Nanostructured MoO3 for Efficient Energy and Environmental Catalysis
Abstract
1. Introduction
2. Phase Structure and Morphology of Nanostructured MoO3
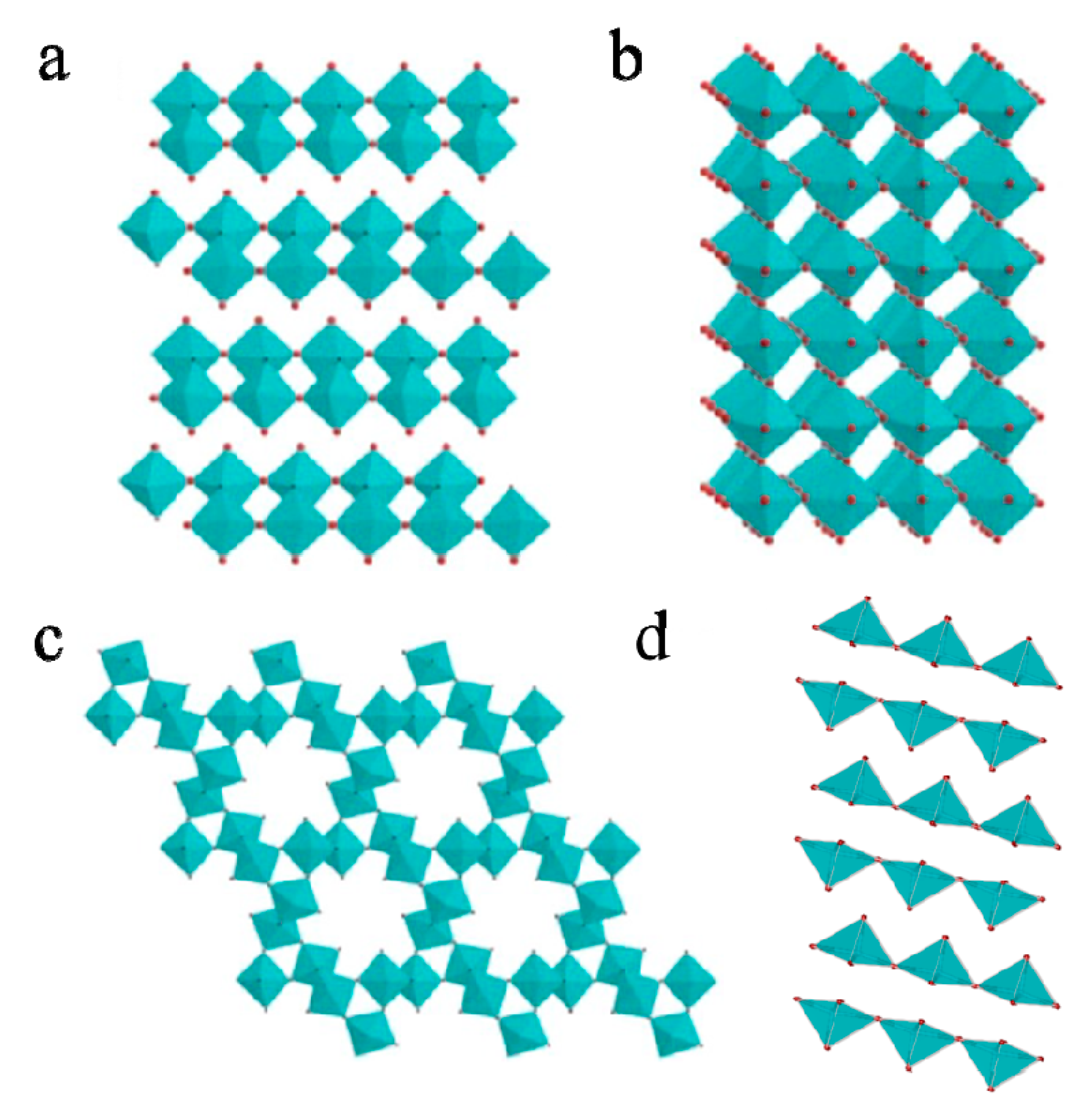
2.1. The Phase Structure of MoO3
2.2. The Morphology of Nanostructured MoO3
3. The Application of MoO3 in Energy Related Catalysis
3.1. Application in Hydrogen Evolution
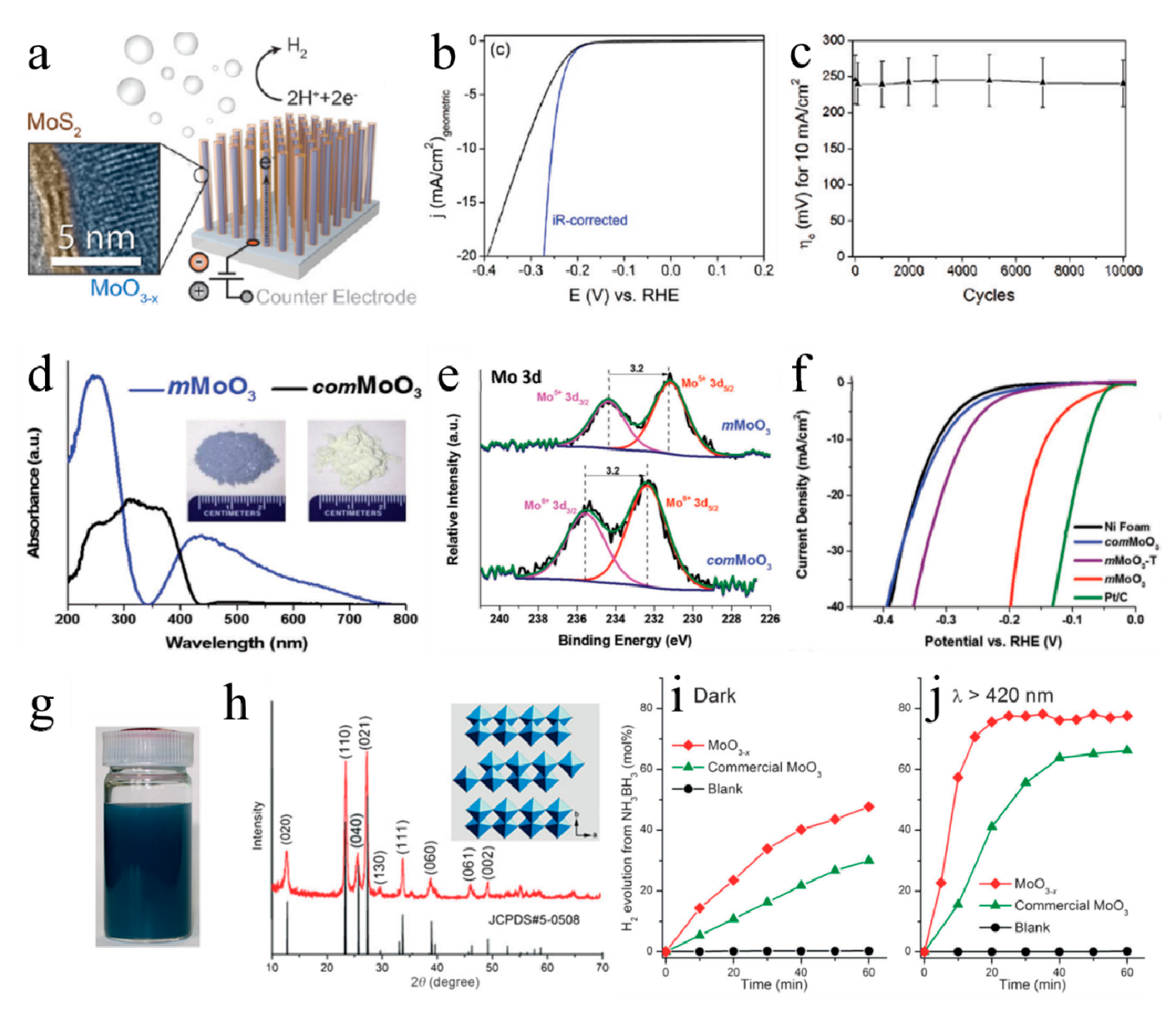
3.1.1. Electrocatalytic Hydrogen Evolution Reactions
3.1.2. Photocatalytic Hydrogen Evolution Reactions
3.1.3. Ammonia Borane Dehydrogenation
3.2. Application in Oxygen Evolution
3.2.1. Electrocatalytic Oxygen Evolution Reactions
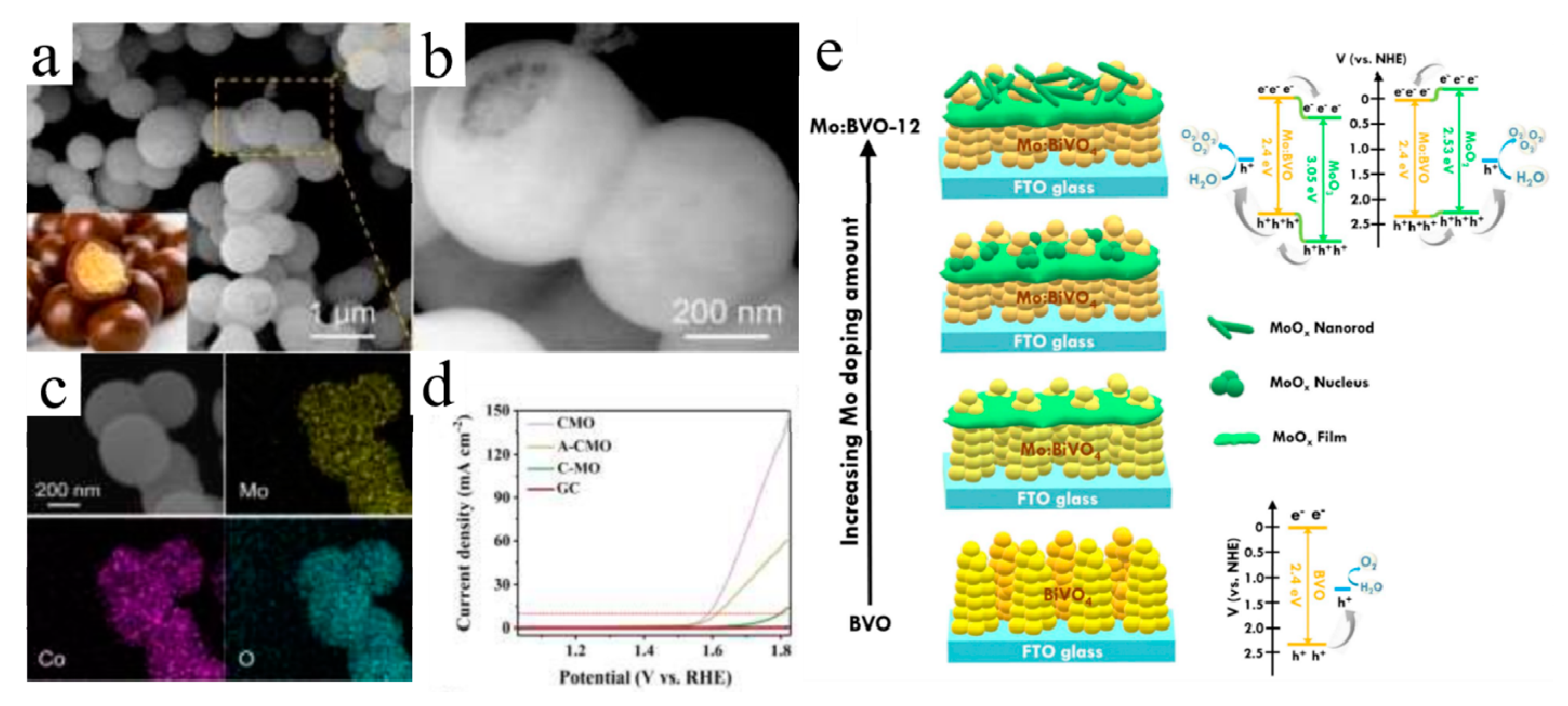
3.2.2. Photoelectrochemical Oxygen Evolution Reactions
3.3. Application in Fuel Cells
3.3.1. Direct Methanol Fuel Cells
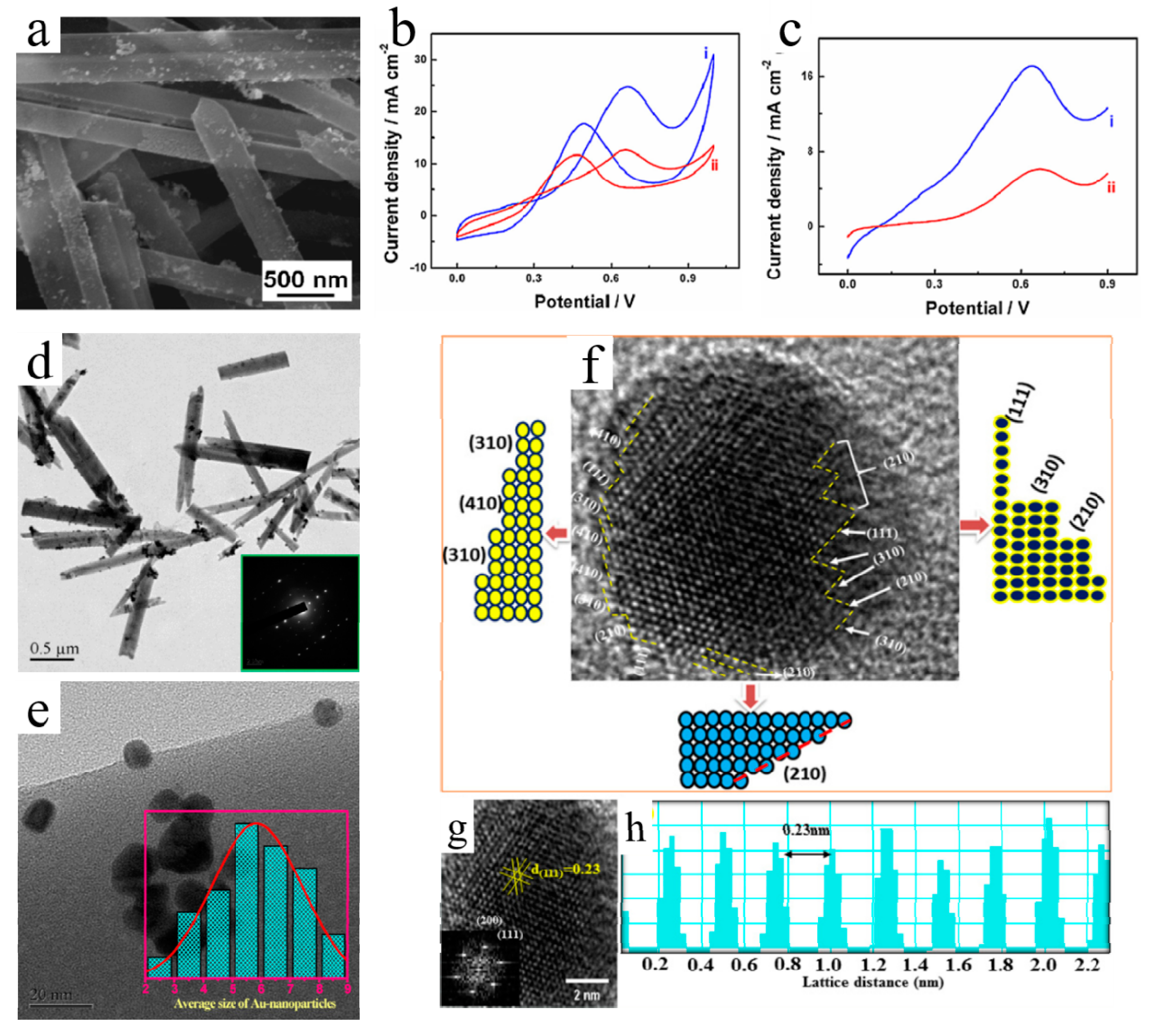
3.3.2. Oxygen-Reduction Reactions
4. MoO3 Applied in Environmental Catalysis
4.1. Photodegradation of Organic Pollutants
4.2. Selective Catalysis to Reduce Air Pollutants
4.2.1. Selective Catalytic Reduction of NOx with NH3
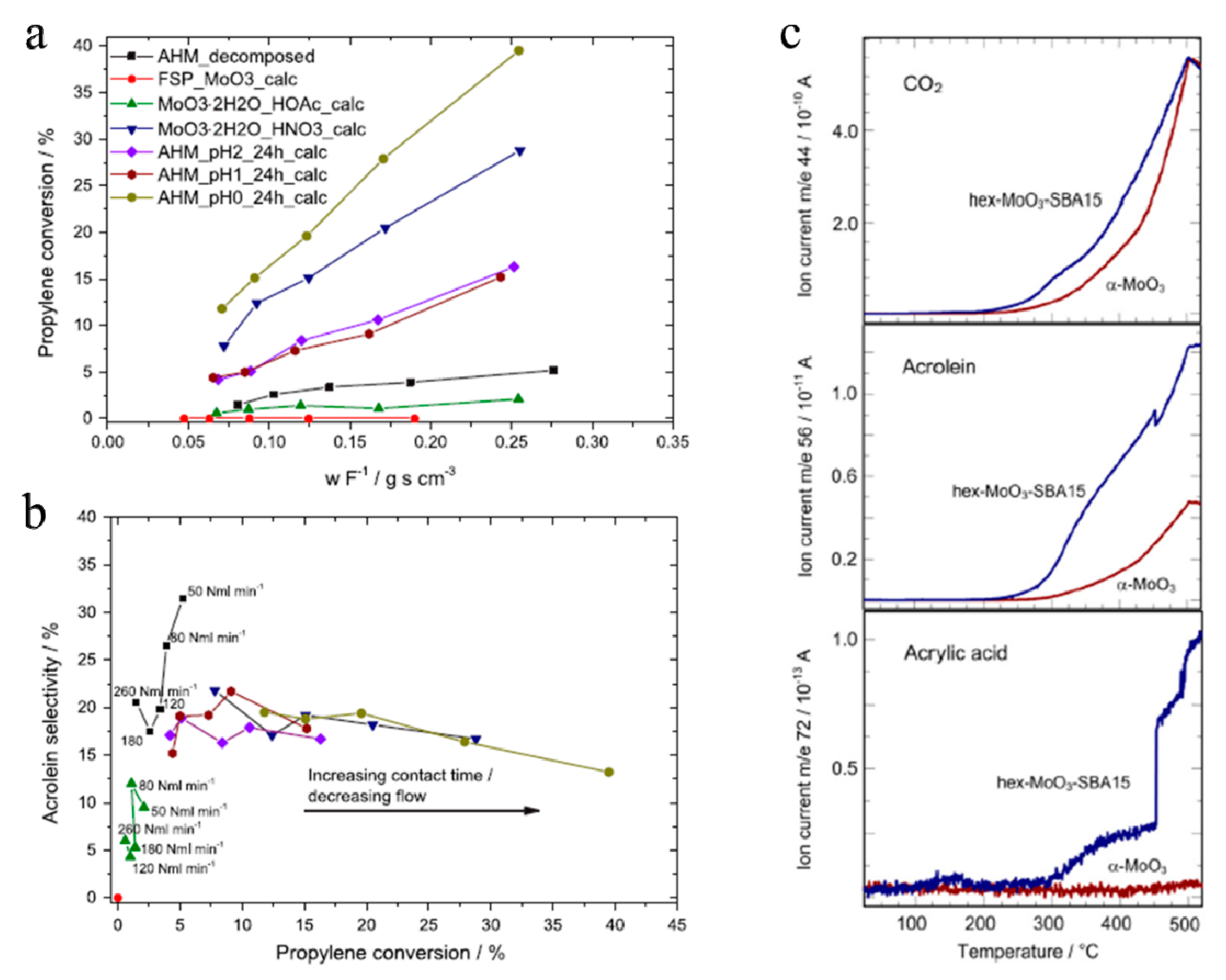
4.2.2. Selective Catalytic Oxidation of Propene
4.2.3. Selective Catalytic Oxidation of Methane
4.2.4. Other Catalysis to Reduce Air Pollutants
5. Summary and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Ibrahim, I.D.; Sadiku, E.R.; Jamiru, T.; Hamam, Y.; Alayli, Y.; Eze, A.A. Prospects of nanostructured composite materials for energy harvesting and storage. J. King Saud Univ. Sci. 2019, (in press). [CrossRef]
- Buller, S.; Strunk, J. Nanostructure in energy conversion. J. Energy Chem. 2016, 25, 171–190. [Google Scholar] [CrossRef]
- Li, Y.M.; Somorjai, G.A. Nanoscale advances in catalysis and energy applications. Nano Lett. 2010, 10, 2289–2295. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.; Wang, D.; Zhang, J.; Lu, F.; Li, Y. Synthesis and applications of graphdiyne-based metal-free catalysts. Adv. Mater. 2019, 31, 1803762. [Google Scholar] [CrossRef]
- Ren, H.; Sun, S.; Cui, J.; Li, X. Synthesis, functional modifications, and diversified applications of molybdenum oxides micro-/nanocrystals: A review. Cryst. Growth Des. 2018, 18, 6326–6369. [Google Scholar] [CrossRef]
- Cui, X.; Tang, C.; Zhang, Q. A review of electrocatalytic reduction of dinitrogen to ammonia under ambient conditions. Adv. Energy Mater. 2018, 8, 1800369. [Google Scholar] [CrossRef]
- Zhang, X.; Lei, J.; Wu, D.; Jing, Y.; Zhou, Z. A Ti-anchored Ti2CO2 monolayer (MXene) as a single-atom catalyst for CO oxidation. J. Mater. Chem. A 2016, 4, 4871–4876. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, X.; Yang, Q.; Liang, S.; Zhang, X.; Yang, Z. Cuprous oxide (Cu2O) crystals with tailored architectures: A comprehensive review on synthesis, fundamental properties, functional modifications and applications. Prog. Mater. Sci. 2018, 96, 111–173. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Y.; Yang, Z.; Yan, Y.; Sun, K. Synthesis of MoS2 and MoO2 for their applications in H2 generation and lithium ion batteries: A review. Sci. Technol. Adv. Mat. 2013, 14, 043501. [Google Scholar] [CrossRef]
- Sun, S.; Liang, S. Morphological zinc stannate: Synthesis, fundamental properties and applications. J. Mater. Chem. A 2017, 5, 20534–20560. [Google Scholar] [CrossRef]
- Sun, S. Recent advances in hybrid Cu2O-based heterogeneous nanostructures. Nanoscale. 2015, 7, 10850–10882. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; Farooqi, S.A.; Wang, X.; Yan, C. Recent progress on molybdenum oxides for rechargeable batteries. ChemSusChem 2019, 12, 755–771. [Google Scholar] [CrossRef] [PubMed]
- Van Pham, D.; Patil, R.A.; Yang, C.; Yeh, W.; Liou, Y. Impact of the crystal phase and 3d-valence conversion on the capacitive performance of one-dimensional MoO2, MoO3, and Magnéli-phase Mo4O11 nanorod-based pseudocapacitors. Nano Energy. 2018, 47, 105–114. [Google Scholar] [CrossRef]
- Tan, X.; Wang, L.; Cheng, C.; Yan, X.; Shen, B.; Zhang, J. Plasmonic MoO3−x@MoO3 nanosheets for highly sensitive SERS detection through nanoshell-isolated electromagnetic enhancement. Chem. Commun. 2016, 52, 2893–2896. [Google Scholar] [CrossRef] [PubMed]
- Sinaim, H.; Ham, D.J.; Lee, J.S.; Phuruangrat, A.; Thongtem, S.; Thongtem, T. Free-polymer controlling morphology of α-MoO3 nanobelts by a facile hydrothermal synthesis, their electrochemistry for hydrogen evolution reactions and optical properties. J. Alloy. Compd. 2012, 512, 172–178. [Google Scholar] [CrossRef]
- Imawan, C.; Steffes, H.; Solzbacher, F.; Obermeier, E. A new preparation method for sputtered MoO3 multilayers for the application in gas sensors. Sens. Actuators B 2001, 78, 119–125. [Google Scholar] [CrossRef]
- Jiang, F.; Li, W.; Zou, R.; Liu, Q.; Xu, K.; An, L.; Hu, J. MoO3/PANI coaxial heterostructure nanobelts by in situ polymerization for high performance supercapacitors. Nano Energy 2014, 7, 72–79. [Google Scholar] [CrossRef]
- Sadhanala, H.K.; Harika, V.K.; Penki, T.R.; Aurbach, D.; Gedanken, A. Ultrafine ruthenium oxide nanoparticles supported on molybdenum oxide nanosheets as highly efficient electrocatalyst for hydrogen evolution in acidic medium. Chemcatchem. 2019, 11, 1495–1502. [Google Scholar] [CrossRef]
- Yang, S.; Wang, Z.; Hu, Y.; Luo, X.; Lei, J.; Zhou, D.; Fei, L.; Wang, Y.; Gu, H. Highly responsive room-temperature hydrogen sensing of α-MoO3 nanoribbon membranes. ACS Appl. Mater. Interfaces 2015, 7, 9247–9253. [Google Scholar] [CrossRef]
- Chen, X.; Lei, W.; Liu, D.; Hao, J.; Cui, Q.; Zou, G. Synthesis and characterization of hexagonal and truncated hexagonal shaped MoO3 nanoplates. J. Phys. Chem. C 2009, 113, 21582–21585. [Google Scholar] [CrossRef]
- Tang, W.; Liu, L.; Tian, S.; Li, L.; Yue, Y.; Wu, Y.; Zhu, K. Aqueous supercapacitors of high energy density based on MoO3 nanoplates as anode material. Chem. Commun. 2011, 47, 10058–10060. [Google Scholar] [CrossRef] [PubMed]
- Di Yao, D.; Field, M.R.; O’Mullane, A.P.; Kalantar-zadeh, K.; Ou, J.Z. Electrochromic properties of TiO2 nanotubes coated with electrodeposited MoO3. Nanoscale 2013, 5, 10353–10359. [Google Scholar] [CrossRef] [PubMed]
- Alsaif, M.M.Y.A.; Field, M.R.; Daeneke, T.; Chrimes, A.F.; Chrimes, W.; Carey, B.J.; Berean, K.J.; Walia, S.; Embden, J.; Zhang, B.; et al. Exfoliation solvent dependent plasmon resonances in two-dimensional sub-stoichiometric molybdenum oxide nanoflakes. ACS Appl. Mater. Interfaces 2016, 8, 3482–3493. [Google Scholar] [CrossRef]
- Navas, I.; Vinodkumar, R.; Lethy, K.J.; Detty, A.P.; Ganesan, V.; Sathe, V.; Mahadevan Pillai, V.P. Growth and characterization of molybdenum oxide nanorods by RF magnetron sputtering and subsequent annealing. J. Phys. D Appl. Phys. 2009, 42, 175305. [Google Scholar] [CrossRef]
- Zheng, L.; Xu, Y.; Jin, D.; Xie, Y. Novel metastable hexagonal MoO3 nanobelts: Synthesis, photochromic, and electrochromic properties. Chem. Mater. 2009, 21, 5681–5690. [Google Scholar] [CrossRef]
- McCarron, E.M., III; Calabrese, J.C. The growth and single crystal structure of a high pressure phase of molybdenum trioxide: MoO3-II. J. Solid State Chem. 1991, 91, 121–125. [Google Scholar] [CrossRef]
- de Castro, I.A.; Datta, R.S.; Ou, J.Z.; Castellanos-Gomez, A.; Sriram, S.; Daeneke, T.; Kalantar-zadeh, K. Molybdenum oxides–from fundamentals to functionality. Adv. Mater. 2017, 29, 1701619. [Google Scholar] [CrossRef]
- Wu, C.; Xie, H.; Li, D.; Liu, D.; Ding, S.; Tao, S.; Chen, H.; Liu, Q.; Chen, S.; Chu, W. Atomically intercalating tin ions into the interlayer of molybdenum oxide nanobelt toward long-cycling lithium battery. J. Phys. Chem. Lett. 2018, 9, 817–824. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, W.; Liu, X.; Mei, Y.; Huang, Y. Nanostructured Mo-based electrode materials for electrochemical energy storage. Chem. Soc. Rev. 2015, 44, 2376–2404. [Google Scholar] [CrossRef]
- Mai, L.Q.; Hu, B.; Chen, W.; Qi, Y.Y.; Lao, C.S.; Yang, R.S.; Dai, Y.; Wang, Z.L. Lithiated MoO3 nanobelts with greatly improved performance for lithium batteries. Adv. Mater. 2007, 19, 3712–3716. [Google Scholar] [CrossRef]
- Chithambararaj, A.; Rajeswari Yogamalar, N.; Bose, A.C. Hydrothermally synthesized h-MoO3 and α-MoO3 nanocrystals: New findings on crystal-structure-dependent charge transport. Cryst. Growth Des. 2016, 16, 1984–1995. [Google Scholar] [CrossRef]
- Mizushima, T.; Fukushima, K.; Ohkita, H.; Kakuta, N. Synthesis of β-MoO3 through evaporation of HNO3-added molybdic acid solution and its catalytic performance in partial oxidation of methanol. Appl. Catal. A 2007, 326, 106–112. [Google Scholar] [CrossRef]
- Pham, T.T.P.; Nguyen, P.H.D.; Vo, T.T.; Nguyen, H.H.P.; Luu, C.L. Facile method for synthesis of nanosized β-MoO3 and their catalytic behavior for selective oxidation of methanol to formaldehyde. Adv. Nat. Sci. Nanosci. Nanotechnol. 2015, 6, 045010. [Google Scholar] [CrossRef]
- Lunk, H.J.; Hartl, H.; Hartl, M.A.; Fait, M.J.; Shenderovich, I.G.; Feist, M.; Frisk, T.A.; Daemen, L.L.; Mauder, D.; Gurinov, A.A. “Hexagonal Molybdenum Trioxide”—known for 100 years and still a fount of new discoveries. Inorg. Chem. 2010, 49, 9400–9408. [Google Scholar] [CrossRef]
- Pan, W.; Tian, R.; Jin, H.; Guo, Y.; Zhang, L.; Wu, X.; Zhang, L.; Han, Z.; Liu, G.; Li, J. Structure, optical, and catalytic properties of novel hexagonal metastable h-MoO3 nano-and microrods synthesized with modified liquid-phase processes. Chem. Mater. 2010, 22, 6202–6208. [Google Scholar] [CrossRef]
- Xu, S.; Li, D.; Wu, P. One-pot, facile, and versatile synthesis of monolayer MoS2/WS2 quantum dots as bioimaging probes and efficient electrocatalysts for hydrogen evolution reaction. Adv. Funct. Mater. 2015, 25, 1127–1136. [Google Scholar] [CrossRef]
- Lu, X.; Wang, R.; Yang, F.; Jiao, W.; Liu, W.; Hao, L.; He, X. Preparation of MoO3 QDs through combining intercalation and thermal exfoliation. J. Mater. Chem. C 2016, 4, 6720–6726. [Google Scholar] [CrossRef]
- Lu, X.; Wang, R.; Hao, L.; Yang, F.; Jiao, W.; Zhang, J.; Peng, P.; Liu, W. Preparation of quantum dots from MoO3 nanosheets by UV irradiation and insight into morphology changes. J. Mater. Chem. C 2016, 4, 11449–11456. [Google Scholar] [CrossRef]
- Meduri, P.; Clark, E.; Kim, J.H.; Dayalan, E.; Sumanasekera, G.U.; Sunkara, M.K. MoO3–x nanowire arrays as stable and high-capacity anodes for lithium ion batteries. Nano Lett. 2012, 12, 1784–1788. [Google Scholar] [CrossRef]
- Prakash, N.G.; Dhananjaya, M.; Narayana, A.L.; Shaik, D.P.; Rosaiah, P.; Hussain, O.M. High performance one dimensional α-MoO3 nanorods for supercapacitor applications. Ceram. Int. 2018, 44, 9967–9975. [Google Scholar] [CrossRef]
- Hu, S.; Wang, X. Single-walled MoO3 nanotubes. Am. Chem. J. 2008, 130, 8126–8127. [Google Scholar] [CrossRef] [PubMed]
- Enyashin, A.N.; Ivanovskaya, V.V.; Ivanovskii, A.L. Electronic properties and chemical bonding of single-walled MoO3 nanotubes. Mendeleev Commun. 2004, 14, 94–95. [Google Scholar] [CrossRef]
- Jiang, J.; Liu, J.; Peng, S.; Qian, D.; Luo, D.; Wang, Q.; Tian, Z.; Liu, Y. Facile synthesis of α-MoO3 nanobelts and their pseudocapacitive behavior in an aqueous Li2SO4 solution. J. Mater. Chem. A 2013, 1, 2588–2594. [Google Scholar] [CrossRef]
- Peng, H.; Ma, G.; Mu, J.; Sun, K.; Lei, Z. Low-cost and high energy density asymmetric supercapacitors based on polyaniline nanotubes and MoO3 nanobelts. J. Mater. Chem. A 2014, 2, 10384–10388. [Google Scholar] [CrossRef]
- Balendhran, S.; Walia, S.; Alsaif, M.; Nguyen, E.P.; Ou, J.Z.; Zhuiykov, S.; Sriram, S.; Bhaskaran, M.; Kalantar-zadeh, K. Field effect biosensing platform based on 2D α-MoO3. ACS Nano 2013, 7, 9753–9760. [Google Scholar] [CrossRef]
- Balendhran, S.; Deng, J.; Ou, J.Z.; Walia, S.; Scott, J.; Tang, J.; Wang, K.L.; Field, M.R.; Russo, S.; Zhuiykov, S.; et al. Enhanced charge carrier mobility in two-dimensional high dielectric molybdenum oxide. Adv. Mater. 2013, 25, 109–114. [Google Scholar] [CrossRef]
- Ji, F.; Ren, X.; Zheng, X.; Liu, Y.; Pang, L.; Jiang, J.; Liu, S.F. 2D-MoO3 nanosheets for superior gas sensors. Nanoscale 2016, 8, 8696–8703. [Google Scholar] [CrossRef]
- Cheng, H.; Kamegawa, T.; Mori, K.; Yamashita, H. Surfactant-free nonaqueous synthesis of plasmonic molybdenum oxide nanosheets with enhanced catalytic activity for hydrogen generation from ammonia borane under visible light. Angew. Chem. Int. Ed. 2014, 53, 2910–2914. [Google Scholar] [CrossRef]
- Sui, L.L.; Xu, Y.M.; Zhang, X.F.; Cheng, X.L.; Gao, S.; Zhao, H.; Cai, Z.; Huo, L.H. Construction of three-dimensional flower-like α-MoO3 with hierarchical structure for highly selective triethylamine sensor. Sens. Actuators B 2015, 208, 406–414. [Google Scholar] [CrossRef]
- Yu, L.; Wu, H.B.; Lou, X.W.D. Self-templated formation of hollow structures for electrochemical energy applications. Acc. Chem. Res. 2017, 50, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Xie, Y.; Chu, B. Use of block copolymer micelles on formation of hollow MoO3 nanospheres. Langmuir 2000, 16, 9015–9022. [Google Scholar] [CrossRef]
- Du, K.; Fu, W.; Wei, R.; Yang, H.; Xu, J.; Chang, L.; Yu, Q.; Zou, G. Ultrasonic-assisted synthesis of highly dispersed MoO3 nanospheres using 3-mercaptopropyltrimethoxysilane. Ultrason. Sonochem. 2008, 15, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Brezesinski, T.; Wang, J.; Tolbert, S.H.; Dunn, B. Ordered mesoporous α-MoO3 with iso-oriented nanocrystalline walls for thin-film pseudocapacitors. Nat. Mater. 2010, 9, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Miao, R.; Huan, T.D.; Mosa, I.M.; Poyraz, A.S.; Zhong, W.; Cloud, J.E.; Kriz, D.A.; Thanneeru, S.; He, J.; et al. Mesoporous MoO3−x material as an efficient electrocatalyst for hydrogen evolution reactions. Adv. Energy Mater. 2016, 6, 1600528. [Google Scholar] [CrossRef]
- Chen, Z.; Cummins, D.; Reinecke, B.N.; Clark, E.; Sunkara, M.K.; Jaramillo, T.F. Core-shell MoO3-MoS2 nanowires for hydrogen evolution: A functional design for electrocatalytic materials. Nano Lett. 2011, 11, 4168–4175. [Google Scholar] [CrossRef]
- Jin, B.; Zhou, X.; Huang, L.; Licklederer, M.; Yang, M.; Schmuki, P. Aligned MoOx/MoS2 core-shell nanotubular structures with a high density of reactive sites based on self-ordered anodic molybdenum oxide nanotubes. Angew. Chem. Int. Ed. 2016, 55, 12252–12256. [Google Scholar] [CrossRef]
- Cummins, D.R.; Martinez, U.; Sherehiy, A.; Kappera, R.; Martinez-Garcia, A.; Schulze, R.K.; Jasinski, J.; Zhang, J.; Gupta, R.K.; Lou, J.; et al. Efficient hydrogen evolution in transition metal dichalcogenides via a simple one-step hydrazine reaction. Nat. Commun. 2016, 7, 11857. [Google Scholar] [CrossRef]
- Liu, W.; Xu, Q.; Yan, P.; Chen, J.; Du, Y.; Chu, S.; Wang, J. Fabrication of a single-atom platinum catalyst for the hydrogen evolution reaction: A new protocol by utilization of HxMoO3−x with plasmon resonance. ChemCatChem 2018, 10, 946–950. [Google Scholar] [CrossRef]
- Li, J.; Cheng, Y.; Zhang, J.; Fu, J.; Yan, W.; Xu, Q. Confining Pd nanoparticles and atomically dispersed Pd into defective MoO3 nanosheet for enhancing electro-and photocatalytic hydrogen evolution performances. ACS Appl. Mater. Interfaces 2019, 11, 27798–27804. [Google Scholar] [CrossRef]
- Datta, R.S.; Haque, F.; Mohiuddin, M.; Carey, B.J.; Syed, N.; Zavabeti, A.; Zhang, B.; Khan, H.; Berean, K.J.; Ou, J.Z. Highly active two dimensional α-MoO3−x for the electrocatalytic hydrogen evolution reaction. J. Mater. Chem. A 2017, 5, 24223–24231. [Google Scholar] [CrossRef]
- Zhang, W.; Li, H.; Firby, C.J.; Al-Hussein, M.; Elezzabi, A.Y. Oxygen vacancy tunable electrochemical properties of electrodeposited molybdenum oxide films. ACS Appl. Mater. Interfaces 2019, 11, 20378–20385. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, R.; Hu, D.; Huang, X.; Liu, Y.; Yan, K. Porous molybdenum trioxide as a bifunctional electrocatalyst for oxygen and hydrogen evolution. J. Electroanal. Chem. 2019, 836, 102–106. [Google Scholar] [CrossRef]
- Li, L.; Zhang, T.; Yan, J.; Cai, X.; Liu, S. P Doped MoO3−x Nanosheets as efficient and stable electrocatalysts for hydrogen evolution. Small 2017, 13, 1700441. [Google Scholar] [CrossRef]
- Haque, F.; Zavabeti, A.; Zhang, B.Y.; Datta, R.S.; Yin, Y.; Yi, Z.; Wang, Y.; Mahmood, N.; Pillai, N.; Syed, N.; et al. Ordered intracrystalline pores in planar molybdenum oxide for enhanced alkaline hydrogen evolution. J. Mater. Chem. A 2019, 7, 257–268. [Google Scholar] [CrossRef]
- Ma, C.; Zhou, J.; Cui, Z.; Wang, Y.; Zou, Z. In situ growth MoO3 nanoflake on conjugated polymer: An advanced photocatalyst for hydrogen evolution from water solution under solar light. Sol. Energy Mater. Sol. Cells 2016, 150, 102–111. [Google Scholar] [CrossRef]
- Pang, X.; Bian, H.; Su, M.; Ren, Y.; Qi, J.; Ma, H.; Wu, D.; Hu, L.; Du, B.; Wei, Q. Photoelectrochemical cytosensing of RAW264.7 macrophage cells based on a TiO2 nanoneedls@MoO3 array. Anal. Chem. 2017, 89, 7950–7957. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, L.; Jin, B.; Huang, L.; Gan, Y. Enhanced photoelectrochemical properties and water splitting activity of self-ordered MoO3-TiO2 nanotubes. Appl. Surf. Sci. 2016, 364, 410–415. [Google Scholar] [CrossRef]
- Garcia-Esparza, A.T.; Shinagawa, T.; Ould-Chikh, S.; Qureshi, M.; Peng, X.; Wei, N.; Anjum, D.H.; Clo, A.; Weng, T.C.; Nordlund, D.; et al. An oxygen-insensitive hydrogen evolution catalyst coated by a molybdenum-based layer for overall water splitting. Angew. Chem. Int. Ed. 2017, 56, 5780–5784. [Google Scholar] [CrossRef]
- Guo, S.; Li, X.; Ren, X.; Yang, L.; Zhu, J.; Wei, B. Optical and electrical enhancement of hydrogen evolution by MoS2@MoO3 core-shell nanowires with designed tunable plasmon resonance. Adv. Funct. Mater. 2018, 28, 1802567. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, P.; Qiu, M.; Dong, J.; Zhang, Y.; Lou, X.W. Ultrasmall MoOx clusters as a novel cocatalyst for photocatalytic hydrogen evolution. Adv. Mater. 2019, 31, 1804883. [Google Scholar]
- Cheng, H.; Qian, X.; Kuwahara, Y.; Mori, K.; Yamashita, H. A plasmonic molybdenum oxide hybrid with reversible tunability for visible-light-enhanced catalytic reactions. Adv. Mater. 2015, 27, 4616–4621. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Kuwahara, Y.; Wen, M.; Navlani-García, M.; Mori, K.; An, T.; Yamashita, H. Room-temperature and aqueous-phase synthesis of plasmonic molybdenum oxide nanoparticles for visible-light-enhanced hydrogen generation. Chem. - Asian J. 2016, 11, 2377–2381. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Kuwahara, Y.; Mori, K.; Cheng, H.; Wen, M.; Yamashita, H. High-surface-area plasmonic MoO3−x: Rational synthesis and enhanced ammonia borane dehydrogenation activity. J. Mater. Chem. A 2017, 5, 8946–8953. [Google Scholar] [CrossRef]
- Lu, D.; Feng, Y.; Ding, Z.; Liao, J.; Zhang, X.; Liu, H.R.; Li, H. MoO3-doped MnCo2O4 microspheres consisting of nanosheets: An inexpensive nanostructured catalyst to hydrolyze ammonia borane for hydrogen generation. Nanomaterials 2019, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Tariq, M.; Zaman, W.Q.; Sun, W.; Zhou, Z.; Wu, Y.; Cao, L.M.; Yang, J. Unraveling the beneficial electrochemistry of IrO2/MoO3 hybrid as a highly stable and efficient oxygen evolution reaction catalyst. ACS Sustain. Chem. Eng. 2018, 6, 4854–4862. [Google Scholar] [CrossRef]
- Illathvalappil, R.; George, L.; Kurungot, S. Coexisting few-layer assemblies of NiO and MoO3 deposited on vulcan carbon as an efficient and durable electrocatalyst for water oxidation. ACS Appl. Energy Mater. 2019, 2, 4987–4998. [Google Scholar] [CrossRef]
- Guo, C.; Sun, X.; Kuang, X.; Gao, L.; Zhao, M.; Qu, L.; Zhang, Y.; Wu, D.; Ren, X.; Wei, Q. Amorphous Co-doped MoOx nanospheres with a core-shell structure toward an effective oxygen evolution reaction. J. Mater. Chem. A 2019, 7, 1005–1012. [Google Scholar] [CrossRef]
- Lou, S.N.; Scott, J.; Iwase, A.; Amal, R.; Ng, Y.H. Photoelectrochemical water oxidation using a Bi2MoO6/MoO3 heterojunction photoanode synthesised by hydrothermal treatment of an anodised MoO3 thin film. J. Mater. Chem. A 2016, 4, 6964–6971. [Google Scholar] [CrossRef]
- He, H.; Zhou, Y.; Ke, G.; Zhong, X.; Yang, M.; Bian, L.; Lv, K.; Dong, F. Improved surface charge transfer in MoO3/BiVO4 heterojunction film for photoelectrochemical water oxidation. Electrochim. Acta 2017, 257, 181–191. [Google Scholar] [CrossRef]
- Yao, Y.; Sun, M.; Zhang, Z.; Lin, X.; Gao, B.; Anandan, S.; Liu, W. In situ synthesis of MoO3/Ag/TiO2 nanotube arrays for enhancement of visible-light photoelectrochemical performance. Int. J. Hydrogen Energy 2019, 44, 9348–9358. [Google Scholar] [CrossRef]
- Chen, Y.S.; Lin, L.Y. Facile synthesis of Bi-functional molybdenum-doped BiVO4/Molybdenum oxide heterojunction as the photocatalyst for water oxidation. J. Power Sources 2019, 434, 226705. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Fachini, E.R.; Cabrera, C.R. Electrochemically codeposited platinum/molybdenum oxide electrode for catalytic oxidation of methanol in acid solution. Electrochem. Solid-State Lett. 1999, 2, 437–439. [Google Scholar] [CrossRef]
- Wang, Y.; Fachini, E.R.; Cruz, G.; Zhu, Y.; Ishikawa, Y.; Colucci, J.A.; Cabrera, C.R. Effect of surface composition of electrochemically codeposited platinum/molybdenum oxide on methanol oxidation. J. Electrochem. Soc. 2001, 148, 222–226. [Google Scholar] [CrossRef]
- Justin, P.; Rao, G.R. Methanol oxidation on MoO3 promoted Pt/C electrocatalyst. Int. J. Hydrogen Energy 2011, 36, 5875–5884. [Google Scholar] [CrossRef]
- Zhang, H.; Yao, G.; Wang, L.; Su, Y.; Yang, W.; Lin, Y. 3D Pt/MoO3 nanocatalysts fabricated for effective electrocatalytic oxidation of alcohol. Appl. Surf. Sci. 2015, 356, 294–300. [Google Scholar] [CrossRef]
- Shao, M.; Chang, Q.; Dodelet, J.P.; Chenitz, R. Recent advances in electrocatalysts for oxygen reduction reaction. Chem. Rev. 2016, 116, 3594–3657. [Google Scholar] [CrossRef]
- Cakar, I.; Özdokur, K.V.; Demir, B.; Yavuz, E.; Demirkol, D.O.; Koçak, S.; Timurb, S.; Ertaş, F.N. Molybdenum oxide/platinum modified glassy carbon electrode: A novel electrocatalytic platform for the monitoring of electrochemical reduction of oxygen and its biosensing applications. Sens. Actuators B 2013, 185, 331–336. [Google Scholar] [CrossRef]
- Liu, X.; Wang, H.; Chen, S.; Qi, X.; Gao, H.; Hui, Y.; Bai, Y.; Guo, L.; Ding, W.; Wei, Z. SO2-tolerant Pt-MoO3/C catalyst for oxygen reduction reaction. J. Energy Chem. 2014, 23, 358–362. [Google Scholar] [CrossRef]
- Yavuz, E.; Özdokur, K.V.; Çakar, İ.; Koçak, S.; Ertaş, F.N. Electrochemical preparation, characterization of molybdenum-oxide/platinum binary catalysts and its application to oxygen reduction reaction in weakly acidic medium. Electrochim. Acta 2015, 151, 72–80. [Google Scholar] [CrossRef]
- Karuppasamy, L.; Chen, C.Y.; Anandan, S.; Wu, J.J. High index surfaces of Au-nanocrystals supported on one-dimensional MoO3-nanorod as a bi-functional electrocatalyst for ethanol oxidation and oxygen reduction. Electrochim. Acta 2017, 246, 75–88. [Google Scholar] [CrossRef]
- Liu, X.; Iocozzia, J.; Wang, Y.; Cui, X.; Chen, Y.; Zhao, S.; Li, Z.; Lin, Z. Noble metal–metal oxide nanohybrids with tailored nanostructures for efficient solar energy conversion, photocatalysis and environmental remediation. Energy Env. Sci. 2017, 10, 402–434. [Google Scholar] [CrossRef]
- Hu, C.; Xu, M.; Zhang, J.; Zhou, Y.; Hu, B.; Yu, G. Recyclable MoO3 nanobelts for photocatalytic degradation of Rhodamine B by near infrared irradiation. Int. J. Chem. Kinet. 2019, 51, 3–13. [Google Scholar] [CrossRef]
- Etman, A.S.; Abdelhamid, H.N.; Yuan, Y.; Wang, L.; Zou, X.; Sun, J. Facile water-based strategy for synthesizing MoO3−x nanosheets: Efficient visible light photocatalysts for dye degradation. ACS Omega 2018, 3, 2193–2201. [Google Scholar] [CrossRef]
- Gao, B.; Fan, H.; Zhang, X.; Zhu, C. Template-free hydrothermal synthesis of α-molybdenum trioxide nanobelts and their photocatalytic activity for degradation of methylene blue. Micro Nano Lett. 2013, 8, 500–503. [Google Scholar] [CrossRef]
- Ma, Y.; Jia, Y.; Jiao, Z.; Wang, L.; Yang, M.; Bi, Y.; Qi, Y. Facile synthesize α-MoO3 nanobelts with high adsorption property. Mater. Lett. 2015, 157, 53–56. [Google Scholar] [CrossRef]
- Szkoda, M.; Trzciński, K.; Siuzdak, K.; Lisowska-Oleksiak, A. Photocatalytical properties of maze-like MoO3 microstructures prepared by anodization of Mo plate. Electrochim. Acta 2017, 228, 139–145. [Google Scholar] [CrossRef]
- Liu, Y.; Feng, P.; Wang, Z.; Jiao, X.; Akhtar, F. Novel fabrication and enhanced photocatalytic MB degradation of hierarchical porous monoliths of MoO3 nanoplates. Sci. Rep. 2017, 7, 1845. [Google Scholar] [CrossRef]
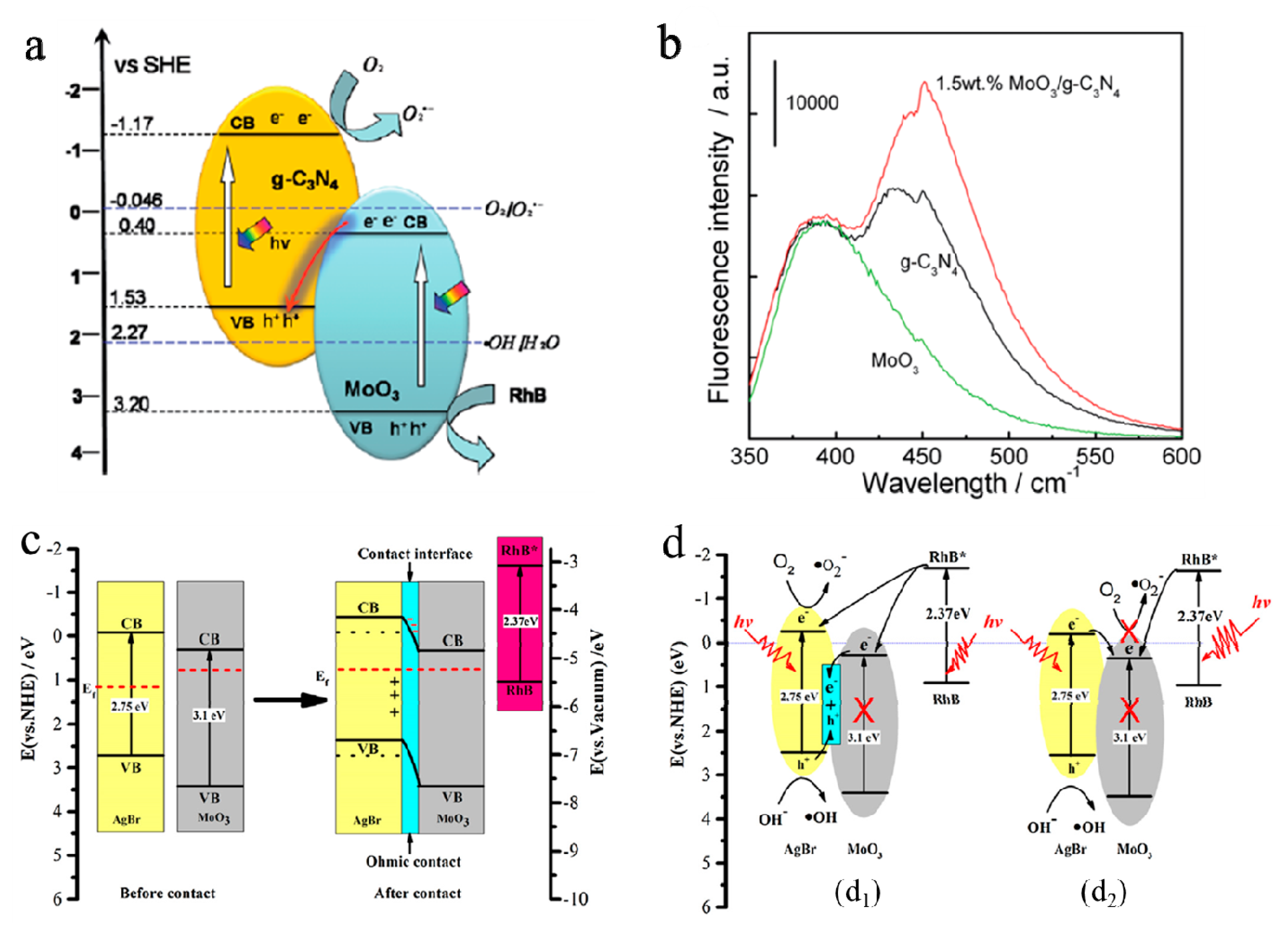
- Feng, B.; Wu, Z.; Liu, J.; Zhu, K.; Li, Z.; Jin, X.; Hou, Y.; Xi, Q.; Cong, M.; Liu, P.; et al. Combination of ultrafast dye-sensitized-assisted electron transfer process and novel Z-scheme system: AgBr nanoparticles interspersed MoO3 nanobelts for enhancing photocatalytic performance of RhB. Appl. Catal. B 2017, 206, 242–251. [Google Scholar] [CrossRef]
- Xi, Q.; Liu, J.; Wu, Z.; Bi, H.; Li, Z.; Zhu, K.; Zhuang, J.; Chen, J.; Lu, S.; Huang, Y.; et al. In-situ fabrication of MoO3 nanobelts decorated with MoO2 nanoparticles and their enhanced photocatalytic performance. Appl. Surf. Sci. 2019, 480, 427–437. [Google Scholar] [CrossRef]
- Phuruangrat, A.; Cheed-Im, U.; Thongtem, T.; Thongtem, S. High visible light photocatalytic activity of Eu-doped MoO3 nanobelts synthesized by hydrothermal method. Mater. Lett. 2016, 172, 166–170. [Google Scholar] [CrossRef]
- Phuruangrat, A.; Cheed-Im, U.; Thongtem, T.; Thongtem, S. Influence of Gd dopant on photocatalytic properties of MoO3 nanobelts. Mater. Lett. 2016, 173, 158–161. [Google Scholar] [CrossRef]
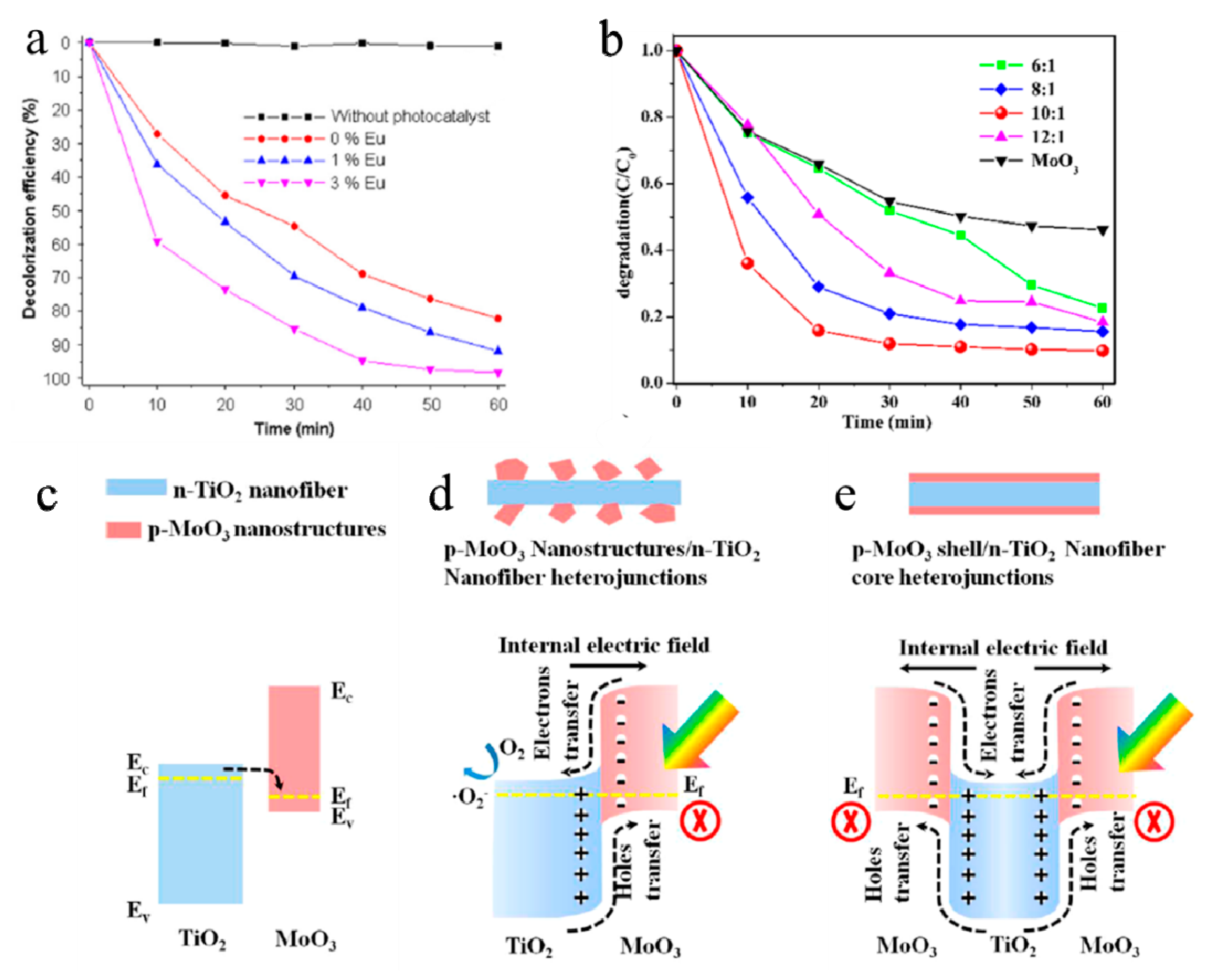
- Lu, M.; Shao, C.; Wang, K.; Lu, N.; Zhang, X.; Zhang, P.; Zhang, M.; Li, X.; Liu, Y. p-MoO3 nanostructures/n-TiO2 nanofiber heterojunctions: Controlled fabrication and enhanced photocatalytic properties. ACS Appl. Mater. Interfaces 2014, 6, 9004–9012. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, B.; Hao, Y.; Yao, Z. MoO3-nanowire membrane and Bi2Mo3O12/MoO3 nano-heterostructural photocatalyst for wastewater treatment. Chem. Eng. J. 2014, 244, 382–390. [Google Scholar] [CrossRef]
- Balendhran, S.; Walia, S.; Nili, H.; Ou, J.Z.; Zhuiykov, S.; Kaner, R.B.; Sriram, S.; Bhaskaran, M.; Kalantar-zadeh, K. Two-dimensional molybdenum trioxide and dichalcogenides. Adv. Funct. Mater. 2013, 23, 3952–3970. [Google Scholar] [CrossRef]
- Li, H.; Yu, K.; Tang, Z.; Fu, H.; Zhu, Z. High photocatalytic performance of a type-II α-MoO3@MoS2 heterojunction: From theory to experiment. Phys. Chem. Chem. Phys. 2016, 18, 14074–14085. [Google Scholar] [CrossRef]
- Liu, H.; Lv, T.; Zhu, C.; Zhu, Z. Direct bandgap narrowing of TiO2/MoO3 heterostructure composites for enhanced solar-driven photocatalytic activity. Sol. Energy Mater. Sol. Cells 2016, 153, 1–8. [Google Scholar] [CrossRef]
- He, Y.; Zhang, L.; Wang, X.; Wu, Y.; Lin, H.; Zhao, L.; Weng, W.; Wan, H.; Fan, M. Enhanced photodegradation activity of methyl orange over Z-scheme type MoO3–gC3N4 composite under visible light irradiation. RSC Adv. 2014, 4, 13610–13619. [Google Scholar] [CrossRef]
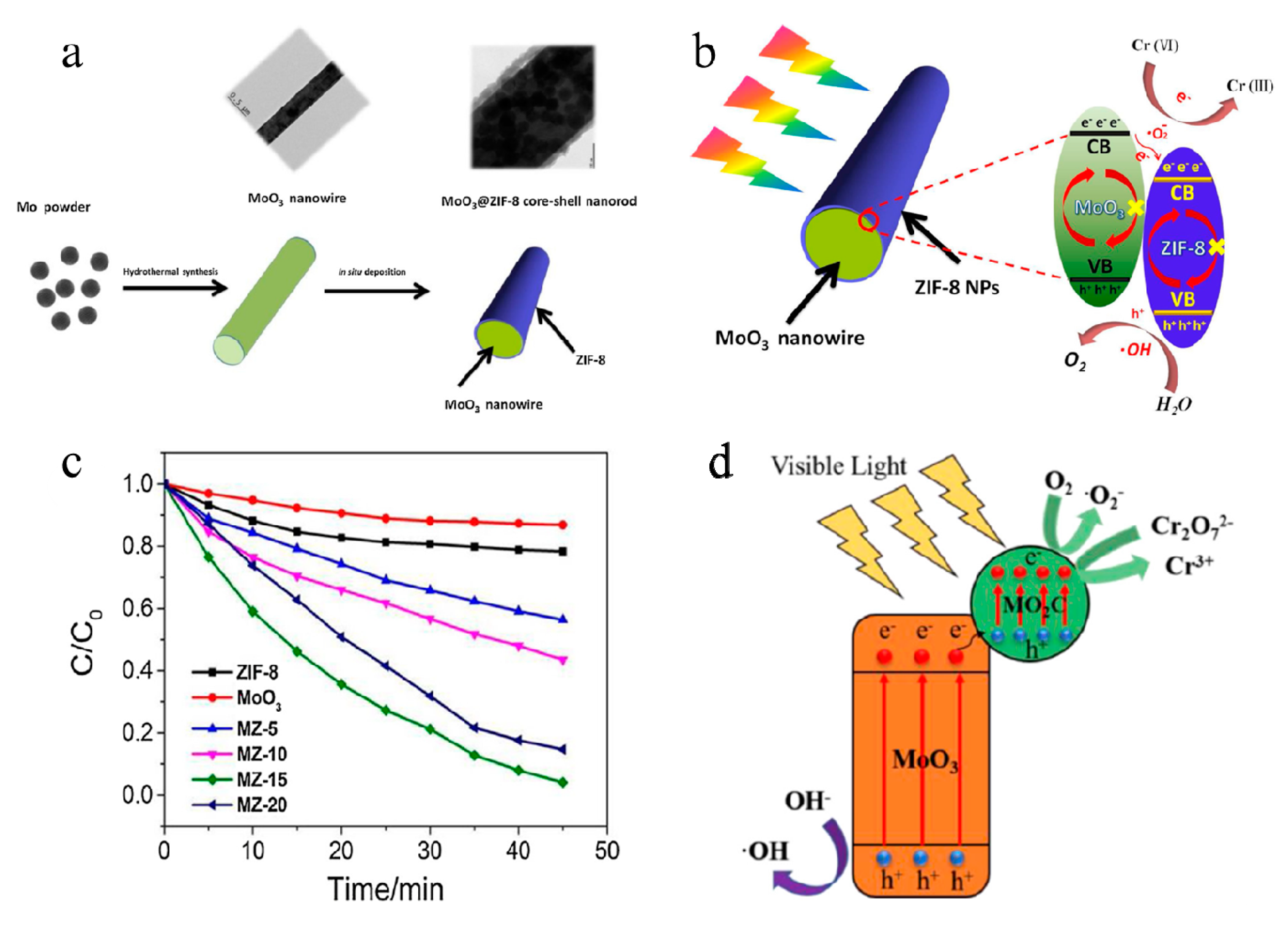
- Zhang, Y.; Park, S.J. Facile construction of MoO3@ ZIF-8 core-shell nanorods for efficient photoreduction of aqueous Cr (VI). Appl. Catal. B 2019, 240, 92–101. [Google Scholar] [CrossRef]
- Jing, X.; Peng, X.; Sun, X.; Zhou, W.; Wang, W.; Wang, S. Design and synthesis of Mo2C/MoO3 with enhanced visible-light photocatalytic performance for reduction of Cr (VI) and degradation of organic pollutants. Mater. Sci. Semicond. Process. 2019, 100, 262–269. [Google Scholar] [CrossRef]
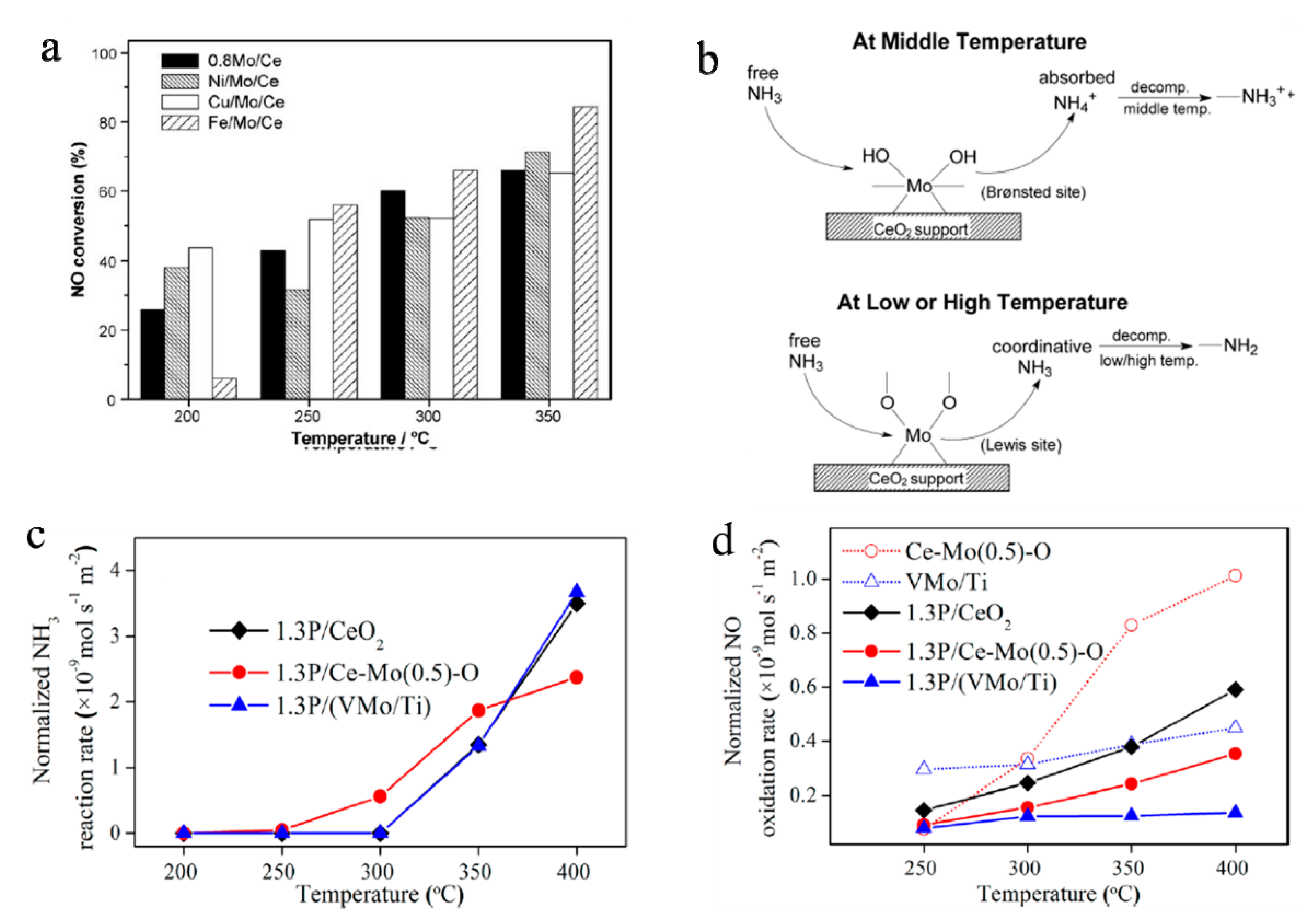
- Jiang, Y.; Bao, C.; Liu, Q.; Liang, G.; Lu, M.; Ma, S. A novel CeO2-MoO3-WO3/TiO2 catalyst for selective catalytic reduction of NO with NH3. Catal. Commun. 2018, 103, 96–100. [Google Scholar] [CrossRef]
- Geng, Y.; Chen, X.; Yang, S.; Liu, F.; Shan, W. Promotional effects of Ti on a CeO2–MoO3 catalyst for the selective catalytic reduction of NOx with NH3. ACS Appl. Mater. Interfaces 2017, 9, 16951–16958. [Google Scholar] [CrossRef]
- Casagrande, L.; Lietti, L.; Nova, I.; Forzatti, P.; Baiker, A. SCR of NO by NH3 over TiO2-supported V2O5–MoO3 catalysts: Reactivity and redox behavior. Appl. Catal. B 1999, 22, 63–77. [Google Scholar] [CrossRef]
- Yu, W.; Zhu, J.; Qi, L.; Sun, C.; Gao, F.; Dong, L.; Chen, Y. Surface structure and catalytic properties of MoO3/CeO2 and CuO/MoO3/CeO2. J. Colloid Interface Sci. 2011, 364, 435–442. [Google Scholar] [CrossRef]
- Chang, H.; Jong, M.T.; Wang, C.; Qu, R.; Du, Y.; Li, J.; Hao, J. Design strategies for P-containing fuels adaptable CeO2–MoO3 catalysts for DeNOx: Significance of phosphorus resistance and N2 selectivity. Env. Sci. Technol. 2013, 47, 11692–11699. [Google Scholar] [CrossRef]
- Zhu, J.; Gao, F.; Dong, L.; Yu, W.; Qi, L.; Wang, Z.; Dong, L.; Chen, Y. Studies on surface structure of MxOy/MoO3/CeO2 system (M = Ni, Cu, Fe) and its influence on SCR of NO by NH3. Appl. Catal. B 2010, 95, 144–152. [Google Scholar] [CrossRef]
- He, C.; Cheng, J.; Zhang, X.; Douthwaite, M.; Pattisson, S.; Hao, Z. Recent advances in the catalytic oxidation of volatile organic compounds: A review based on pollutant sorts and sources. Chem. Rev. 2019, 119, 4471–4568. [Google Scholar] [CrossRef]
- Schuh, K.; Kleist, W.; Høj, M.; Jensen, A.D.; Beato, P.; Patzke, G.R.; Grunwaldt, J.D. Systematic study on the influence of the morphology of α-MoO3 in the selective oxidation of propylene. J. Solid State Chem. 2015, 228, 42–52. [Google Scholar] [CrossRef]
- Volta, J.C.; Tatibouet, J.M. Structure sensitivity of MoO3 in mild oxidation of propylene. J. Catal. 1985, 93, 467–470. [Google Scholar] [CrossRef]
- Volta, J.C.; Forissier, M.; Theobald, F.; Pham, T.P. Dependence of selectivity on surface structure of MoO3 catalysts. Faraday Discuss. Chem. Soc. 1981, 72, 225–233. [Google Scholar] [CrossRef]
- Ressler, T.; Walter, A.; Huang, Z.D.; Bensch, W. Structure and properties of a supported MoO3–SBA-15 catalyst for selective oxidation of propene. J. Catal. 2008, 254, 170–179. [Google Scholar] [CrossRef]
- Khatib, S.J.; Oyama, S.T. Direct oxidation of propylene to propylene oxide with molecular oxygen: A review. Catal. Rev. 2015, 57, 306–344. [Google Scholar] [CrossRef]
- Jin, G.; Lu, G.; Guo, Y.; Guo, Y.; Wang, J.; Liu, X. Epoxidation of propylene by molecular oxygen over modified Ag–MoO3 catalyst. Catal. Lett. 2003, 87, 249–252. [Google Scholar] [CrossRef]
- Jin, G.; Lu, G.; Guo, Y.; Guo, Y.; Wang, J.; Liu, X. Direct epoxidation of propylene with molecular oxygen over Ag–MoO3/ZrO2 catalyst. Catal. Today. 2004, 93–95, 173–182. [Google Scholar] [CrossRef]
- Nguyen, T.K.L.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Nghiem, L.D.; Liu, Y.; Ni, B.; Hai, F.I. Insight into greenhouse gases emissions from the two popular treatment technologies in municipal wastewater treatment processes. Sci. Total Env. 2019, 671, 1302–1313. [Google Scholar] [CrossRef]
- Han, B.; Yang, Y.; Xu, Y.; Etim, U.J.; Qiao, K.; Xu, B.; Yan, Z. A review of the direct oxidation of methane to methanol. Chin. J. Catal. 2016, 37, 1206–1215. [Google Scholar] [CrossRef]
- Liu, R.S.; Iwamoto, M.; Lunsford, J.H. Partial oxidation of methane by nitrous oxide over molybdenum oxide supported on silica. J. Chem. Soc. Chem. Commun. 1982, 1, 78–79. [Google Scholar] [CrossRef]
- Smith, M.R.; Ozkan, U.S. The partial oxidation of methane to formaldehyde: Role of different crystal planes of MoO3. J. Catal. 1993, 141, 124–139. [Google Scholar] [CrossRef]
- Arena, F.; Giordano, N.; Parmaliana, A. Working mechanism of oxide catalysts in the partial oxidation of methane to formaldehyde. II. Redox properties and reactivity of SiO2, MoO3/SiO2, V2O5/SiO2, TiO2, and V2O5/TiO2 systems. J. Catal. 1997, 167, 66–76. [Google Scholar] [CrossRef]
- Taylor, S.H.; Hargreaves, J.S.; Hutchings, G.J.; Joyner, R.W.; Lembacher, C.W. The partial oxidation of methane to methanol: An approach to catalyst design. Catal. Today 1998, 42, 217–224. [Google Scholar] [CrossRef]
- Mohamed, M.; Katib, S.M.A. Structural and catalytic characteristics of MoO3/CeO2 catalysts: CO oxidation activity. Appl. Catal. A 2005, 287, 236–243. [Google Scholar] [CrossRef]
- Wang, C.H.; Lin, S.S.; Liou, S.B.; Weng, H.S. The promoter effect and a rate expression of the catalytic incineration of (CH3)2S2 over an improved CuO–MoO3/γ-Al2O3 catalyst. Chemosphere 2002, 49, 389–394. [Google Scholar] [CrossRef]
- Huang, X.; Peng, Y.; Liu, X.; Li, K.; Deng, Y.; Li, J. The promotional effect of MoO3 doped V2O5/TiO2 for chlorobenzene oxidation. Catal. Commun. 2015, 69, 161–164. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Yao, Y.; Luo, Z.; Pan, C.; Yang, J.; Fang, Y.; Deng, H.; Liu, C.; Tan, Q.; Liu, F.; et al. Nanostructured MoO3 for Efficient Energy and Environmental Catalysis. Molecules 2020, 25, 18. https://doi.org/10.3390/molecules25010018
Zhu Y, Yao Y, Luo Z, Pan C, Yang J, Fang Y, Deng H, Liu C, Tan Q, Liu F, et al. Nanostructured MoO3 for Efficient Energy and Environmental Catalysis. Molecules. 2020; 25(1):18. https://doi.org/10.3390/molecules25010018
Chicago/Turabian StyleZhu, Yuhua, Yuan Yao, Zhu Luo, Chuanqi Pan, Ji Yang, Yarong Fang, Hongtao Deng, Changxiang Liu, Qi Tan, Fudong Liu, and et al. 2020. "Nanostructured MoO3 for Efficient Energy and Environmental Catalysis" Molecules 25, no. 1: 18. https://doi.org/10.3390/molecules25010018
APA StyleZhu, Y., Yao, Y., Luo, Z., Pan, C., Yang, J., Fang, Y., Deng, H., Liu, C., Tan, Q., Liu, F., & Guo, Y. (2020). Nanostructured MoO3 for Efficient Energy and Environmental Catalysis. Molecules, 25(1), 18. https://doi.org/10.3390/molecules25010018







