MCR Scaffolds Get Hotter with 18F-Labeling
Abstract
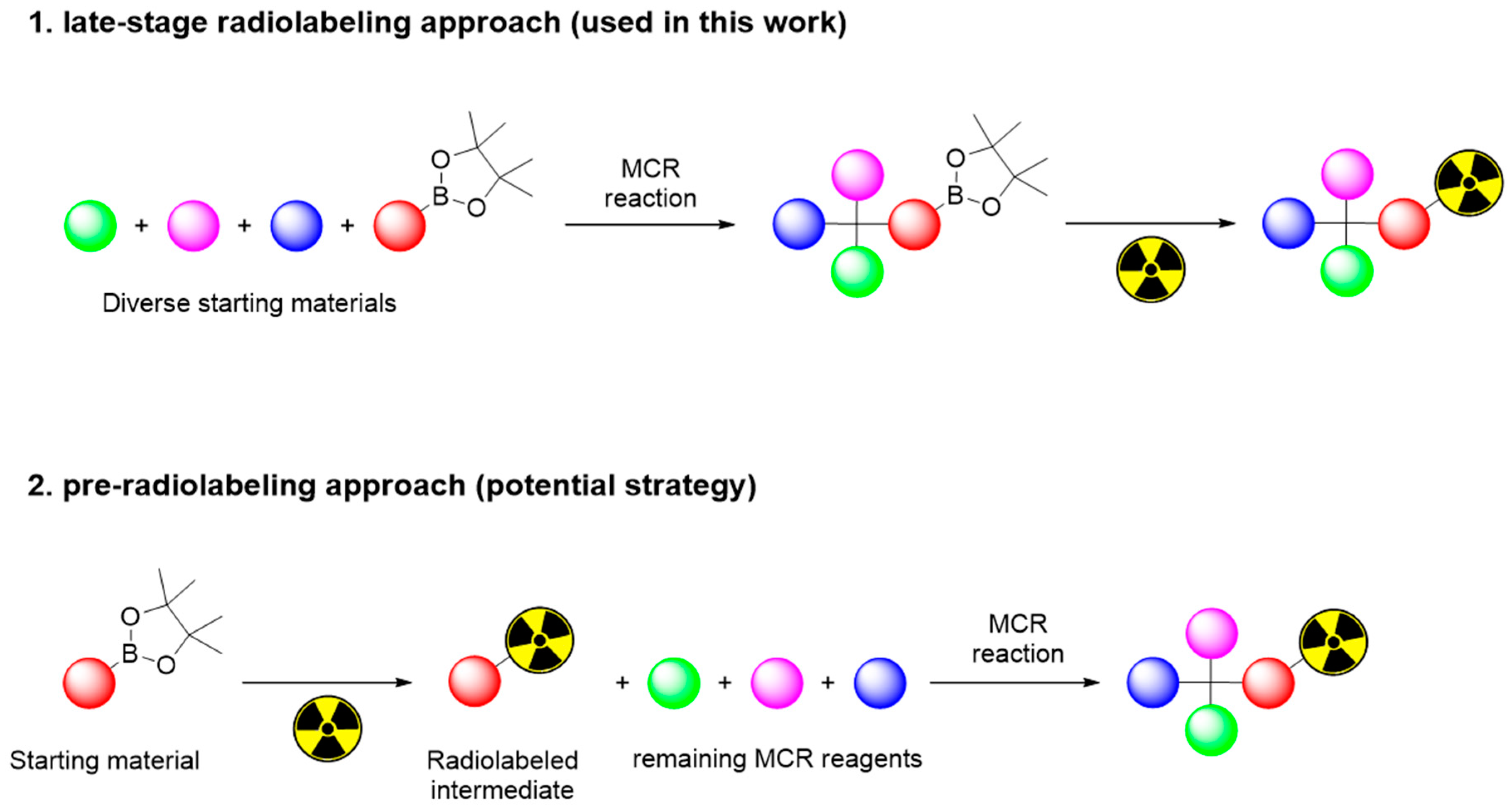
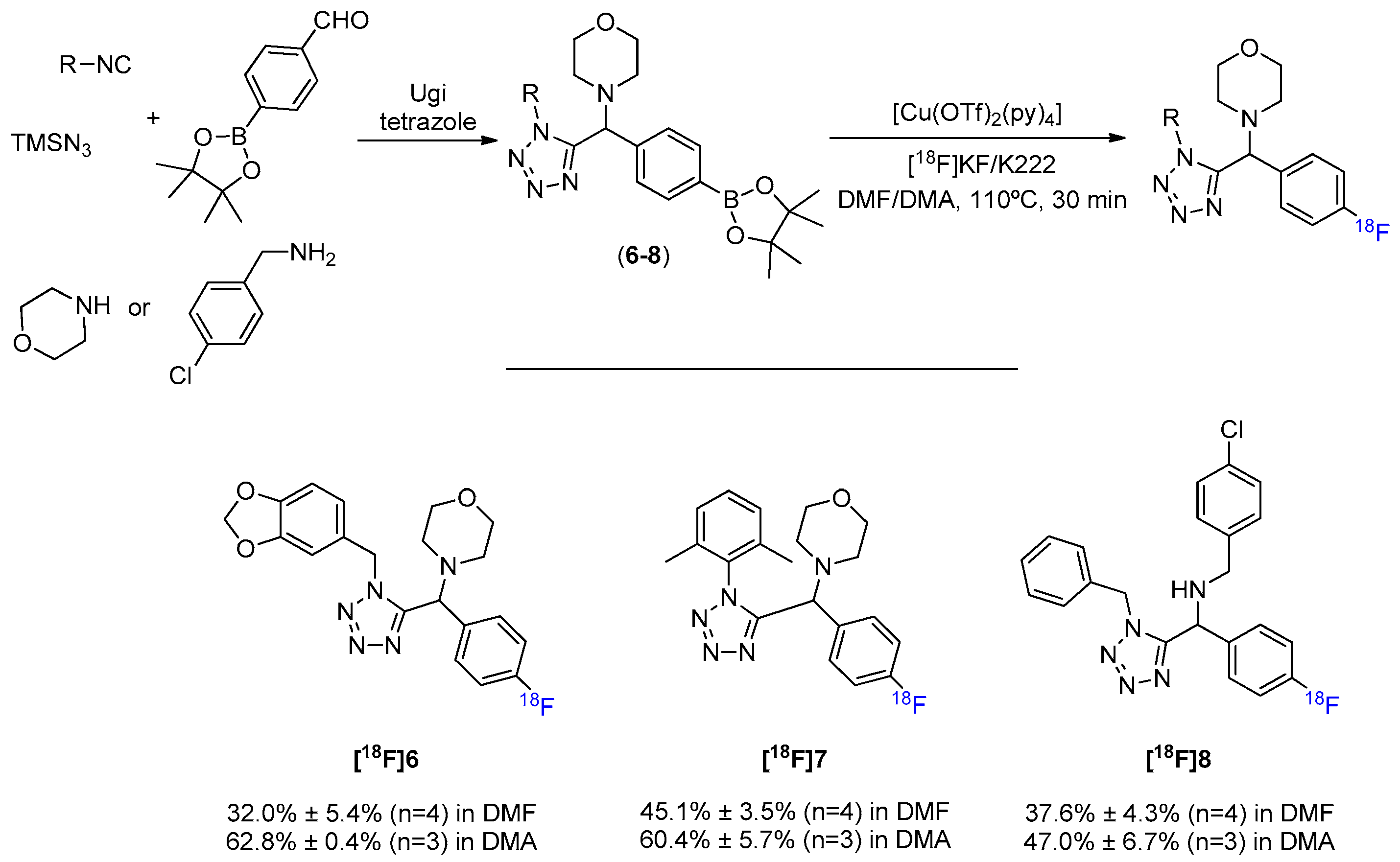
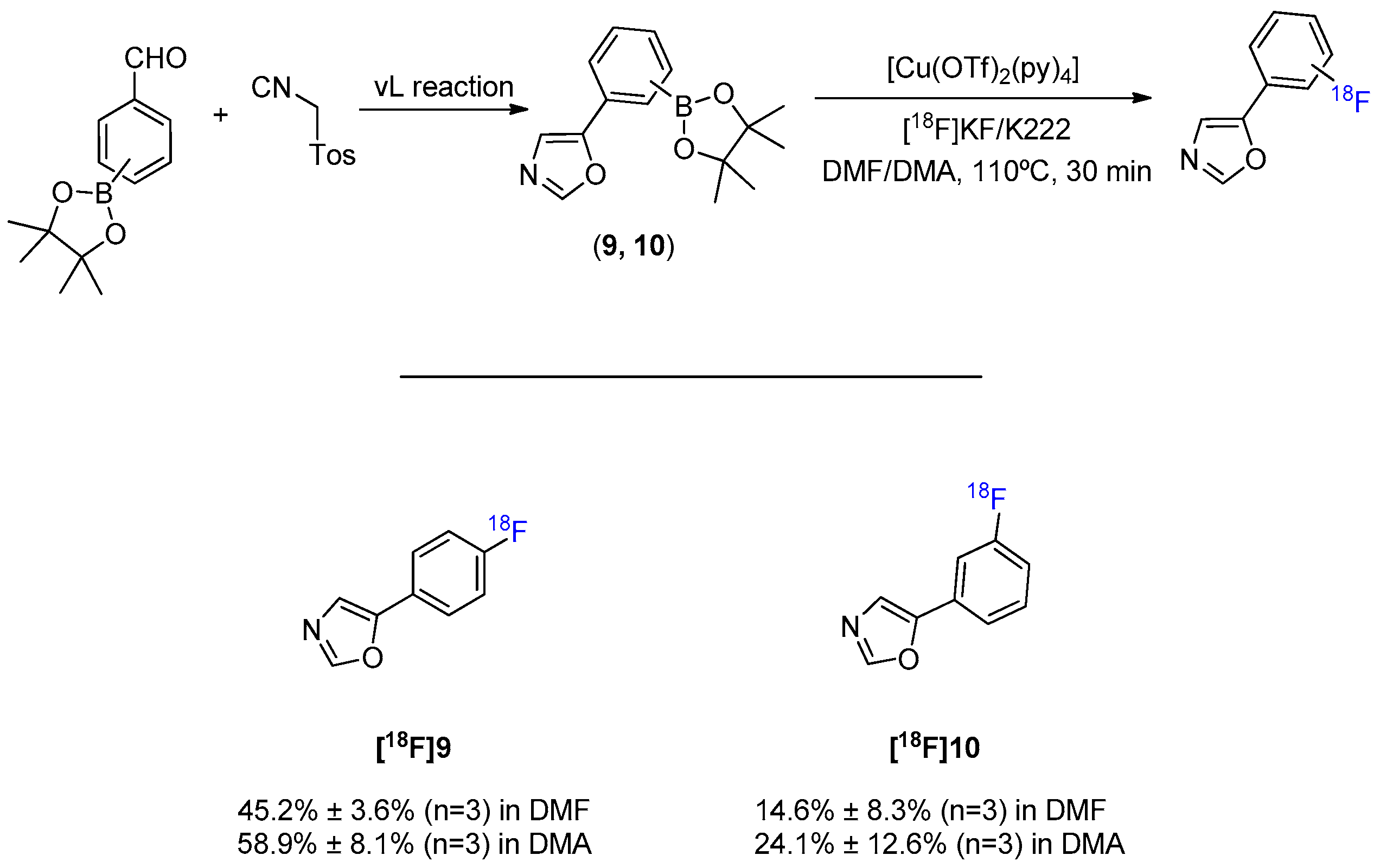
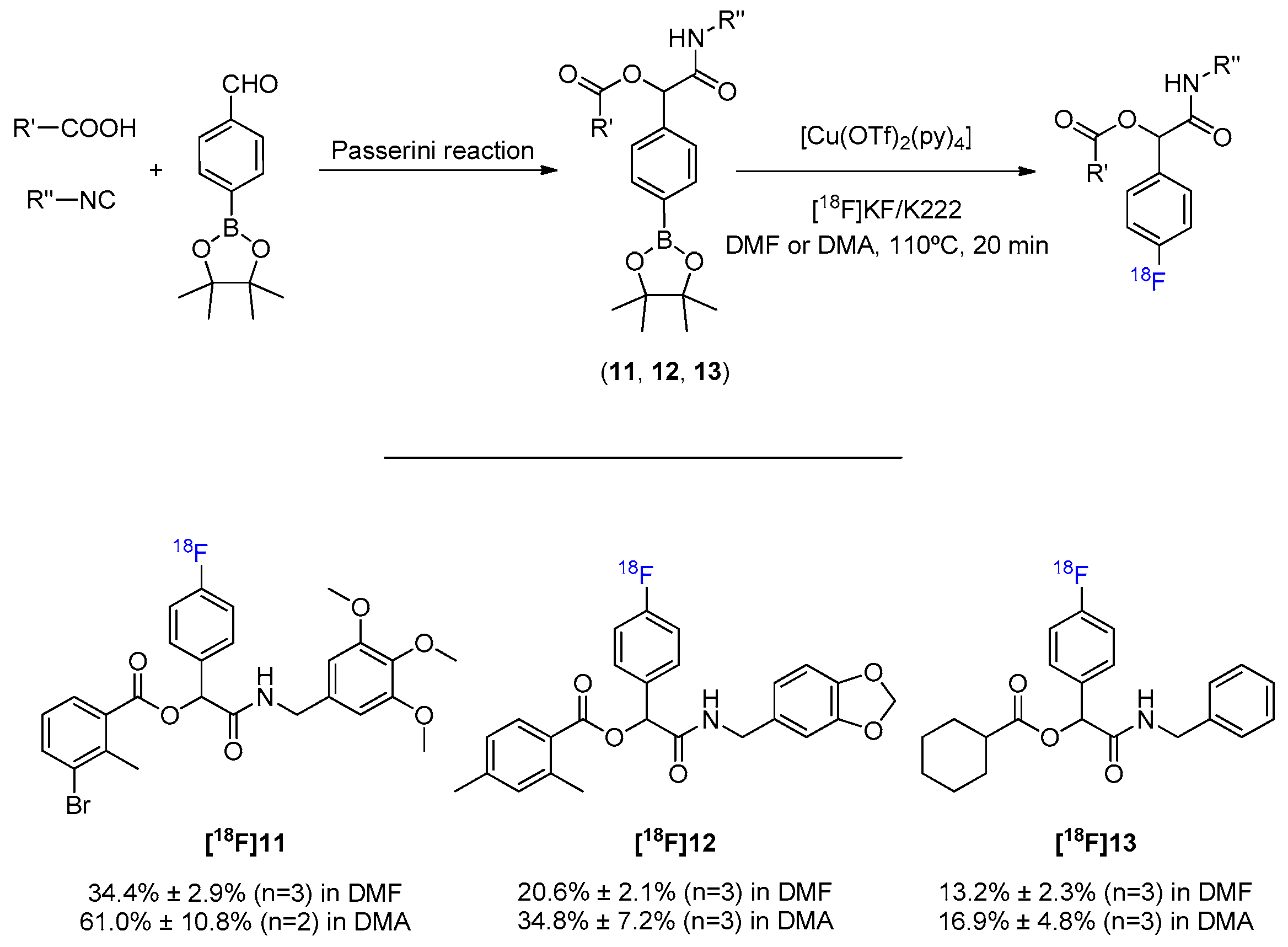
1. Introduction
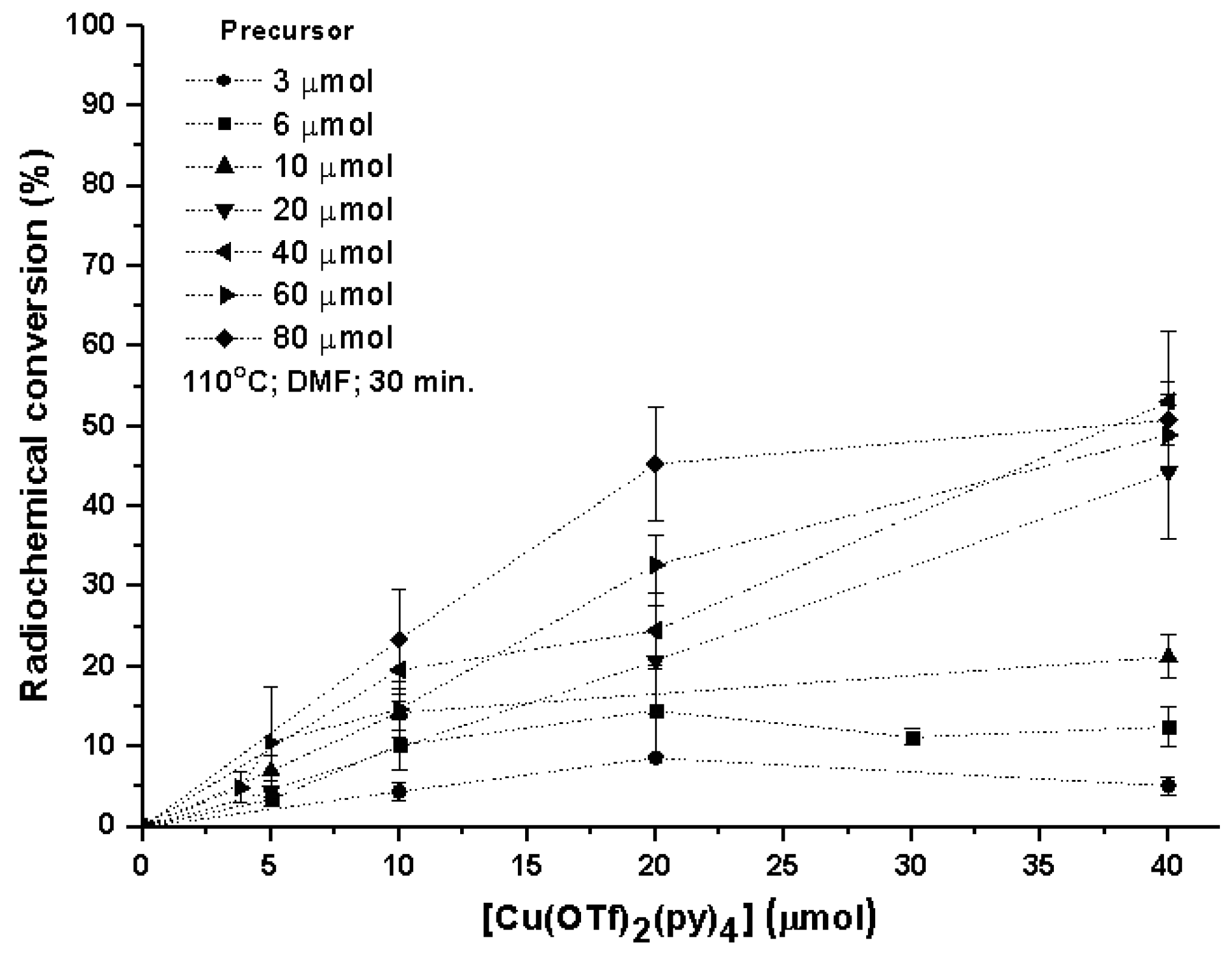
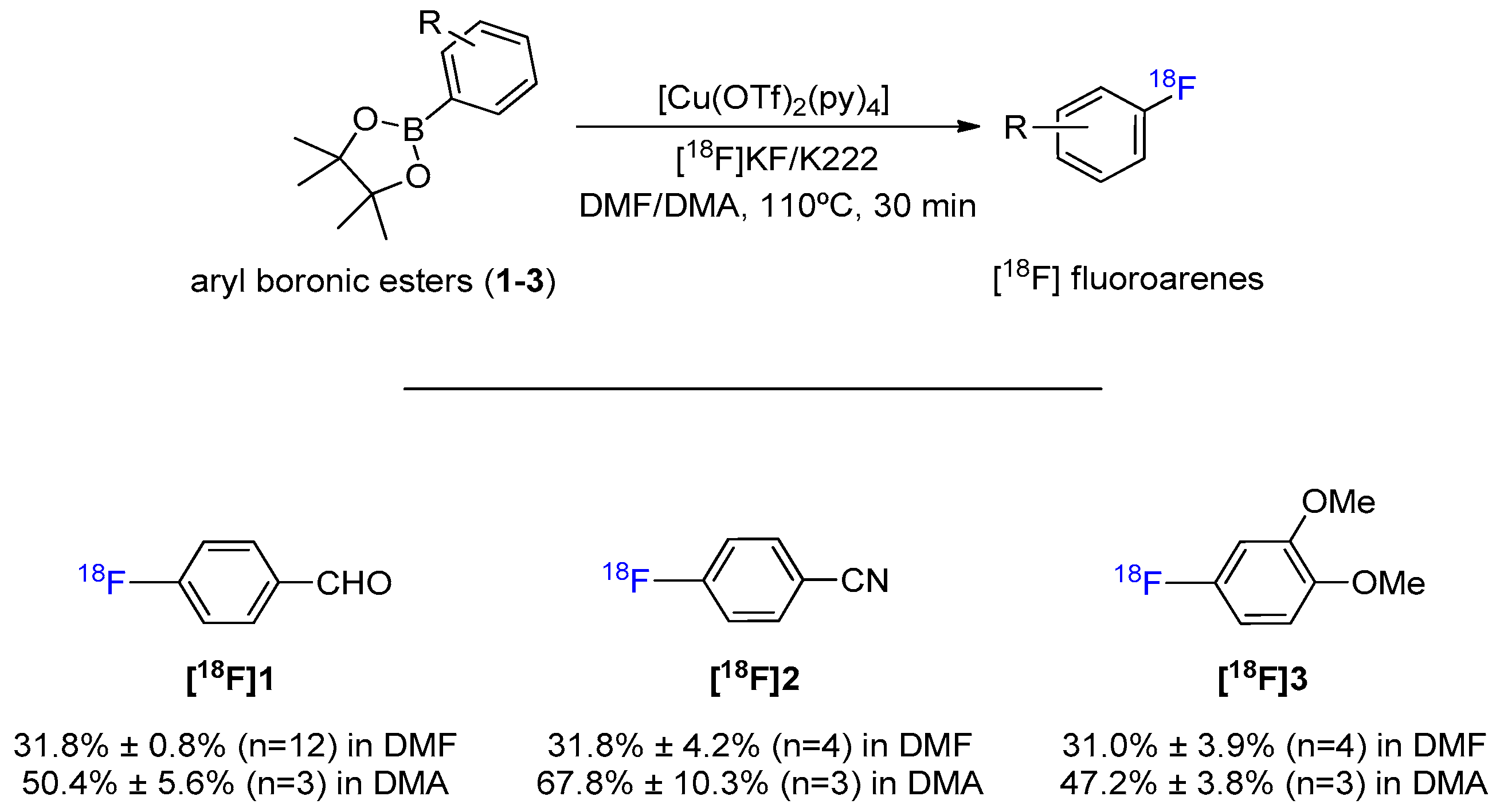
2. Results
3. Discussion
4. Materials and Methods
4.1. General Information
4.2. General Procedure for the Synthesis of Compounds
4.3. Characterization Data
4.4. General Procedure for the Radiolabeling Chemistry
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Matthews, P.M.; Rabiner, E.A.; Passchier, J.; Gunn, R.N. Positron emission tomography molecular imaging for drug development. Brit. J. Clin. Pharmacol. 2012, 73, 175–186. [Google Scholar] [CrossRef]
- Fernandes, E.; Barbosa, Z.; Clemente, G.; Alves, F.; Abrunhosa, A.J. Positron emitting tracers in pre-clinical drug development. Curr. Rad. 2012, 5, 90–98. [Google Scholar] [CrossRef]
- Patel, S.; Schmidt, K.; Hesterman, J.; Hoppin, J. Advancing Drug Discovery and Development Using Molecular Imaging (ADDMI): An Interest Group of the World Molecular Imaging Society and an Inaugural Session on Positron Emission Tomography (PET). Mol. Imaging Biol. 2017, 19, 348–356. [Google Scholar] [CrossRef]
- Kalinski, C.; Umkehrer, M.; Weber, L.; Kolb, J.; Burdack, C.; Ross, G. On the industrial applications of MCRs: Molecular diversity in drug discovery and generic drug synthesis. Mol. Divers. 2010, 14, 513–522. [Google Scholar] [CrossRef]
- Slobbe, P.; Ruijter, E.; Orru, R.V.A. Recent applications of multicomponent reactions in medicinal chemistry. MedChemComm 2012, 3, 1189–1218. [Google Scholar] [CrossRef]
- Zarganes-Tzitzikas, T.; Domling, A. Modern multicomponent reactions for better drug syntheses. Org. Chem. Front. 2014, 1, 834–837. [Google Scholar] [CrossRef]
- Ruijter, E.; Scheffelaar, R.; Orru, R.V.A. Multicomponent Reaction Design in the Quest for Molecular Complexity and Diversity. Angew. Chem. Int. Ed. 2011, 50, 6234–6246. [Google Scholar] [CrossRef]
- Dömling, A.; Wang, W.; Wang, K. Chemistry and Biology Of Multicomponent Reactions. Chem. Rev. 2012, 112, 3083–3135. [Google Scholar] [CrossRef]
- De Graaff, C.; Ruijter, E.; Orru, R.V.A. Recent developments in asymmetric multicomponent reactions. Chem. Soc. Rev. 2012, 41, 3969–4009. [Google Scholar] [CrossRef]
- Dömling, A.; AlQahtani, A.D. Multicomponent Reactions in Organic Synthesis. In Multicomponent Reactions in Organic Synthesis, 1st ed.; Zhu, J., Wang, Q., Wang, M., Eds.; Wiley-VCH Verlag GmbH & Co. KgaA: Weinheim, Germany, 2014; pp. 1–12. [Google Scholar]
- Alegre-Requena, J.V.; Marqués-López, E.; Herrera, R.P. Introduction: Multicomponent Startegies. In Multicomponent Reactions: Concepts and Applications for Design and Synthesis, 1st ed.; Herrera, R.P., Marqués-López, E., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 1–15. [Google Scholar]
- Zhu, Q.; Yuan, Q.; Chen, M.; Guo, M.; Huang, H. Multicomponent Reactions with Cyclic Tertiary Amines Enabled by Facile C−N Bond Cleavage. Angew. Chem. Int. Ed. 2017, 56, 5101–5105. [Google Scholar] [CrossRef]
- Wang, K.; Nguyen, K.; Huang, Y.; Dömling, A. Cyanoacetamide Multicomponent Reaction (I): Parallel Synthesis Of Cyanoacetamides. J. Comb. Chem. 2009, 11, 920–927. [Google Scholar] [CrossRef]
- Cao, H.; Liu, H.; Dömling, A. Efficient Multicomponent Reaction Synthesis of the Schistosomiasis Drug Praziquantel. Chem. Eur. J. 2010, 16, 12296–12298. [Google Scholar] [CrossRef]
- Aillaud, I.; Haurena, C.; Gall, E.L.; Martens, T.; Ricci, G. 2-Chlorophenyl Zinc Bromide: A Convenient Nucleophile for the Mannich-Related Multicomponent Synthesis of Clopidogrel and Ticlopidine. Molecules 2010, 15, 8144–8155. [Google Scholar] [CrossRef]
- Liu, H.; William, S.; Herdtweck, E.; Botros, S.; Dömling, A. MCR Synthesis of Praziquantel Derivatives. Chem. Biol. Drug Des. 2012, 79, 470–477. [Google Scholar] [CrossRef]
- Khoury, K.; Sinha, M.K.; Nagashima, T.; Herdtweck, E.; Dömling, A. Efficient assembly of iminodicarboxamides by a truly four component reaction. Angew. Chem. Int. Ed. 2012, 51, 10280–10283. [Google Scholar] [CrossRef]
- Neochoritis, C.G.; Stotani, S.; Mishra, B.; Dömling, A. Efficient Isocyanide-less Isocyanide-Based Multicomponent Reactions. Org. Let. 2015, 17, 2002–2005. [Google Scholar] [CrossRef]
- Wehlan, H.; Oehme, J.; Schäfer, A.; Rossen, K. Development of Scalable Conditions for the Ugi Reaction—Application to the Synthesis of (R)-Lacosamide. Org. Process Res. Dev. 2015, 19, 1980–1986. [Google Scholar] [CrossRef]
- Li, L.; Hopkinson, M.N.; Yona, R.L.; Bejot, R.; Gee, A.D.; Gouverneur, V. Convergent 18F radiosynthesis: A new dimension for radiolabelling. Chem. Sci. 2011, 2, 123–131. [Google Scholar] [CrossRef]
- Qu, W.; Zha, Z.; Ploessl, K.; Lieberman, B.P.; Zhu, L.; Wise, D.R.; Thompson, C.B.; Kung, H.F. Synthesis of Optically Pure 4-Fluoro-Glutamines as Potential Metabolic Imaging Agents for Tumors. J. Am. Chem. Soc. 2011, 133, 1122–1133. [Google Scholar] [CrossRef]
- Preshlock, S.; Calderwood, S.; Verhoog, S.; Tredwell, M.; Huiban, M.; Hienzsch, A.; Gruber, S.; Wilson, T.C.; Taylor, N.J.; Cailly, T.; et al. Enhanced copper-mediated 18F-fluorination of aryl boronic esters provides eight radiotracers for PET applications. Chem. Commun. 2016, 52, 8361–8364. [Google Scholar] [CrossRef]
- Schäfer, D.; Weiß, P.; Ermert, J.; Castillo Meleán, J.; Zarrad, F.; Neumaier, B. Preparation of No-Carrier-Added 6-[18F]Fluoro-l-tryptophan via Cu-Mediated Radiofluorination. Eur. J. Org. Chem. 2016, 2016, 4621–4628. [Google Scholar] [CrossRef]
- Giglio, B.C.; Fei, H.; Wang, M.; Wang, H.; He, L.; Feng, H.; Wu, Z.; Lu, H.; Li, Z. Synthesis of 5-[(18)F]Fluoro-α-methyl Tryptophan: New Trp Based PET Agents. Theranostics 2017, 7, 1524–1530. [Google Scholar] [CrossRef]
- Taylor, N.J.; Emer, E.; Preshlock, S.; Schedler, M.; Tredwell, M.; Verhoog, S.; Mercier, J.; Genicot, C.; Gouverneur, V. Derisking the Cu-Mediated 18F-Fluorination of Heterocyclic Positron Emission Tomography Radioligands. J. Am. Chem. Soc. 2017, 139, 8267–8276. [Google Scholar] [CrossRef]
- Lu, J.; Guan, Z.-Z.; Gao, J.-W.; Zhang, Z.-H. An improved procedure for the synthesis of arylboronates by palladium-catalyzed coupling reaction of aryl halides and bis(pinacolato)diboron in polyethylene glycol. Appl. Org. Chem. 2011, 25, 537–541. [Google Scholar] [CrossRef]
- Tredwell, M.; Preshlock, S.M.; Taylor, N.J.; Gruber, S.; Huiban, M.; Passchier, J.; Mercier, J.; Génicot, C.; Gouverneur, V. A General Copper-Mediated Nucleophilic 18F Fluorination of Arenes. Angew. Chem. Int. Ed. 2014, 53, 7751–7755. [Google Scholar] [CrossRef]
- Zlatopolskiy, B.D.; Zischler, J.; Krapf, P.; Zarrad, F.; Urusova, E.A.; Kordys, E.; Endepols, H.; Neumaier, B. Copper-Mediated Aromatic Radiofluorination Revisited: Efficient Production of PET Tracers on a Preparative Scale. Chem. Eur. J. 2015, 21, 5972–5979. [Google Scholar] [CrossRef] [PubMed]
- Mossine, A.V.; Brooks, A.F.; Makaravage, K.J.; Miller, J.M.; Ichiishi, N.; Sanford, M.S.; Scott, P.J.H. Synthesis of [18F]Arenes via the Copper-Mediated [18F]Fluorination of Boronic Acids. Org. Lett. 2015, 17, 5780–5783. [Google Scholar] [CrossRef] [PubMed]
- Vitaku, E.; Smith, D.T.; Njardarson, J.T. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. [Google Scholar] [CrossRef]
- Altıntop, M.D.; Sever, B.; Akalın Çiftçi, G.; Kucukoglu, K.; Özdemir, A.; Soleimani, S.S.; Nadaroglu, H.; Kaplancıklı, Z.A. Synthesis and evaluation of new benzodioxole-based dithiocarbamate derivatives as potential anticancer agents and hCA-I and hCA-II inhibitors. Eur. J. Med. Chem. 2017, 125, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Naim, M.J.N.; Alam, O.; Alam, M.J.; Alam, P.; Shrivastava, N. A review on pharmacological profile of Morpholine derivatives. Int. J. Pharm. Pharm. Sci. 2015, 3, 40–51. [Google Scholar]
- Raju, G.N.; Suresh, P.V.; Nadendla, R.R.; Anusha, K. Synthesis, characterization and antimicrobial evaluation of isoxazole derivatives. Der. Pharm. Chem. 2015, 7, 346–352. [Google Scholar]
- Swellmeen, L. 1,3-Oxazole Derivatives: A Review of Biological Activities as Antipathogenic. Der. Pharm. Chem. 2016, 8, 269–286. [Google Scholar]
- Joshi, S.; Bisht, A.S.; Juyal, D. Systematic scientific study of 1, 3-oxazole derivatives as a useful lead for pharmaceuticals: A review. J. Pharm. Innov. 2017, 6, 109–117. [Google Scholar]
- Reza Kazemizadeh, A.; Ramazani, A. Synthetic Applications of Passerini Reaction. Curr. Org. Chem. 2012, 16, 418–450. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zarganes-Tzitzikas, T.; Clemente, G.S.; Elsinga, P.H.; Dömling, A. MCR Scaffolds Get Hotter with 18F-Labeling. Molecules 2019, 24, 1327. https://doi.org/10.3390/molecules24071327
Zarganes-Tzitzikas T, Clemente GS, Elsinga PH, Dömling A. MCR Scaffolds Get Hotter with 18F-Labeling. Molecules. 2019; 24(7):1327. https://doi.org/10.3390/molecules24071327
Chicago/Turabian StyleZarganes-Tzitzikas, Tryfon, Gonçalo S. Clemente, Philip H. Elsinga, and Alexander Dömling. 2019. "MCR Scaffolds Get Hotter with 18F-Labeling" Molecules 24, no. 7: 1327. https://doi.org/10.3390/molecules24071327
APA StyleZarganes-Tzitzikas, T., Clemente, G. S., Elsinga, P. H., & Dömling, A. (2019). MCR Scaffolds Get Hotter with 18F-Labeling. Molecules, 24(7), 1327. https://doi.org/10.3390/molecules24071327