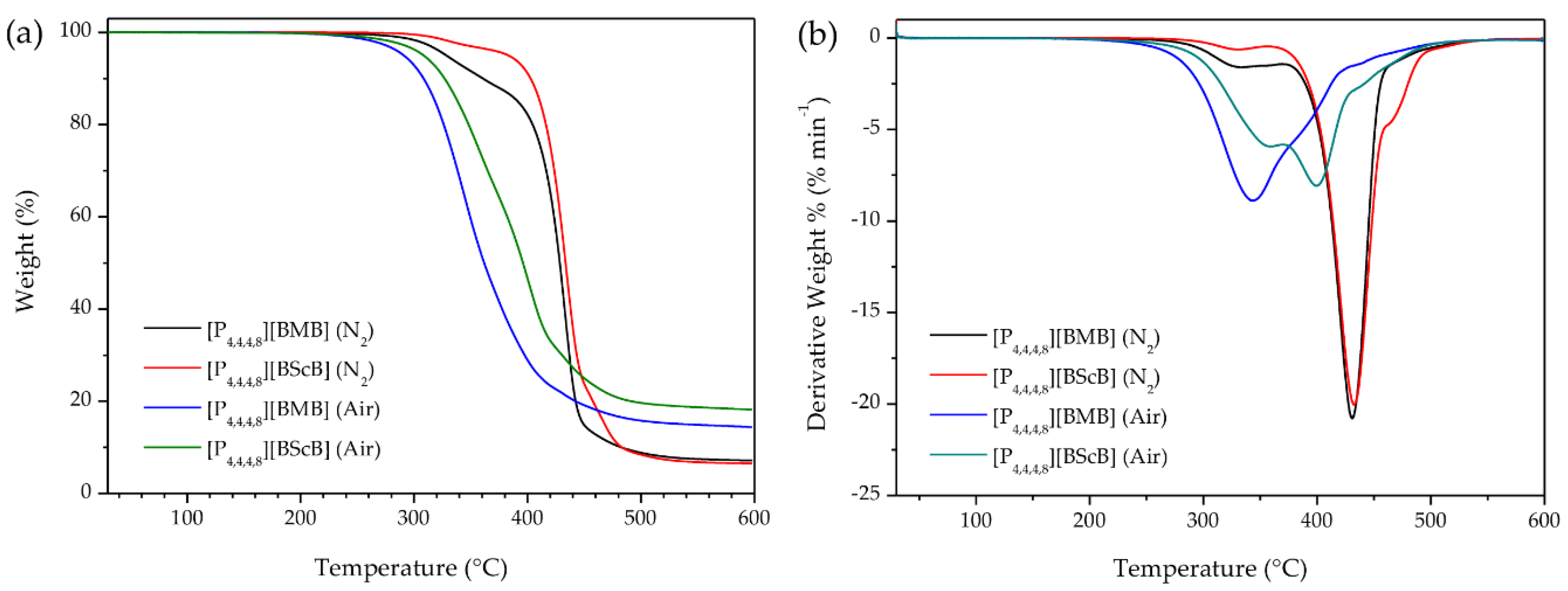
2.1. Thermal Stability
2.2. Ionic Conductivity
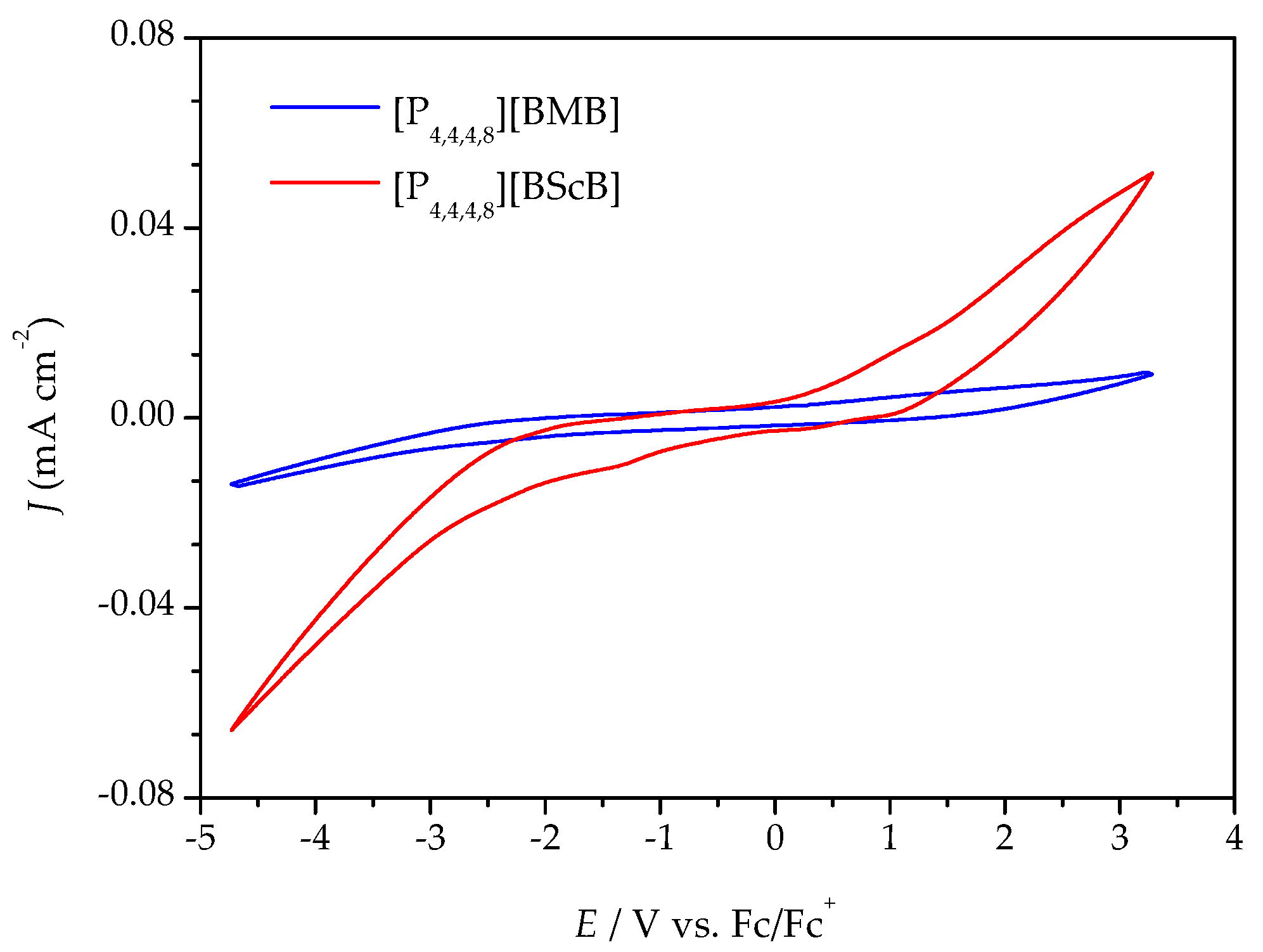
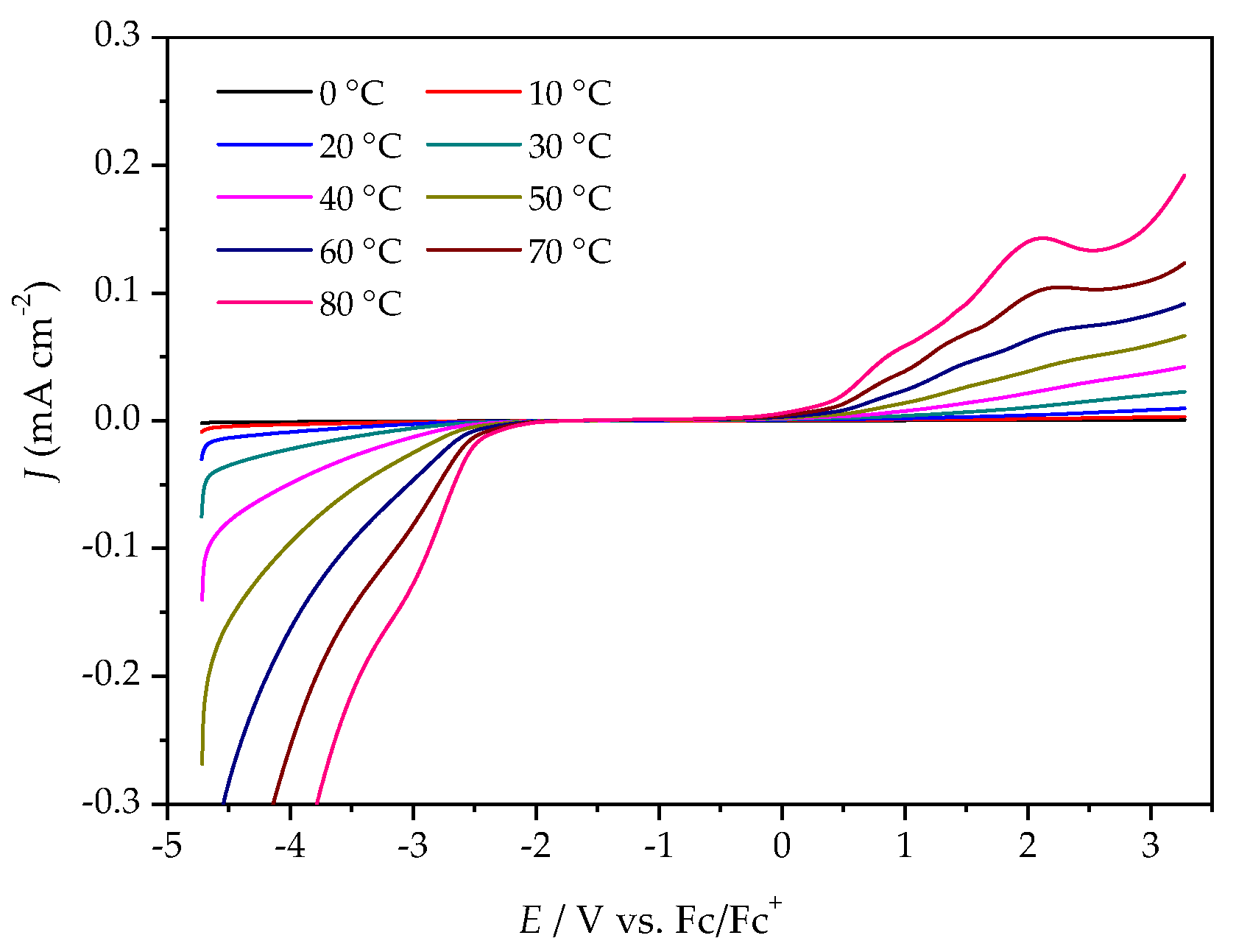
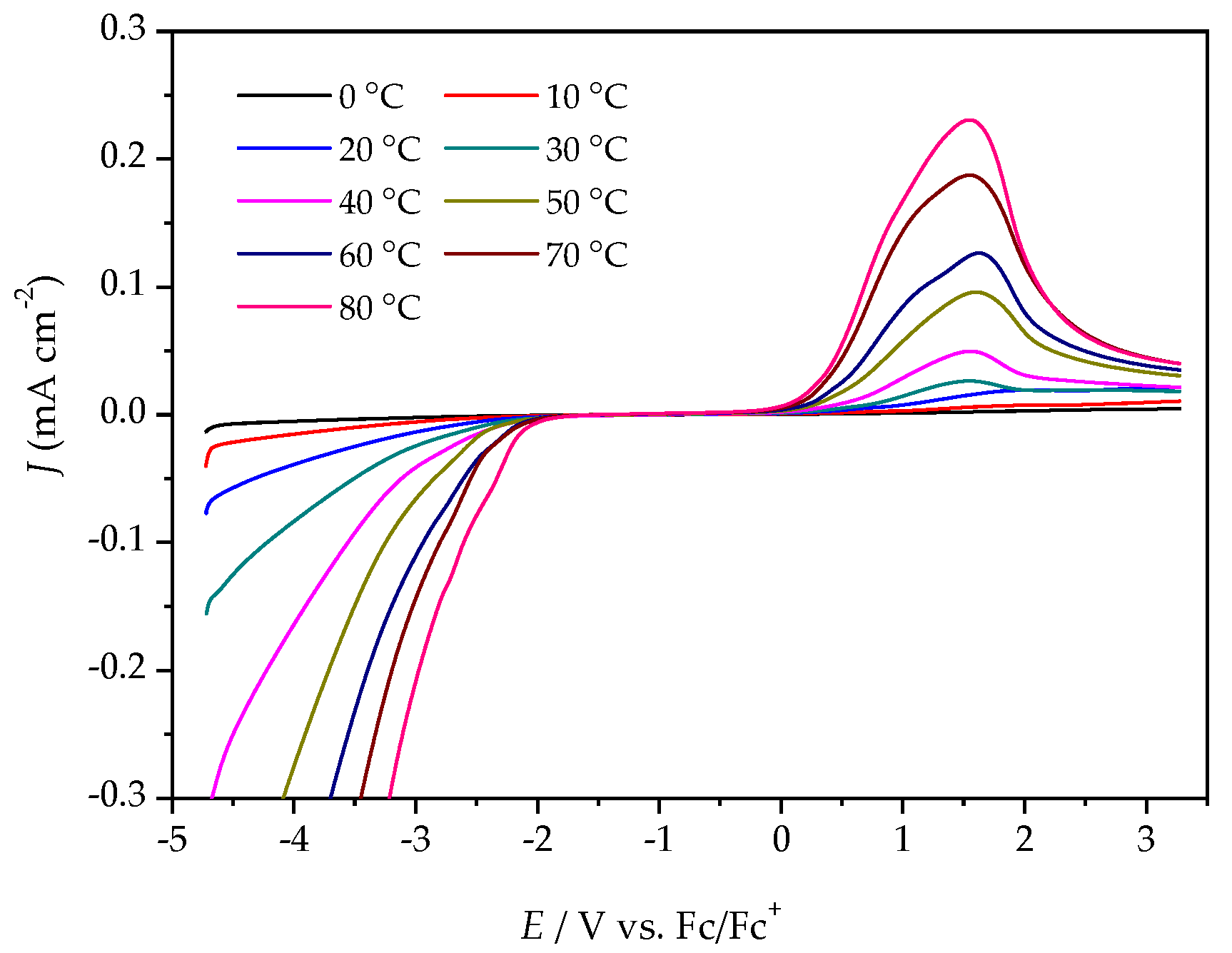
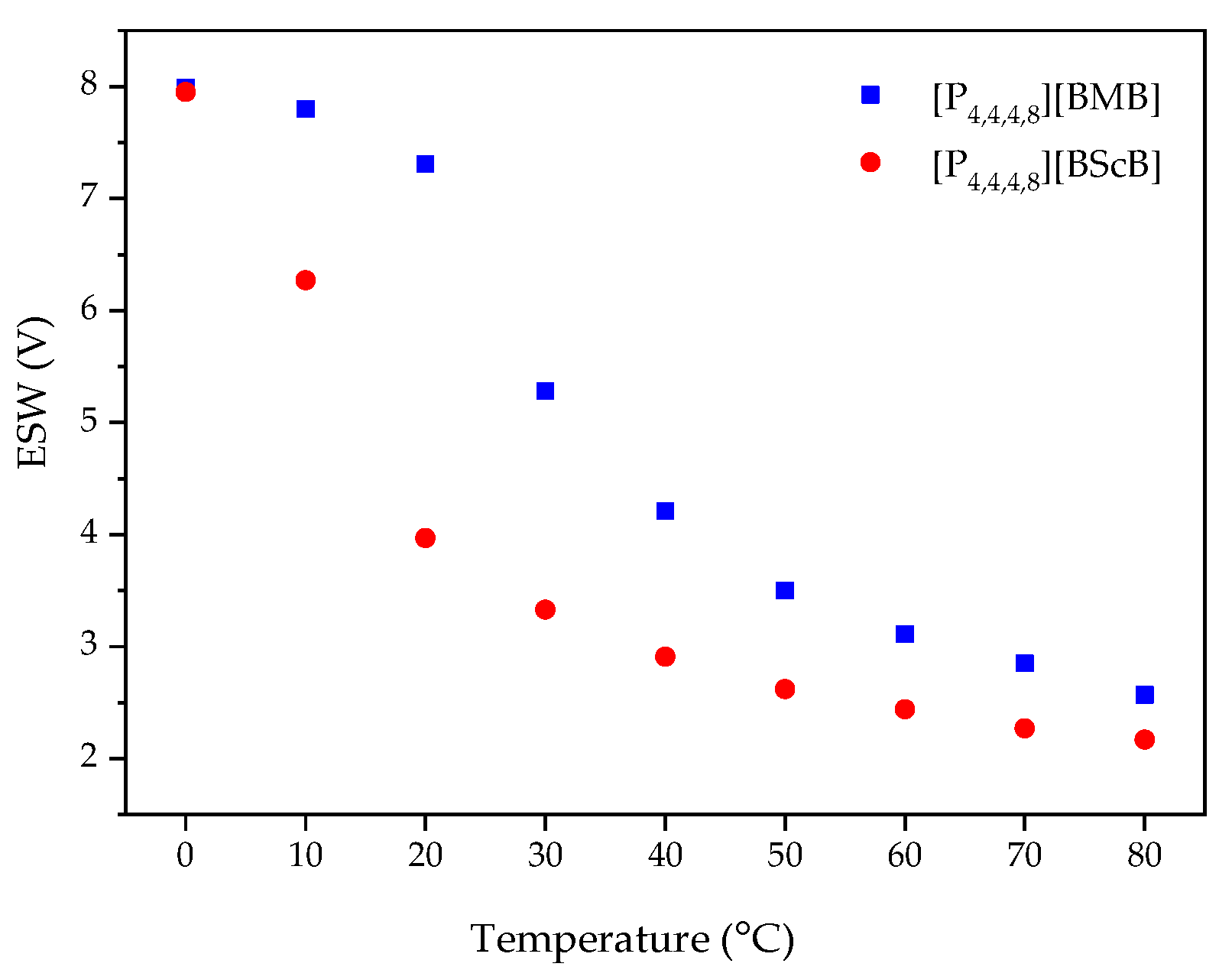
2.3. Electrochemical Stability
3. Materials and Methods
3.1. Materials
3.2. Thermal Stability
3.3. Ionic Conductivity
3.4. Electrochemical Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Welton, T. Room-temperature ionic liquids. Solvents for synthesis and catalysis. Chem. Rev. 1999, 99, 2071–2083. [Google Scholar] [CrossRef]
- MacFarlane, D.R.; Pringle, J.M.; Howlett, P.C.; Forsyth, M. Ionic liquids and reactions at the electrochemical interface. Phys. Chem. Chem. Phys. 2010, 12, 1659–1669. [Google Scholar] [CrossRef]
- Petkovic, M.; Seddon, K.; Pereira, C. Ionic liquids: A pathway to environmental acceptability. Chem. Soc. Rev. 2011, 40, 1383–1403. [Google Scholar] [CrossRef]
- Shah, F.U.; Glavatskih, S.; Dean, P.M.; MacFarlane, D.R.; Forsyth, M.; Antzutkin, O.A. Halogen-free chelated orthoborate ionic liquids and organic ionic plastic crystals. J. Mater. Chem. 2012, 22, 6928–6938. [Google Scholar] [CrossRef]
- Hallett, J.; Welton, T. Room-temperature ionic liquids: Solvents for synthesis and catalysis. Chem. Rev. 2011, 111, 3508–3576. [Google Scholar] [CrossRef]
- Plechkova, N.; Seddon, K. Applications of ionic liquids in the chemical industry. Chem. Soc. Rev. 2008, 37, 123–150. [Google Scholar] [CrossRef]
- Handy, S.T.; Okello, M. Homogeneous supported synthesis using ionic liquid supports: Tunable separation properties. J. Org. Chem. 2005, 70, 2874–2877. [Google Scholar] [CrossRef]
- Parvulescu, V.I.; Hardacre, C. Catalysis in ionic liquids. Chem. Rev. 2007, 207, 2615–2665. [Google Scholar] [CrossRef]
- Brown, R.J.; Dyson, P.J.; Ellis, D.; Welton, T. 1-Butyl-3-methylimidazolium cobalt tetracarbonyl [bmim][Co(CO)4]: A catalytically active organometallic ionic liquid. Chem. Commun. 2001, 1862–1863. [Google Scholar] [CrossRef]
- Shah, F.U.; Glavatskih, S.; Antzutkin, O.N. Boron in tribology: From borates to ionic liquids. Tribol. Lett. 2013, 51, 281–301. [Google Scholar] [CrossRef]
- Shimpi, M.R.; Velaga, S.P.; Shah, F.U.; Antzutkin, O.N. Pharmaceutical crystal engineering using ionic liquid anion-solute interactions. Cryst. Growth Des. 2017, 17, 1729–1734. [Google Scholar] [CrossRef]
- MacFarlane, D.R.; Forsyth, M.; Howlett, P.C.; Pringle, J.M.; Sun, J.; Annat, G.; Neil, W.; Izgorodina, E.I. Ionic liquids in electrochemical devices and processes: Managing interfacial electrochemistry. Acc. Chem. Res. 2007, 40, 1165–1173. [Google Scholar] [CrossRef]
- Scheers, J.; Fantini, S.; Johansson, P. A review of electrolytes for lithium-sulphur batteries. J. Power Sources 2014, 255, 204–218. [Google Scholar] [CrossRef]
- Zhong, C.; Deng, Y.; Hu, W.; Qiao, J.; Zhang, L.; Zhang, J. A review of electrolyte materials and compositions for electrochemical supercapacitors. Chem. Soc. Rev. 2015, 44, 7484–7539. [Google Scholar] [CrossRef]
- Dietz, M.L.; Dzielawa, J.A. Ion-exchange as a mode of cation transfer into room temperature ionic liquids containing crown ethers: Implications for the ‘greenness’ of ionic liquids as diluents in liquid–liquid extraction. Chem. Commun. 2001, 2124–2125. [Google Scholar] [CrossRef]
- Luo, H.; Dai, S.; Bonnesen, P.V. Solvent extraction of Sr2+ and Cs+ based on room temperature ionic liquids containing monoaza-substituted crown ethers. Anal. Chem. 2004, 76, 2773–2779. [Google Scholar] [CrossRef]
- Chun, S.; Dzyuba, S.V.; Bartsch, R.A. Influence of structural variation in room temperature ionic liquids on the selectivity and efficiency of competitive alkali metal salt extraction by a crown ether. Anal. Chem. 2001, 73, 3737–3741. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Filippov, A.; Shah, F.U. Insights into the effect of CO2 absorption on the ionic mobility of ionic liquids. Phys. Chem. Chem. Phys. 2016, 18, 28617–28625. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Shah, F.U. Ether functionalized choline tethered amino acid ionic liquids for enhanced CO2 capture. ACS Sustain. Chem. Eng. 2016, 4, 5441–5449. [Google Scholar] [CrossRef]
- Maton, C.; Vos, N.D.; Stevens, C.V. Ionic liquid thermal stabilities: Decomposition mechanisms and analysis tools. Chem. Soc. Rev. 2013, 42, 5963–5977. [Google Scholar] [CrossRef]
- Kosmulski, M.; Gustafsson, J.; Rosenholm, J.B. Thermal stability of low temperature ionic liquids revisited. Thermochim. Acta 2004, 412, 47–53. [Google Scholar] [CrossRef]
- Del Sesto, R.E.; McCleskey, T.M.; Macomber, C.; Ott, K.C.; Koppisch, A.T.; Baker, G.A.; Burrell, A.K. Limited thermal stability of imidazolium and pyrrolidinium ionic liquids. Thermochim. Acta 2009, 491, 118–120. [Google Scholar] [CrossRef]
- Tsunashima, K.; Niwa, E.; Kodama, S.; Sugiya, M.; Ono, Y. Thermal and transport properties of ionic liquids based on benzyl-substituted phosphonium cations. J. Phys. Chem. B 2009, 113, 15870–15874. [Google Scholar] [CrossRef]
- Ferreira, A.F.; Simões, P.N.; Ferreira, A.G.M. Quaternary phosphonium-based ionic liquids: Thermal stability and heat capacity of the liquid phase. J. Chem. Thermodyn. 2012, 45, 16–27. [Google Scholar] [CrossRef]
- Barrosse-Antle, L.E.; Bond, A.M.; Compton, R.G.; O’Mahony, A.M.; Rogers, E.I.; Silvester, D.S. Voltammetry in room temperature ionic liquids: Comparisons and contrasts with conventional electrochemical solvents. Chem. Asian J. 2010, 5, 202–230. [Google Scholar] [CrossRef]
- Kroon, M.C.; Buijs, W.; Peters, C.J.; Witkamp, G.J. Decomposition of ionic liquids in electrochemical processing. Green Chem. 2006, 8, 241–245. [Google Scholar] [CrossRef]
- Shah, F.U.; Holmgren, A.; Rutland, M.W.; Glavatskih, S.; Antzutkin, O.N. Interfacial behavior of orthoborate ionic liquids at inorganic oxide surfaces probed by NMR, IR and Raman spectroscopy. J. Phys. Chem. C 2018, 122, 19687–19698. [Google Scholar] [CrossRef]
- Ranke, J.; Stolte, S.; Störmann, R.; Arning, J.; Jastorff, B. Design of sustainable chemical products—The example of ionic liquids. Chem. Rev. 2007, 107, 2183–2206. [Google Scholar] [CrossRef]
- Deferm, C.; den Bossche, A.V.; Luyten, J.; Oosterhof, H.; Fransaer, J.; Binnemans, K. Thermal stability of trihexyl(tetradecyl)phosphonium chloride. Phys. Chem. Chem. Phys. 2018, 20, 2444–2456. [Google Scholar] [CrossRef]
- Corbridge, D.E.C. Phosphorus: An Outline of Its Chemistry, Biochemistry, and Technology; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Ngo, H.L.; LeCompte, K.; Hargens, L.; McEwen, A.B. Thermal properties of imidazolium ionic liquids. Thermochim. Acta 2000, 357–358, 97–102. [Google Scholar] [CrossRef]
- Tsunashima, K.; Kodama, S.; Sugiya, M.; Kunugi, Y. Physical and electrochemical properties of room-temperature dicyanamide ionic liquids based on quaternary phosphonium cations. Electrochimi. Acta 2010, 56, 762–766. [Google Scholar] [CrossRef]
- MacFarlane, D.R.; Forsyth, M.; Izgorodina, E.I.; Abbott, A.P.; Annat, G.; Fraser, K. On the concept of ionicity in ionic liquids. Phys. Chem. Chem. Phys. 2009, 11, 4962–4967. [Google Scholar] [CrossRef]
- Ueno, K.; Tokuda, H.; Watanabe, M. Ionicity in ionic liquids: Correlation with ionic structure and physicochemical properties. Phys. Chem. Chem. Phys. 2010, 12, 1649–1658. [Google Scholar] [CrossRef]
- Nilsson-Hallén, J.; Ahlström, B.; Marczewski, M.; Johansson, P. Ionic liquids: A simple model to predict ion conductivity based on DFT derived physical parameters. Front. Chem. 2019, 7, 00126. [Google Scholar] [CrossRef]
- Shah, F.U.; Glavatskih, S.; MacFarlane, D.R.; Forsyth, M.; Antzutkin, O.N. Novel halogen-free chelated orthoborate-phosphonium ionic liquids: Synthesis and tribophysical properties. Phys. Chem. Chem. Phys. 2011, 13, 12865–12873. [Google Scholar] [CrossRef]
- Tsuzuki, S.; Tokuda, H.; Hayamizu, K.; Watanabe, M. Magnitude and directionality of interaction in ion pairs of ionic liquids: Relationship with ionic conductivity. J. Phys. Chem. B 2005, 109, 16474–16481. [Google Scholar] [CrossRef]
- Galinski, M.; Lewandowski, A.; Stepniak, I. Ionic liquids as electrolytes. Electrochim. Acta 2006, 51, 5567–5580. [Google Scholar]
- O’Mahony, A.M.; Silvester, D.S.; Aldous, L.; Hardacre, C.; Compton, R.G. Effect of water on the electrochemical window and potential limits of room-temperature ionic liquids. J. Chem. Eng. Data 2008, 53, 2884–2891. [Google Scholar] [CrossRef]
- Chavan, S.N.; Tiwari, A.; Nagaiah, T.C.; Mandal, D. Ether and siloxane functionalized ionic liquids and their mixtures as electrolyte for lithium-ion batteries. Phys. Chem. Chem. Phys. 2016, 18, 16116–16126. [Google Scholar] [CrossRef]
- Schröder, U.; Wadhawan, J.D.; Compton, R.G.; Marken, F.; Suarez, P.A.Z.; Consorti, C.S.; de Souza, R.F.; Dupont, J. Water-induced accelerated ion diffusion: Voltammetric studies in 1-methyl-3-[2,6-(S)-dimethylocten-2-yl]-imidazolium tetrafluoroborate, 1-butyl-3-methyl-imidazolium tetrafluoroborate and hexafluorophosphate ionic liquids. New J. Chem. 2000, 24, 1009–1015. [Google Scholar] [CrossRef]
- Wang, Y.L.; Sarman, S.; Kloo, L.; Antzutkin, O.N.; Glavatskih, S.; Laaksonen, A. Solvation structures of water in trihexyltetradecylphosphonium-orthoborate ionic liquids. J. Chem. Phys. 2016, 145, 064507. [Google Scholar] [CrossRef]
- Silvester, D.S.; Compton, R.G. Electrochemistry in room temperature ionic liquids: A review and some possible applications. Z. Phys. Chem. 2006, 220, 1247–1274. [Google Scholar] [CrossRef]
- Mun, J.; Jung, Y.S.; Yim, T.; Lee, H.Y.; Kim, H.; Kim, Y.G.; Oh, S.M. Electrochemical stability of bis(trifluoromethanesulfonyl)imide-based ionic liquids at elevated temperature as a solvent for a titanium oxide bronze electrode. J. Power Sources 2009, 194, 1068–1074. [Google Scholar] [CrossRef]
- Xue, Z.; Qin, L.; Jiang, J.; Mu, T.; Gao, G. Thermal, electrochemical and radiolytic stabilities of ionic liquids. Phys. Chem. Chem. Phys. 2018, 20, 8382–8402. [Google Scholar] [CrossRef]
Sample Availability: Samples of the ionic liquids are available from the authors. |
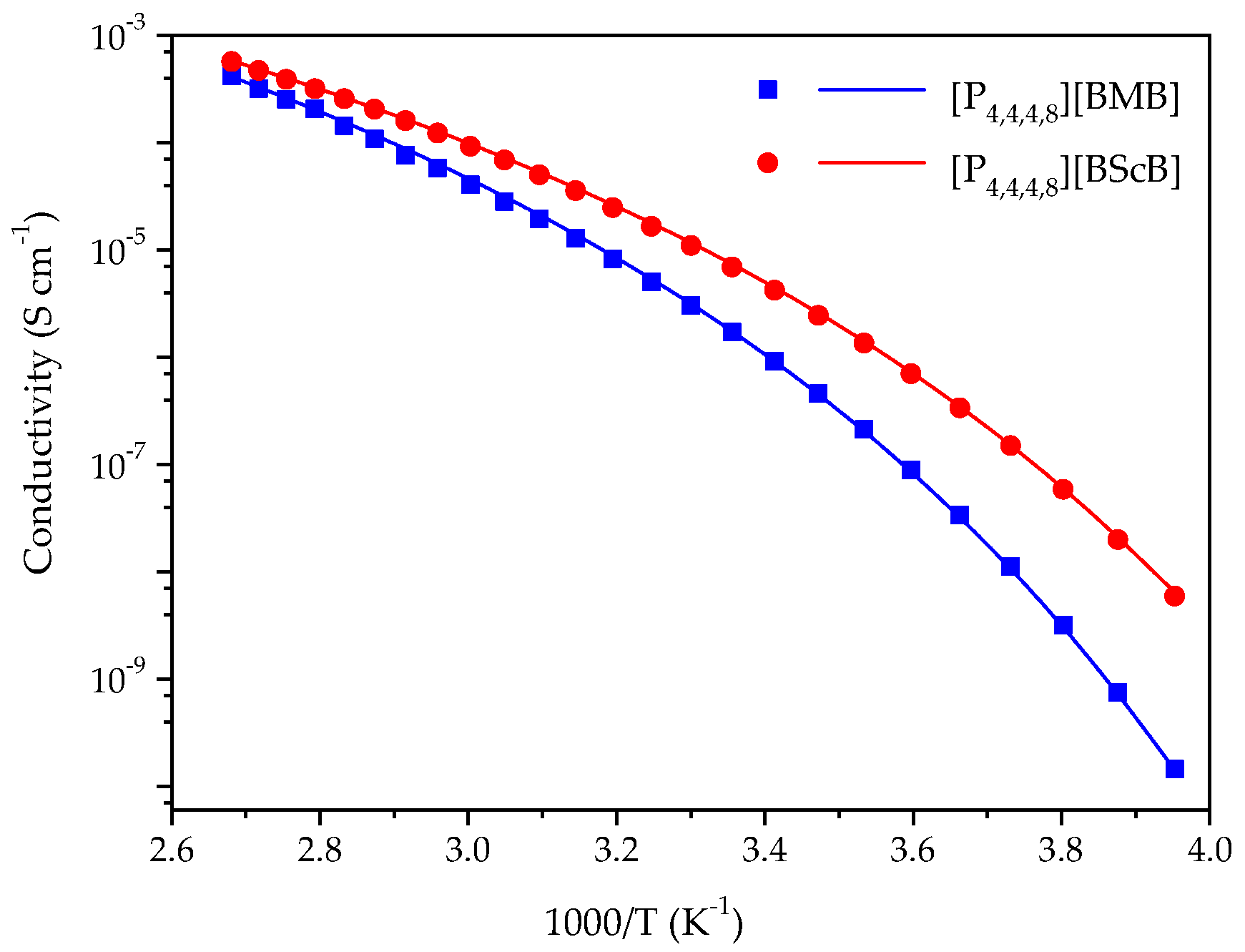
| IL | σ0 (S cm−1) | B (K) | T0 (K) | Eσ (kJ mol−1 K−1) |
|---|---|---|---|---|
| [P4,4,4,8][BMB] | 1.15 | 1460 | 189 | 12.1 |
| [P4,4,4,8][BScB] | 0.50 | 1290 | 182 | 10.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, F.U.; Khan, I.A.; Johansson, P. Comparing the Thermal and Electrochemical Stabilities of Two Structurally Similar Ionic Liquids. Molecules 2020, 25, 2388. https://doi.org/10.3390/molecules25102388
Shah FU, Khan IA, Johansson P. Comparing the Thermal and Electrochemical Stabilities of Two Structurally Similar Ionic Liquids. Molecules. 2020; 25(10):2388. https://doi.org/10.3390/molecules25102388
Chicago/Turabian StyleShah, Faiz Ullah, Inayat Ali Khan, and Patrik Johansson. 2020. "Comparing the Thermal and Electrochemical Stabilities of Two Structurally Similar Ionic Liquids" Molecules 25, no. 10: 2388. https://doi.org/10.3390/molecules25102388
APA StyleShah, F. U., Khan, I. A., & Johansson, P. (2020). Comparing the Thermal and Electrochemical Stabilities of Two Structurally Similar Ionic Liquids. Molecules, 25(10), 2388. https://doi.org/10.3390/molecules25102388







