Formation of Ethyl Carbamate during the Production Process of Cantonese Soy Sauce
Abstract
1. Introduction
2. Results
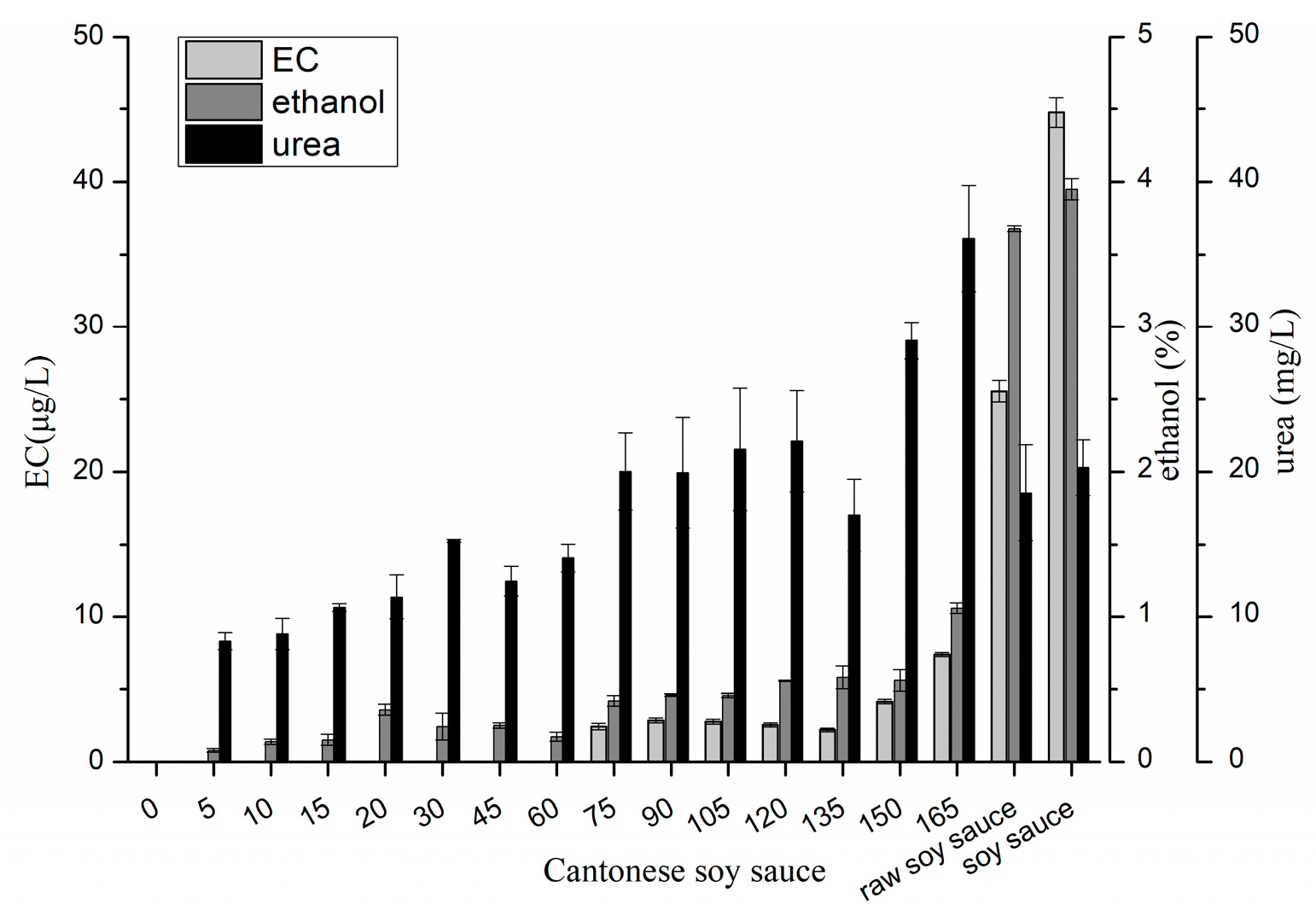
2.1. Changes in EC, Ethanol and Urea in Cantonese Soy Sauce
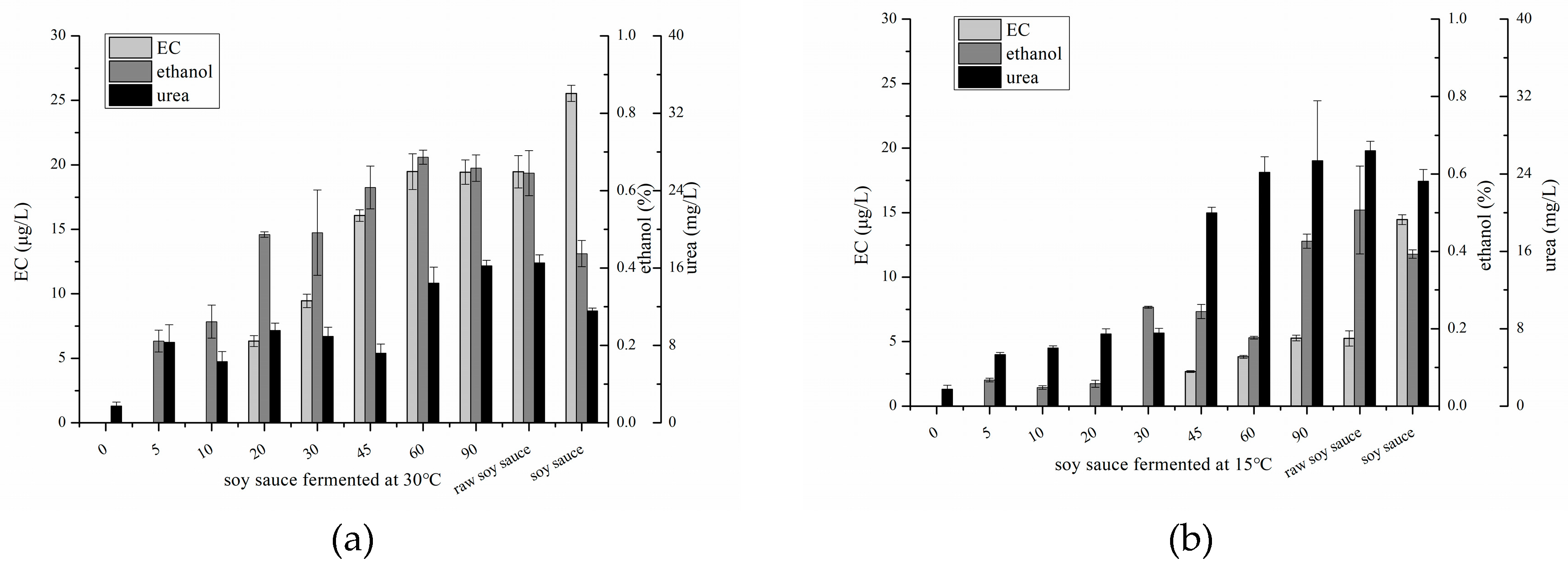
2.2. Changes of EC, Ethanol and Urea in Soy Sauce Produced in Laboratory
2.3. Metal Content, Cyanide, Free Amino Acids and pH
2.4. Correlation between Ethyl Carbamate and Its Precursors
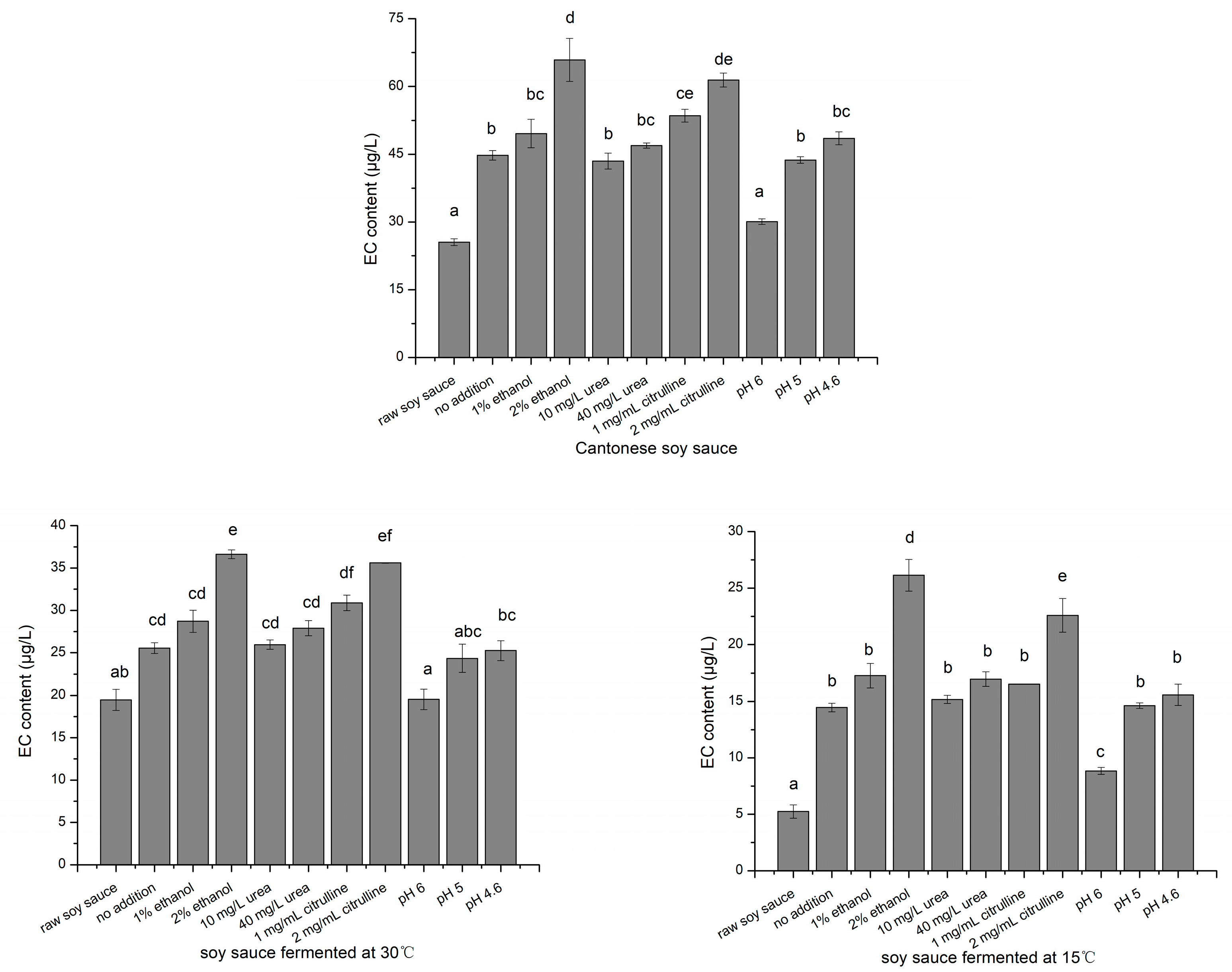
2.5. EC Formation with Ethanol, Urea, Citrulline Addition and pH Adjustment
3. Discussion
3.1. Precursors of EC in Soy Sauce
3.2. Critical Accumulation Stages of EC and Its Influencing Factors
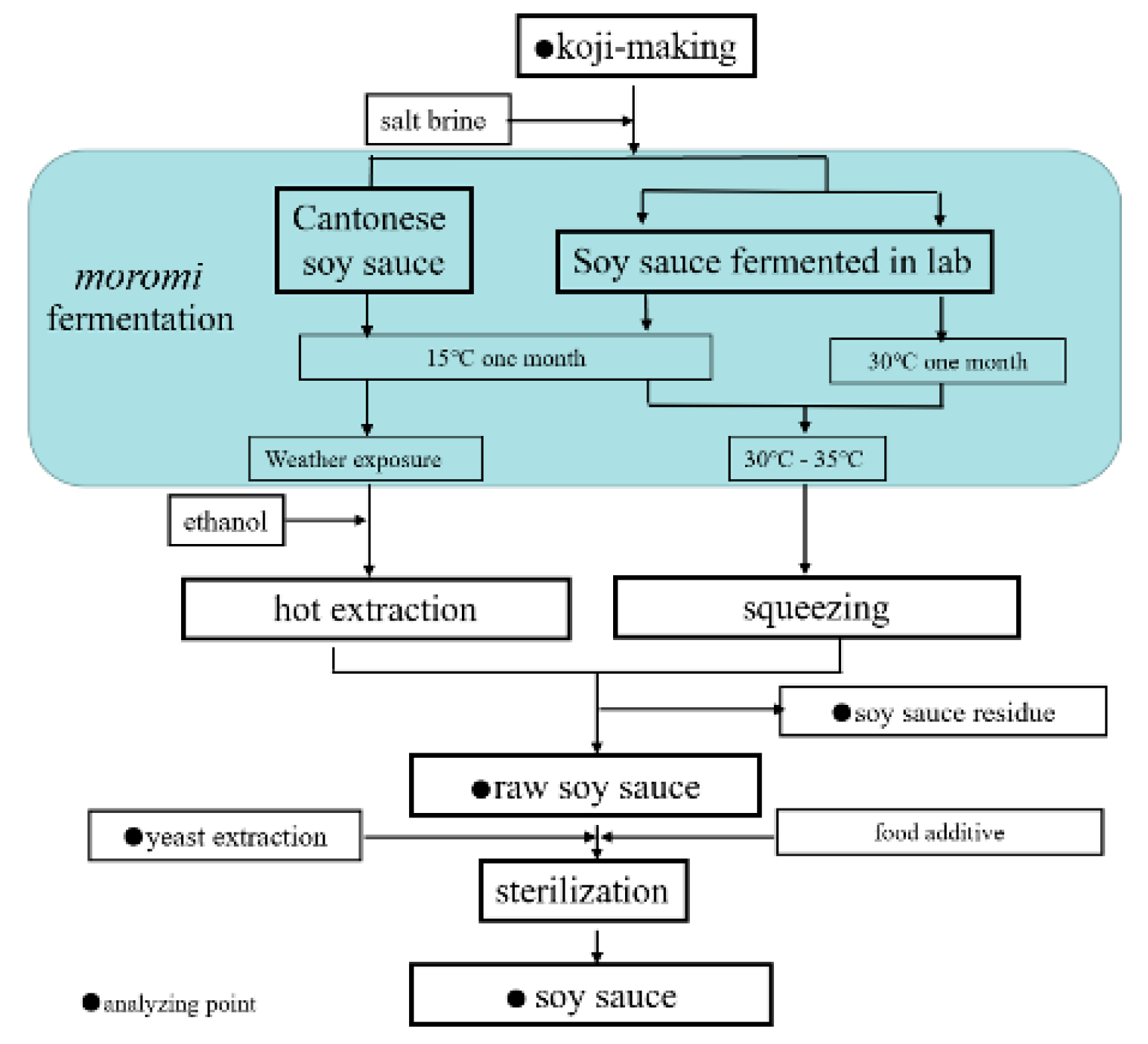
4. Materials and Methods
4.1. Reagents
4.2. Sample Preparation
4.3. Heat Treatment of Soy Sauce
4.4. Determination of EC
4.5. Determination of Urea
4.6. Determination of Free Amino Acid
4.7. Determination of Alcohol and Cyanide Content
4.8. Measurement of Metal Content and pH
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Peng, M.; Liu, J.; Liu, Z.; Fu, B.; Hu, Y.; Zhou, M.; Fu, C.; Gao, B.; Wang, C.; Li, D.; et al. Effect of citrus peel on phenolic compounds, organic acids and antioxidant activity of soy sauce. LWT-Food Sci. Technol. 2018, 90, 627–635. [Google Scholar] [CrossRef]
- Chen, T.; Lu, L.; Xu, C.; Lin, X.; Leung, Y.-k.; Ho, S.-M.; Ruan, X.Z.; Lian, X. Inhibition Role of Atherogenic Diet on Ethyl Carbamate Induced Lung Tumorigenesis in C57BL/6J Mice. Sci. Rep. 2017, 7, 4723. [Google Scholar] [CrossRef]
- Liu, H.; Cui, B.; Xu, Y.; Hu, C.; Liu, Y.; Qu, G.; Li, D.; Wu, Y.; Zhang, D.; Quan, S. Ethyl carbamate induces cell death through its effects on multiple metabolic pathways. Chem. Biol. Interact. 2017, 277, 21–32. [Google Scholar] [CrossRef]
- Wu, P.; Pan, X.; Wang, L.; Shen, X.; Yang, D. A survey of ethyl carbamate in fermented foods and beverages from Zhejiang, China. Food Control 2012, 23, 286–288. [Google Scholar] [CrossRef]
- Koh, E.; Kwon, H. Quantification of ethyl carbamate in soy sauce consumed in Korea and estimated daily intakes by age. J. Sci. Food Agric. 2007, 87, 98–102. [Google Scholar] [CrossRef]
- Choi, B.; Ryu, D.; Kim, C.; Lee, J.; Choi, A.; Koh, E. Probabilistic dietary exposure to ethyl carbamate from fermented foods and alcoholic beverages in the Korean population. Food Addit. Contam. 2017, 34, 1885–1892. [Google Scholar] [CrossRef]
- Park, S.-R.; Ha, S.-D.; Yoon, J.-H.; Lee, S.-Y.; Hong, K.-P.; Lee, E.-H.; Yeom, H.-J.; Yoon, N.-G.; Bae, D.-H. Exposure to ethyl carbamate in alcohol-drinking and nondrinking adults and its reduction by simple charcoal filtration. Food Control 2009, 20, 946–952. [Google Scholar] [CrossRef]
- Xia, Q.; Yang, C.; Wu, C.; Zhou, R.; Li, Y. Quantitative strategies for detecting different levels of ethyl carbamate (EC) in various fermented food matrices: An overview. Food Control 2018, 84, 499–512. [Google Scholar] [CrossRef]
- Weber, J.; Sharypov, V. Ethyl carbamate in foods and beverages: A review. Environ. Chem. Lett. 2009, 7, 233–247. [Google Scholar] [CrossRef]
- Wu, P.; Cai, C.; Shen, X.; Wang, L.; Zhang, J.; Tan, Y.; Jiang, W.; Pan, X. Formation of ethyl carbamate and changes during fermentation and storage of yellow rice wine. Food Chem. 2014, 152, 108–112. [Google Scholar] [CrossRef]
- Galinaro, C.A.; Ohe, T.H.; da Silva, A.C.; da Silva, S.C.; Franco, D.W. Cyanate as an active precursor of ethyl carbamate formation in sugar cane spirit. J. Agric. Food. Chem. 2015, 63, 7415–7420. [Google Scholar] [CrossRef]
- Choi, B.; Koh, E. Changes of ethyl carbamate and its precursors in maesil (Prunus mume) extract during one-year fermentation. Food Chem. 2016, 209, 318–322. [Google Scholar] [CrossRef]
- Feng, Y.; Cai, Y.; Su, G.; Zhao, H.; Wang, C.; Zhao, M. Evaluation of aroma differences between high-salt liquid-state fermentation and low-salt solid-state fermentation soy sauces from China. Food Chem. 2014, 145, 126–134. [Google Scholar] [CrossRef]
- Zhou, K.; Liu, Y.; Li, W.-Q.; Liu, G.-L.; Wei, N.; Sun, Y.-M.; Bai, W.-D.; Xu, Z.-L. An improved HPLC-FLD for fast and simple detection of ethyl carbamate in soy sauce and prediction of precursors. Food Anal. Method. 2017, 10, 3856–3865. [Google Scholar] [CrossRef]
- Sun, S.Y.; Jiang, W.G.; Zhao, Y.P. Profile of volatile compounds in 12 Chinese soy sauces produced by a high-salt-diluted state fermentation. J. Instit. Brewing 2010, 116, 316–328. [Google Scholar] [CrossRef]
- Luh, B.S. Industrial production of soy sauce. J. Ind. Microbiol. 1995, 14, 467–471. [Google Scholar] [CrossRef]
- Fang, F.; Zhang, J.; Zhou, J.; Zhou, Z.; Li, T.; Lu, L.; Zeng, W.; Du, G.; Chen, J. Accumulation of Citrulline by Microbial Arginine Metabolism during Alcoholic Fermentation of Soy Sauce. J. Agric. Food. Chem. 2018, 66, 2108–2113. [Google Scholar] [CrossRef]
- Tanaka, Y.; Watanabe, J.; Mogi, Y. Monitoring of the microbial communities involved in the soy sauce manufacturing process by PCR-denaturing gradient gel electrophoresis. Food Microbiol. 2012, 31, 100–106. [Google Scholar] [CrossRef]
- Bruno, S.N.F.; Vaitsman, D.S.; Kunigami, C.N.; Brasil, M.G. Influence of the distillation processes from Rio de Janeiro in the ethyl carbamate formation in Brazilian sugar cane spirits. Food Chem. 2007, 104, 1345–1352. [Google Scholar] [CrossRef]
- Xia, Q.; Yuan, H.; Wu, C.; Zheng, J.; Zhang, S.; Shen, C.; Yi, B.; Zhou, R. An improved and validated sample cleanup method for analysis of ethyl carbamate in Chinese liquor. J. Food Sci. 2014, 79, 1854–1860. [Google Scholar] [CrossRef]
- Matsudo, T.; Aoki, T.; Abe, K.; Fukuta, N.; Higuchi, T.; Sasaki, M.; Uchida, K. Determination of ethyl carbamate in soy sauce and its possible precursor. J. Agric. Food. Chem. 1993, 41, 352–356. [Google Scholar] [CrossRef]
- Zhang, J.; Fang, F.; Chen, J.; Du, G. The arginine deiminase pathway of koji bacteria is involved in ethyl carbamate precursor production in soy sauce. FEMS Microbiol. Lett. 2014, 358, 91–97. [Google Scholar] [CrossRef]
- Gowd, V.; Su, H.; Karlovsky, P.; Chen, W. Ethyl carbamate: An emerging food and environmental toxicant. Food Chem. 2018, 248, 312–321. [Google Scholar] [CrossRef]
- Zhao, X.; Du, G.; Zou, H.; Fu, J.; Zhou, J.; Chen, J. Progress in preventing the accumulation of ethyl carbamate in alcoholic beverages. Trends Food Sci. Technol. 2013, 32, 97–107. [Google Scholar] [CrossRef]
- Van Der Sluis, C.; Tramper, J.; Wijffels, R.H. Enhancing and accelerating flavour formation by salt-tolerant yeasts in Japanese soy-sauce processes. Trends Food Sci. Technol. 2001, 12, 322–327. [Google Scholar] [CrossRef]
- Lee, W.-J.; Cho, D.-H. Microbiological studies of Korean native soy-sauce fermentation-A study on the microflora changes during Korean native soy-sauce fermentation. Appl. Biol. Chem. 1971, 14, 137–148. [Google Scholar]
- Shukla, S.; Kim, M. Determination of biogenic amines and total aflatoxins: Quality index of starter culture soy sauce samples. Food Sci. Biotechnol. 2016, 25, 1221–1224. [Google Scholar] [CrossRef]
- Zhou, W.; Fang, R.; Chen, Q. Effect of gallic and protocatechuic acids on the metabolism of ethyl carbamate in Chinese yellow rice wine brewing. Food Chem. 2017, 233, 174–181. [Google Scholar] [CrossRef]
- Cui, C.; Zhao, M.; Li, D.; Zhao, H.; Sun, W. Biochemical changes of traditional Chinese-type soy sauce produced in four seasons during processing. CyTA-J. Food 2014, 12, 166–175. [Google Scholar] [CrossRef]
- Huang, Z.; Pan, X.D.; Wu, P.G.; Chen, Q.; Han, J.L.; Shen, X.H. Validation (in-house and collaboratory) of the quantification method for ethyl carbamate in alcoholic beverages and soy sauce by GC-MS. Food Chem. 2013, 141, 4161. [Google Scholar] [CrossRef]
- Clark, S.; Francis, P.S.; Conlan, X.A.; Barnett, N.W. Determination of urea using high-performance liquid chromatography with fluorescence detection after automated derivatisation with xanthydrol. J. Chromatogr. A 2007, 1161, 207. [Google Scholar] [CrossRef]
- Williams, M.B.; Reese, H.D. Colorimetric determination of ethyl alcohol. Anal. Chem. 1950, 22, 1556–1561. [Google Scholar] [CrossRef]
- Haskins, F.A.; Gorz, H.J.; Hill, R.M. Colorimetric determination of cyanide in enzyme-hydrolyzed extracts of dried sorghum leaves. J. Agric. Food Chem. 1988, 36, 775–778. [Google Scholar] [CrossRef]
- Shang, S.; Wang, H. Flame atomic absorption spectrometric determination of copper, zinc, calcium, magnesium and iron in fresh eggs using microvolume injection. Talanta 1997, 44, 269–274. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available. |
| Cantonese Soy Sauce | Soy Sauce Fermented at 30 °C | Soy Sauce Fermented at 15 °C | |||
|---|---|---|---|---|---|
| days | pH | days | pH | days | pH |
| 5 | 5.87 ± 0.01 | 5 | 6.02 ± 0.01 | 5 | 6.02 ± 0.01 |
| 10 | 5.20 ± 0.10 | 10 | 5.15 ± 0.21 | 10 | 5.61 ± 0.14 |
| 15 | 5.29 ± 0.03 | 20 | 4.88 ± 0.03 | 20 | 5.51 ± 0.11 |
| 20 | 5.24 ± 0.04 | 30 | 4.82 ± 0.05 | 30 | 5.01 ± 0.06 |
| 30 | 5.17 ± 0.02 | 45 | 4.70 ± 0.02 | 45 | 4.88 ± 0.06 |
| 45 | 4.88 ± 0.01 | 60 | 4.65 ± 0.04 | 60 | 4.66 ± 0.03 |
| 60 | 4.85 ± 0.01 | 90 | 4.65 ± 0.04 | 90 | 4.69 ± 0.04 |
| 75 | 4.89 ± 0.02 | Raw soy sauce | 4.65 ± 0.02 | Raw soy sauce | 4.69 ± 0.04 |
| 90 | 4.83 ± 0.01 | Soy sauce | 4.80 ± 0.02 | Soy sauce | 4.77 ± 0.02 |
| 105 | 4.81 ± 0.01 | ||||
| 120 | 4.69 ± 0.02 | ||||
| 135 | 4.63 ± 0.02 | ||||
| 150 | 4.48 ± 0.04 | ||||
| 160 | 4.55 ± 0.01 | ||||
| Raw soy sauce | 4.53 ± 0.02 | ||||
| Soy sauce | 4.72 ± 0.03 | ||||
| Cantonese Soy Sauce | |||||||
| Days | 5 | 20 | 60 | 90 | 135 | 165 | Soy sauce |
| citrulline | 308 ± 20 | 574 ± 21 | 1093 ± 15 | 1794 ± 112 | 2234 ± 62 | 2358 ± 64 | 1222 ± 62 |
| ornithine | 172 ± 10 | 1045 ± 102 | 66 ± 42 | 100 ± 50 | 612 ± 18 | 548 ± 12 | 484 ± 16 |
| arginine | 1076 ± 83 | 1332 ± 138 | 3725 ± 35 | 3790 ± 230 | 3230 ± 270 | 3475 ± 275 | 2804 ± 185 |
| Soy Sauce Fermented at 30 °C | |||||||
| Days | 5 | 20 | 45 | 90 | Soy sauce | ||
| citrulline | 163 ± 34 | 807 ± 46 | 903 ± 82 | 1428 ± 27 | 1054 ± 55 | ||
| ornithine | 186 ± 20 | 633 ± 33 | 74 ± 55 | 2984 ± 234 | 1588 ± 84 | ||
| arginine | 574 ± 138 | 1610 ± 122 | 2118 ± 184 | 1060 ± 46 | 1020 ± 140 | ||
| Soy Sauce Fermented at 15 °C | |||||||
| Days | 5 | 20 | 45 | 90 | Soy sauce | ||
| citrulline | 254 ± 28 | 518 ± 62 | 705 ± 55 | 922 ± 64 | 768 ± 66 | ||
| ornithine | 34 ± 12 | 1800 ± 160 | 74 ± 22 | 385 ± 52 | 392 ± 83 | ||
| arginine | 718 ± 86 | 472 ± 106 | 2052 ± 210 | 3390 ± 180 | 2135 ± 185 | ||
| Soy Sauce Sample | Item | Ethanol | Cyanide | Urea | Citrulline | Ornithine | Arginine | pH |
|---|---|---|---|---|---|---|---|---|
| Cantonese soy sauce | EC | 0.97 ** | 0.04 | 0.26 | −0.12 | 0.07 | −0.17 | 0.70 ** |
| ethanol | 0.08 | 0.297 | −0.10 | 0.14 | −0.19 | 0.74 ** | ||
| cyanide | −0.30 | −0.23 | 0.38 | −0.86 * | 0.25 | |||
| Urea | 0.78 * | −0.05 | 0.15 | −0.81 * | ||||
| citrulline | −0.03 | 0.43 | −0.97 * | |||||
| ornithine | −0.61 | −0.09 | ||||||
| arginine | −0.46 | |||||||
| Soy sauce fermented at 30 °C | EC | 0.79 ** | 0.52 | 0.76 * | 0.82 | 0.61 | 0.20 | −0.79 * |
| ethanol | 0.71 * | 0.83 ** | 0.89 * | 0.50 | 0.65 | −0.87 * | ||
| cyanide | 0.37 | 0.49 | −0.04 | 0.80 | −0.71 | |||
| urea | 0.75 | 0.99 ** | −0.36 | −0.45 | ||||
| citrulline | 0.80 | 0.31 | −0.90 * | |||||
| ornithine | −0.32 | −0.45 | ||||||
| arginine | −0.73 | |||||||
| Soy sauce fermented at 15 °C | EC | 0.69 * | −0.22 | 0.72 * | 0.59 | −0.24 | 0.53 | −0.80 * |
| ethanol | 0.15 | 0.85 ** | 0.90 * | −0.38 | 0.94 * | −0.79 * | ||
| cyanide | −0.03 | 0.36 | −0.15 | 0.25 | −0.37 | |||
| urea | 0.95 * | −0.36 | 0.94 * | −0.86 * | ||||
| citrulline | −0.06 | 0.89 * | −0.98 * | |||||
| ornithine | −0.44 | 0.13 | ||||||
| arginine | −0.87 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, K.; Siroli, L.; Patrignani, F.; Sun, Y.; Lanciotti, R.; Xu, Z. Formation of Ethyl Carbamate during the Production Process of Cantonese Soy Sauce. Molecules 2019, 24, 1474. https://doi.org/10.3390/molecules24081474
Zhou K, Siroli L, Patrignani F, Sun Y, Lanciotti R, Xu Z. Formation of Ethyl Carbamate during the Production Process of Cantonese Soy Sauce. Molecules. 2019; 24(8):1474. https://doi.org/10.3390/molecules24081474
Chicago/Turabian StyleZhou, Kai, Lorenzo Siroli, Francesca Patrignani, Yuanming Sun, Rosalba Lanciotti, and Zhenlin Xu. 2019. "Formation of Ethyl Carbamate during the Production Process of Cantonese Soy Sauce" Molecules 24, no. 8: 1474. https://doi.org/10.3390/molecules24081474
APA StyleZhou, K., Siroli, L., Patrignani, F., Sun, Y., Lanciotti, R., & Xu, Z. (2019). Formation of Ethyl Carbamate during the Production Process of Cantonese Soy Sauce. Molecules, 24(8), 1474. https://doi.org/10.3390/molecules24081474






