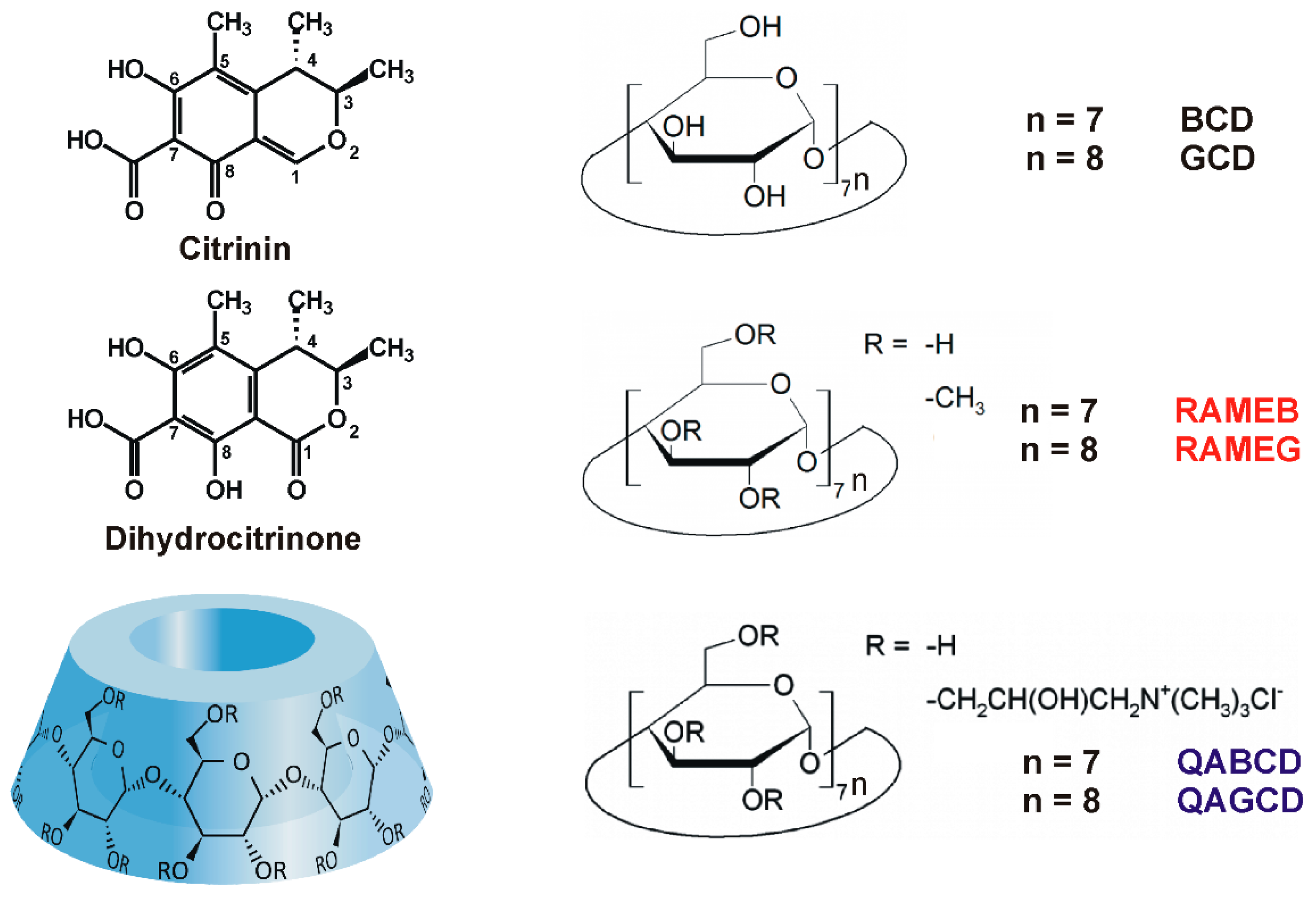
Interaction of Dihydrocitrinone with Native and Chemically Modified Cyclodextrins
Abstract
1. Introduction
2. Results and Discussion
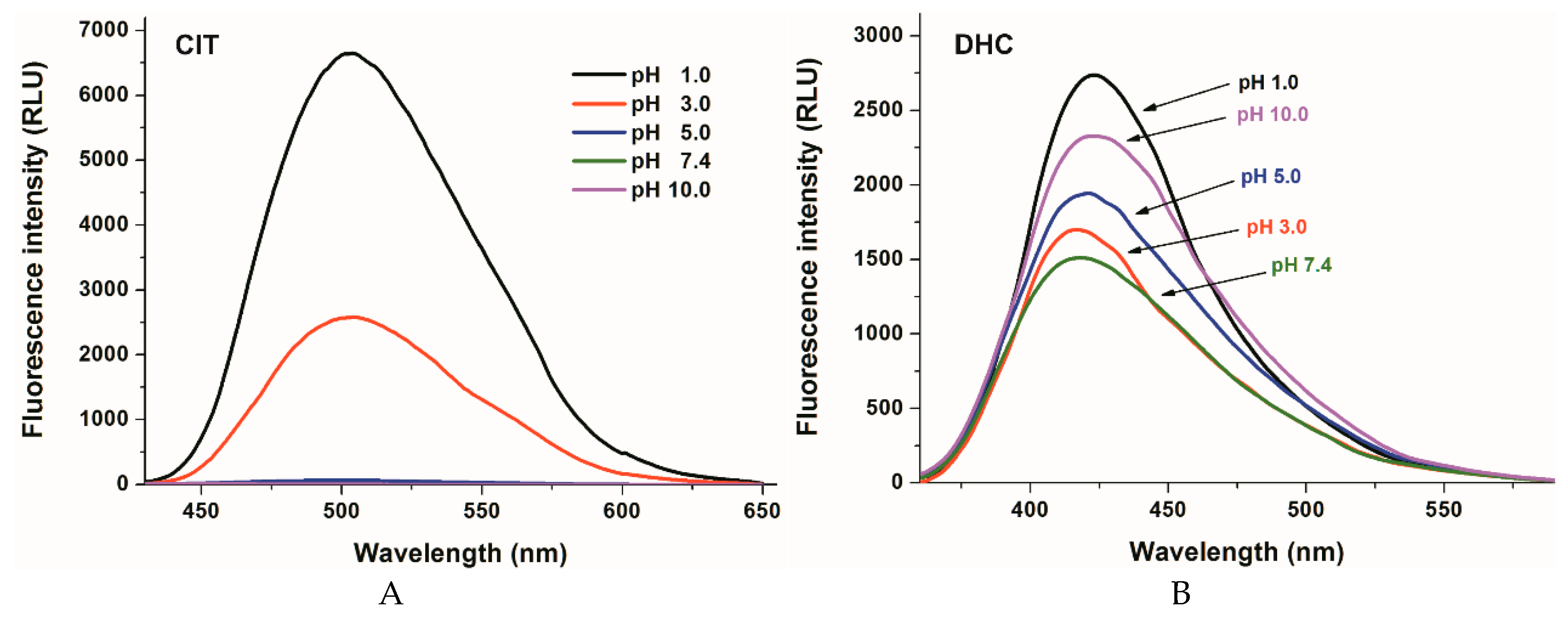
2.1. Fluorescence Properties of DHC and CIT in Different Buffers
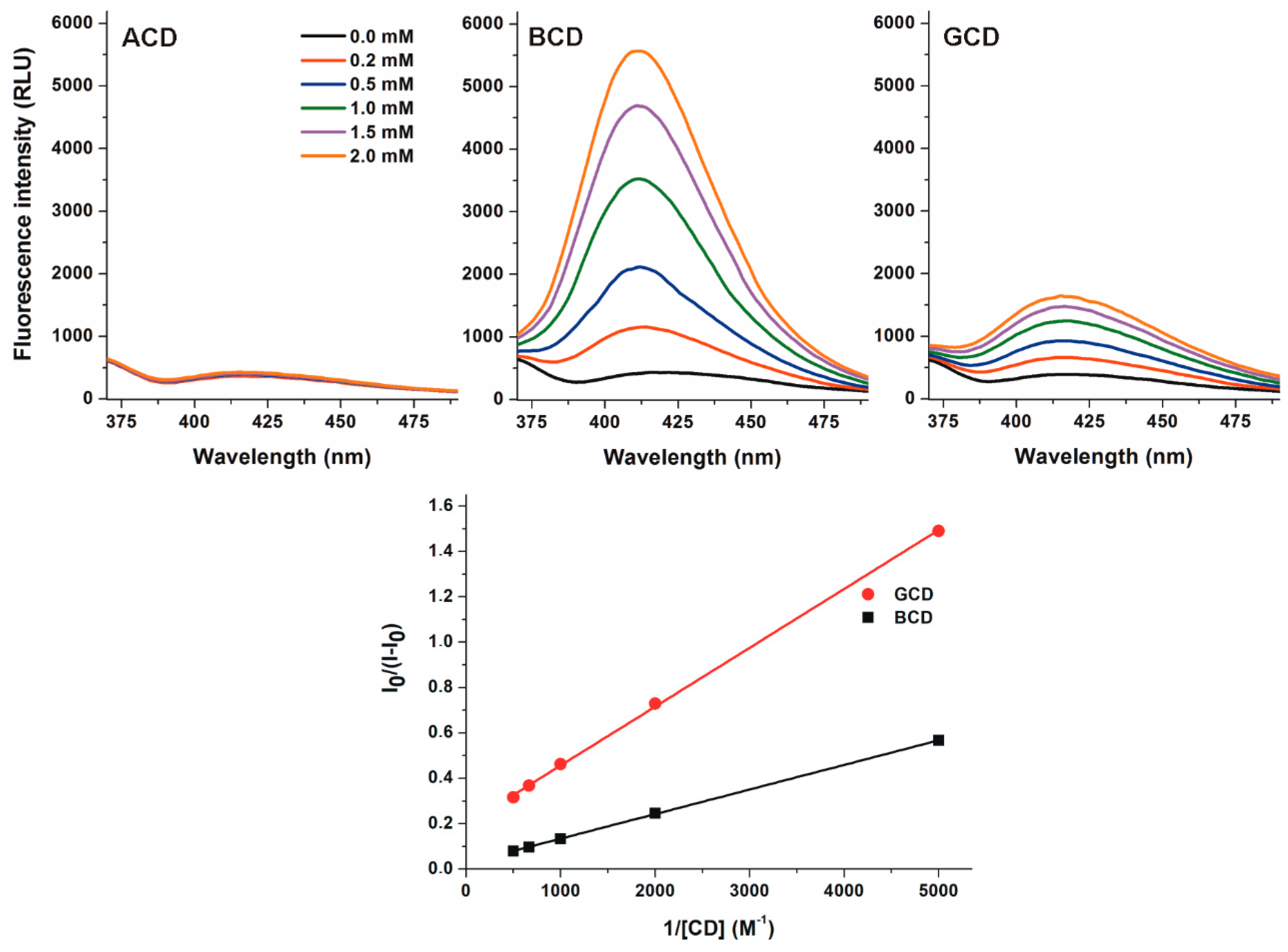
2.2. Effects of Native α-, β-, and γ-CDs on the Fluorescence Signal of DHC
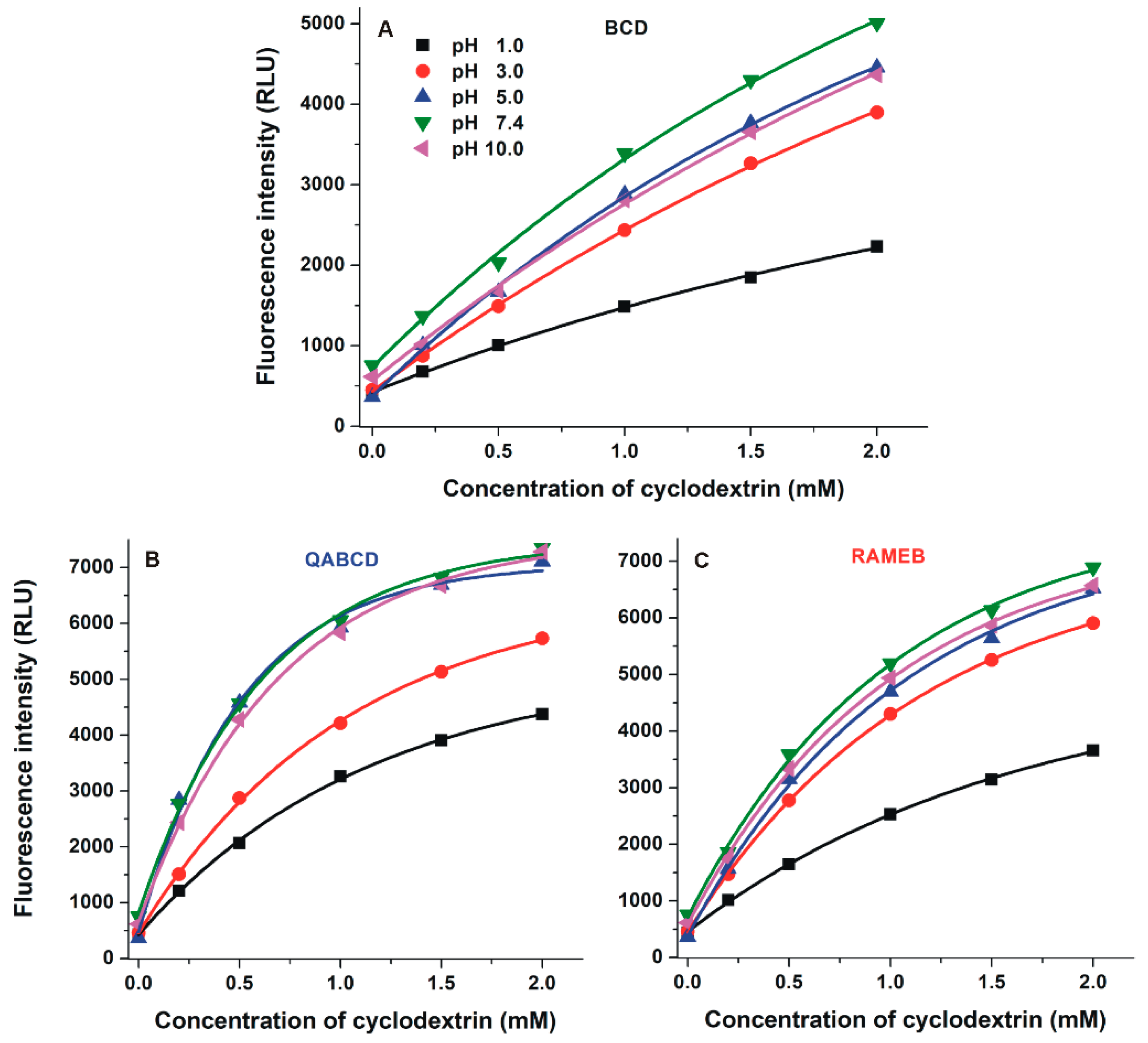
2.3. Interaction of DHC with β-Cyclodextrins
2.4. Interaction of DHC with γ-Cyclodextrins
2.5. Modeling Studies
3. Materials and Methods
3.1. Reagents
3.2. Fluorescence Spectroscopic Measurements
3.3. Modeling Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Geisen, R.; Schmidt-Heydt, M.; Touhami, N.; Himmelsbach, A. New aspects of ochratoxin A and citrinin biosynthesis in Penicillium. Curr. Opin. Food Sci. 2018, 23, 23–31. [Google Scholar] [CrossRef]
- da Rocha, M.E.B.; Freire, F.C.O.; Maia, F.E.F.; Guedes, M.I.F.; Rondina, D. Mycotoxins and their effects on human and animal health. Food Control 2014, 36, 59–165. [Google Scholar] [CrossRef]
- de Oliveira Filho, J.W.G.; Islam, M.T.; Ali, E.S.; Uddin, S.J.; Santos, J.V.O.; de Alencar, M.V.O.B.; Júnior, A.L.G.; Paz, M.F.C.J.; de Brito, M.D.R.M.; E Sousa, J.M.C.; et al. A comprehensive review on biological properties of citrinin. Food Chem. Toxicol. 2017, 110, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Kitabatake, N.; Trivedi, A.B.; Doi, E. Thermal Decomposition and Detoxification of Citrinin under Various Moisture Conditions. J. Agric. Food Chem. 1991, 39, 2240–2244. [Google Scholar] [CrossRef]
- Dunn, B.B.; Stack, M.E.; Park, D.L.; Joshi, A.; Friedman, L.; King, R.L. Isolation and identification of dihydrocitrinone, a urinary metabolite of citrinin in rats. J. Toxicol. Environ. Health 1983, 12, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Degen, G.H.; Ali, N.; Gundert-Remy, U. Preliminary data on citrinin kinetics in humans and their use to estimate citrinin exposure based on biomarkers. Toxicol. Lett. 2018, 282, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Föllmann, W.; Behm, C.; Degen, G.H. Toxicity of the mycotoxin citrinin and its metabolite dihydrocitrinone and of mixtures of citrinin and ochratoxin A in vitro. Arch. Toxicol. 2014, 88, 1097–1107. [Google Scholar] [CrossRef]
- Faisal, Z.; Vörös, V.; Lemli, B.; Derdák, D.; Kunsági-Máté, S.; Bálint, M.; Hetényi, C.; Csepregi, R.; Kőszegi, T.; Bergmann, D.; Sueck, F.; Humpf, H.-U.; Hübner, F.; Poór, M. Interaction of the mycotoxin metabolite dihydrocitrinone with serum albumin. Mycotoxin Res. 2018. [Google Scholar] [CrossRef]
- Gerding, J.; Ali, N.; Schwartzbord, J.; Cramer, B.; Brown, D.L.; Degen, G.H.; Humpf, H.-U. A comparative study of the human urinary mycotoxin excretion patterns in Bangladesh, Germany and Haiti using a rapid and sensitive LC-MS/MS approach. Mycotoxin Res. 2015, 31, 127–136. [Google Scholar] [CrossRef]
- Ali, N.; Blaszkewicz, M.; Degen, G.H. Occurrence of the mycotoxin citrinin and its metabolite dihydrocitrinone in urines of German adults. Arch. Toxicol. 2015, 89, 573–578. [Google Scholar] [CrossRef]
- Ali, N.; Hossain, K.; Degen, G.H. Blood plasma biomarkers of citrinin and ochratoxin A exposure in young adults in Bangladesh. Mycotoxin Res. 2018, 34, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Szente, L.; Szejtli, J. Highly soluble cyclodextrin derivatives: Chemistry, properties, and trends in development. Adv. Drug Deliv. Rev. 1999, 36, 17–28. [Google Scholar] [CrossRef]
- Szente, L.; Szemán, J. Cyclodextrins in Analytical Chemistry: Host−Guest Type Molecular Recognition. Anal. Chem. 2013, 85, 8024–8030. [Google Scholar] [CrossRef] [PubMed]
- Crini, G. Review: A history of cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. [Google Scholar] [CrossRef] [PubMed]
- Aghamohammadi, M.; Alizadeh, N. Fluorescence enhancement of the aflatoxin B1 by forming inclusion complexes with some cyclodextrins and molecular modeling study. J. Lumin. 2007, 127, 575–582. [Google Scholar] [CrossRef]
- Amadasi, A.; Dall’asta, C.; Ingletto, G.; Pela, R.; Marchelli, R.; Cozzini, P. Explaining cyclodextrin–mycotoxin interactions using a ’natural’ force field. Bioorg. Med. Chem. 2007, 15, 4585–4594. [Google Scholar] [CrossRef]
- Verrone, R.; Catucci, L.; Cosma, P.; Fini, P.; Agostiano, A.; Lippolis, V.; Pascale, M. Effect of b-cyclodextrin on spectroscopic properties of ochratoxin A in aqueous solution. J. Incl. Phenom. Macrocycl. Chem. 2007, 57, 475–479. [Google Scholar] [CrossRef]
- Dall’Asta, C.; Faccini, A.; Galaverna, G.; Corradini, R.; Dossena, A.; Marchelli, R. Complexation of zearalenone and zearalenols with native and modified b-cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 2009, 64, 331–340. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, J.; Dong, L.; Lu, L.; Chen, F.; Hu, D.; Wang, X. A study of fluorescence properties of citrinin in b-cyclodextrin aqueous solution and different solvents. J. Lumin. 2012, 132, 1437–1445. [Google Scholar] [CrossRef]
- Poór, M.; Kunsági-Máté, S.; Sali, N.; Kőszegi, T.; Szente, L.; Peles-Lemli, B. Interactions of zearalenone with native and chemically modified cyclodextrins and their potential utilization. J. Photochem. Photobiol. B Biol. 2015, 151, 63–68. [Google Scholar] [CrossRef]
- Poór, M.; Kunsági-Máté, S.; Szente, L.; Matisz, G.; Secenji, G.; Czibulya, Z.; Kőszegi, T. Interaction of ochratoxin A with quaternary ammonium beta-cyclodextrin. Food Chem. 2015, 172, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Poór, M.; Zand, A.; Szente, L.; Lemli, B.; Kunsági-Máté, S. Interaction of α- and β-zearalenols with β-cyclodextrins. Molecules 2017, 22, 1910. [Google Scholar] [CrossRef]
- Maragos, C.M.; Appell, M.; Lippolis, V.; Visconti, A.; Catucci, L.; Pascale, M. Use of cyclodextrins as modifiers of fluorescence in the detection of mycotoxins. Food Addit. Contam. 2008, 25, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Dall’Asta, C.; Faccini, A.; Galaverna, G.; Corradini, R.; Dossena, A.; Marchelli, R. Complexation of the mycotoxin zearalenone with β-cyclodextrin: Study of the interaction and first promising applications. Mycotoxin Res. 2008, 24, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Galaverna, G.; Dall’Asta, C.; Corradini, R.; Dossena, A.; Marchelli, R. Cyclodextrins as selectors for mycotoxin recognition. World Mycotoxin J. 2008, 1, 397–406. [Google Scholar] [CrossRef]
- Appell, M.; Jackson, M.A. Synthesis and evaluation of cyclodextrin-based polymers for patulin extraction from aqueous solutions. J. Incl. Phenom. Macrocycl. Chem. 2010, 68, 117–122. [Google Scholar] [CrossRef]
- Appell, M.; Jackson, M.A. Sorption of ochratoxin A from aqueous solutions using β-cyclodextrin–polyurethane polymer. Toxins 2012, 4, 98–109. [Google Scholar] [CrossRef]
- Poór, M.; Faisal, Z.; Zand, A.; Bencsik, T.; Lemli, B.; Kunsági-Máté, S.; Szente, L. Removal of Zearalenone and Zearalenols from Aqueous Solutions Using Insoluble Beta-Cyclodextrin Bead Polymer. Toxins 2018, 10, 216. [Google Scholar] [CrossRef]
- Poór, M.; Matisz, G.; Kunsági-Máté, S.; Derdák, D.; Szente, L.; Lemli, B. Fluorescence spectroscopic investigation of the interaction of citrinin with native and chemically modified cyclodextrins. J. Lumin. 2016, 172, 23–28. [Google Scholar] [CrossRef]
- Tölgyesi, Á.; Stroka, J.; Tamosiunas, V.; Zwickel, T. Simultaneous analysis of Alternaria toxins and citrinin in tomato: An optimised method using liquid chromatography-tandem mass spectrometry. Food Addit. Contam. Part A Chem. 2015, 32, 1512–1522. [Google Scholar] [CrossRef]
- Dobretsov, G.E.; Syrejschikova, T.I.; Smolina, N.V. On mechanisms of fluorescence quenching by water. Biophysics 2014, 59, 183–188. [Google Scholar] [CrossRef]
- Bergmann, D.; Hübner, F.; Wibbeling, B.; Daniliuc, C.; Cramer, B.; Humpf, H.-U. Large-scale total synthesis of 13C3-labeled citrinin and its metabolite dihydrocitrinone. Mycotoxin Res. 2018, 34, 141–150. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
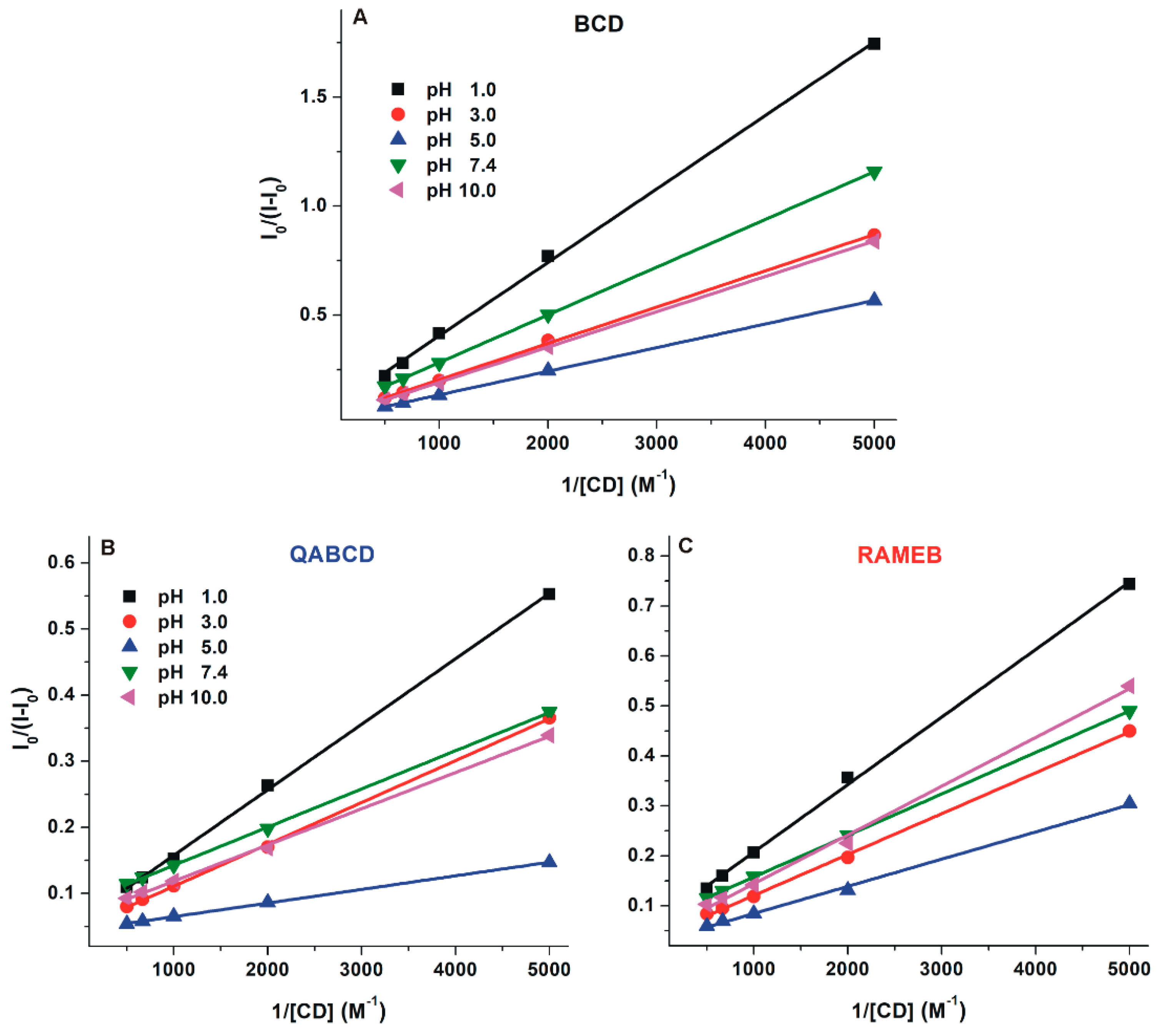
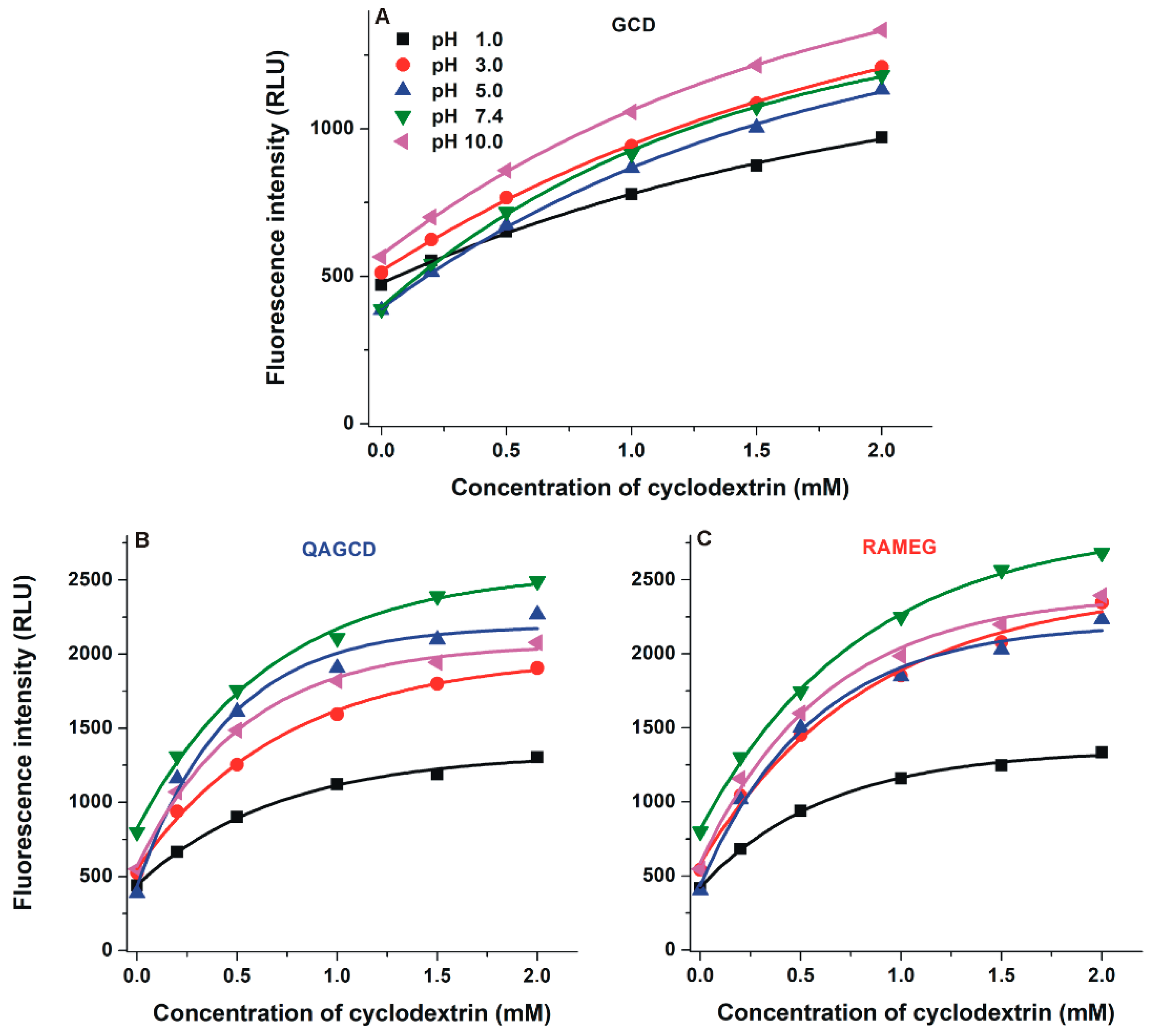
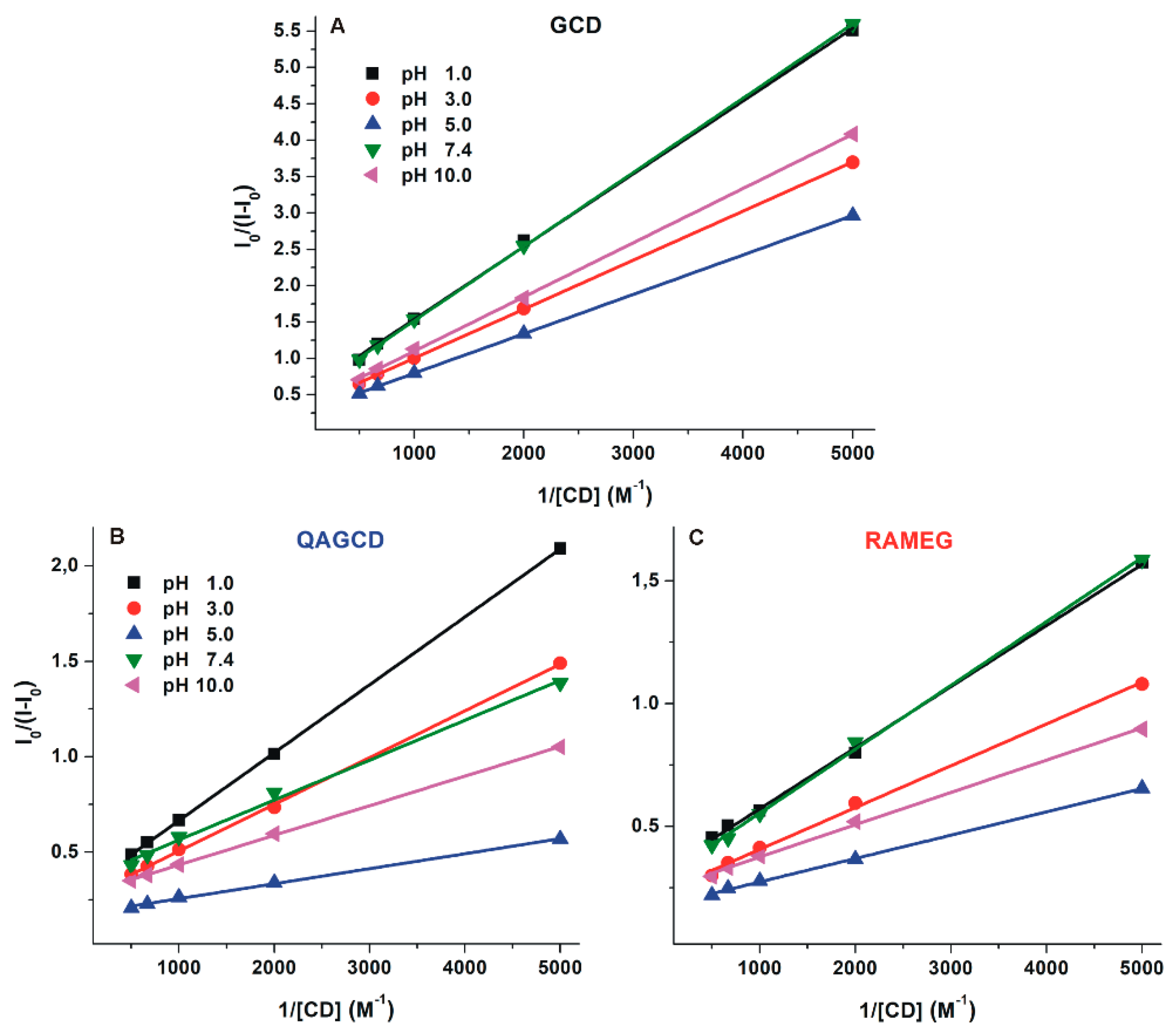
| pH | DHC–BCD | DHC–QABCD | DHC–RAMEB | |||
|---|---|---|---|---|---|---|
| logK ± SEM | I/I0 ± SEM | logK ± SEM | I/I0 ± SEM | logK ± SEM | I/I0 ± SEM | |
| 1.0 | 2.29 ± 0.04 | 5.59 ± 0.12 | 2.76 ± 0.01 | 10.17 ± 0.11 | 2.76 ± 0.02 | 6.98 ± 0.11 |
| 3.0 | 2.34 ± 0.04 | 9.23 ± 0.35 | 2.81 ± 0.02 | 12.89 ± 0.18 | 2.79 ± 0.04 | 12.89 ± 0.19 |
| 5.0 | 2.47 ± 0.05 | 13.14 ± 0.63 | 3.23 ± 0.05 | 19.53 ± 0.06 | 2.78 ± 0.02 | 17.59 ± 0.12 |
| 7.4 | 2.42 ± 0.06 | 6.75 ± 0.06 | 3.21 ± 0.02 | 9.92 ± 0.07 | 2.90 ± 0.03 | 9.31 ± 0.14 |
| 10.0 | 2.22 ± 0.02 | 7.40 ± 0.36 | 3.03 ± 0.02 | 14.22 ± 0.34 | 2.85 ± 0.04 | 12.74 ± 0.34 |
| pH | DHC–GCD | DHC–QAGCD | DHC–RAMEG | |||
|---|---|---|---|---|---|---|
| logK ± SEM | I/I0 ± SEM | logK ± SEM | I/I0 ± SEM | logK ± SEM | I/I0 ± SEM | |
| 1.0 | 2.69 ± 0.03 | 2.09 ± 0.03 | 2.98 ± 0.03 | 2.96 ± 0.06 | 3.12 ± 0.01 | 3.12 ± 0.08 |
| 3.0 | 2.78 ± 0.05 | 2.41 ± 0.10 | 3.13 ± 0.05 | 3.70 ± 0.12 | 3.20 ± 0.06 | 4.21 ± 0.09 |
| 5.0 | 2.73 ± 0.04 | 3.05 ± 0.07 | 3.38 ± 0.04 | 6.01 ± 0.09 | 3.22 ± 0.03 | 5.64 ± 0.08 |
| 7.4 | 2.75 ± 0.03 | 2.00 ± 0.01 | 3.18 ± 0.02 | 3.48 ± 0.16 | 3.05 ± 0.02 | 3.46 ± 0.05 |
| 10.0 | 2.74 ± 0.06 | 2.32 ± 0.07 | 3.24 ± 0.02 | 3.83 ± 0.02 | 3.18 ± 0.05 | 4.42 ± 0.03 |
| Ionic State of DHC | DHC–BCD | DHC–GCD | ||||||
| logK | ΔG | ΔH | ΔS | logK | ΔG | ΔH | ΔS | |
| 0 | 2.3 | −18.65 | −14.60 | 13.6 | 2.7 | −21.90 | −22.85 | −3.19 |
| −1 | 2.5 | −20.28 | −16.02 | 14.3 | 2.7 | −21.94 | −26.45 | −15.13 |
| −2 | 2.2 | −17.84 | −13.19 | 15.6 | 2.7 | −21.97 | −28.19 | −20.86 |
| DHC–RAMEB | DHC–QABCD | |||||||
| logK | ΔG | ΔH | ΔS | logK | ΔG | ΔH | ΔS | |
| 0 | 2.7 | −21.90 | −17.85 | 13.6 | 2.7 | −21.90 | −17.85 | 13.6 |
| −1 | 2.8 | −22.71 | −18.45 | 14.3 | 3.3 | −26.76 | −25.07 | 5.7 |
| −2 | 2.8 | −22.71 | −18.06 | 15.6 | 3.0 | −24.33 | −22.49 | 6.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faisal, Z.; Kunsági-Máté, S.; Lemli, B.; Szente, L.; Bergmann, D.; Humpf, H.-U.; Poór, M. Interaction of Dihydrocitrinone with Native and Chemically Modified Cyclodextrins. Molecules 2019, 24, 1328. https://doi.org/10.3390/molecules24071328
Faisal Z, Kunsági-Máté S, Lemli B, Szente L, Bergmann D, Humpf H-U, Poór M. Interaction of Dihydrocitrinone with Native and Chemically Modified Cyclodextrins. Molecules. 2019; 24(7):1328. https://doi.org/10.3390/molecules24071328
Chicago/Turabian StyleFaisal, Zelma, Sándor Kunsági-Máté, Beáta Lemli, Lajos Szente, Dominik Bergmann, Hans-Ulrich Humpf, and Miklós Poór. 2019. "Interaction of Dihydrocitrinone with Native and Chemically Modified Cyclodextrins" Molecules 24, no. 7: 1328. https://doi.org/10.3390/molecules24071328
APA StyleFaisal, Z., Kunsági-Máté, S., Lemli, B., Szente, L., Bergmann, D., Humpf, H.-U., & Poór, M. (2019). Interaction of Dihydrocitrinone with Native and Chemically Modified Cyclodextrins. Molecules, 24(7), 1328. https://doi.org/10.3390/molecules24071328







