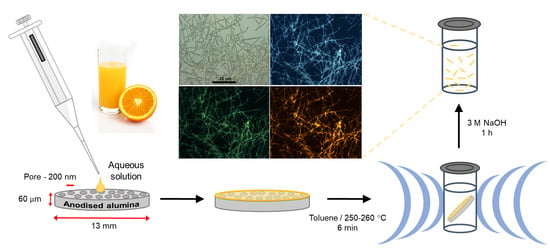
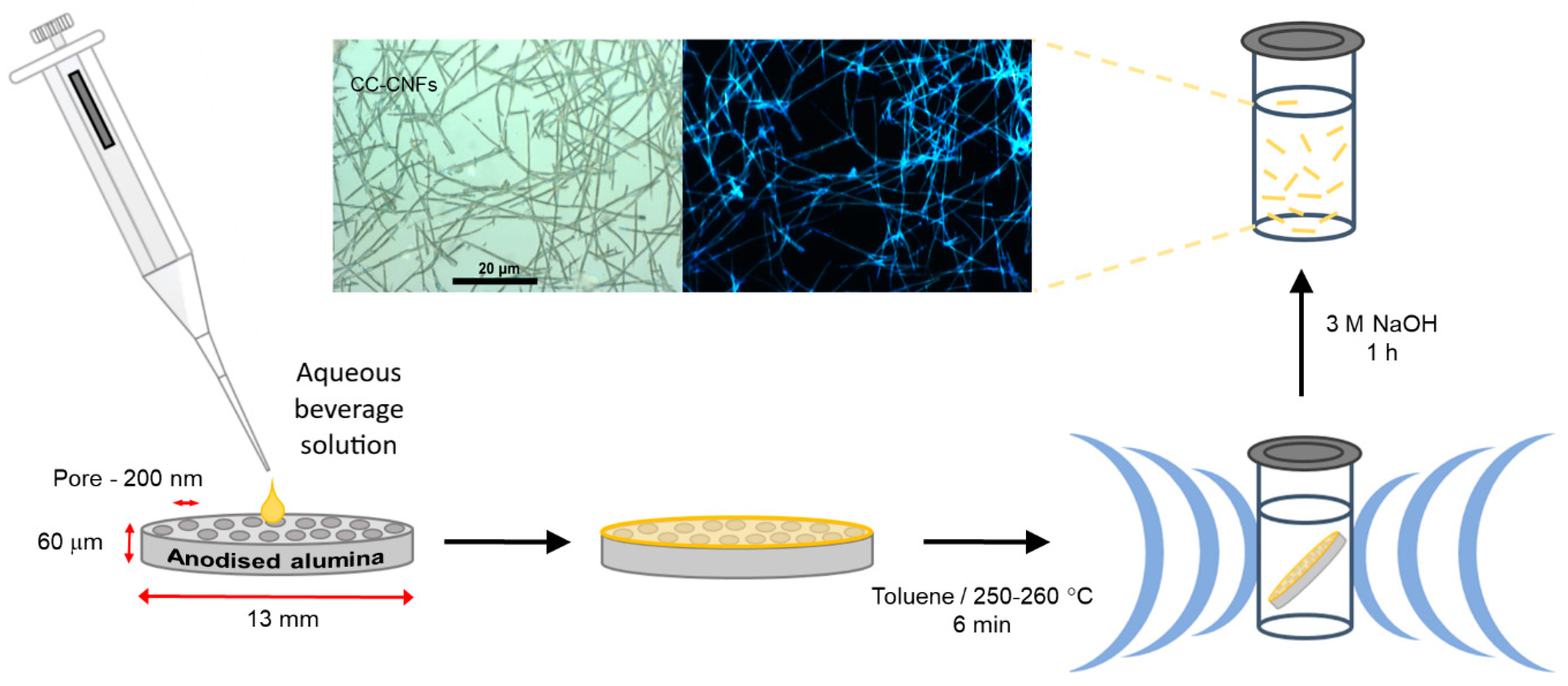
Template-Assisted Synthesis of Luminescent Carbon Nanofibers from Beverage-Related Precursors by Microwave Heating
Abstract
:1. Introduction
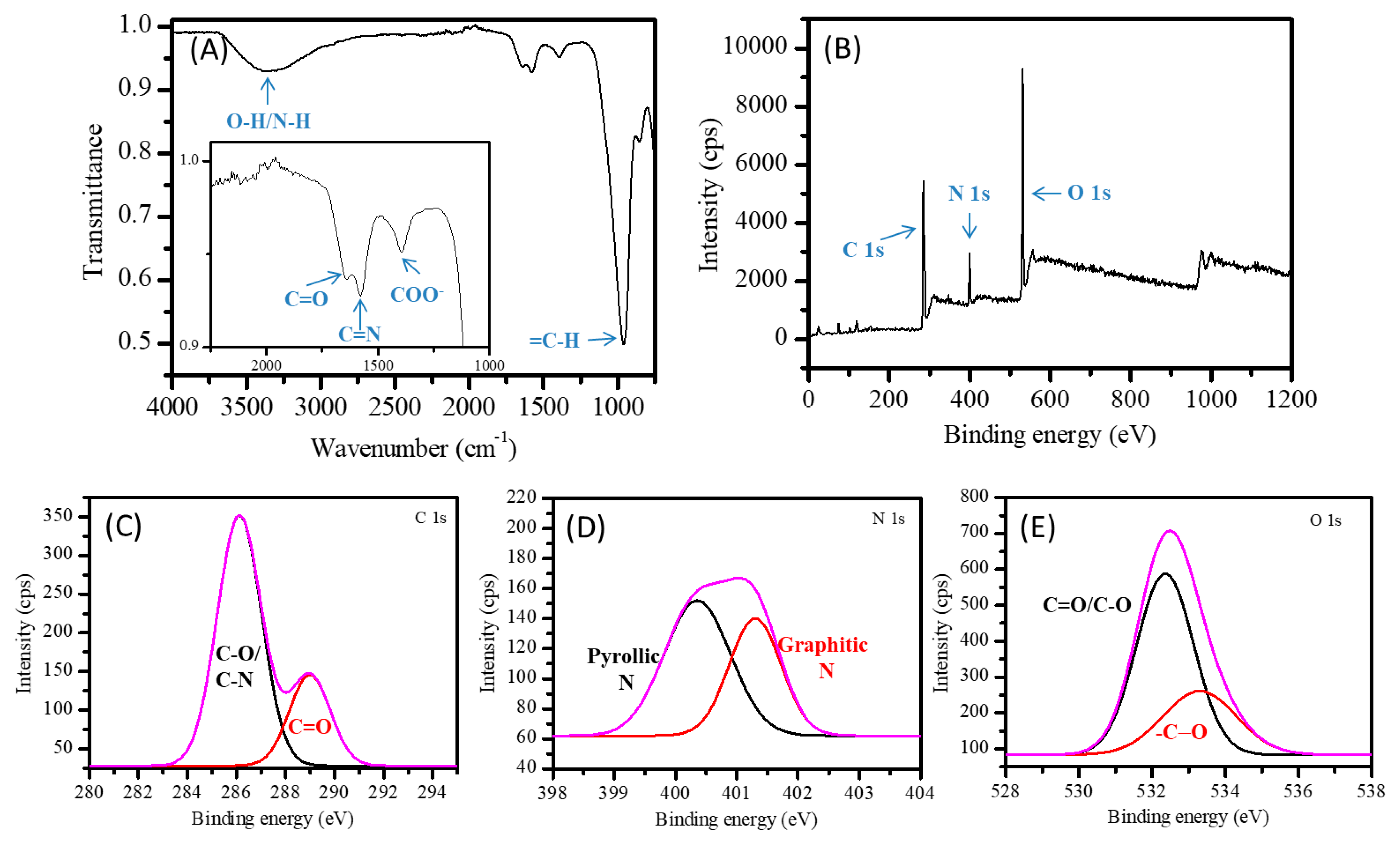
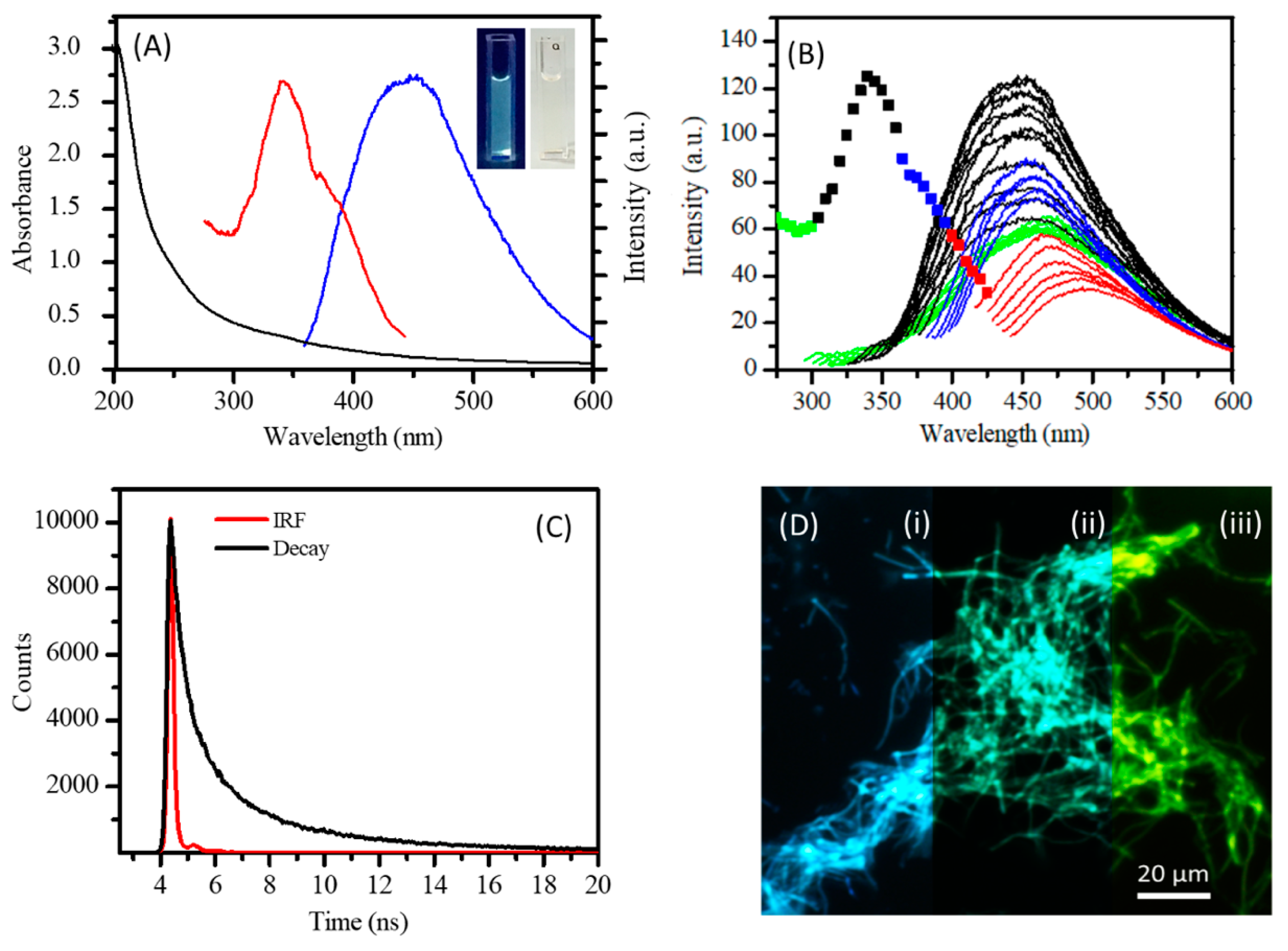
2. Results and Discussion
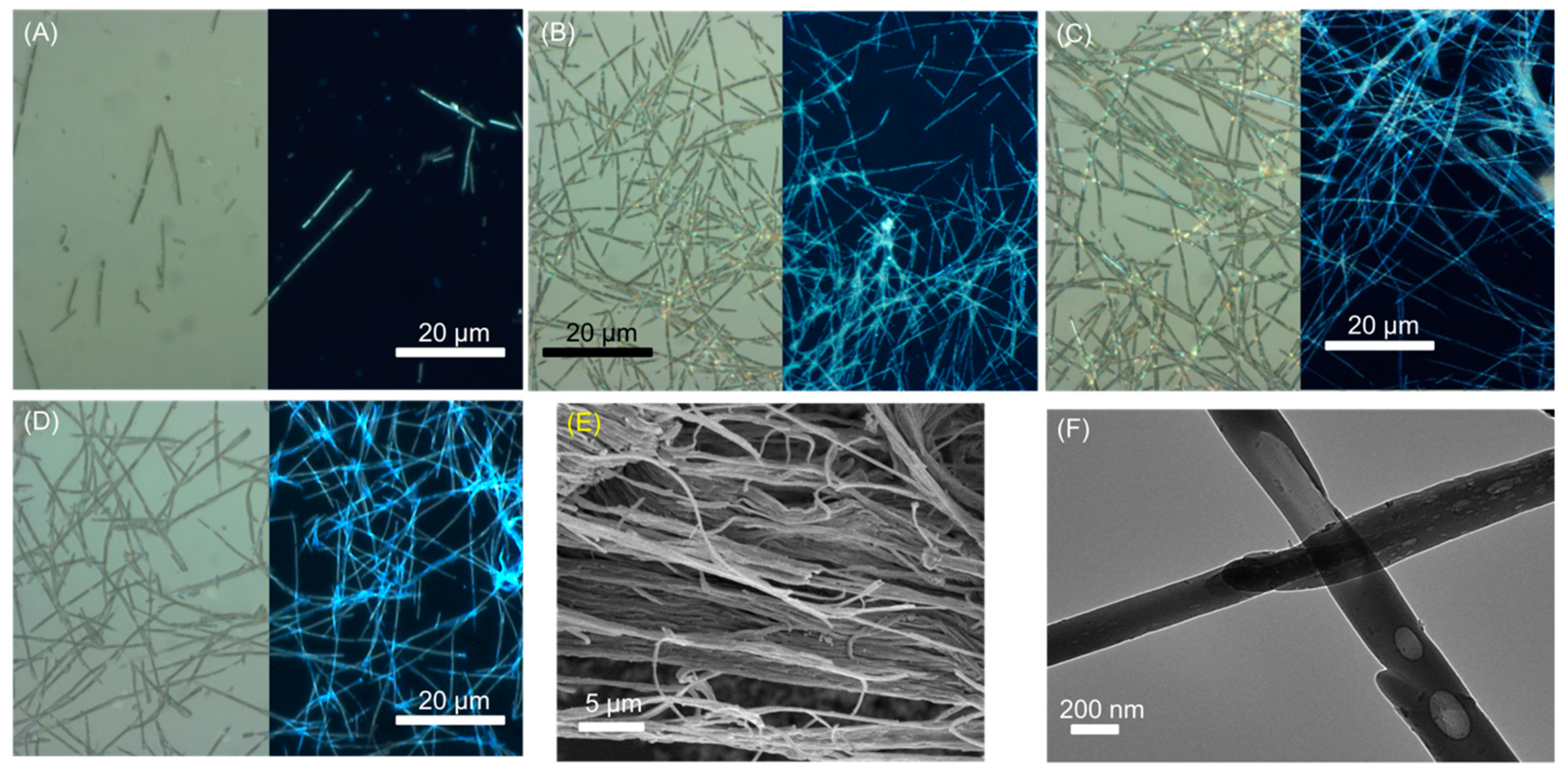
2.1. Carbon Nanofibers Prepared from Malic Acid (MA-CNF)
2.2. Carbon Nanofibers Prepared from Commercially Available Beverages
3. Materials and Methods
3.1. Characterisation of Dispersed Carbon Fibers
3.2. Characterisation of Deposited Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schwenke, A.M.; Hoeppener, S.; Schubert, U.S. Synthesis and Modification of Carbon Nanomaterials utilizing Microwave Heating. Adv. Mater. 2015, 27, 4113–4141. [Google Scholar] [CrossRef]
- Hong, E.; Lee, K.-H.; Oh, S.; Park, C.-G. Synthesis of Carbon Nanotubes Using Microwave Radiation. Adv. Funct. Mater. 2003, 13, 961–966. [Google Scholar] [CrossRef]
- Bajpai, R.; Wagner, H.D. Fast growth of carbon nanotubes using a microwave oven. Carbon 2015, 82, 327–336. [Google Scholar] [CrossRef]
- Chen, K.; Wang, C.; Ma, D.; Huang, W.; Bao, X. Graphitic carbon nanostructures via a facile microwave-induced solid-state process. Chem. Commun. 2008, 2765, 2765–2767. [Google Scholar] [CrossRef]
- Lin, J.; Huang, Y.; Wang, S.; Chen, G. Microwave-Assisted Rapid Exfoliation of Graphite into Graphene by Using Ammonium Bicarbonate as the Intercalation Agent. Ind. Eng. Chem. Res. 2017, 56, 9341–9346. [Google Scholar] [CrossRef]
- Brunetti, F.G.; Herrero, M.A.; Muñoz, J.D.M.; Díaz-Ortiz, A.; Alfonsi, J.; Meneghetti, M.; Prato, M.; Vázquez, E. Microwave-Induced Multiple Functionalization of Carbon Nanotubes. J. Am. Chem. Soc. 2008, 130, 8094–8100. [Google Scholar] [CrossRef] [PubMed]
- Rubio, N.; Herrero, M.A.; Meneghetti, M.; Díaz-Ortiz, A.; Schiavon, M.; Prato, M.; Vázquez, E. Efficient functionalization of carbon nanohorns via microwave irradiation. J. Mater. Chem. 2009, 19, 4407. [Google Scholar] [CrossRef]
- Melucci, M.; Treossi, E.; Ortolani, L.; Giambastiani, G.; Morandi, V.; Klar, P.; Casiraghi, C.; Samorì, P.; Palermo, V. Facile covalent functionalization of graphene oxide using microwaves: Bottom-up development of functional graphitic materials. J. Mater. Chem. 2010, 20, 9052–9060. [Google Scholar] [CrossRef]
- Jiang, K.; Sun, S.; Zhang, L.; Lu, Y.; Wu, A.; Cai, C.; Lin, H. Red, Green, and Blue Luminescence by Carbon Dots: Full-Color Emission Tuning and Multicolor Cellular Imaging. Angew. Chem. Int. Ed. 2015, 54, 5360–5363. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zhang, L.; Jiang, K.; Wu, A.; Lin, H. Toward High-Efficient Red Emissive Carbon Dots: Facile Preparation, Unique Properties, and Applications as Multifunctional Theranostic Agents. Chem. Mater. 2016, 28, 8659–8668. [Google Scholar] [CrossRef]
- Tian, J.; Qin, X.; Asiri, A.M.; Al-Youbi, A.O.; Liu, S.; Wang, L.; Zhang, Y.; Luo, Y.; Sun, X. Hydrothermal Treatment of Grass: A Low-Cost, Green Route to Nitrogen-Doped, Carbon-Rich, Photoluminescent Polymer Nanodots as an Effective Fluorescent Sensing Platform for Label-Free Detection of Cu(II) Ions. Adv. Mater. 2012, 24, 2037–2041. [Google Scholar]
- Sahu, S.; Behera, B.; Maiti, T.K.; Mohapatra, S. Simple one-step synthesis of highly luminescent carbon dots from orange juice: Application as excellent bio-imaging agents. Chem. Commun. 2012, 48, 8835–8837. [Google Scholar] [CrossRef] [PubMed]
- Sk, M.P.; Jaiswal, A.; Paul, A.; Ghosh, S.S.; Chattopadhyay, A. Presence of Amorphous Carbon Nanoparticles in Food Caramels. Sci. Rep. 2012, 2, 383. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liao, H.; Wu, H.; Zhao, H.; Tan, M. Fluorescent carbon dots from beer for breast cancer cell imaging and drug delivery. Anal. Methods 2015, 7, 8911–8917. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, N.; Gong, N.; Wang, H.; Shi, X.; Gu, W.; Ye, L. One-step microwave-assisted polyol synthesis of green luminescent carbon dots as optical nanoprobes. Carbon 2014, 68, 258–264. [Google Scholar] [CrossRef]
- Song, Y.; Zhu, S.; Zhang, S.; Fu, Y.; Wang, L.; Zhao, X.; Yang, B. Investigation from chemical structure to photoluminescent mechanism: A type of carbon dots from the pyrolysis of citric acid and an amine. J. Mater. Chem. C 2015, 3, 5976–5984. [Google Scholar] [CrossRef]
- Li, X.; Zhang, S.; Kulinich, S.A.; Liu, Y.; Zeng, H. Engineering surface states of carbon dots to achieve controllable luminescence for solid-luminescent composites and sensitive Be2+ detection. Sci. Rep. 2015, 4, 4976. [Google Scholar] [CrossRef]
- Liao, H.; Jiang, C.; Vera, J.M.; Seni, O.D.; Demera, K.; Liu, W.; Yu, C.; Tan, M. Fluorescent Nanoparticles from Several Commercial Beverages: Their Properties and Potential Application for Bioimaging. J. Agric. Chem. 2015, 63, 8527–8533. [Google Scholar] [CrossRef]
- Jiang, C.; Wu, H.; Song, X.; Ma, X.; Wang, J.; Tan, M. Presence of photoluminescent carbon dots in Nescafe® original instant coffee: Applications to bioimaging. Talanta 2014, 127, 68–74. [Google Scholar] [CrossRef]
- Wang, R.; Lu, K.-Q.; Tang, Z.-R.; Xu, Y.-J. Recent progress in carbon quantum dots: Synthesis, properties and applications in photocatalysis. J. Mater. Chem. A 2017, 5, 3717–3734. [Google Scholar] [CrossRef]
- Sharma, V.; Tiwari, P.; Mobin, S.M. Sustainable carbon-dots: Recent advances in green carbon dots for sensing and bioimaging. J. Mater. Chem. B 2017, 5, 8904–8924. [Google Scholar] [CrossRef]
- Park, S.Y.; Lee, H.U.; Park, E.S.; Lee, S.C.; Lee, J.-W.; Jeong, S.W.; Kim, C.H.; Lee, Y.-C.; Huh, Y.S.; Lee, J. Photoluminescent Green Carbon Nanodots from Food-Waste-Derived Sources: Large-Scale Synthesis, Properties, and Biomedical Applications. ACS Appl. Mater. Interfaces 2014, 6, 3365–3370. [Google Scholar] [CrossRef]
- Puvvada, N.; Kumar, B.N.P.; Konar, S.; Kalita, H.; Mandal, M.; Pathak, A. Synthesis of biocompatible multicolor luminescent carbon dots for bioimaging applications. Sci. Technol. Adv. Mater. 2012, 13, 45008. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qu, K.; Xu, B.; Ren, J.; Qu, X. Microwave assisted one-step green synthesis of cell-permeable multicolor photoluminescent carbon dots without surface passivation reagents. J. Mater. Chem. 2011, 21, 2445. [Google Scholar] [CrossRef]
- Zhai, X.; Zhang, P.; Liu, C.; Bai, T.; Li, W.; Dai, L.; Liu, W. Highly luminescent carbon nanodots by microwave-assisted pyrolysis. Chem. Commun. 2012, 48, 7955. [Google Scholar] [CrossRef] [PubMed]
- Schwenke, A.M.; Hoeppener, S.; Schubert, U.S. Microwave synthesis of carbon nanofibers—The influence of MW irradiation power, time, and the amount of catalyst. J. Mater. Chem. A 2015, 3, 23778–23787. [Google Scholar] [CrossRef]
- Hammel, E.; Tang, X.; Trampert, M.; Schmitt, T.; Mauthner, K.; Eder, A.; Pötschke, P. Carbon nanofibers for composite applications. Carbon 2004, 42, 1153–1158. [Google Scholar] [CrossRef]
- Qie, L.; Chen, W.-M.; Wang, Z.-H.; Shao, Q.-G.; Yuan, L.-X.; Hu, X.-L.; Zhang, W.-X.; Huang, Y.-H.; Chen, W.; Wang, Z.; et al. Nitrogen-Doped Porous Carbon Nanofiber Webs as Anodes for Lithium Ion Batteries with a Superhigh Capacity and Rate Capability. Adv. Mater. 2012, 24, 2047–2050. [Google Scholar] [CrossRef] [PubMed]
- AbdelGawad, A.M.; Hudson, S.M.; Rojas, O.J. Antimicrobial wound dressing nanofiber mats from multicomponent (chitosan/silver-NPs/polyvinyl alcohol) systems. Carbohydr. Polym. 2014, 100, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Deeney, C.; Wang, S.; Belhout, S.A.; Gowen, A.; Rodriguez, B.J.; Redmond, G.; Quinn, S.J. Templated microwave synthesis of luminescent carbon nanofibers. RSC Adv. 2018, 8, 12907–12917. [Google Scholar] [CrossRef]
- Dadras, S.; Faraji, M. Improved carbon nanotube growth inside an anodic aluminum oxide template using microwave radiation. J. Phys. Chem. Solids 2018, 116, 203–208. [Google Scholar] [CrossRef]
- Li, L.; Yu, B.; You, T. Nitrogen and sulfur co-doped carbon dots for highly selective and sensitive detection of Hg (II) ions. Biosens. Bioelectron. 2015, 74, 263–269. [Google Scholar] [CrossRef]
- Zhi, B.; Cui, Y.; Wang, S.; Frank, B.P.; Williams, D.N.; Brown, R.P.; Rosenzweig, Z.; Fairbrother, D.H.; Orr, G.; Haynes, C.L.; et al. Malic Acid Carbon Dots: From Super-resolution Live-Cell Imaging to Highly Efficient Separation. ACS Nano 2018, 12, 5741–5752. [Google Scholar] [CrossRef]
- Mondal, T.K.; Gupta, A.; Ghorai, U.K.; Saha, S.K.; Shaw, B.K. Highly luminescent N-doped carbon quantum dots from lemon juice with porphyrin-like structures surrounded by graphitic network for sensing applications. RSC Adv. 2016, 6, 59927–59934. [Google Scholar] [CrossRef]
- Li, F.; Lin, F.; Chen, Q.; Cai, Z.; Xu, W.; Wang, Y. Fluorescent carbon nanodots facilely extracted from Coca Cola for temperature sensing. Methods Appl. Fluoresc. 2017, 5, 44002. [Google Scholar] [CrossRef]
- Liu, J.; Liu, X.; Luo, H.; Gao, Y. One-step preparation of nitrogen-doped and surface-passivated carbon quantum dots with high quantum yield and excellent optical properties. RSC Adv. 2014, 4, 7648. [Google Scholar] [CrossRef]
- Strauss, V.; Margraf, J.T.; Dölle, C.; Butz, B.; Nacken, T.J.; Walter, J.; Bauer, W.; Peukert, W.; Spiecker, E.; Clark, T.; et al. Carbon Nanodots: Toward a Comprehensive Understanding of Their Photoluminescence. J. Am. Chem. Soc. 2014, 136, 17308–17316. [Google Scholar] [CrossRef]
- Zhi, B.; Gallagher, M.J.; Frank, B.P.; Lyons, T.Y.; Qiu, T.A.; Da, J.; Mensch, A.C.; Hamers, R.J.; Rosenzweig, Z.; Fairbrother, D.H.; et al. Investigation of phosphorous doping effects on polymeric carbon dots: Fluorescence, photostability, and environmental impact. Carbon 2018, 129, 438–449. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B.; Solis, M.S. Chemical and Structural Properties of Carbonaceous Products Obtained by Hydrothermal Carbonization of Saccharides. Chem. A Eur. J. 2009, 15, 4195–4203. [Google Scholar] [CrossRef]
- Jurmanová, J.; Štěpánová, V.; Slavíček, P.; Stupavská, M.; Černák, M. Surface chemical changes of atmospheric pressure plasma treated rabbit fibres important for felting process. Appl. Surf. Sci. 2015, 355, 1037–1043. [Google Scholar]
- Zhang, Z.; Fang, Y.; Yi, C.; Pan, Y.; Chen, J. Tuning photoluminescence and surface properties of carbon nanodots for chemical sensing. Nanoscale 2016, 8, 500–507. [Google Scholar] [CrossRef]
- Li, F.; Liu, C.; Yang, J.; Wang, Z.; Liu, W.; Tian, F. Mg/N double doping strategy to fabricate extremely high luminescent carbon dots for bioimaging. RSC Adv. 2014, 4, 3201–3205. [Google Scholar] [CrossRef]
- Baker, S.N.; Baker, G.A. Luminescent Carbon Nanodots: Emergent Nanolights. Angew. Chem. Int. Ed. 2010, 49, 6726–6744. [Google Scholar] [CrossRef]
- Schneider, J.; Reckmeier, C.J.; Xiong, Y.; Von Seckendorff, M.; Susha, A.S.; Kasák, P.; Rogach, A.L. Molecular Fluorescence in Citric Acid-Based Carbon Dots. J. Phys. Chem. C 2017, 121, 2014–2022. [Google Scholar] [CrossRef]
- Sciortino, A.; Van Dam, B.; Marino, E.; Schall, P.; Cannas, M.; Messina, F. Solvatochromism Unravels the Emission Mechanism of Carbon Nanodots. J. Phys. Chem. Lett. 2016, 7, 3419–3423. [Google Scholar] [CrossRef]
- Demchenko, A.P.; Dekaliuk, M. The origin of emissive states of carbon nanoparticles derived from ensemble-averaged and single-molecular studies. Nanoscale 2016, 8, 14057–14069. [Google Scholar] [CrossRef]
- Nie, H.; Li, M.; Li, Q.; Liang, S.; Tan, Y.; Sheng, L.; Shi, W.; Zhang, S.X.-A. Carbon Dots with Continuously Tunable Full-Color Emission and Their Application in Ratiometric pH Sensing. Chem. Mater. 2014, 26, 3104–3112. [Google Scholar] [CrossRef]
- Sun, X.; Lei, Y. Fluorescent carbon dots and their sensing applications. TrAC Anal. Chem. 2017, 89, 163–180. [Google Scholar] [CrossRef]
- Lin, Z.; Xue, W.; Chen, H.; Lin, J.-M. Peroxynitrous-Acid-Induced Chemiluminescence of Fluorescent Carbon Dots for Nitrite Sensing. Anal. Chem. 2011, 83, 8245–8251. [Google Scholar] [CrossRef]
- Krysmann, M.J.; Kelarakis, A.; Dallas, P.; Giannelis, E.P. Formation Mechanism of Carbogenic Nanoparticles with Dual Photoluminescence Emission. J. Am. Chem. Soc. 2012, 134, 747–750. [Google Scholar] [CrossRef]
- Dhenadhayalan, N.; Lin, K.-C. Chemically Induced Fluorescence Switching of Carbon-Dots and Its Multiple Logic Gate Implementation. Sci. Rep. 2015, 5, 10012. [Google Scholar] [CrossRef]
Sample Availability: Samples of the fibers may be made available from the authors. |



| Sample | Length (μm) | N | Sample | Length (μm) | n |
|---|---|---|---|---|---|
| Lj-CNF | 30 ± 11 | 208 | Lj-CNF (no PEI) | N/A * | |
| Oj-CNF | 24 ± 10 | 200 | Oj-CNF (no PEI) | 32 ± 11 | 200 |
| Gj-CNF | 30 ± 10 | 200 | Gj-CNF (no PEI) | 37 ± 10 | 220 |
| CC-CNF | 21 ± 12 | 200 | CC-CNF (no PEI) | 19 ± 11 | 200 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deeney, C.; McKiernan, E.P.; Belhout, S.A.; Rodriguez, B.J.; Redmond, G.; Quinn, S.J. Template-Assisted Synthesis of Luminescent Carbon Nanofibers from Beverage-Related Precursors by Microwave Heating. Molecules 2019, 24, 1455. https://doi.org/10.3390/molecules24081455
Deeney C, McKiernan EP, Belhout SA, Rodriguez BJ, Redmond G, Quinn SJ. Template-Assisted Synthesis of Luminescent Carbon Nanofibers from Beverage-Related Precursors by Microwave Heating. Molecules. 2019; 24(8):1455. https://doi.org/10.3390/molecules24081455
Chicago/Turabian StyleDeeney, Clara, Eoin P. McKiernan, Samir A. Belhout, Brian J. Rodriguez, Gareth Redmond, and Susan J. Quinn. 2019. "Template-Assisted Synthesis of Luminescent Carbon Nanofibers from Beverage-Related Precursors by Microwave Heating" Molecules 24, no. 8: 1455. https://doi.org/10.3390/molecules24081455
APA StyleDeeney, C., McKiernan, E. P., Belhout, S. A., Rodriguez, B. J., Redmond, G., & Quinn, S. J. (2019). Template-Assisted Synthesis of Luminescent Carbon Nanofibers from Beverage-Related Precursors by Microwave Heating. Molecules, 24(8), 1455. https://doi.org/10.3390/molecules24081455