Photochromic and Photocatalytic Properties of Ultra-Small PVP-Stabilized WO3 Nanoparticles
Abstract
1. Introduction
2. Results
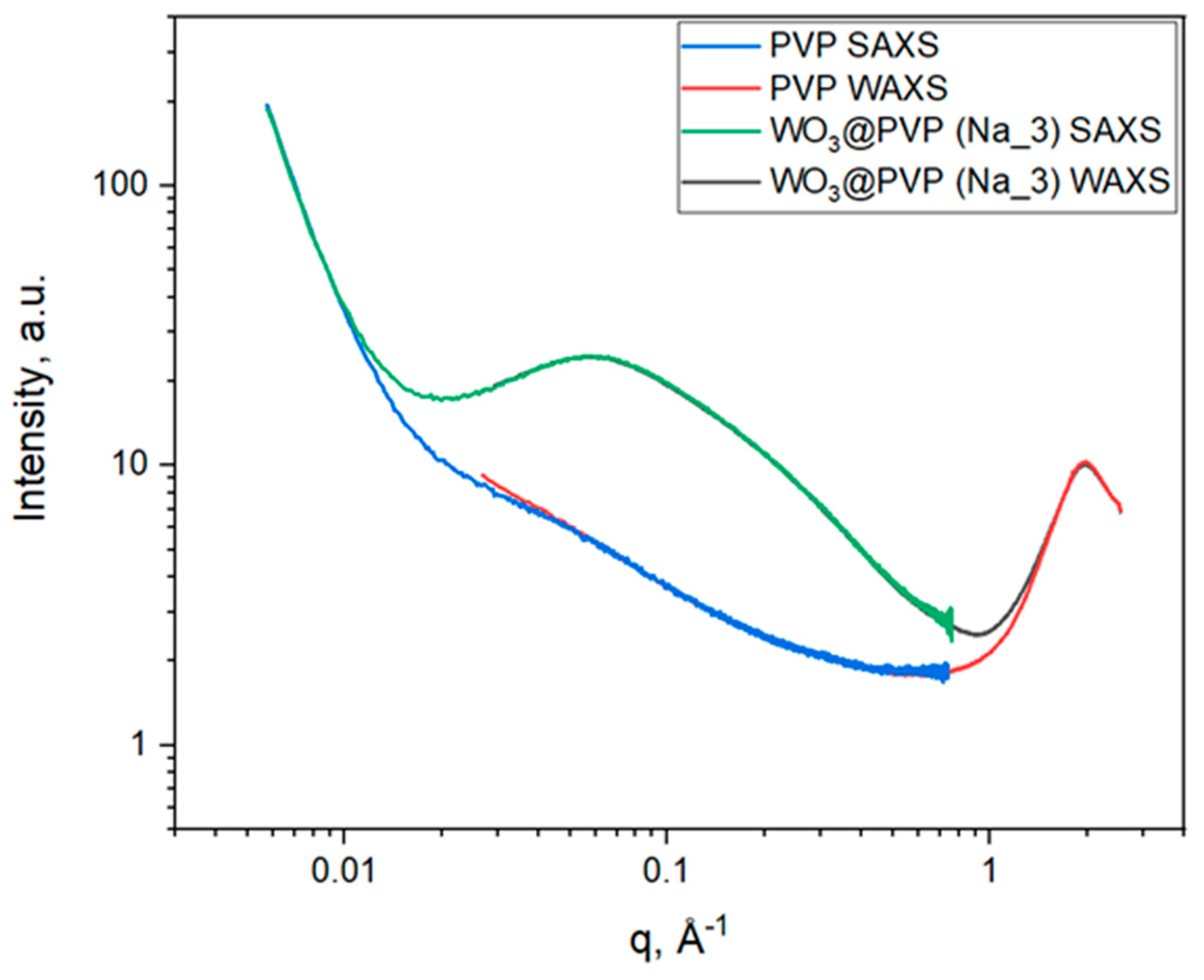
2.1. Small- and Wide-Angle X-ray Scattering
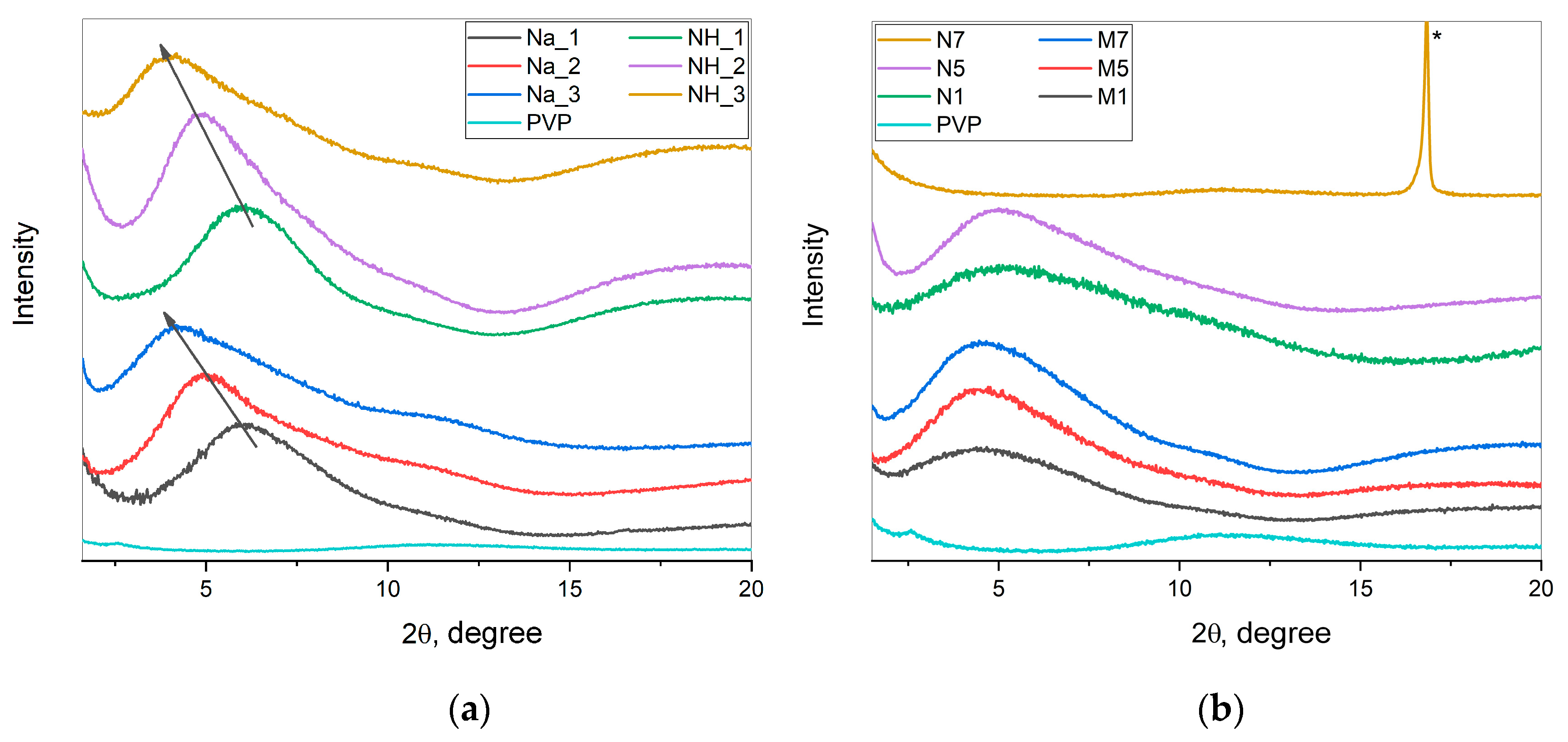
2.2. X-ray Diffraction
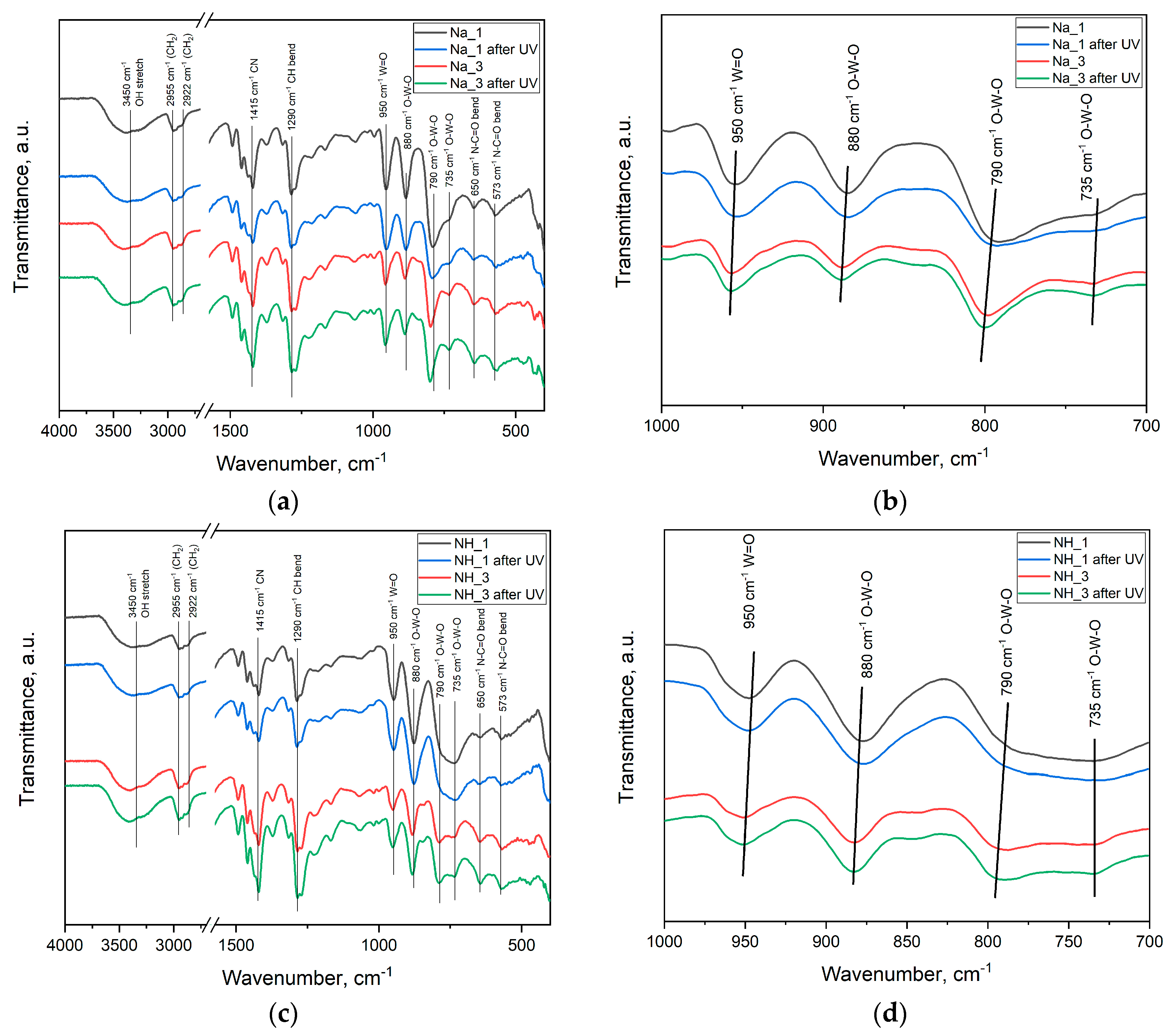
2.3. Fourier-Transform Infrared Spectroscopy
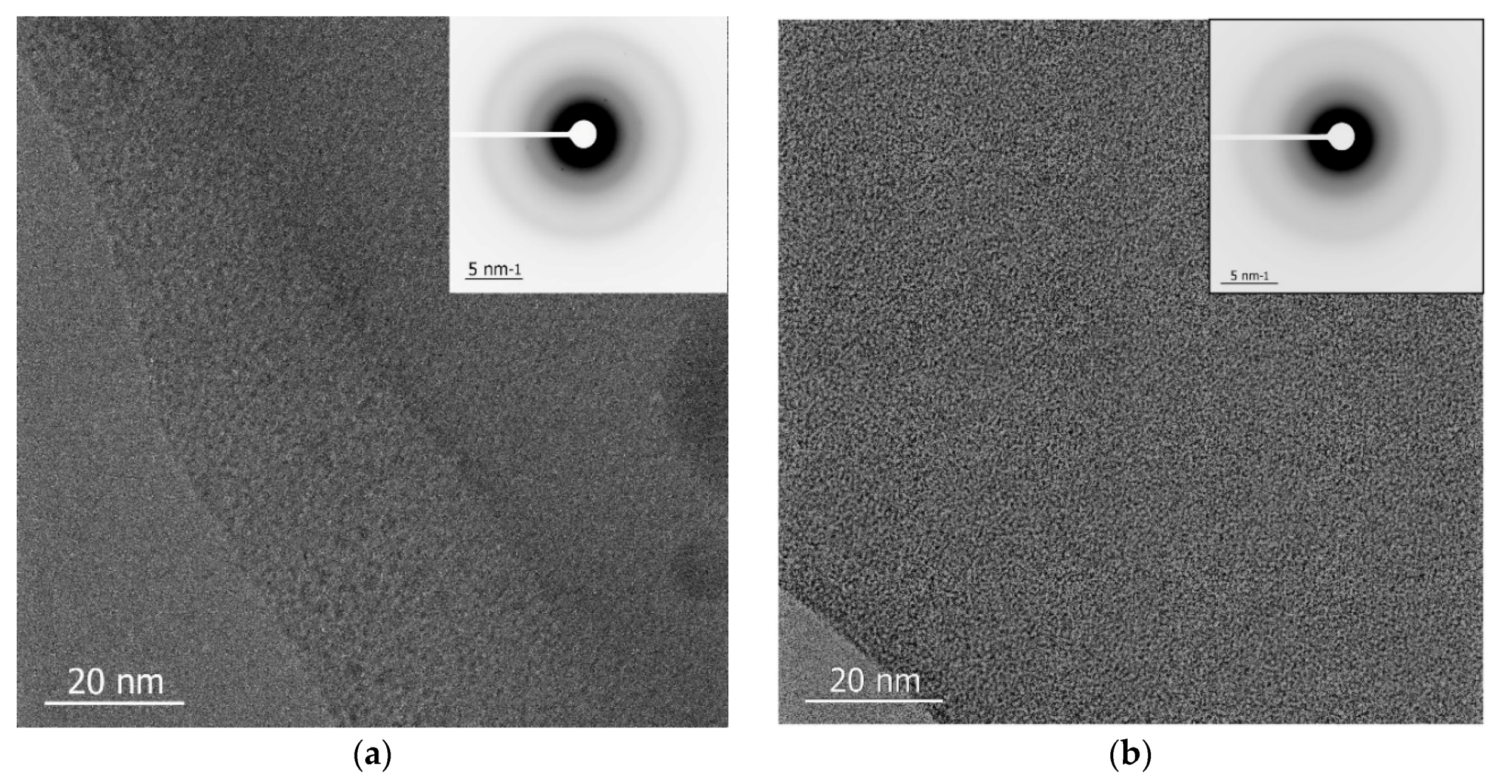
2.4. Transmission Electron Microscopy
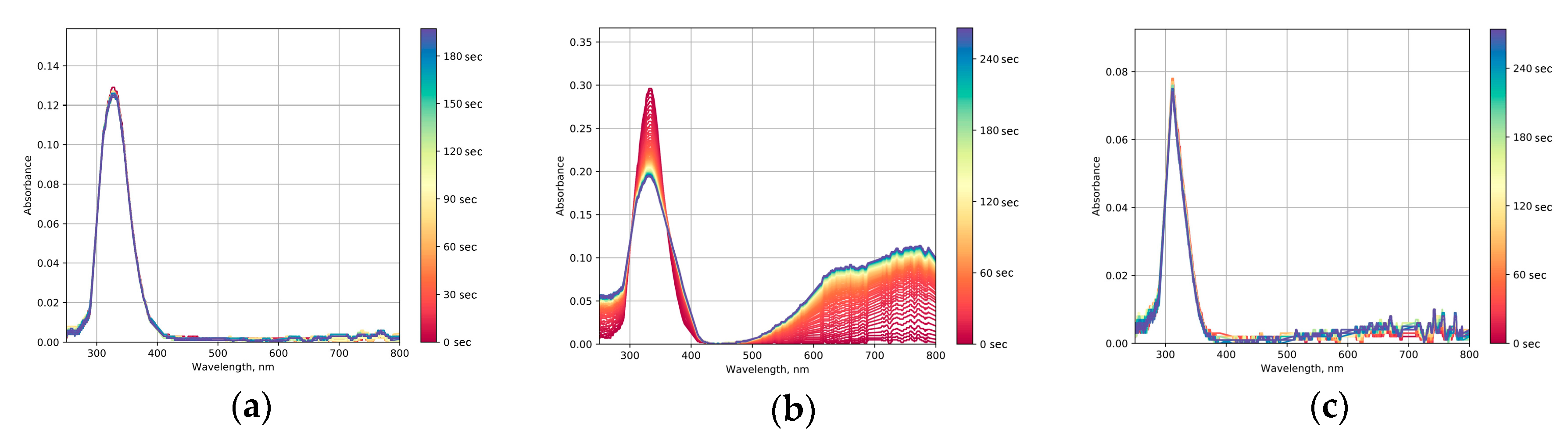
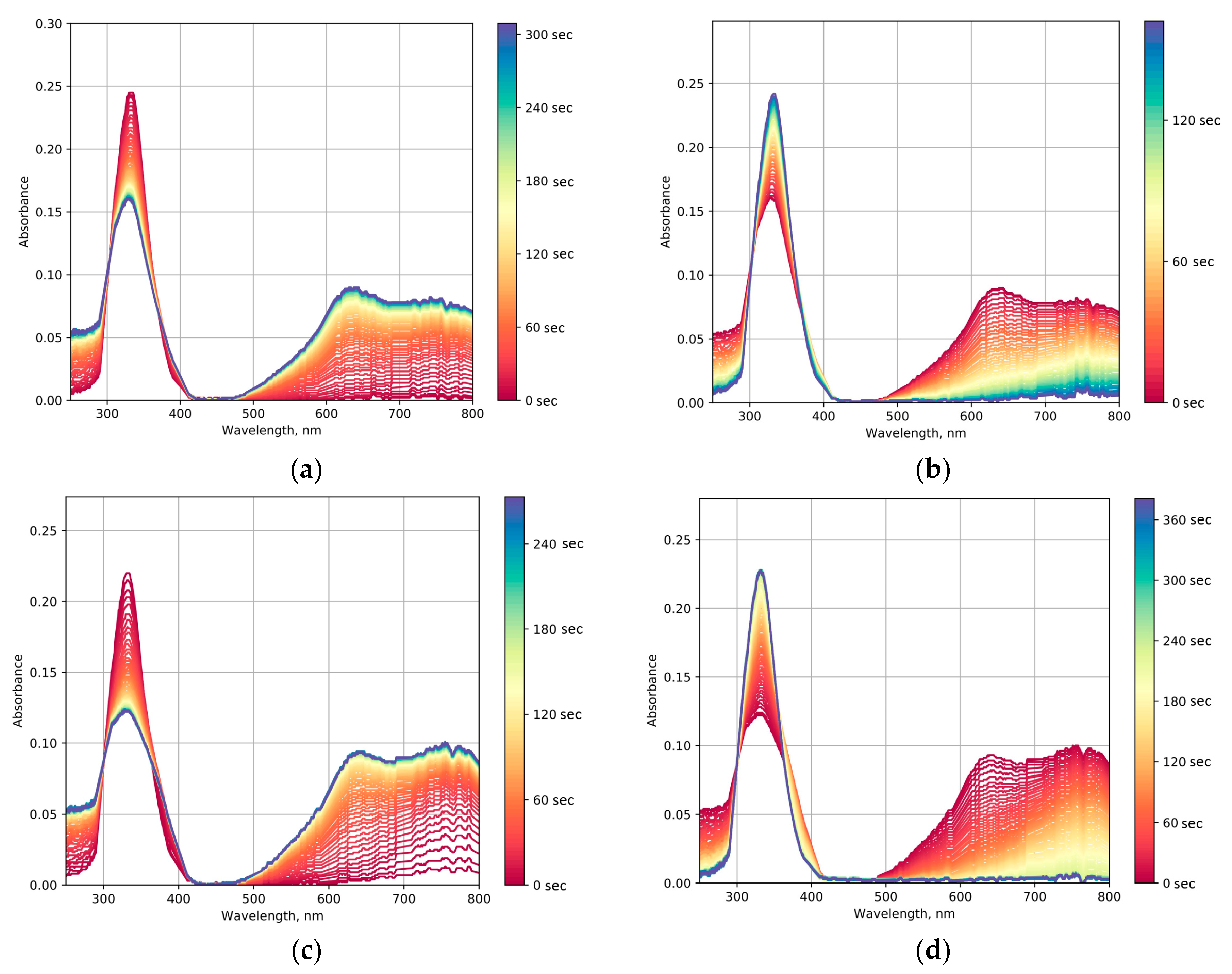
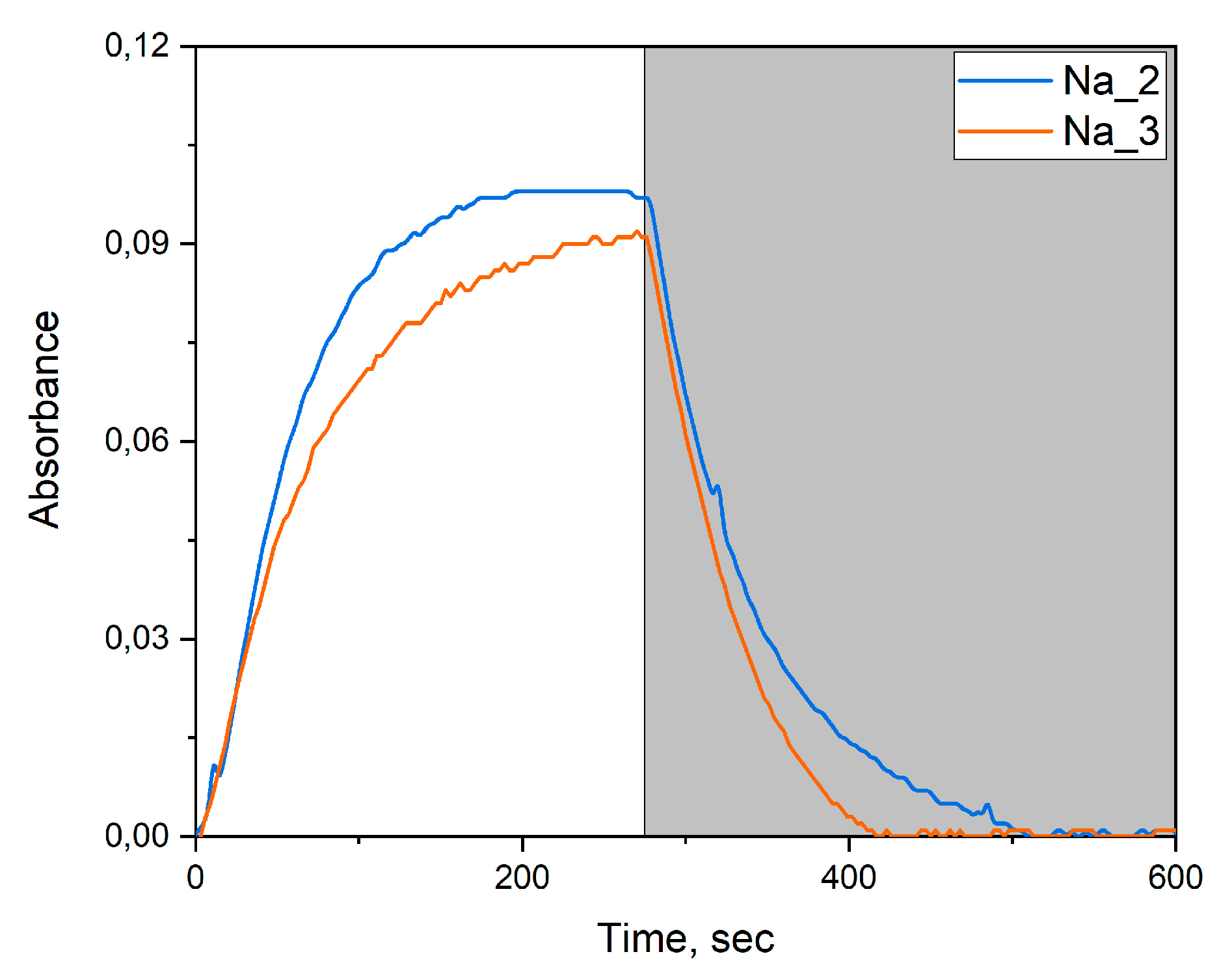
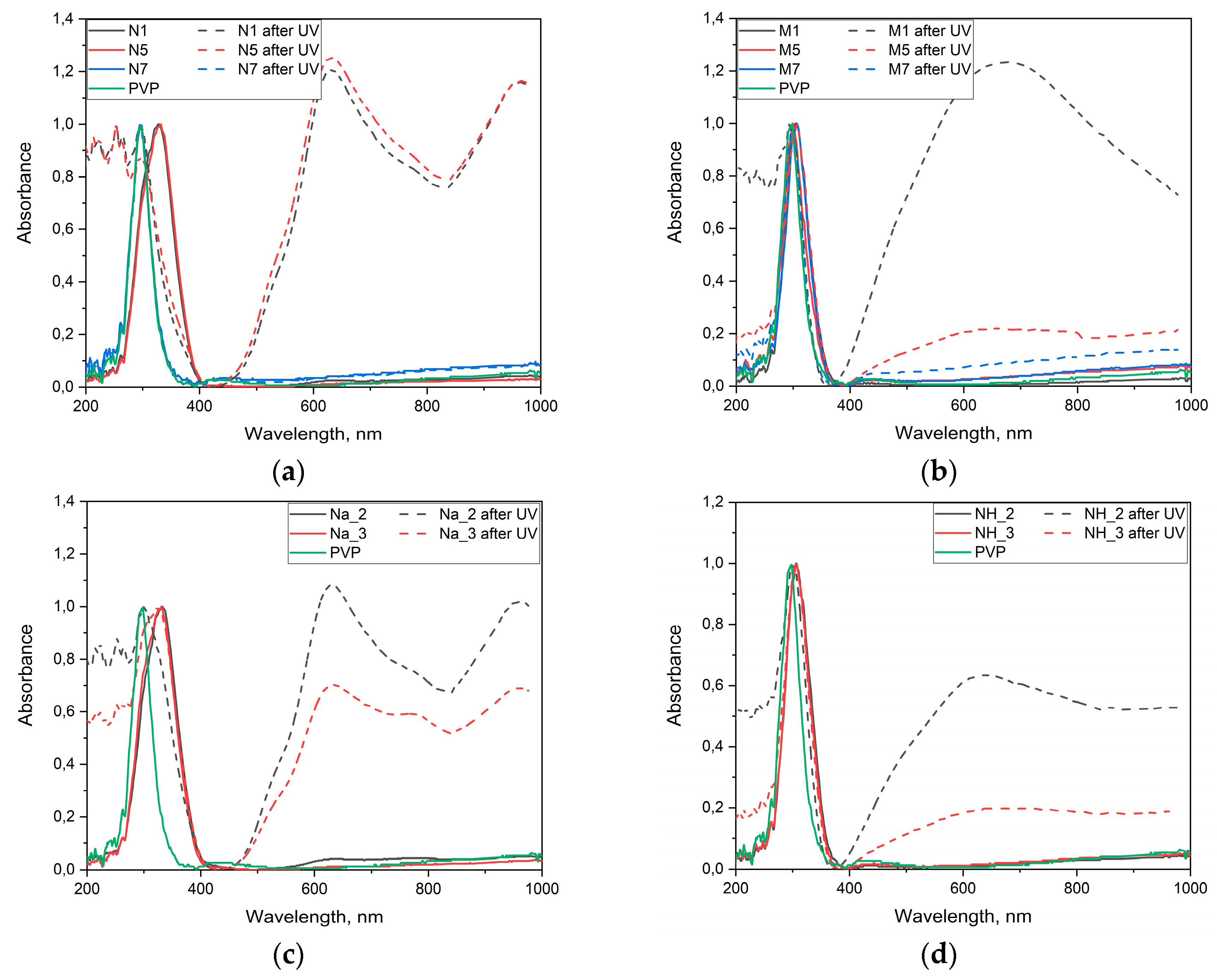
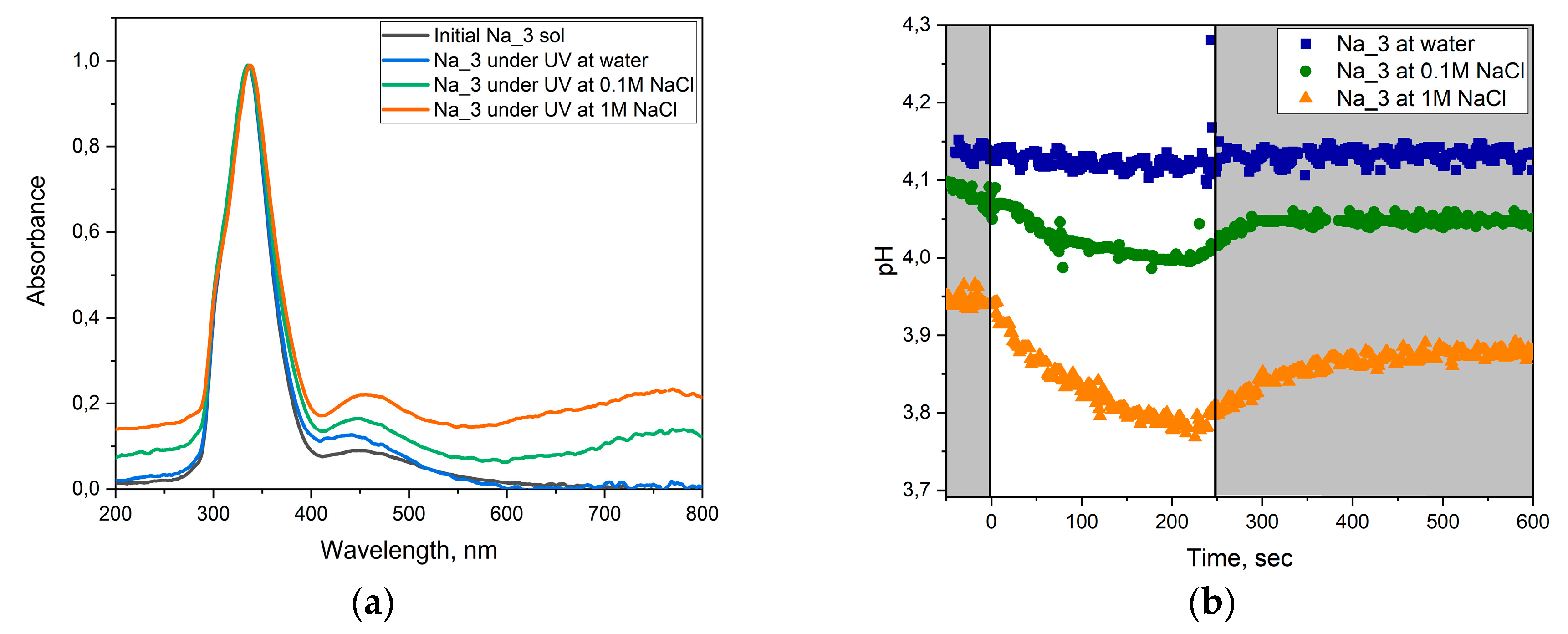
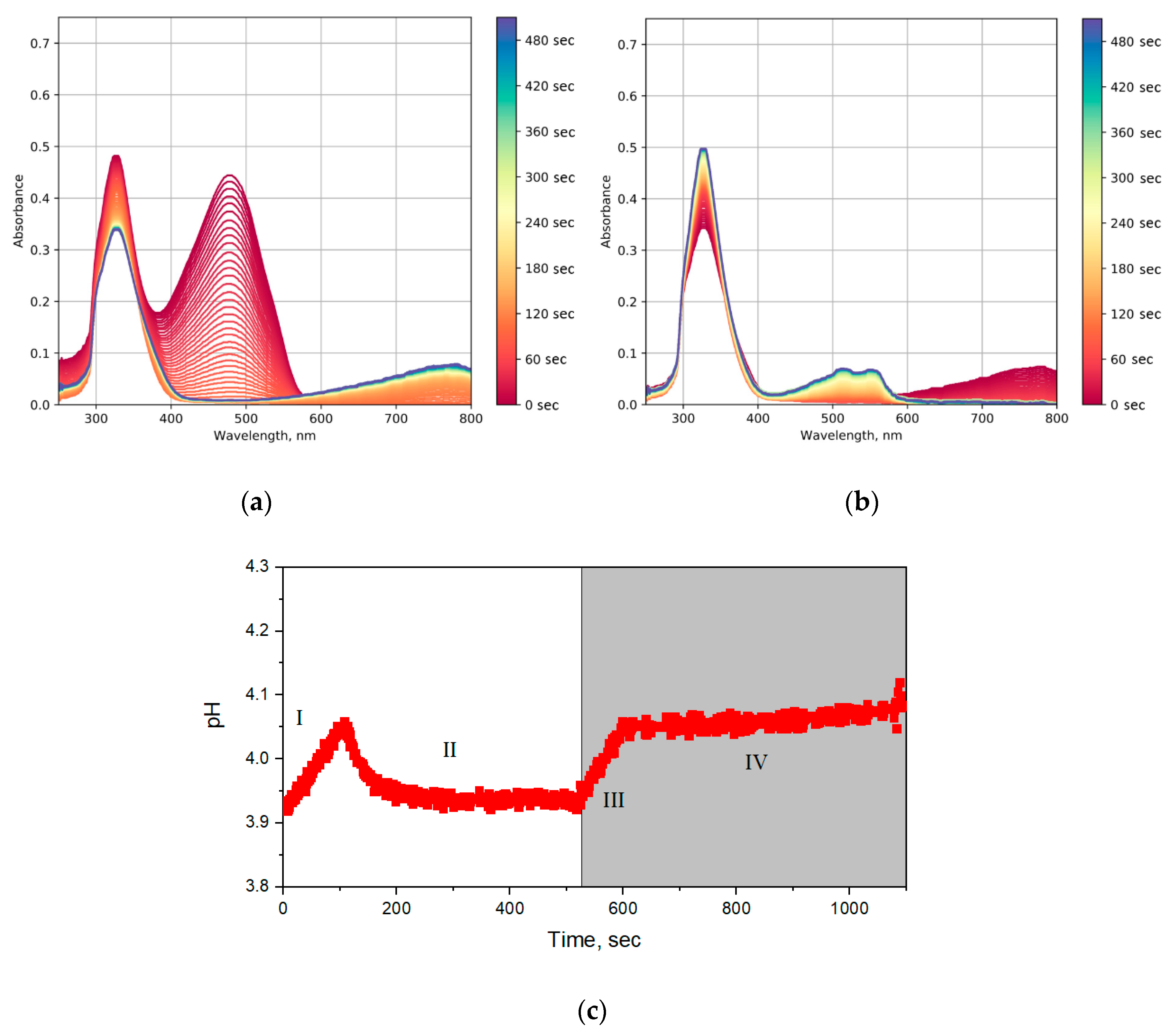
2.5. Photochromic Tests
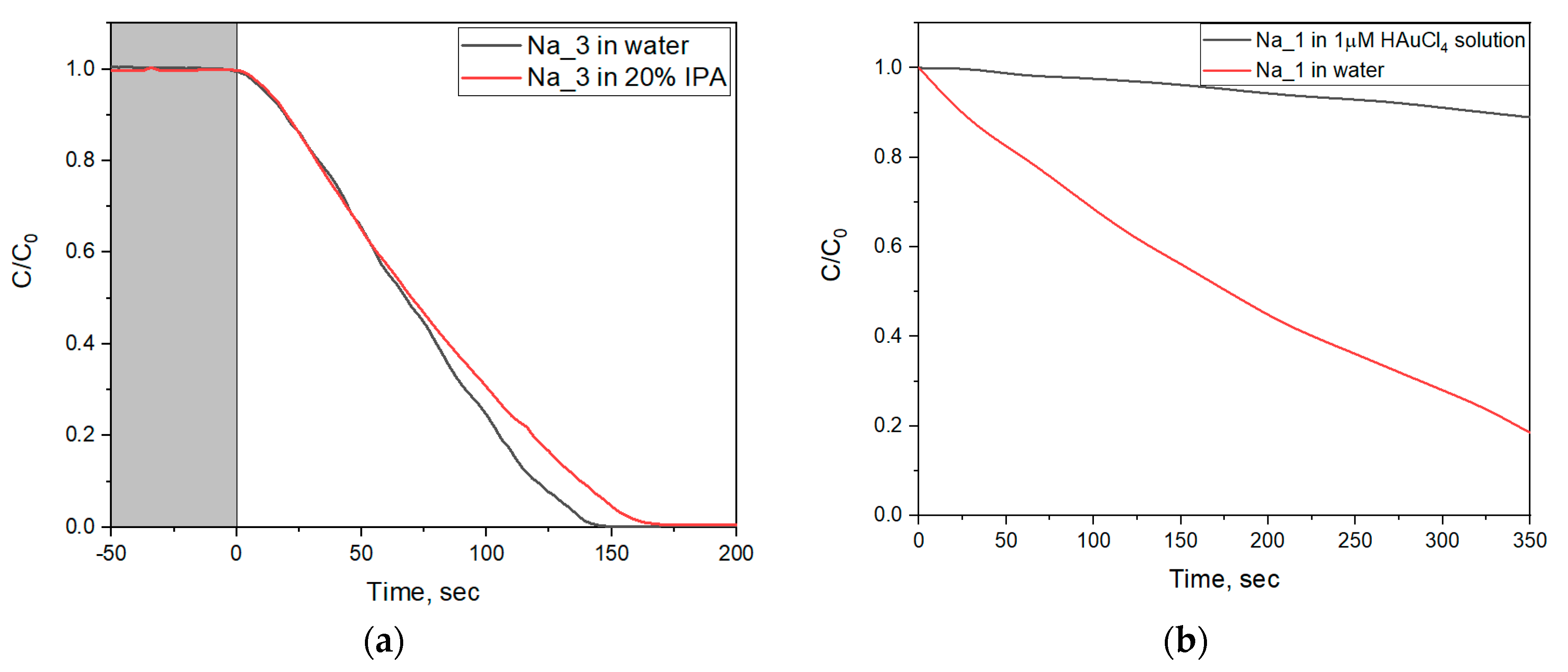
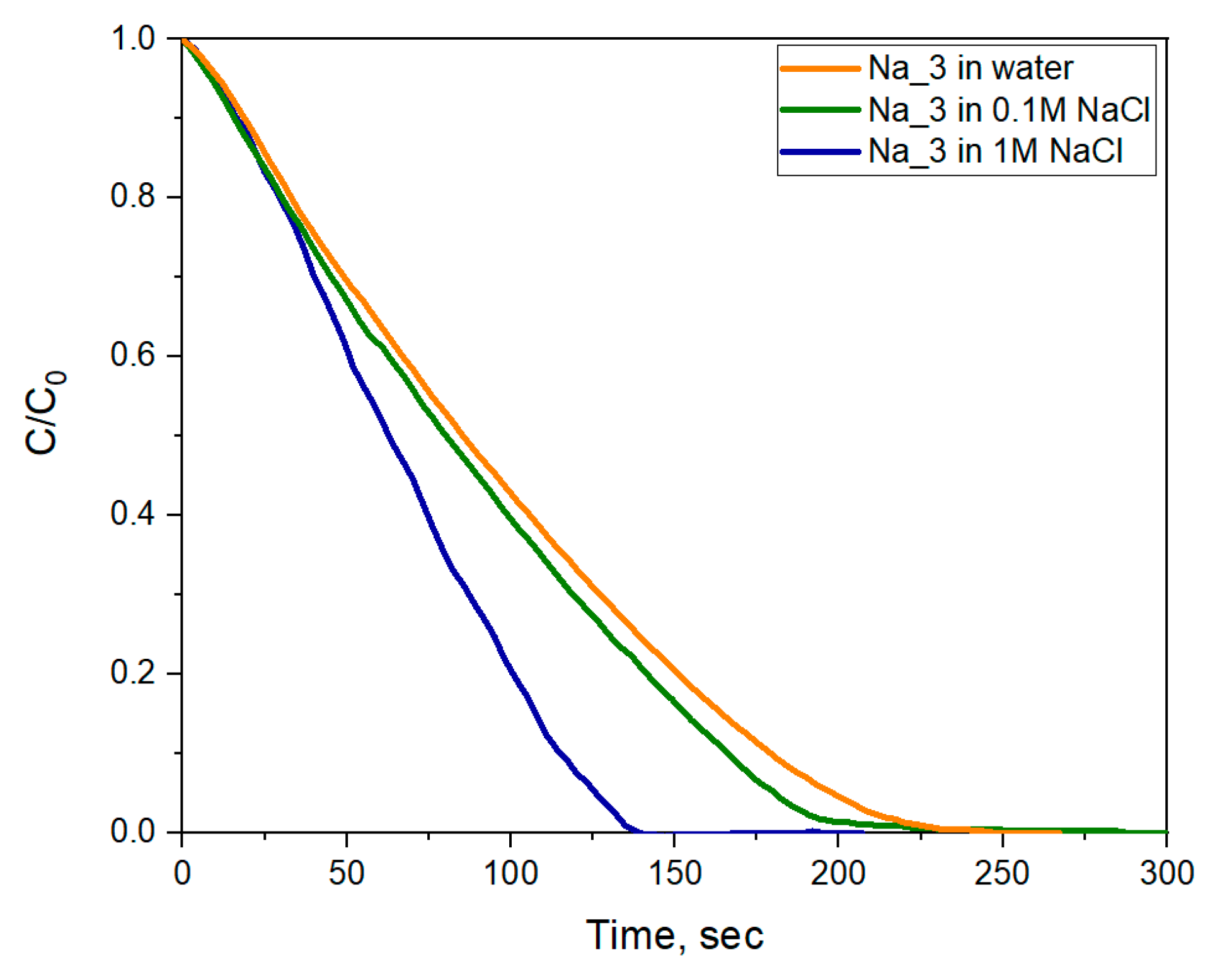
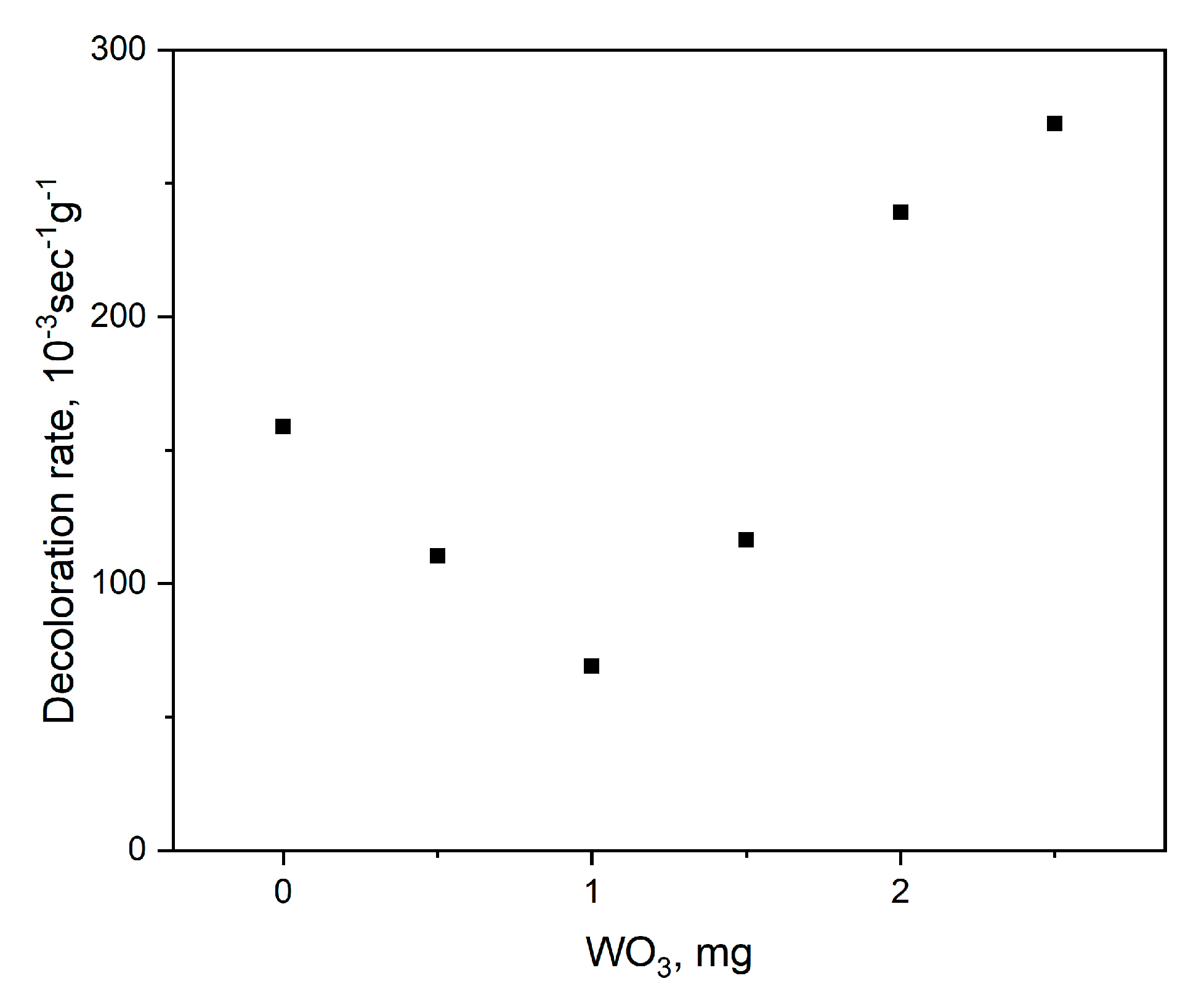
2.6. Photocatalytic Dye Discoloration
3. Materials and Methods
3.1. Materials
3.2. Preparation of Sols
3.3. Characterization
3.3.1. XRD
3.3.2. SAXS
3.3.3. TEM
3.3.4. FTIR
3.3.5. Photochromic and Photocatalytic Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, D.; Ye, J. Hierarchical WO3 hollow shells: Dendrite, sphere, dumbbell, and their photocatalytic properties. Adv. Funct. Mater. 2008, 18, 1922–1928. [Google Scholar] [CrossRef]
- Baeck, S.-H.; Choi, K.-S.; Jaramillo, T.F.; Stucky, G.D.; McFarland, E.W. Enhancement of photocatalytic and electrochromic properties of electrochemically fabricated mesoporous WO3 thin films. Adv. Mater. 2003, 15, 1269–1273. [Google Scholar] [CrossRef]
- Hosseini, F.; Rasuli, R.; Jafarian, V. Immobilized WO3 nanoparticles on graphene oxide as a photo-induced antibacterial agent against UV-resistant Bacillus pumilus. J. Phys. D. Appl. Phys. 2018, 51, 145403. [Google Scholar] [CrossRef]
- Han, B.; Popov, A.L.; Shekunova, T.O.; Kozlov, D.A.; Ivanova, O.S.; Rumyantsev, A.A.; Shcherbakov, A.B.; Popova, N.R.; Baranchikov, A.E.; Ivanov, V.K. Highly Crystalline WO3 Nanoparticles Are Nontoxic to Stem Cells and Cancer Cells. J. Nanomater. 2019, 2019, 5384132. [Google Scholar] [CrossRef]
- Hariharan, V.; Radhakrishnan, S.; Parthibavarman, M.; Dhilipkumar, R.; Sekar, C. Synthesis of polyethylene glycol (PEG) assisted tungsten oxide (WO3) nanoparticles for l-dopa bio-sensing applications. Talanta 2011, 85, 2166–2174. [Google Scholar] [CrossRef] [PubMed]
- Popov, A.L.; Ermakov, A.M.; Shekunova, T.O.; Shcherbakov, A.B.; Ermakova, O.N.; Ivanova, O.S.; Popova, N.R.; Baranchikov, A.Y.; Ivanov, V.K. PVP-stabilized tungsten oxide nanoparticles inhibit proliferation of NCTC L929 mouse fibroblasts via induction of intracellular oxidative stress. Nanosyst. Phys. Chem. Math. 2019, 10, 92–101. [Google Scholar] [CrossRef]
- Popov, A.L.; Han, B.; Ermakov, A.M.; Savintseva, I.V.; Ermakova, O.N.; Popova, N.R.; Shcherbakov, A.B.; Shekunova, T.O.; Ivanova, O.S.; Kozlov, D.A.; et al. PVP-stabilized tungsten oxide nanoparticles: pH sensitive anti-cancer platform with high cytotoxicity. Mater. Sci. Eng. C 2020, 108, 110494. [Google Scholar] [CrossRef]
- Li, R.; Zhou, Y.; Shao, Z.; Zhao, S.; Chang, T.; Huang, A.; Li, N.; Ji, S.; Jin, P. Enhanced Coloration/Bleaching Photochromic Performance of WO3 Based on PVP/PU Composite Matrix. ChemistrySelect 2019, 4, 9817–9821. [Google Scholar] [CrossRef]
- Li, N.; Zhao, Y.; Wang, Y.; Lu, Y.; Song, Y.; Huang, Z.; Li, Y.; Zhao, J. Aqueous synthesis and visible-light photochromism of metastable h-WO3 hierarchical nanostructures. Eur. J. Inorg. Chem. 2015, 2015, 2804–2812. [Google Scholar] [CrossRef]
- Deb, S.K. Optical and photoelectric properties and colour centres in thin films of tungsten oxide. Philos. Mag. 1973, 27, 801–822. [Google Scholar] [CrossRef]
- DeJournett, T.J.; Spicer, J.B. Photoinduced Silver Precursor Decomposition for Particle Modification in Tungsten Oxide—Polymer Matrix Nanocomposites. J. Phys. Chem. C 2014, 118, 9820–9831. [Google Scholar] [CrossRef]
- Duncan, R.C.; Faughnan, B.W.; Phillips, W. Photochromics and Cathodochromics Inorganic Photochromic and Cathodochromic Recording Materials. Appl. Opt. 1970, 9, 2236–2243. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wang, J.; Wu, J.; Li, H.; Wang, H.; Li, Z.; Zhou, M.; Zuo, T. Proton exchange growth to mesoporous WO3·0.33 H2O structure with highly photochromic sensitivity. Mater. Lett. 2013, 91, 334–337. [Google Scholar] [CrossRef]
- Bechinger, C.; Burdis, M.S.; Zhang, J.-G. Comparison between electrochromic and photochromic coloration efficiency of tungsten oxide thin films. Solid State Commun. 1997, 101, 753–756. [Google Scholar] [CrossRef]
- Bechinger, C.; Oefinger, G.; Herminghaus, S.; Leiderer, P. On the fundamental role of oxygen for the photochromic effect of WO3. J. Appl. Phys. 1993, 74, 4527–4533. [Google Scholar] [CrossRef]
- Wagner, W.; Rauch, F.; Ottermann, C.; Bange, K. In-depth profiling of hydrogen in oxidic multilayer systems. Surf. Interface Anal. 1990, 16, 331–334. [Google Scholar] [CrossRef]
- Gavrilyuk, A.I. Aging of the nanosized photochromic WO3 films and the role of adsorbed water in the photochromism. Appl. Surf. Sci. 2016, 364, 498–504. [Google Scholar] [CrossRef]
- Wang, S.; Fan, W.; Liu, Z.; Yu, A.; Jiang, X. Advances on tungsten oxide based photochromic materials: Strategies to improve their photochromic properties. J. Mater. Chem. C 2018, 6, 191–212. [Google Scholar] [CrossRef]
- Yamase, T. Photo-and electrochromism of polyoxometalates and related materials. Chem. Rev. 1998, 98, 307–326. [Google Scholar] [CrossRef]
- Jing, X.; Zou, D.; Meng, Q.; Zhang, W.; Zhang, F.; Feng, W.; Han, X. Fabrication and visible-light photochromism of novel hybrid inorganic—Organic film based on polyoxometalates and ethyl cellulose. Inorg. Chem. Commun. 2014, 46, 149–154. [Google Scholar] [CrossRef]
- Popov, A.L.; Zholobak, N.M.; Balko, O.I.; Balko, O.B.; Shcherbakov, A.B.; Popova, N.R.; Ivanova, O.S.; Baranchikov, A.E.; Ivanov, V.K. Photo-induced toxicity of tungsten oxide photochromic nanoparticles. J. Photochem. Photobiol. B Biol. 2018, 178, 395–403. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Yao, J. Photochromism in composite and hybrid materials based on transition-metal oxides and polyoxometalates. Prog. Mater. Sci. 2006, 51, 810–879. [Google Scholar] [CrossRef]
- Wei, J.; Jiao, X.; Wang, T.; Chen, D. Electrospun photochromic hybrid membranes for flexible rewritable media. ACS Appl. Mater. Interfaces 2016, 8, 29713–29720. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wei, J.; Dong, S.; Li, H.; Xia, Y.; Jiao, X.; Wang, T.; Chen, D. Novel PVP/HTA hybrids for multifunctional rewritable paper. ACS Appl. Mater. Interfaces 2018, 10, 1701–1706. [Google Scholar] [CrossRef]
- Hirai, M.; Hagiwara, Y.; Takeuchi, K.; Kimura, R.; Onai, T.; Kawai-Hirai, R.; Tohta, N.; Sugiyama, M. Thermal unfolding and refolding of protein under osmotic pressure clarified by wide-angle X-ray scattering. Thermochim. Acta 2012, 532, 15–21. [Google Scholar] [CrossRef]
- Basha, M.A.-F. Magnetic and optical studies on polyvinylpyrrolidone thin films doped with rare earth metal salts. Polym. J. 2010, 42, 728–734. [Google Scholar] [CrossRef]
- He, G.-H.; Liang, C.-J.; Ou, Y.-D.; Liu, D.-N.; Fang, Y.-P.; Xu, Y.-H. Preparation of novel Sb2O3/WO3 photocatalysts and their activities under visible light irradiation. Mater. Res. Bull. 2013, 48, 2244–2249. [Google Scholar] [CrossRef]
- Balzer, R.; Drago, V.; Schreiner, W.H.; Probst, L.F.D. Synthesis and structure-activity relationship of a WO3 catalyst for the total oxidation of BTX. J. Braz. Chem. Soc. 2014, 25, 2026–2031. [Google Scholar]
- Kumar, V.B.; Mohanta, D. Formation of nanoscale tungsten oxide structures and colouration characteristics. Bull. Mater. Sci. 2011, 34, 435–442. [Google Scholar] [CrossRef]
- Shannon, R.D.T.; Prewitt, C.T. Revised values of effective ionic radii. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1970, 26, 1046–1048. [Google Scholar] [CrossRef]
- Sidey, V. On the effective ionic radii for ammonium. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2016, 72, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.T.; Darwent, J.R. Photoreduction of methyl orange sensitized by colloidal titanium dioxide. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1984, 80, 1631–1643. [Google Scholar] [CrossRef]
- Gehlen, M.H.; Ferreira, M.; Neumann, M.G. Interaction of methyl orange with cationic micelles and its effect on dye photochemistry. J. Photochem. Photobiol. A Chem. 1995, 87, 55–60. [Google Scholar] [CrossRef]
- Hsiao, P.-H.; Li, T.-C.; Chen, C.-Y. ZnO/Cu2O/Si Nanowire Arrays as Ternary Heterostructure-Based Photocatalysts with Enhanced Photodegradation Performances. Nanoscale Res. Lett. 2019, 14, 244. [Google Scholar] [CrossRef] [PubMed]
- David, G.; Pérez, J. Combined sampler robot and high-performance liquid chromatography: A fully automated system for biological small-angle X-ray scattering experiments at the Synchrotron SOLEIL SWING beamline. J. Appl. Crystallogr. 2009, 42, 892–900. [Google Scholar] [CrossRef]
- Lebedev, V.A.; Sudin, V.V.; Kozlov, D.A.; Garshev, A.V. Photocatalytic properties of nanocrystalline TiO2 modified with CuO and WO3. Nanotechnol. Russ. 2016, 11, 20–28. [Google Scholar] [CrossRef]
- Tang, C.-H.; Chen, K.-Y.; Chen, C.-Y. Solution-processed ZnO/Si based heterostructures with enhanced photocatalytic performance. New J. Chem. 2018, 42, 13797–13802. [Google Scholar] [CrossRef]
- Tang, C.-H.; Hsiao, P.-H.; Chen, C.-Y. Efficient Photocatalysts Made by Uniform Decoration of Cu2O Nanoparticles on Si Nanowire Arrays with Low Visible Reflectivity. Nanoscale Res. Lett. 2018, 13, 312. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Hsiao, P.-H.; Wei, T.-C.; Chen, T.-C.; Tang, C.-H. Well incorporation of carbon nanodots with silicon nanowire arrays featuring excellent photocatalytic performances. Phys. Chem. Chem. Phys. 2017, 19, 11786–11792. [Google Scholar] [CrossRef]
- Lebedev, V.A.; Kozlov, D.A.; Kolesnik, I.V.; Poluboyarinov, A.S.; Becerikli, A.E.; Grünert, W.; Garshev, A.V. The amorphous phase in titania and its influence on photocatalytic properties. Appl. Catal. B Environ. 2016, 195, 39–47. [Google Scholar] [CrossRef]
- Jones, E.; Oliphant, T.; Peterson, P. SciPy: Open Source Scientific Tools for Python. 2001. Available online: https://www.scipy.org (accessed on 28 December 2019).
- Hunter, J.D. Matplotlib: A 2D Graphics Environment. Comput. Sci. Eng. 2007, 9, 90–95. [Google Scholar] [CrossRef]
Sample Availability: All samples listed in the paper are available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozlov, D.A.; Shcherbakov, A.B.; Kozlova, T.O.; Angelov, B.; Kopitsa, G.P.; Garshev, A.V.; Baranchikov, A.E.; Ivanova, O.S.; Ivanov, V.K. Photochromic and Photocatalytic Properties of Ultra-Small PVP-Stabilized WO3 Nanoparticles. Molecules 2020, 25, 154. https://doi.org/10.3390/molecules25010154
Kozlov DA, Shcherbakov AB, Kozlova TO, Angelov B, Kopitsa GP, Garshev AV, Baranchikov AE, Ivanova OS, Ivanov VK. Photochromic and Photocatalytic Properties of Ultra-Small PVP-Stabilized WO3 Nanoparticles. Molecules. 2020; 25(1):154. https://doi.org/10.3390/molecules25010154
Chicago/Turabian StyleKozlov, Daniil A., Alexander B. Shcherbakov, Taisiya O. Kozlova, Borislav Angelov, Gennady P. Kopitsa, Alexey V. Garshev, Alexander E. Baranchikov, Olga S. Ivanova, and Vladimir K. Ivanov. 2020. "Photochromic and Photocatalytic Properties of Ultra-Small PVP-Stabilized WO3 Nanoparticles" Molecules 25, no. 1: 154. https://doi.org/10.3390/molecules25010154
APA StyleKozlov, D. A., Shcherbakov, A. B., Kozlova, T. O., Angelov, B., Kopitsa, G. P., Garshev, A. V., Baranchikov, A. E., Ivanova, O. S., & Ivanov, V. K. (2020). Photochromic and Photocatalytic Properties of Ultra-Small PVP-Stabilized WO3 Nanoparticles. Molecules, 25(1), 154. https://doi.org/10.3390/molecules25010154








