A Combined Mechanochemical and Calcination Route to Mixed Cobalt Oxides for the Selective Catalytic Reduction of Nitrophenols
Abstract
1. Introduction
2. Results and Discussion
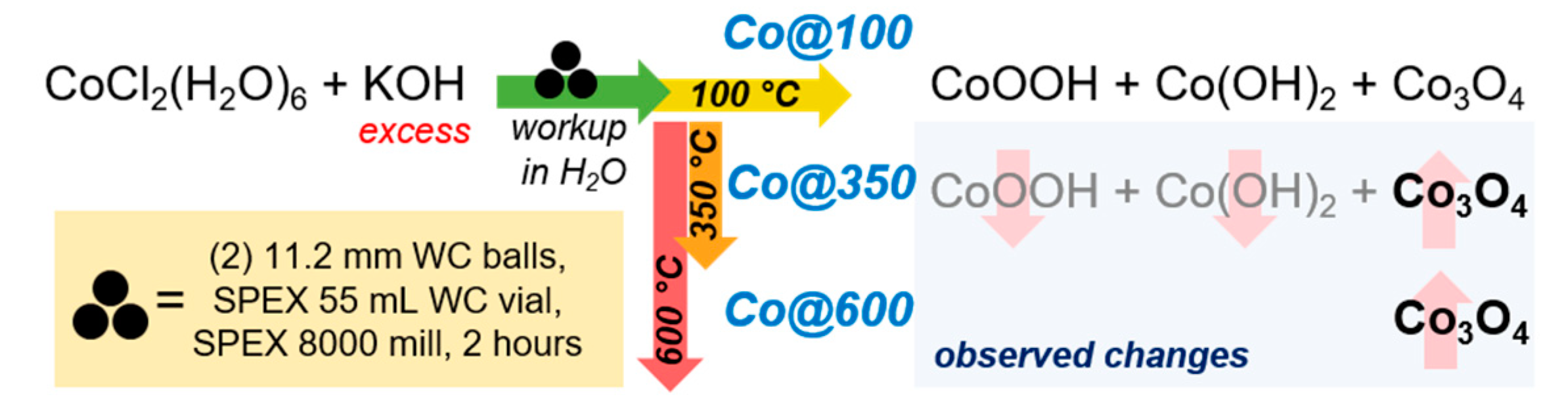
2.1. Catalyst Synthesis
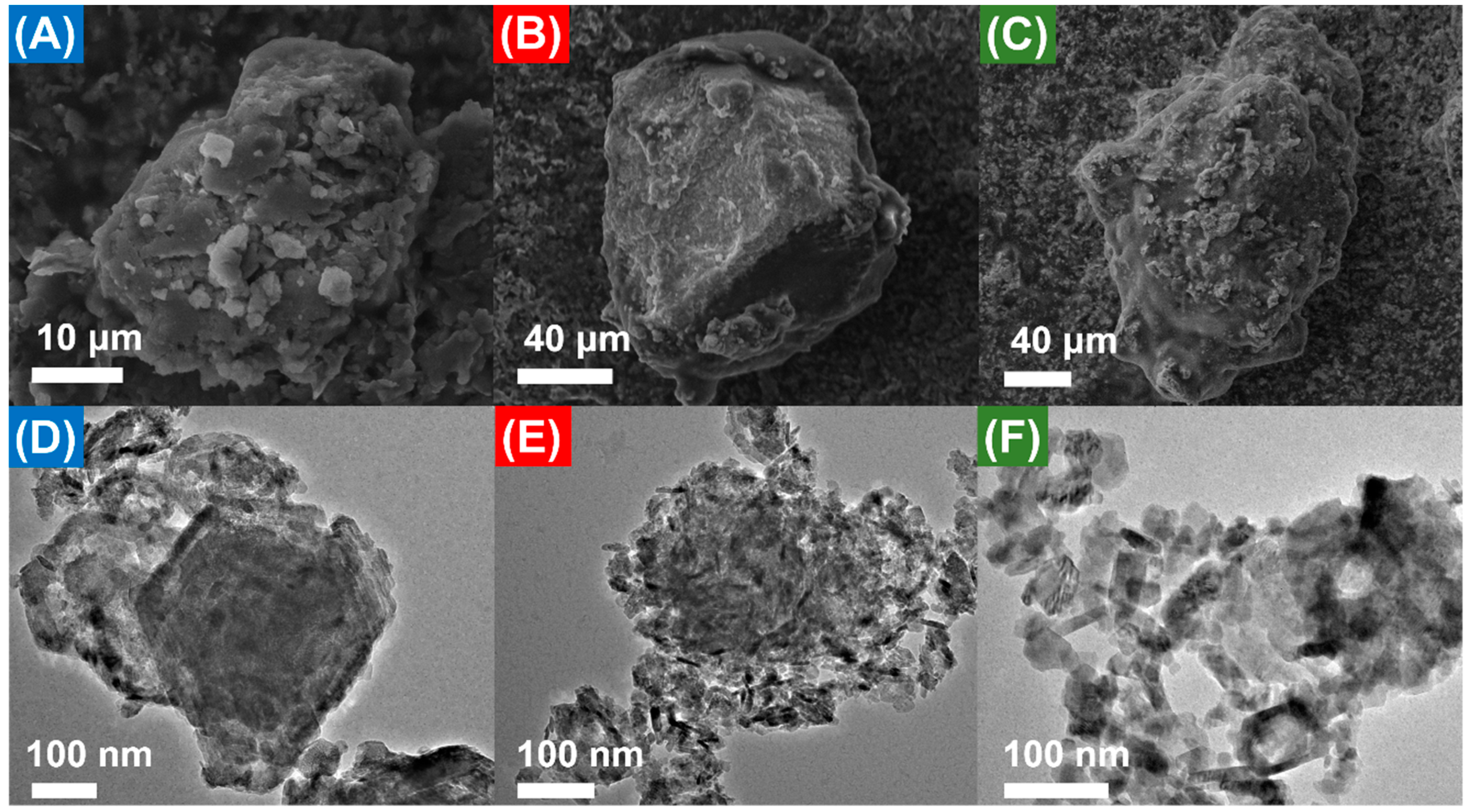
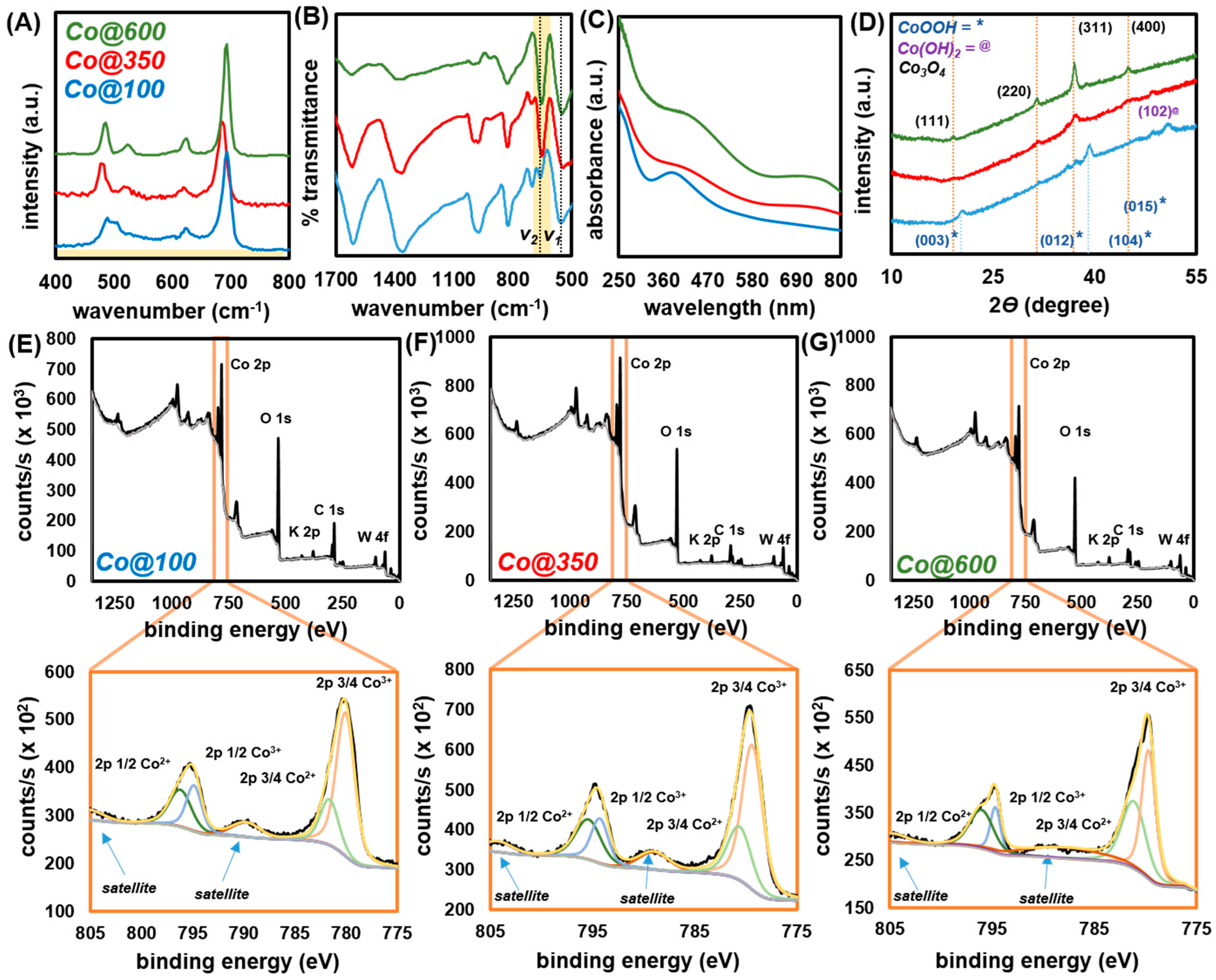
2.2. Catalyst Characterization
2.3. Catalysis
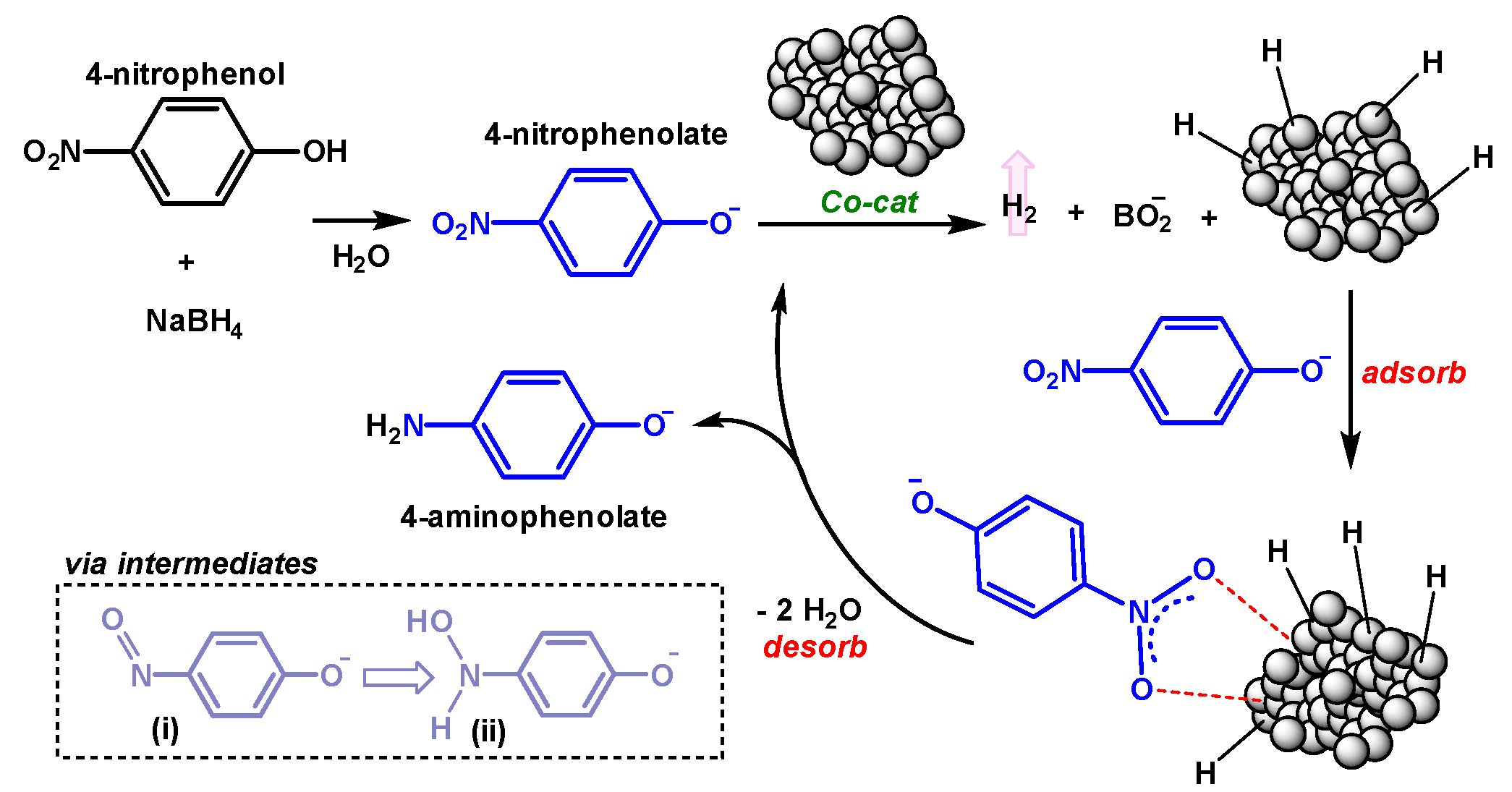
2.3.1. Catalytic Reduction of 4-Nitrophenol
2.3.2. Recyclability of Co@350
2.3.3. Testing Applicability of Co@350 in a Flow Process
2.3.4. Catalytic Reduction of Amino-Nitrophenols
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yi, S.; Zhuang, W.-Q.; Wu, B.; Tiong-Lee, S.; Tay, J.-H. Biodegradation of p-nitrophenol by aerobic granules in a sequencing batch reactor. Environ. Sci. Technol. 2006, 40, 2396–2401. [Google Scholar] [CrossRef] [PubMed]
- Podeh, M.R.H.; Bhattacharya, S.K.; Qu, M. Effects of nitrophenol on acetate utilizing methanogenic systems. Wat. Res. 1995, 29, 391–399. [Google Scholar] [CrossRef]
- Xia, J.; He, G.; Zhang, L.; Sun, X.; Wang, X. Hydrogenation of nitrophenols catalyzed by carbon black-supported nickel nanoparticles under mild conditions. Appl. Catal. B 2016, 180, 408–415. [Google Scholar] [CrossRef]
- Yu, S.; Hu, J.; Wang, J. Gamma radiation-induced degradation of p-nitrophenol (PNP) in the presence of hydrogen peroxide (H2O2) in aqueous solution. J. Hazard. Mater. 2010, 177, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Ju, K.-S.; Parales, R.E. Nitroaromatic compounds, from synthesis to biodegradation. Microbiol. Mol. Biol. Rev. 2010, 74, 250–272. [Google Scholar] [CrossRef]
- Zhao, H.; Kong, C.-H. Enhanced removal of p-nitrophenol in a microbial fuel cell after a long-term operation and the catabolic versatility of its microbial community. Chem. Eng. J. 2018, 339, 424–431. [Google Scholar] [CrossRef]
- Sun, M.; Reible, D.D.; Lowry, G.V.; Gregory, K.B. Effect of applied voltage, initial concentration, and natural organic matter on sequential reduction/oxidation of nitrobenzene by graphite electrodes. Environ. Sci. Technol. 2012, 46, 6174–6181. [Google Scholar] [CrossRef]
- Downing, R.S.; Kunkeler, P.J.; van Bekkum, H. Catalytic synthesis of aromatic amines. Catal. Today 1997, 37, 121–136. [Google Scholar] [CrossRef]
- Wegener, G.; Brandt, M.; Duda, L.; Hofmann, J.; Klesczewski, B.; Koch, D.; Kumpf, R.-J.; Orzesek, H.; Pirkl, H.-G.; Six, C.; et al. Trends in industrial catalysis in the polyurethane industry. Appl. Catal. A 2001, 22, 303–335. [Google Scholar] [CrossRef]
- Nomura, K. Transition metal catalyzed hydrogenation of reduction in water. J. Mol. Catal. A 1998, 130, 1–28. [Google Scholar] [CrossRef]
- Espinosa Bosch, M.; Ruiz Sánchez, A.J.; Sánchez Rojas, F.; Bosh Ojeda, C. Determination of paracetamol: Historical evolution. J. Pharm. Biomed. Anal. 2006, 42, 291–321. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Zhang, H.; Zhang, W.; Lai, B.; Yao, G. Removal of nitrophenols and their derivatives by chemical redox: A review. Chem. Eng. J. 2019, 359, 13–31. [Google Scholar] [CrossRef]
- Aditya, T.; Pal, A.; Pal, T. Nitroarene reduction: A trusted model reaction to test nanoparticle catalysts. Chem. Commun. 2015, 51, 9410–9431. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Feng, X.; Huang, D.; Yang, G.; Astruc, D. Basic concepts and recent advances in nitrophenol reduction by gold- and other transition metal nanoparticles. Coord. Chem. Rev. 2015, 287, 114–136. [Google Scholar] [CrossRef]
- Hervés, P.; Pérez-Lorenzo, M.; Liz-Marzán, L.M.; Dzubiella, J.; Lu, Y.; Ballauff, M. Catalysis by metallic nanoparticles in aqueous solution: Model reactions. Chem. Soc. Rev. 2012, 41, 5577–5587. [Google Scholar] [CrossRef]
- Menumerov, E.; Hughes, R.A.; Golze, S.D.; Neal, R.D.; Demille, T.B.; Campanaro, J.C.; Kotesky, K.C.; Rouvimov, S.; Neretiva, S. Identifying the true catalyst in the reduction of 4-nitrophenol: A case study showing the effect of leaching and oxidative etching using Ag catalysts. ACS Catal. 2018, 8, 8879–8888. [Google Scholar] [CrossRef]
- Menumerov, E.; Hughes, R.A.; Neretina, S. One-step catalytic reduction of 4-nitrophenol through the direct injection of metal salts into oxygen-depleted reactants. Catal. Sci. Technol. 2017, 7, 1460–1464. [Google Scholar] [CrossRef]
- Dolatkhah, A.; Jani, P.; Wilson, L.D. Redox-responsive polymer template as an advanced multifunctional catalyst support for silver nanoparticles. Langmuir 2018, 34, 10560–10568. [Google Scholar] [CrossRef]
- Hsia, C.-F.; Madasu, M.; Huang, M. Aqueous phase synthesis of Au-Cu core-shell nanocubes and octahedra with tunable sizes and noncentrally located cores. Chem. Mater. 2016, 28, 3073–3079. [Google Scholar] [CrossRef]
- Bari, N.K.; Kumar, G.; Bhatt, A.; Hazra, J.P.; Garg, A.; Ali, M.E.; Sinha, S. Nanoparticle fabrication on bacterial microcompartment surface for the development of hybrid enzyme-inorganic catalyst. ACS Catal. 2018, 8, 7742–7748. [Google Scholar] [CrossRef]
- Wang, F.; Li, W.; Feng, X.; Liu, D.; Zhang, Y. Decoration of Pt on Cu/Co double-doped CeO2 nanospheres and their greatly enhanced catalytic activity. Chem. Sci. 2016, 7, 1867–1873. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.A.; Makis, J.J.; Marvin, K.A.; Rodenbusch, S.E.; Stevenson, K.J. Size-dependant hydrogenation of p-nitrophenol with Pd nanoparticles synthesized with poly(amido)amine dendrimer templates. J. Phys. Chem. C. 2013, 117, 22644–22651. [Google Scholar] [CrossRef]
- Jadbabaei, N.; Slobodjian, R.J.; Shuai, D.; Zhang, H. Catalytic reduction of 4-nitrophenol by palladium-resin composites. Appl. Catal. 2017, 543, 209–217. [Google Scholar] [CrossRef]
- Islam, M.T.; Saenz-Arana, R.; Wang, H.; Bernal, R.; Noveron, J.C. Green synthesis of gold, silver, platinum, and palladium nanoparticles reduced and stabilized by sodium rhodizonate and their catalytic reduction of 4-nitrophenol and methyl orange. New J. Chem. 2018, 42, 6472–6478. [Google Scholar] [CrossRef]
- Liu, A.; Traulsen, C.H.-H.; Cornelissen, J.J.L.M. Immobilization of catalytic virus-like particles in a flow reactor. Chem. Commun. 2017, 53, 7632–7634. [Google Scholar] [CrossRef]
- Koklioti, M.A.; Skaltsas, T.; Sato, Y.; Suenaga, K.; Stergiou, A.; Tagmatarchis, N. Mechanistic insights into the photocatalytic properties of metal nanocluster/graphene ensembles. Examining the role of visible light in the reduction of 4-nitrophenol. Nanoscale 2017, 9, 9685–9692. [Google Scholar] [CrossRef]
- Bingwa, N.; Meijboom, R. Kinetic evaluation of dendrimer-encapsulated palladium nanoparticles in the 4-nitrophenol reduction reaction. J. Phys. Chem. C. 2014, 118, 19849–19858. [Google Scholar] [CrossRef]
- Islam, M.T.; Jing, H.; Yang, T.; Zubia, E.; Goos, A.G.; Bernal, R.A.; Botez, C.E.; Narayan, M.; Chan, C.K.; Noveron, J.C. Fullerene stabilized gold nanoparticles supported on titanium dioxide for enhanced photocatalytic degradation of methyl orange and catalytic reduction of 4-nitrophenol. J. Environ. Chem. Eng. 2018, 6, 3827–3836. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Huang, C.P.; Doong, R.-A. Enhanced catalytic reduction of nitrophenols by sodium borohydride over highly recyclable Au@graphitic carbon nitride nanocomposites. Appl. Catal. B 2019, 240, 337–347. [Google Scholar] [CrossRef]
- Wu, Y.; Wen, M.; Wu, Q.-S.; Fang, H. Ni/graphene nanostructure and its electron-enhanced catalytic action for hydrogenation reaction of nitrophenol. J. Phys. Chem. C 2014, 118, 6307–6313. [Google Scholar] [CrossRef]
- Wang, X.; Lu, J.; Zhao, Y.; Wang, X.; Lin, Z.; Liu, X.; Wu, R.; Yang, C.; Su, X. Facile fabrication of nickel/heazlewoodite@carbon nanosheets and their superior catalytic performance of 4-nitrophenol reduction. ChemCatChem 2018, 10, 4143–4153. [Google Scholar] [CrossRef]
- Mandlimath, T.R.; Gopal, B. Catalytic activity of first row transition metal oxides in the conversion of p-nitrophenol to p-aminophenol. J. Mol. Catal. A 2011, 350, 9–15. [Google Scholar] [CrossRef]
- Mohamed, M.J.S.; Denthaje, K.B. Novel RGO-ZnWO4-Fe3O4 nanocomposites as an efficient catalyst for rapid reduction of 4-nitrophenol to 4-aminophenol. Ind. Eng. Chem. Res. 2016, 55, 7267–7272. [Google Scholar] [CrossRef]
- Qiao, X.-Q.; Zhang, Z.-W.; Hou, D.-F.; Li, D.-S.; Liu, Y.; Lan, Y.-Q.; Zhang, J.; Feng, P.; Bu, X. Tunable MoS2/SnO2 P-N heterojunctions for an efficient trimethylamine gas sensor and 4-nitrophenol reduction catalyst. ACS Sustain. Chem. Eng. 2018, 6, 12375–12384. [Google Scholar] [CrossRef]
- Ma, Y.; Ni, Y.; Guo, F.; Xiang, N. Flowerlike copper(II)-based coordination polymers particles: Rapid room-temperature fabrication, influencing factors, and transformation toward CuO microstructures with good catalytic activity for the reduction of 4-nitrophenol. Cryst. Growth Des. 2015, 15, 2243–2252. [Google Scholar] [CrossRef]
- Shtansky, D.V.; Firestein, K.L.; Goldberg, D.V. Fabrication and application of BN nanoparticles, nanosheets and their nanohybrids. Nanoscale 2018, 10, 17477–17493. [Google Scholar] [CrossRef]
- Huang, C.; Ye, W.; Liu, Q.; Qiu, X. Dispersed Cu2O octahedrons on h-BN nanosheets for p-nitrophenol reduction. ACS Appl. Mater. Interfaces 2014, 6, 14469–14476. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, Y.; Deng, J.; Xie, S.; Lin, H.; Zhao, X.; Yang, J.; Han, Z.; Dai, H. Fe2O3/3DOM BiVO4: High-performance photocatalysts for the visible light-driven degradation of 4-nitrophenol. Appl. Catal. B 2017, 202, 569–579. [Google Scholar] [CrossRef]
- Kong, X.-K.; Sun, Z.-Y.; Chen, M.; Chen, C.; Chen, Q. Metal-free catalytic reduction of 4-nitrophenol to 4-aminophenol by N-doped grapheme. Energy Environ. Sci. 2013, 6, 3260–3266. [Google Scholar] [CrossRef]
- Gao, L.; Li, R.; Sui, X.; Li, R.; Chen, C.; Chen, Q. Conversion of chicken feather waste to N-doped carbon nanotubes for the catalytic reduction of 4-nitrophenol. Environ. Sci. Technol. 2014, 48, 10191–10197. [Google Scholar] [CrossRef]
- Wu, Q.; Liang, M.; Zhang, S.; Liu, X.; Wang, F. Development of function black phosphorus nanosheets with remarkable catalytic and antibacterial performance. Nanoscale 2018, 10, 10428–10435. [Google Scholar] [CrossRef] [PubMed]
- Raza, W.; Krupanidhi, S.B. Engineering defects in graphene oxide for selective ammonia and enzyme-free glucose sensing and excellent catalytic performance for para-nitrophenol reduction. ACS Appl. Mater. Interfaces 2018, 10, 25285–25294. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, F.; Yang, Y.; Guo, S.; Zhang, J. Composites of graphene quantum dots and reduced graphene oxide as catalysts for nitroarene reduction. ACS Omega 2017, 2, 7293–7298. [Google Scholar] [CrossRef] [PubMed]
- Stergiou, A.; Tagmatarchis, N. Fluorene-perylene diimide arrays onto graphene sheets for photocatalysis. ACS Appl. Mater. Interfaces 2016, 8, 21576–21584. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Chen, N.; Zhang, J.; Qu, L. Graphene/graphitic carbon nitride hybrids for catalysis. Mater. Horiz. 2017, 4, 832–850. [Google Scholar] [CrossRef]
- Wang, Z.; Su, R.; Wang, D.; Shi, J.; Wang, J.-X.; Pu, Y.; Chen, J.-F. Sulfurized graphene as efficient metal-free catalysts for reduction of 4-nitrophenol to 4-aminophenol. Ind. Eng. Chem. Res. 2017, 56, 13610–13617. [Google Scholar] [CrossRef]
- Pan, L.; Tang, J.; Wang, F. Synthesis and electrocatalytic performance for p-nitrophenol reduction of rod-like Co3O4 and Ag/Co3O4 composites. Mater. Res. Bull. 2013, 48, 2648–2653. [Google Scholar] [CrossRef]
- Mogudi, B.M.; Ncube, P.; Meijboom, R. Catalytic activity of mesoporous cobalt oxides with controlled porosity and crystallite sizes: Evaluation using the reduction of 4-nitrophenol. Appl. Catal. B 2016, 74, 74–82. [Google Scholar] [CrossRef]
- Mogudi, B.M.; Ncube, P.; Bingwa, N.; Mawila, N.; Mathebula, S.; Meijboom, R. Promotion effects of alkali- and alkaline earth metals on catalytic activity of mesoporous Co3O4 for 4-nitrophenol reduction. Appl. Catal. B 2017, 218, 240–248. [Google Scholar] [CrossRef]
- Chen, H.; Yang, M.; Tao, S.; Chen, G. Oxygen vacancy enhanced catalytic activity of reduced Co3O4 towards p-nitrophenol reduction. Appl. Catal. B 2017, 209, 648–656. [Google Scholar] [CrossRef]
- Song, T.; Ren, P.; Duan, Y.; Wang, Z.; Chen, X.; Yang, Y. Cobalt nanocomposites on N-doped hierarchical porous carbon for highly selective formation of anilines and imines from nitroarenes. Green Chem. 2018, 20, 4629–4637. [Google Scholar] [CrossRef]
- Chinnappan, A.; Eshkalak, S.K.; Baskar, C.; Khatibzadeh, M.; Kowsari, E.; Ramakrishna, S. Flower-like 3-dimensional hierarchical Co3O4/NiO microspheres for 4-nitrophenol reduction reaction. Nanoscale Adv. 2019, 1, 305–313. [Google Scholar] [CrossRef]
- Park, J.-W.; Park, C.-M. Mechanochemically induced transformation of CoO(OH) into Co3O4 nanoparticles and their highly reversible Li storage characteristics. RSC Adv. 2017, 7, 10618–10623. [Google Scholar] [CrossRef]
- Shaw, T.E.; Shultz, L.R.; Garayeva, L.R.; Blair, R.G.; Noll, B.C.; Jurca, T. Mechanochemical routes for the synthesis of acetyl- and bis-(imino)pyridine ligands and organometallics. Dalton Trans. 2018, 47, 16876–16884. [Google Scholar] [CrossRef]
- He, G.; Ding, J.; Zhang, J.; Hao, Q.; Chen, H. One-step ball-milling preparation of highly photocatalytic active CoFe2O4-reduced graphene oxide heterojunctions for organic dye removal. Ind. Eng. Chem. Res. 2015, 54, 2862–2867. [Google Scholar] [CrossRef]
- Lyu, H.; Gao, B.; He, F.; Ding, C.; Tang, J.; Crittenden, J.C. Ball-milled carbon nanomaterials for energy and environmental applications. ACS Sustain. Chem. Eng. 2017, 5, 9568–9585. [Google Scholar] [CrossRef]
- Peters, D.W.; Blair, R.G. Mechanochemical synthesis of an organometallic compound: A high volume manufacturing method. Faraday Discuss. 2014, 170, 83–91. [Google Scholar] [CrossRef]
- Restrepo, D.; Hick, S.M.; Griebel, C.; Alarcón, J.; Giesler, K.; Chen, Y.; Orlovskaya, N.; Blair, R.G. Size controlled mechanochemical synthesis of ZrSi2. Chem. Commun. 2013, 49, 707–709. [Google Scholar] [CrossRef]
- Zhao, B.; Zheng, Y.; Ye, F.; Deng, X.; Xu, X.; Liu, M.; Shao, Z. Multifunctional iron oxide nanoflake/graphene composites derived from mechanochemical synthesis for enhanced lithium storage and electrocatalysis. ACS Appl. Mater. Interfaces 2015, 7, 14446–14455. [Google Scholar] [CrossRef]
- Muñoz-Batista, M.J.; Rodriquez-Padron, D.; Puente-Santiago, A.R.; Luque, R. Mechanochemistry: Toward sustainable design of advanced nanomaterials for electrochemical energy storage and catalytic applications. ACS Sustain. Chem. Eng. 2018, 6, 9530–9544. [Google Scholar] [CrossRef]
- Saitow, K.; Wang, Y.; Takahashi, S. Mechano-synthesized orange TiO2 shows significant photocatalysis under visible light. Sci. Rep. 2018, 8, 15549. [Google Scholar] [CrossRef] [PubMed]
- Jeon, I.-Y.; Choi, H.-J.; Choi, M.; Seo, J.-M.; Jung, S.-M.; Kim, M.-J.; Zhang, S.; Zhang, L.; Xia, Z.; Dai, L.; et al. Facile, scalable synthesis of edge-halogenated graphene nanoplatelets as efficient metal-free electrocatalysis for oxygen reduction reaction. Sci. Rep. 2013, 3, 1810. [Google Scholar] [CrossRef] [PubMed]
- Prochowicz, D.; Nawrocki, J.; Terlecki, M.; Marynowski, W.; Lewiński, J. Facile mechanosynthesis of the archetypal Zn-based metal-organic frameworks. Inorg. Chem. 2018, 57, 13437–13442. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, H.; Martens, W.N.; Frost, R.L. Synthesis and characterization of cobalt hydroxide, cobalt oxyhydroxide, and cobalt oxide nanodiscs. J. Phys. Chem. C 2010, 114, 111–119. [Google Scholar] [CrossRef]
- Solsona, B.; Davies, T.E.; Garcia, T.; Vásquez, I.; Dejoz, A.; Taylor, S.H. Total oxidation of propane using nanocrystalline cobalt oxide and supported cobalt oxide catalysts. Appl. Catal. B 2008, 84, 176–184. [Google Scholar] [CrossRef]
- Liu, F.; Su, H.; Jin, L.; Zhang, H.; Chu, X.; Yang, W. Facile synthesis of ultrafine cobalt oxide nanoparticles for high-performance supercapacitors. J. Colloid Interface Sci. 2017, 505, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Boudard, M.; Rapenne, L.; Chaix-Pluchery, O.; Carmezim, M.J.; Montemor, M.F. Structural evolution, magnetic properties and electrochemical response of MnCo2O4 nanosheet films. RSC. Adv. 2015, 5, 27844–27852. [Google Scholar] [CrossRef]
- Hadjiev, V.G.; Iliev, M.N.; Vergilov, I.V. The Raman spectra of Co3O4. J. Phys. C Solid State Phys 1988, 21, L199–L201. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Koza, J.A.; Switzer, J.A. Conversion of electrodeposited Co(OH)2 to CoOOH and Co3O4, and comparison of their catalytic activity for the oxygen evolution reaction. Electrochim. Acta 2014, 140, 359–365. [Google Scholar] [CrossRef]
- Alrehaily, L.M.; Joseph, J.M.; Wren, J.C. Radiation-induced formation of Co3O4 nanoparticles from Co2+(aq): Probing the kinetics using radical scavengers. Phys. Chem. Chem. Phys. 2015, 17, 24138–24150. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, W.; Dou, M.; Wang, F.; Liu, J.; Li, Z.; Li, J. Nanorod-constructed porous Co3O4 nanowires: Highly sensitive sensors for the detection of hydrazine. Analyst 2015, 140, 1686–1692. [Google Scholar] [CrossRef]
- Pandey, B.K.; Shahi, A.K.; Gopal, R. Magnetic colloid by PLA: Optical, magnetic and thermal transport properties. Appl. Surf. Sci. 2015, 347, 461–470. [Google Scholar] [CrossRef]
- Li, N.; Zhu, Y.D.; Liu, T.; Liu, S.G.; Lin, S.M.; Shi, Y.; Luo, H.Q.; Li, N.B. Turn-on fluorescence detection of pyrophosphate anion based on DNA-attached cobalt oxyhydroxide. New J. Chem. 2017, 41, 1993–1996. [Google Scholar] [CrossRef]
- Nie, Q.; Cai, Q.; Xu, H.; Qiao, Z.; Li, Z. A facile colorimetric method for hightly sensitive ascorbic acid detection by using CoOOH nanosheets. Anal. Methods 2018, 10, 2623–2628. [Google Scholar] [CrossRef]
- Mahmoud, W.E.; Al-Agel, F.A. A novel strategy to synthesize cobalt hydroxide and Co3O4 nanowires. J. Phys. Chem. Solids 2011, 72, 904–907. [Google Scholar] [CrossRef]
- Alburquenque, D.; Vargas, E.; Denardin, J.C.; Escrig, J.; Marco, J.F.; Ortiz, J.; Gautier, J.L. Physical and electrochemical study of cobalt oxide nano- and microparticles. Mater. Charact. 2014, 93, 191–197. [Google Scholar] [CrossRef]
- He, T.; Chen, D.R.; Jiao, X.L.; Wang, Y.L. Co3O4 nanoboxes: Surfactant-templated fabrication and microstructure characterization. Adv. Mater. 2006, 18, 1078–1082. [Google Scholar] [CrossRef]
- Taibi, M.; Ammar, S.; Jouini, N.; Fiévet, F.; Molinié, P.; Drillon, M. Layered nickel hydroxide salt: Synthesis, characterization and magnetic behaviour in relation to the basal spacing. J. Mater. Chem. 2002, 12, 3238–3244. [Google Scholar] [CrossRef]
- Wang, L.; Lin, C.; Zhang, F.; Jin, J. Phase transformation guided single-layer β-Co(OH)2 nanosheets for pseudocapacitive electrodes. ACS Nano. 2014, 8, 3724–3734. [Google Scholar] [CrossRef] [PubMed]
- George, G.; Anandhan, S. A comparative study on physico-chemical properties of sol-gel electrospun cobalt oxide nanofibers from two difference polymeric binders. RSC Adv. 2015, 5, 81429–81437. [Google Scholar] [CrossRef]
- Roquerol, F.; Roquerol, J.; Sing, K. Adsorption by Powders and Porous Solids: Principles, Methodology and Applications; Academic Press: San Diego, CA, USA, 1999. [Google Scholar]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Physical Electronics Press: Eden Prairie, MN, USA, 1995. [Google Scholar]
- Rodella, C.B.; Barrett, D.H.; Moya, S.F.; Figueroa, S.J.A.; Pimenta, M.T.B.; Curvelo, A.A.S.; Silva, V.T.D. Physical and Chemical Studies of Tungsten Carbide Catalysts: Effects of Ni Promotion and Sulphonated Carbon. RSC Adv. 2015, 30, 23874–23885. [Google Scholar] [CrossRef]
- Nguyen-Huy, C.; Lee, J.; Seo, J.H.; Yang, E.; Lee, J.; Choi, K.; Lee, H.; Kim, J.H.; Lee, M.S.; Joo, S.H.; et al. An Structure-Dependent Catalytic Properties of Mesoporous Cobalt Oxides in Furfural Hydrogenation. Appl. Catal. A Gen. 2019, 583, 117125. [Google Scholar] [CrossRef]
- Li, Y.; Tan, B.; Wu, Y. Mesoporous Co3O4 Nanowire Arrays for Lithium Ion Batteries with High Capacity and Rate Capability. Nano Lett. 2008, 8, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Damjanović, L.; Majchrzak, M.; Bennici, S.; Auroux, A. Determination of the heat evolved during sodium borohydride hydrolysis catalyzed by Co3O4. Int. J. Hydrog. Energy 2011, 36, 1991–1997. [Google Scholar] [CrossRef]
- Wei, L.; Dong, X.; Ma, M.; Lu, Y.; Wang, D.; Zhang, S.; Zhao, D.; Wang, Q. Co3O4 hollow fiber: An efficient catalyst precursor for hydrolysis of sodium borohydride to generate hydrogen. Int. J. Hydrog. Energy 2018, 43, 1529–1533. [Google Scholar] [CrossRef]
- Demirci, U.B.; Miele, P. Cobalt in NaBH4 hydrolysis, Phys. Chem. Chem. Phys. 2012, 12, 14651–14665. [Google Scholar] [CrossRef]
- Antonels, N.C.; Meijboom, R. Preparation of well-defined dendrimer encapsulated ruthenium nanoparticles and their evaluation in the reduction of 4-nitrophenol according to the Lanmuir-Hinshelwood approach. Langmuir 2013, 29, 13433–13442. [Google Scholar] [CrossRef]
- Wunder, S.; Lu, Y.; Albrecht, M.; Ballauf, M. Catalytic activity of faceted gold nanoparticles studied by a model reaction: Evidence for substrate-induced surface restructuring. ACS Catal. 2011, 1, 908–916. [Google Scholar] [CrossRef]
- Wunder, S.; Polzer, F.; Lu, Y.; Mei, Y.; Ballauf, M. Kinetic analysis of catalytic reduction of 4-nitrophenol by metallic nanoparticles immobilized in spherical polyelectrolyte brushes. J. Phys. Chem. C 2010, 114, 8814–8820. [Google Scholar] [CrossRef]
- Gu, S.; Kaiser, J.; Marzun, G.; Ott, Lu, A.Y.; Ballauff, M.; Zaccone, A.; Barcikowski, S.; Wagener, P. Ligand-free bold nanoparticles as a reference material for kinetics modelling of catalytic reduction of 4-nitrophenol. Catal. Lett. 2015, 145, 1105–1112. [Google Scholar] [CrossRef]
- Nasaruddin, R.R.; Chen, T.; Li, J.; Goswami, N.; Zhang, J.; Yan, N.; Xie, J. Ligands modulate reaction pathway in the hydrogenation of 4-nitrophenol catalyzed by gold nanoclusters. ChemCatChem 2018, 10, 395–402. [Google Scholar] [CrossRef]
- Kong, X.; Zhu, H.; Chen, C.; Huang, G.; Chen, Q. Insights into the reduction of 4-nitrophenol to 4-aminophenol on catalysts. Chem. Phys. Lett. 2017, 684, 148–152. [Google Scholar] [CrossRef]
- Kästner, C.; Thünemann, A. Catalytic reduction of 4-nitrophenol using silver nanoparticles with adjustable activity. Langmuir 2016, 32, 7383–7391. [Google Scholar] [CrossRef] [PubMed]
- Lara, L.R.S.; Zottis, A.D.; Elias, W.C.; Faggion, D., Jr.; de Campos, C.E.M.; Acuña, J.J.S.; Domingos, J.B. The catalytic evaluation of in situ grown Pd nanoparticles on the surface of Fe3O4@dextran particles in the p-nitrophenol reduction reaction. RSC Adv. 2015, 5, 8289–8296. [Google Scholar] [CrossRef]
- Jiang, Z.; Xie, J.; Jiang, D.; Wei, X.; Chen, M. Modifiers-assisted formation of nickel nanoparticles and their catalytic application to p-nitrophenol reduction. Cryst. Eng. Comm. 2013, 15, 560–569. [Google Scholar] [CrossRef]
- Jin, L.; Zhao, X.; Ye, J.; Qian, X.; Dong, M. MOF-derived magnetic Ni-carbon submicrorods for the catalytic reduction of 4-nitrophenol. Catal. Commun. 2018, 107, 43–47. [Google Scholar] [CrossRef]
- Lin, F.-H.; Doong, R.-A. Bifunctional Au-Fe3O4 heterostructures for magnetically recyclable catalysis of nitrophenol reduction. J. Phys. Chem. C 2011, 115, 6591–6598. [Google Scholar] [CrossRef]
- Eley, D.; Rideal, E.K. Parahydrogen Conversion on Tungsten. Nature 1940, 146, 401–402. [Google Scholar] [CrossRef]
- Khalavka, Y.; Becker, J.; Sönnichsen, C. Synthesis of rod-shaped gold nanorattles with improved plasmon sensitivity and catalytic activity. J. Am. Chem. Soc. 2009, 131, 1871–1875. [Google Scholar] [CrossRef]
- Panacek, A.; Prucek, R.; Hrbac, J.; Nevecna, T.; Steffkova, J.; Zboril, R.; Kvitek, L. Polyacrylate-assisted size control of silver nanoparticles and their catalytic activity. Chem. Mater. 2014, 26, 1332–1339. [Google Scholar] [CrossRef]
- Xu, W.; Kong, J.S.; Yeh, Y.-T.E.; Chen, P. Single-molecule nanocatalysis reveals heterogeneous reaction pathways and catalytic dynamics. Nat. Mater. 2008, 7, 992–996. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Xu, W.; Liu, G.; Panda, D.; Chen, P. Size-dependant catalytic activity and dynamics of gold nanoparticles at the single-molecule level. J. Am. Chem. Soc. 2010, 132, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Ansar, S.M.; Kitchens, C.L. Impact of gold nanoparticle stabilizing ligands on the colloidal catalytic reduction of 4-nitropenol. ACS Catal. 2016, 6, 5553–5560. [Google Scholar] [CrossRef]
- Mei, Y.; Sharma, G.; Lu, Y.; Ballauff, M.; Drechsler, M.; Irrgang, T.; Kempe, R. High catalytic activity of platinum nanoparticles immobilized on spherical polyelectrolyte brushes. Langmuir 2005, 21, 12229–12234. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Mei, Y.; Walker, R.; Ballauff, M.; Drechsler, M. ‘Nano-tree’-type spherical polymer brush particles as templates for metallic nanoparticles. Polymer 2006, 47, 4985–4995. [Google Scholar] [CrossRef]
- Mei, Y.; Lu, Y.; Polzer, F.; Ballauff, M. Catalytic activity of palladium nanoparticles encapsulated in spherical polyelectrolyte brushes and core-shell microgels. Chem. Mater. 2007, 19, 1062–1069. [Google Scholar] [CrossRef]
- Kalekar, A.M.; Sharma, K.K.K.; Lehoux, A.; Audonnnet, F.; Remita, H.; Saha, A.; Sharma, G.K. Investigation into catalytic activity of porous platinum nanostructures. Langmuir 2013, 29, 11431–11439. [Google Scholar] [CrossRef]
- Menumerov, E.; Hughes, R.A.; Neretina, S. Catalytic reduction of 4-nitrophenol: A quantitative assessment of the role of dissolved oxygen in determining the induction time. Nano Lett. 2016, 16, 7791–7797. [Google Scholar] [CrossRef]
- Farhadi, S.; Kazem, M.; Siadatnasab, F. NiO nanoparticles prepared via thermal decomposition of the bis(dimethylglyoximato)nickel(II) complex: A novel reusable heterogeneous catalyst for fast and efficient microwave-assisted reduction of nitroarenes with ethanol. Polyhedron 2011, 30, 606–613. [Google Scholar] [CrossRef]
- Li, M.; Chen, G. Revisiting catalytic model reaction p-nitrophenol/NaBH4 using metallic nanoparticles coated on polymeric spheres. Nanoscale 2013, 5, 11919–11927. [Google Scholar] [CrossRef]
- Cho, K.Y.; Seo, H.Y.; Yeom, Y.S.; Kumar, P.; Lee, A.S.; Baek, K.-Y.; Yoon, H.G. STable 2D-structured supports incorporating ionic block copolymer-wrapped carbon nanotubes wit graphene oxide toward compact decoration of metal nanoparticles and high-performance nano-catalysis. Carbon 2016, 105, 340–352. [Google Scholar] [CrossRef]
- Hwang, J.; Siddique, A.B.; Kim, Y.J.; Lee, H.; Maeng, J.H.; Ahn, Y.; Lee, J.S.; Kim, H.S.; Lee, H. Ionic cellulose-stabilized gold nanoparticles and their application in the catalytic reduction of 4-nitrophenol. RSC Adv. 2018, 8, 1758–1763. [Google Scholar] [CrossRef]
- Ballarin, B.; Barreca, D.; Boanini, E.; Bonansegna, E.; Cassani, M.C.; Carraro, G.; Fazzini, S.; Mignani, A.; Nanni, D.; Pinelli, D. Functionalization of silica through thiol-yne radical chemistry: A catalytic system based on gold nanoparticles support on amino-sulfide-branched silica. RSC Adv. 2016, 6, 25780–25788. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Z.; Li, T.; Ye, J.; Shen, L.; She, Z.; Liu, F. Catalytic PVDF membrane for continuous reduction and separation of p-nitrophenol and methylene blue in emulsified oil solution. Chem. Eng. J. 2018, 334, 579–586. [Google Scholar] [CrossRef]
- Javaid, R.; Ksawasaki, S.-i.; Suzuki, A.; Suzuki, T.M. Simple and rapid hydrogenation of p-nitrophenol with aqueous formic acid in catalytic flow reactors. Beilstein J. Org. Chem. 2013, 9, 1156–1163. [Google Scholar]
- Huang, R.; Zhu, H.; Su, R.; Qi, W.; He, Z. Catalytic membrane reactor immobilized with alloy nanoparticle-loaded protein fibrils for continuous reduction of 4-nitrophenol. Environ. Sci. Technol. 2016, 50, 11263–11273. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Mondal, K.; Mukhopadhyay, K.; Prasad, N.E.; Sharma, A. Facile reduction of para-nitrophenol: Catalytic efficiency of silver nanoferns in batch and continuous flow reactors. RSC Adv. 2016, 6, 113981–113990. [Google Scholar] [CrossRef]
- Xu, D.; Wang, F.; Yu, G.; Zhao, H.; Yang, J.; Yuan, M.; Zhang, X.; Dong, Z. Animal-based hypercrosslinked polymer modified with small palladium nanoparticles for efficiently catalytic reduction of nitroarenes. ChemCatChem 2018, 10, 4569–4577. [Google Scholar] [CrossRef]
- Liu, Y.; Guerrouache, M.; Kebe, S.I.; Carbonnier, B.; Le Droumaguet, B. Gold nanoparticles-supported histamine-grafted monolithic capillaries as efficient microreactors for flow-through reduction of nitro-containing compounds. J. Mater. Chem. A 2017, 5, 11805–11814. [Google Scholar] [CrossRef]
- Wu, X.-Q.; Wu, X.-W.; Shen, J.-S.; Zhang, H.-W. In situ formed metal nanoparticle systems for catalytic reduction of nitroaromatic compounds. RSC Adv. 2014, 4, 49287–49294. [Google Scholar] [CrossRef]
- Wu, X.-Q.; Wu, X.-W.; Huang, Q.; Shen, J.-S.; Zhang, H.-W. In situ synthesized gold nanoparticles in hydrogels for catalytic reduction of nitroaromatic compounds. Appl. Surf. Sci. 2015, 331, 210–218. [Google Scholar] [CrossRef]
- Tsao, Y.-C.; Rej, S.; Chiu, C.-Y.; Huang, M.H. Aqueous phase synthesis of Au-Ag core-shell nanocrystals with tunable shapes and their optical and catalytic properties. J. Am. Chem. Soc. 2014, 136, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Shultz, L.R.; Hu, L.; Preradovic, K.; Beazley, M.J.; Feng, X.; Jurca, T. A broader-scope analysis of the catalytic reduction of nitrophenols and azo dyes with noble metal nanoparticles. ChemCatChem 2019, 11, 2590–2595. [Google Scholar] [CrossRef]
Sample Availability: Not available. |

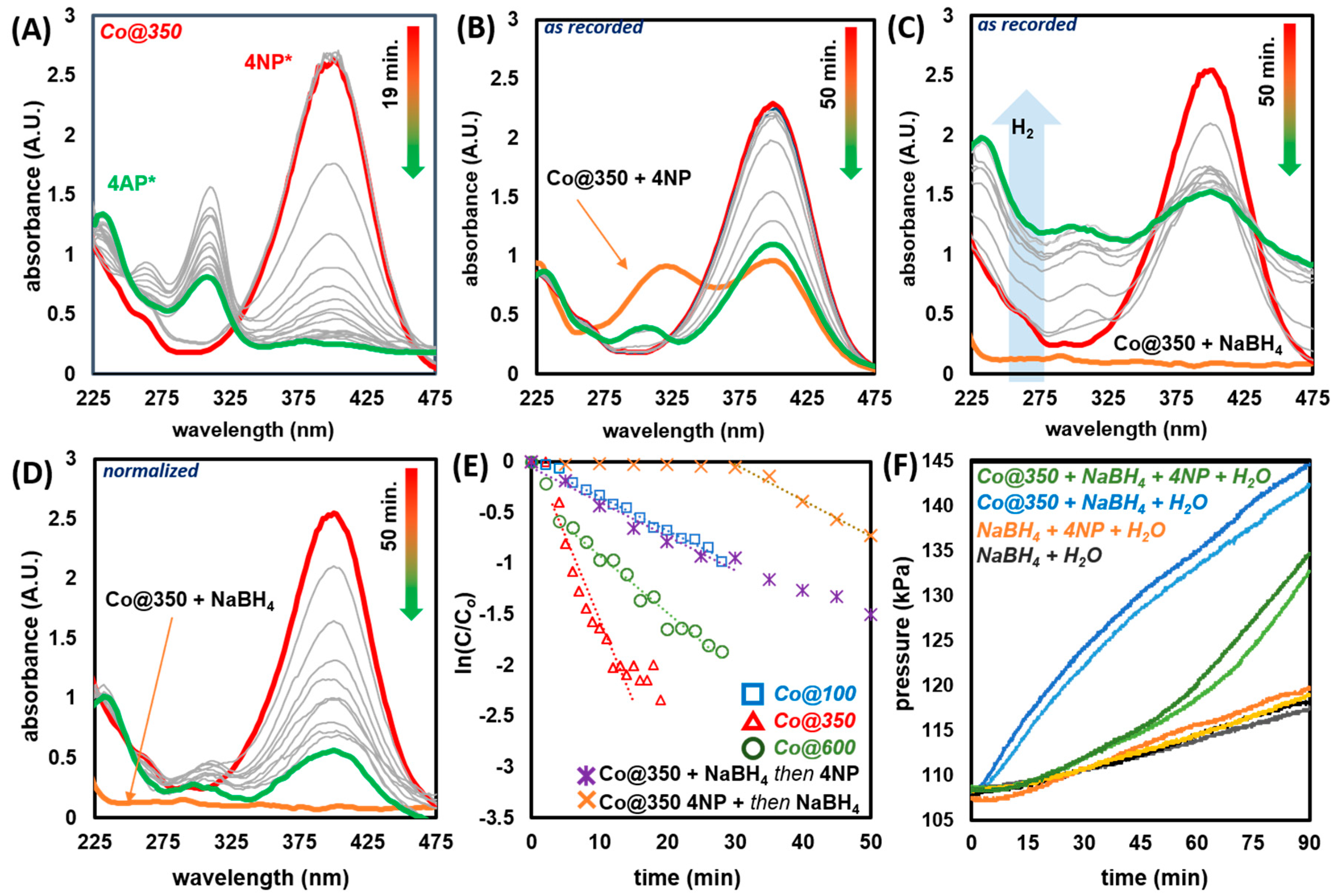
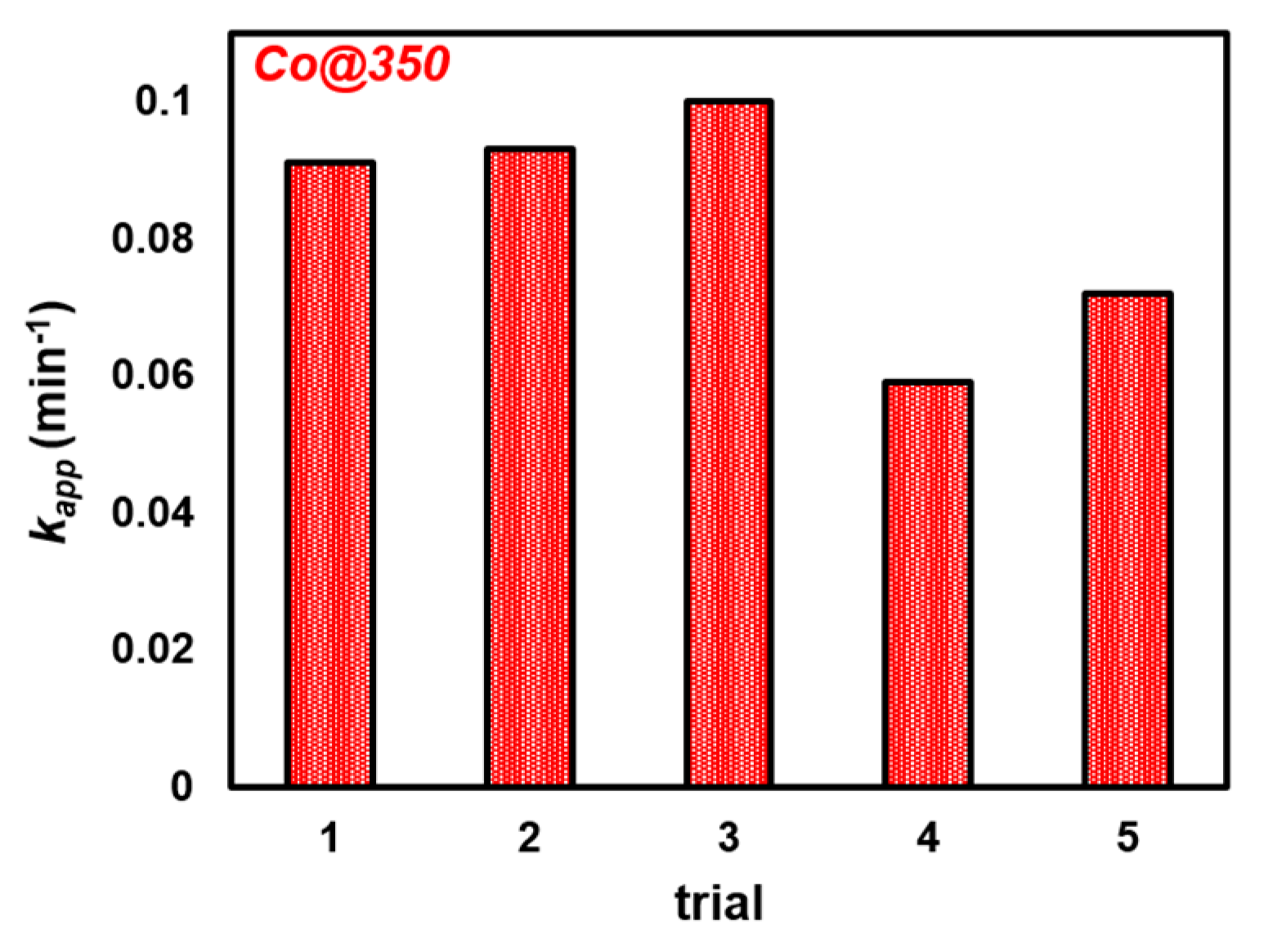

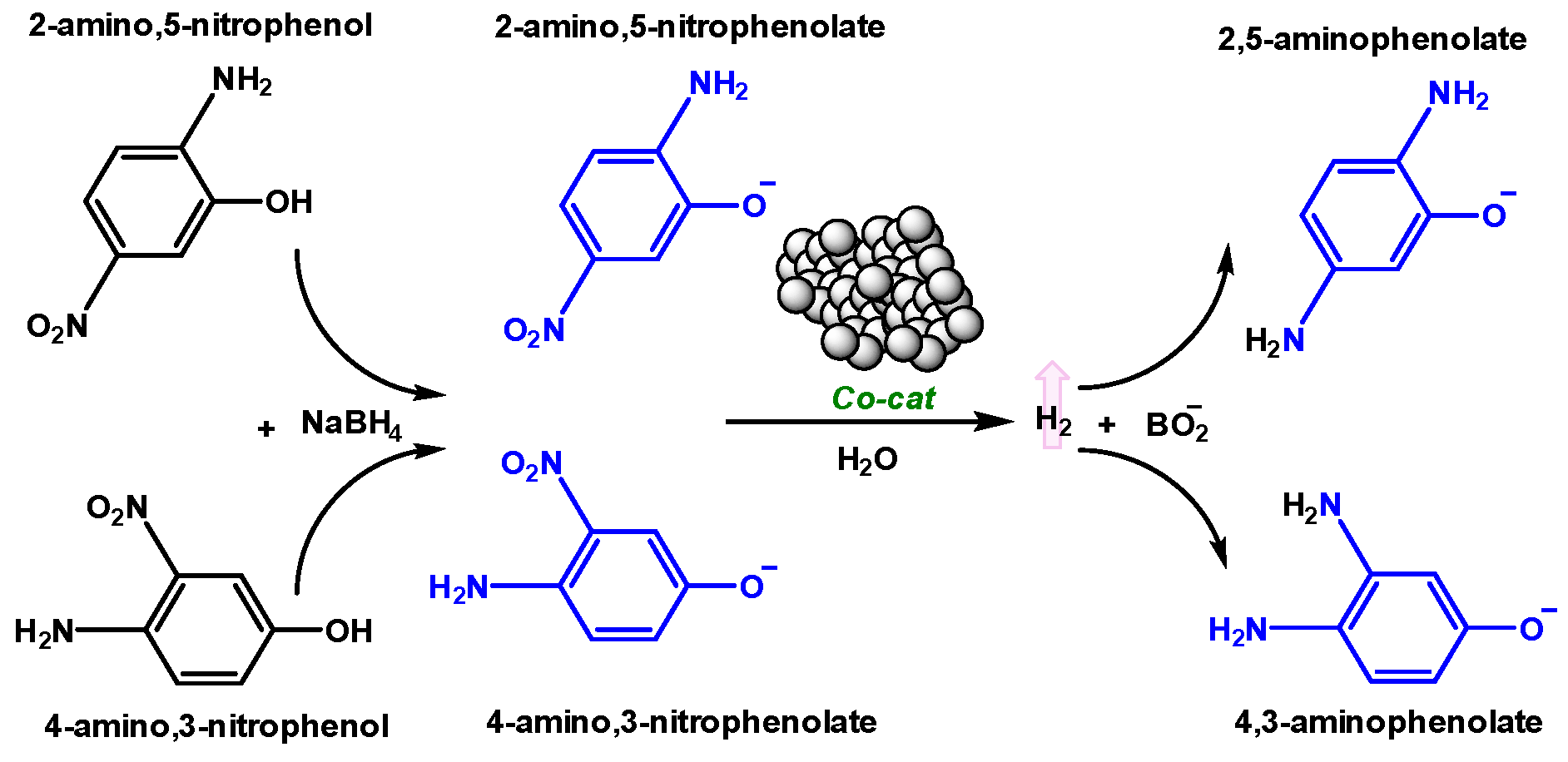
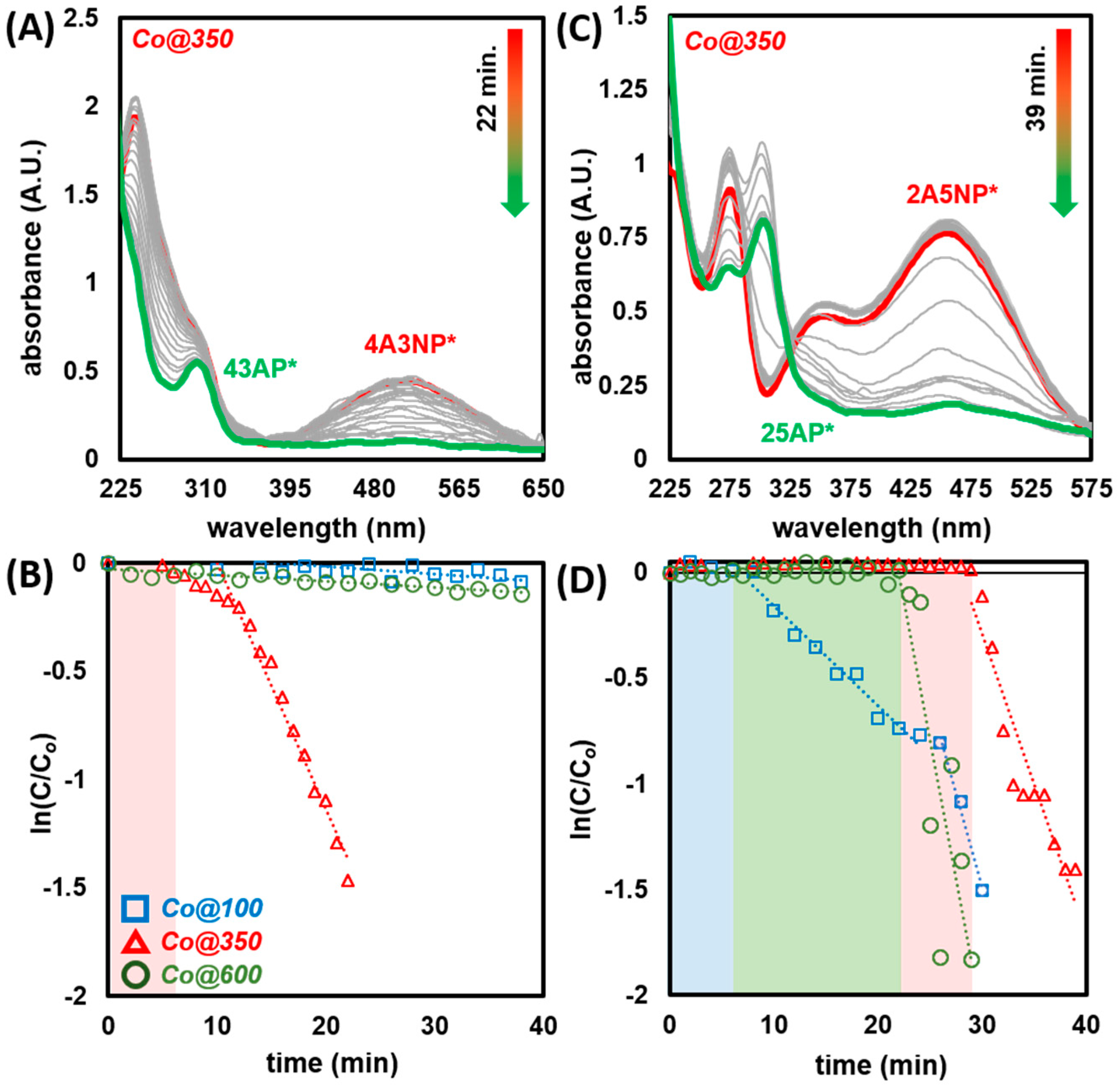
| Cat. | Substrate. | Change | kapp (min−1) | Induction (min) |
|---|---|---|---|---|
| -- | 4NP | -- | no rxn | -- |
| Co@100 | 4NP | -- | 0.033 | 2 |
| Co@350 | 4NP | -- | 0.189 | 2 |
| Co@600 | 4NP | -- | 0.057 | <2 |
| Co@100 | 4NP | no NaBH4 | no rxn | -- |
| Co@350 | 4NP | no NaBH4 | no rxn | -- |
| Co@600 | 4NP | no NaBH4 | no rxn | -- |
| Co@350 | 4NP | +1 mg KOH | 0.132 | 1 |
| Co@350 | 4NP | +NaBH4 last | 0.035 | 30 |
| Co@350 | 4NP | +4NP last | 0.032 | <5 |
| KOH | 4NP | -- | no rxn | -- |
| Co3O4 | 4NP | -- | no rxn | -- |
| Co@100 | 4A3NP | -- | 0.004 | 30 |
| Co@350 | 4A3NP | -- | 0.114 | 6 |
| Co@600 | 4A3NP | -- | 0.003 | 16 |
| Co@100 | 2A5NP | -- | 0.047/0.175 | 8 |
| Co@350 | 2A5NP | -- | 0.143 | 28 |
| Co@600 | 2A5NP | -- | 0.264 | 22 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shultz, L.R.; McCullough, B.; Newsome, W.J.; Ali, H.; Shaw, T.E.; Davis, K.O.; Uribe-Romo, F.J.; Baudelet, M.; Jurca, T. A Combined Mechanochemical and Calcination Route to Mixed Cobalt Oxides for the Selective Catalytic Reduction of Nitrophenols. Molecules 2020, 25, 89. https://doi.org/10.3390/molecules25010089
Shultz LR, McCullough B, Newsome WJ, Ali H, Shaw TE, Davis KO, Uribe-Romo FJ, Baudelet M, Jurca T. A Combined Mechanochemical and Calcination Route to Mixed Cobalt Oxides for the Selective Catalytic Reduction of Nitrophenols. Molecules. 2020; 25(1):89. https://doi.org/10.3390/molecules25010089
Chicago/Turabian StyleShultz, Lorianne R., Bryan McCullough, Wesley J. Newsome, Haider Ali, Thomas E. Shaw, Kristopher O. Davis, Fernando J. Uribe-Romo, Matthieu Baudelet, and Titel Jurca. 2020. "A Combined Mechanochemical and Calcination Route to Mixed Cobalt Oxides for the Selective Catalytic Reduction of Nitrophenols" Molecules 25, no. 1: 89. https://doi.org/10.3390/molecules25010089
APA StyleShultz, L. R., McCullough, B., Newsome, W. J., Ali, H., Shaw, T. E., Davis, K. O., Uribe-Romo, F. J., Baudelet, M., & Jurca, T. (2020). A Combined Mechanochemical and Calcination Route to Mixed Cobalt Oxides for the Selective Catalytic Reduction of Nitrophenols. Molecules, 25(1), 89. https://doi.org/10.3390/molecules25010089









