Optimized Prediction of Reducing Sugars and Dry Matter of Potato Frying by FT-NIR Spectroscopy on Peeled Tubers
Abstract
1. Introduction
2. Results and Discussion
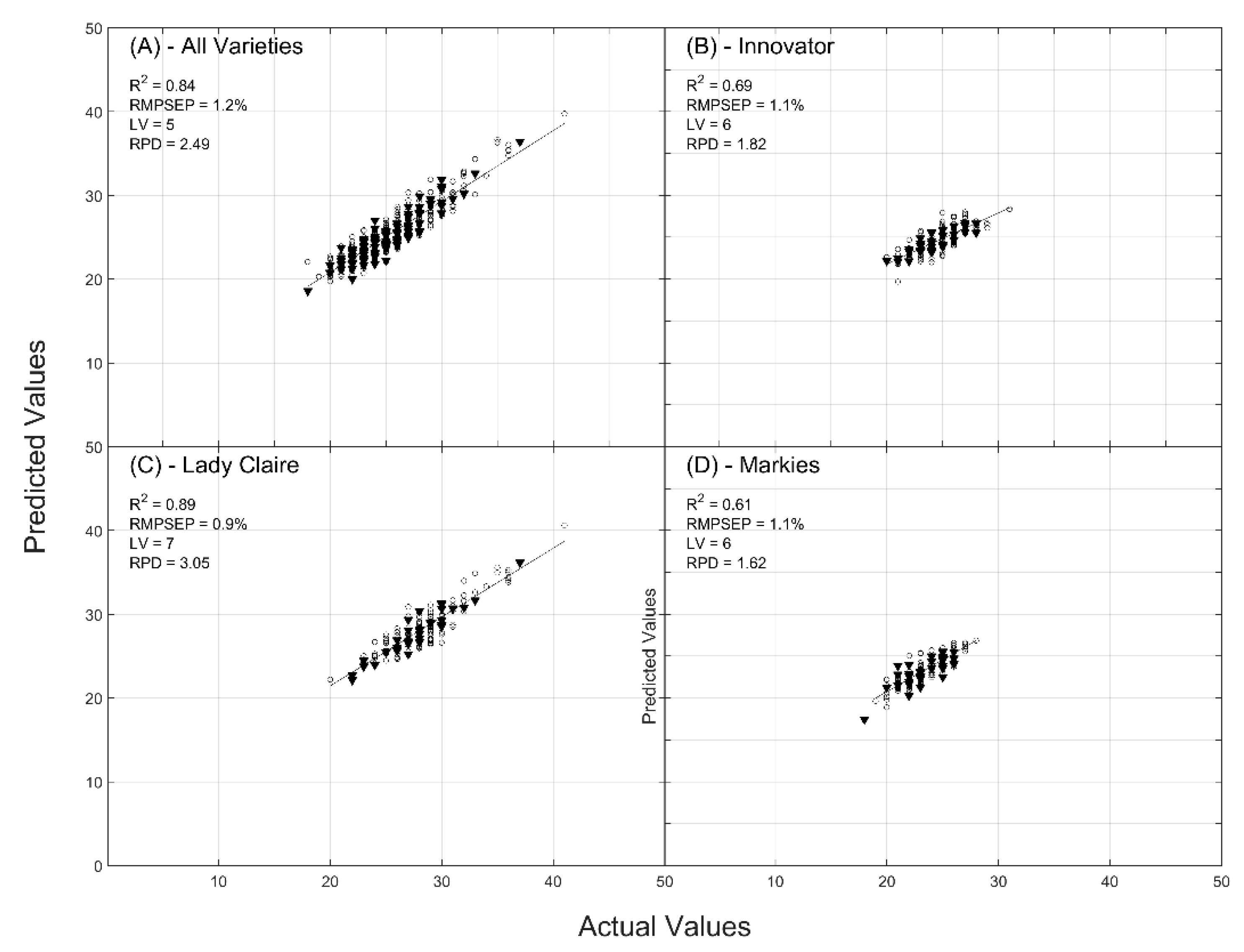
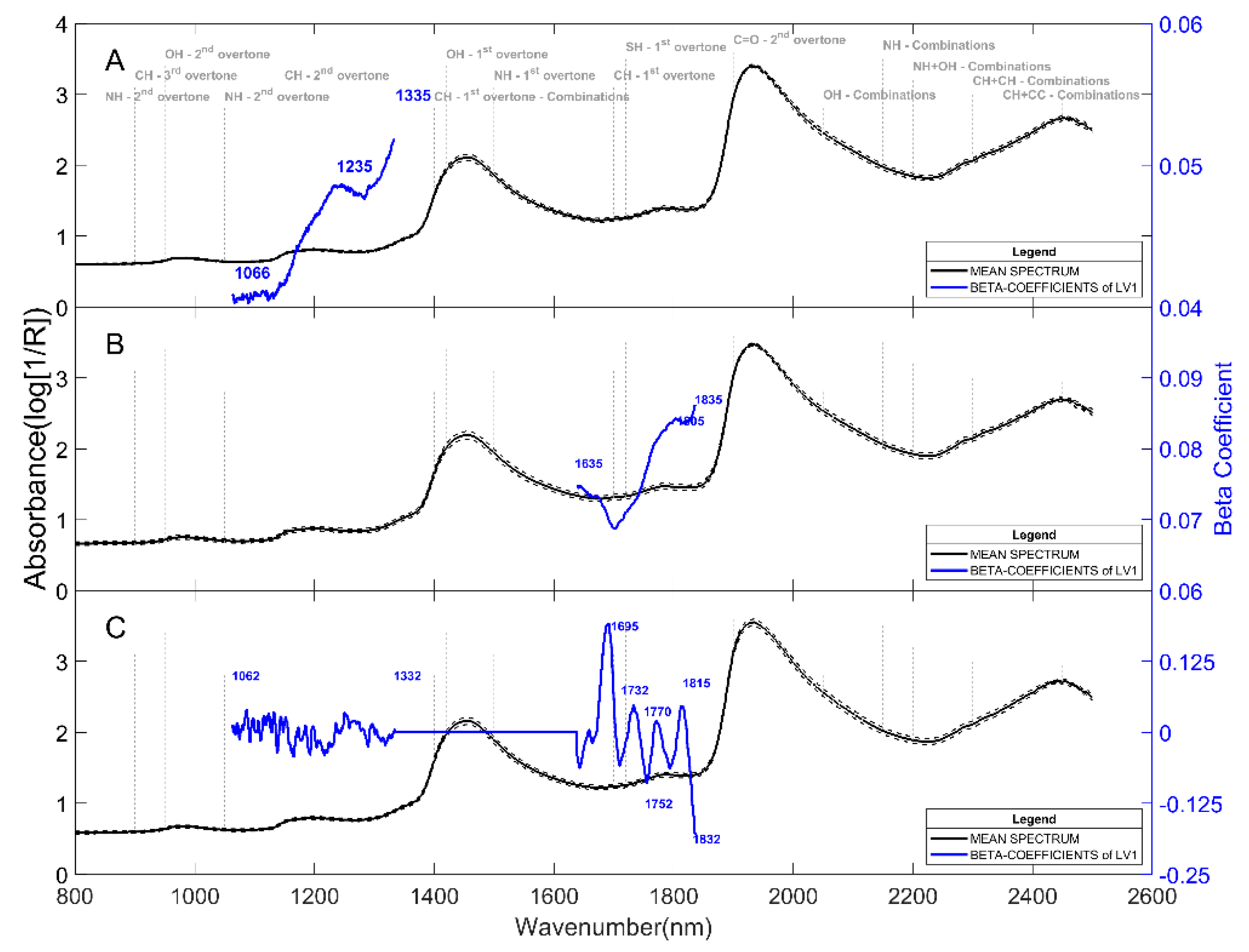
2.1. Prediction of Dry Matter Content
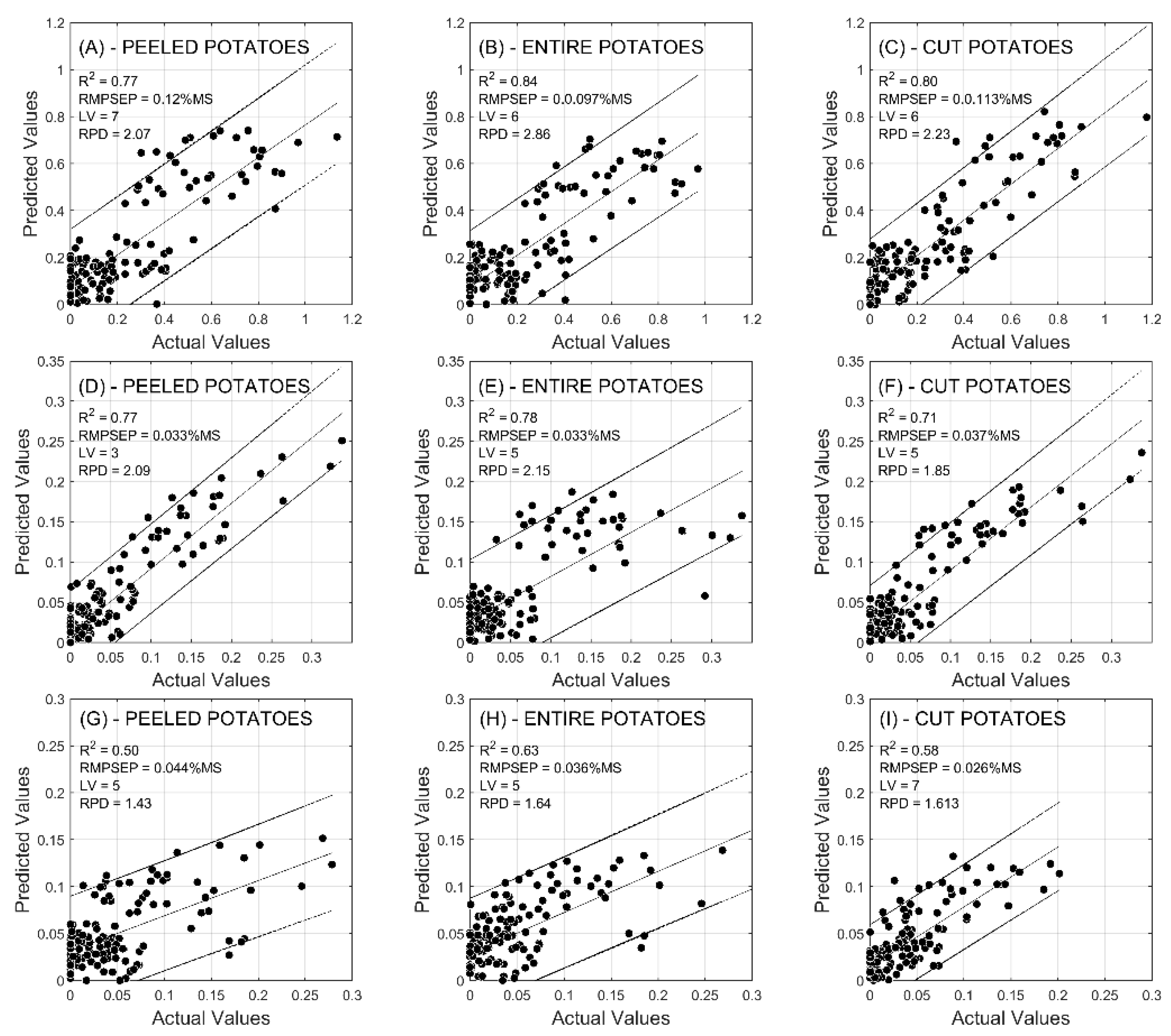
2.2. Prediction of Reducing Sugars
3. Materials and Methods
3.1. Potatoes
3.2. Dry Matter Content
3.3. Reducing Sugars Analyses
3.3.1. Extraction
3.3.2. Quantification of Free Sugars
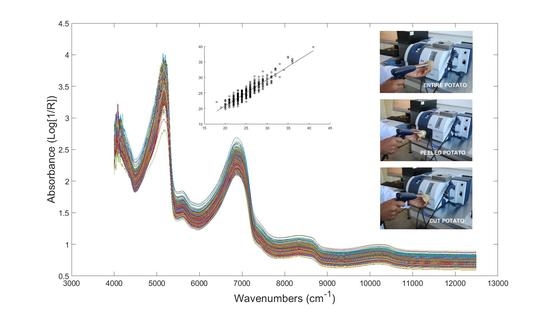
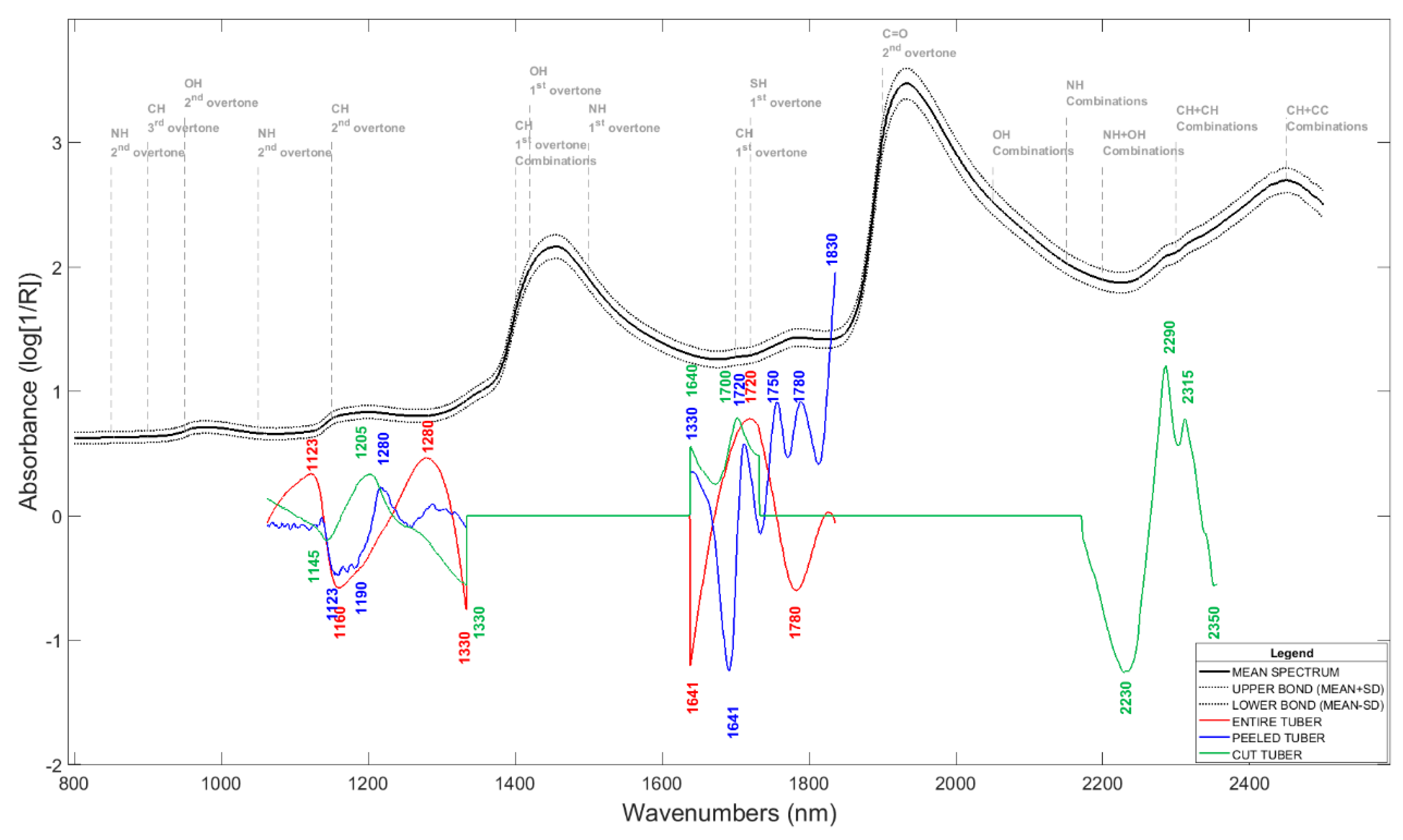
3.4. FT-NIR Spectroscopy
3.5. Chemometric
Partial Least Square Regression
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- FAOSTAT Food and Agriculture Organization of the United Nations. Available online: http://www.fao.org/faostat/fr/#data/QC (accessed on 7 January 2019).
- Maillard, L.C. Action des acides aminés sur les sucres: formation des mélanoïdines par voie méthodique. C. R. Hebd. Séances Acad. Sci. 1912, 154, 66–68. [Google Scholar]
- FoodDrinkEurope. A “Toolbox” for the Reduction of Acrylamide in Fried Potato Crisps. Available online: https://www.fooddrinkeurope.eu/uploads/publications_documents/crisps-EN-final.pdf (accessed on 7 January 2019).
- Matthäus, B.; Haase, N.U.; Vosmann, K. Factors affecting the concentration of acrylamide during deep-fat frying of potatoes. Eur. J. Lipid Sci. Technol. 2004, 106, 793–801. [Google Scholar] [CrossRef]
- Williams, J.S.E. Influence of variety and processing conditions on acrylamide levels in fried potato crisps. Food Chem. 2005, 90, 875–881. [Google Scholar] [CrossRef]
- McGlone, V.A.; Jordan, R.B.; Seelye, R.; Clark, C.J. Dry-matter-a better predictor of the post-storage soluble solids in apples? Postharv. Biol. Technol. 2003, 28, 431–435. [Google Scholar] [CrossRef]
- Peirs, A.S.N.; Touchant, K.; Nicolaï, B.M. Comparison of Fourier transform and dispersive near-infrared reflectance spectroscopy for apple quality measurements. Biosys. Engineer. 2002, 81, 305–311. [Google Scholar] [CrossRef]
- Garcia-Jares, C.M.; Medina, B. Application of multivariate calibration to the simultaneous routine determination of ethanol, glycerol, fructose, glucose and total residual sugars in botrytized-grape sweet wines by means of near-infrared reflectance spectroscopy. Fresen. J. Anal. Chem. 1997, 357, 86–91. [Google Scholar] [CrossRef]
- Camps, C.; Christen, D. Non-destructive assessment of apricot fruit quality by portable visible-near infrared spectroscopy. Lwt-Food Sci. Technol. 2009, 42, 1125–1131. [Google Scholar] [CrossRef]
- Mehrübeoǧlu, M.; Coté, G.L. Determination of total reducing sugars inpotato samples using near-infrared spectroscopy. Cereal Food. World 1997, 42, 409–413. [Google Scholar]
- Van Dijk, C.; Fischer, M.; Holm, J.; Beekhuizen, J.G.; Stolle-Smits, T.; Boeriu, C. Texture of cooked potatoes (Solanum tuberosum). 1. Relationships between dry matter content, sensory-perceived texture, and near-infrared spectroscopy. J. Agric. Food Chem. 2002, 50, 5082–5088. [Google Scholar] [CrossRef]
- Chen, J.Y.; Miao, Y.; Zhang, H.; Matsunaga, R. Non-destructive determination of carbohydrate content in potatoes using near infrared spectroscopy. J. Near Infrared Spec. 2004, 12, 311–314. [Google Scholar] [CrossRef]
- Haase, N.U. Prediction of Potato Processing Quality by near Infrared Reflectance Spectroscopy of Ground Raw Tubers. J. Near Infrared Spec. 2011, 19, 37–45. [Google Scholar] [CrossRef]
- Rady, A.M.; Guyer, D.E. Evaluation of sugar content in potatoes using NIR reflectance and wavelength selection techniques. Postharvest Biol. Tec. 2015, 103, 17–26. [Google Scholar] [CrossRef]
- Jacquinot, P. How the search for a throughput advantage led to Fourier transform spectroscopy. Infrared Phys. 1984, 24, 99–101. [Google Scholar] [CrossRef]
- White, R. Chromatography/Fourier Transform Infrared Spectroscopy and Its Applications; CRC Press: New York, NY, USA, 1989; Volume 10. [Google Scholar]
- Fernández Pierna, J.A.; Manley, M.; Dardenne, P.; Downey, G.; Baeten, V. Spectroscopic Technique: Fourier Transform (FT) Near-Infrared Spectroscopy (NIR) and Microscopy (NIRM). In Modern Techniques for Food Authentication, 2nd ed.; Sun, D.-W., Ed.; Academic Press: London, UK, 2018; pp. 103–138. [Google Scholar]
- Elmore, J.S.; Briddon, A.; Dodson, A.T.; Muttucumaru, N.; Halford, N.G.; Mottram, D.S. Acrylamide in potato crisps prepared from 20 UK-grown varieties: Effects of variety and tuber storage time. Food Chem. 2015, 182, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Helgerud, T.; Wold, J.P.; Pedersen, M.B.; Liland, K.H.; Ballance, S.; Knutsen, S.H.; Rukke, E.O.; Afseth, N.K. Towards on-line prediction of dry matter content in whole unpeeled potatoes using near-infrared spectroscopy. Talanta 2015, 143, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, R.; Büning-Pfaue, H. NIR determination of potato constituents. Potato Res. 1998, 41, 327–334. [Google Scholar] [CrossRef]
- Subedi, P.P.; Walsh, K.B. Assessment of Potato Dry Matter Concentration Using Short-Wave Near-Infrared Spectroscopy. Potato Res. 2009, 52, 67–77. [Google Scholar] [CrossRef]
- Stark, J.C.; Love, S.L. Tuber quality. Potato Prod. Sys. 2003, 329–343. [Google Scholar]
- Storey, R.M.J. The harvested crop. In Potato Biology and Biotechnology Advances and Perspectives; Vreugdenhil, D., Ed.; Elsvier: London, UK, 2007; pp. 441–450. [Google Scholar]
- Chen, J.Y.; Zhang, H.; Miao, Y.; Asakura, M. Nondestructive determination of sugar content in potato tubers using visible and near infrared spectroscopy. Jpn. J. Food Eng. 2010, 11, 59–64. [Google Scholar]
- Yaptenco, K.F.; Suzuki, T.; Kawakami, S.; Sato, H.; Takano, K.; Kozima, T.T. Nondestructive determination of sugar content in ’Danshaku’ potato (Solanum tuberosum L.) by near infrared spectroscopy. J. Agric. Sci. 2000, 44, 284–294. [Google Scholar]
Sample Availability: Samples are not available from the authors. |
| PLS Parameters | PDTE | PDTP | PDTC | |
|---|---|---|---|---|
| Spectra (n) | CAL/VAL | 420/105 | 417/104 | 417/103 |
| Averaged spectra (n) | CAL/VAL | 140 (140) 1/35 | 139 (140) 1/35 | 140 (140) 1/35 |
| Wavenumber range (cm−1) | 9403.8–7498.4 6102.1–5446.3 | 9403.8–7498.4 6102.1–5446.3 | 9403.8–7498.4 6102.1–5774.2 4601.6–4246.8 | |
| LV | CAL/VAL | 7/7 | 7/7 | 7/7 |
| R2 | CAL/VAL | 0.76/0.70 | 0.84/0.83 | 0.83/0.78 |
| RMSE (%) | CAL/VAL | 1.59/1.66 | 1.27/1.23 | 1.34/1.40 |
| RPD | CAL/VAL | 2.05/1.85 | 2.55/2.49 | 2.45/2.17 |
| Data preprocessing | CAL/VAL | LOS | D1 + SNV | SNV |
| DMC values (min–max) (gDW/100 gFW) | CAL/VAL | 18–41/18–37 | 18–41/18–37 | 18–40/18–37 |
| DMC Standard error (%) | CAL/VAL | 3.22/3.07 | 3.22/3.07 | 3.22/3.07 |
| Duration (Days) | Temp. (°C) | Glucose (% FW) | Fructose (% FW) | RS (Glucose + Fructose) (% FW) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IN | LC | MA | IN | LC | MA | IN | LC | MA | ||
| 0 | - | 0.07 a,b | 0.03 c | 0.02 b | 0.05 b,c | 0.01 b,c | 0.01 b | 0.11 a,b | 0.04 b | 0.04 b |
| 6 | 5 | 0.11 b | 0.03 c | 0.02 b | 0.09 c | 0.03 c,d | 0.02 b | 0.20 b | 0.06 b | 0.04 b |
| 24 | 5 | 0.30 c | 0.05 c,d | 0.08 c | 0.27 d | 0.05 d,e | 0.10 c | 0.57 c | 0.10 b,c | 0.18 c |
| 48 | 5 | 0.37 c | 0.07 d | 0.10 c | 0.33 d | 0.07 e | 0.11 c | 0.70 c | 0.14 c | 0.21 c |
| 6 | 8 | 0.08 a | 0.02 b,c | 0.02 b | 0.06 b,c | 0.01 c,d | 0.01 b | 0.14 a,b | 0.03 b | 0.03 b |
| 62 | 8 | 0.03 a | 0.01 a,b | 0.01 a,b | 0.02 a,b | 0.00 a,b | 0.01 a,b | 0.06 a | 0.01 a | 0.01 a,b |
| 195 | 8 | 0.04 a | 0.00 a | 0.00 a | 0.01 a | 0.00 a | 0.00 a | 0.05 a | 0.01 a | 0.00 a |
| p-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| PLS Score | Glucose | Fructose | Fructose + Glucose | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PDTE | PDTP | PDTC | PDTE | PDTP | PDTC | PDTE | PDTP | PDTC | ||
| Spectra | CAL | 420 | 405 | 405 | 402 | 402 | 405 | 405 | 405 | 408 |
| VAL | 105 | 102 | 99 | 99 | 105 | 99 | 102 | 105 | 99 | |
| Averaged spectra | CAL | 140 (140) 1 | 135 (140) | 135 (140) | 134 (140) | 134 (140) | 135 (140) | 135 (140) | 135 (140) | 136 (140) |
| VAL | 35 (35) | 34 (35) | 33 (35) | 33 (35) | 35 (35) | 33 (35) | 34 (35) | 35 (35) | 33 (35) | |
| WL (cm−1) | 9403.8–7498.4; 6102.1–5446.3 | 9403.8–7498.4; 5774.2–5446.3 | 9403.8–7498.4 | 8451.1–7498.4; 5774.2–5446.3 | 9403.8–7498.4 | 9403.8–7498.4 | 9403.8–7498.4; 6102.1–5446.3 | 9403.8–7498.4 | 9403.8–7498.4 | |
| LV | 7 | 6 | 7 | 8 | 6 | 6 | 7 | 6 | 6 | |
| R2 | CAL | 0.56 | 0.71 | 0.82 | 0.72 | 0.71 | 0.77 | 0.68 | 0.69 | 0.76 |
| VAL | 0.70 | 0.82 | 0.79 | 0.84 | 0.82 | 0.85 | 0.77 | 0.84 | 0.80 | |
| RMSE (%) | CAL | 0.108 | 0.076 | 0.061 | 0.065 | 0.071 | 0.061 | 0.149 | 0.151 | 0.129 |
| VAL | 0.084 | 0.059 | 0.066 | 0.050 | 0.050 | 0.049 | 0.120 | 0.097 | 0.113 | |
| RPD | CAL | 1.51 | 1.87 | 2.35 | 1.9 | 1.87 | 2.08 | 1.75 | 1.8 | 2.05 |
| VAL | 1.89 | 2.34 | 2.25 | 2.5 | 2.43 | 2.59 | 2.07 | 2.86 | 2.23 | |
| Data Preprocessing | SNV | SNV | LOS | MMN | None | LOS | MMN | None | LOS | |
| Sugar values (% FW) | CAL | 0–0.709 | 0–0.596 | 0–0.613 | 0–0.417 | 0–0.539 | 0–0.564 | 0–0.969 | 0–1.135 | 0–1.178 |
| VAL | 0–0.494 | 0–0.476 | 0–0.493 | 0–0.39 | 0–0.39 | 0–0.39 | 0–0.779 | 0–0.779 | 0–0.779 | |
| PLS Score | Glucose | Fructose | Fructose + Glucose | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PDTE | PDTP | PDTC | PDTE | PDTP | PDTC | PDTE | PDTP | PDTC | ||
| Spectra | CAL | 402 | 396 | 405 | 405 | 405 | 405 | 411 | 405 | 405 |
| VAL | 102 | 102 | 102 | 102 | 99 | 99 | 102 | 99 | 102 | |
| Averaged spectra | CAL | 134 (140) 1 | 132 (140) | 135 (140) | 135 (140) | 135 (140) | 135 (140) | 137 (140) | 135 (140) | 135 (140) |
| VAL | 34 (35) | 34 (35) | 34 (35) | 34 (35) | 33 (35) | 33 (35) | 34 (35) | 33 (35) | 34 (35) | |
| WL (cm−1) | 8451.1–7498.4 | 9403.8–8451.1; 6102.1–5774.2 | 9403.8–8451.1; 6102.1–5446.3 | 8451.1–7498.4 | 9403.8–8451.1; 6102.1–5774.2 | 9403.8–7498.4; 5774.2–5446.3 | 8451.1–7498.4 | 9403.8–7498.4; 6102.1–5446.3 | 9403.8–7498.4; 5774.2–5446.3 | |
| LV | 5 | 9 | 4 | 3 | 9 | 9 | 3 | 5 | 5 | |
| R2 | CAL | 0.71 | 0.83 | 0.72 | 0.61 | 0.83 | 0.88 | 0.55 | 0.81 | 0.78 |
| VAL | 0.80 | 0.75 | 0.70 | 0.75 | 0.81 | 0.77 | 0.77 | 0.78 | 0.71 | |
| RMSE (%) | CAL | 0.019 | 0.013 | 0.019 | 0.022 | 0.016 | 0.013 | 0.051 | 0.031 | 0.034 |
| VAL | 0.015 | 0.017 | 0.019 | 0.018 | 0.016 | 0.017 | 0.033 | 0.033 | 0.037 | |
| RPD | CAL | 1.86 | 2.4 | 1.89 | 1.6 | 2.39 | 2.94 | 1.5 | 2.31 | 2.14 |
| VAL | 2.29 | 2.14 | 1.83 | 2.01 | 2.4 | 2.11 | 2.09 | 2.15 | 1.85 | |
| Data Preprocessing | SLS | None | MSC | 1st der. | None | None | 1st der. | D1 + MSC | MSC | |
| Sugar values (% FW) | CAL | 0–0.165 | 0–0.128 | 0–0.165 | 0–0.173 | 0–0.173 | 0–0.173 | 0–0.338 | 0–0.338 | 0–0.338 |
| VAL | 0–0.115 | 0–0.115 | 0–0.115 | 0–0.119 | 0–0.119 | 0–0.119 | 0–0.235 | 0–0.235 | 0–0.235 | |
| PLS Score | Glucose | Fructose | Fructose + Glucose | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PDTE | PDTP | PDTC | PDTE | PDTP | PDTC | PDTE | PDTP | PDTC | ||
| Spectra | CAL | 396 | 393 | 408 | 408 | 411 | 411 | 414 | 411 | 393 |
| VAL | 96 | 105 | 102 | 99 | 105 | 102 | 102 | 105 | 96 | |
| Averaged spectra | CAL | 132 (140) 1 | 131 (140) | 136 (140) | 136 (140) | 137 (140) | 137 (140) | 138 (140) | 137 (140) | 131 (140) |
| VAL | 32 (35) | 35 (35) | 34 (35) | 33 (35) | 35 (35) | 34 (35) | 34 (35) | 35 (35) | 32 (35) | |
| WL (cm−1) | 7502.2–6098.2 | 9403.8–7498.4 | 9403.8–7498.4; 6102.1–5446.3 | 9403.8–6098.2 | 9403.8–7498.4; 4601.6–4246.8 | 9403.8–7498.4; 6102.1–5446.3 | 9403.8–7498.4 | 6102.1–5446.3 | 9403.8–7498.4; 6102.1–5446.3 | |
| LV | 6 | 5 | 6 | 9 | 14 | 6 | 5 | 5 | 7 | |
| R2 | CAL | 0.55 | 0.57 | 0.61 | 0.57 | 0.64 | 0.59 | 0.45 | 0.38 | 0.64 |
| VAL | 0.57 | 0.52 | 0.61 | 0.67 | 0.70 | 0.63 | 0.50 | 0.63 | 0.58 | |
| RMSE (%) | CAL | 0.015 | 0.018 | 0.018 | 0.020 | 0.019 | 0.018 | 0.045 | 0.047 | 0.028 |
| VAL | 0.016 | 0.014 | 0.018 | 0.016 | 0.017 | 0.018 | 0.044 | 0.036 | 0.026 | |
| RPD | CAL | 1.49 | 1.52 | 1.6 | 1.74 | 1.68 | 1.56 | 1.35 | 1.27 | 1.66 |
| VAL | 1.56 | 1.47 | 1.62 | 1.52 | 1.83 | 1.66 | 1.43 | 1.64 | 1.61 | |
| Data Preprocessing | D1 + SNV | LOS | SLS | MMN | D1 | SNV | SNV | None | LOS | |
| Sugar values (% FW) | CAL | 0–0.103 | 0–0.131 | 0–0.131 | 0–0.138 | 0–0.147 | 0–0.138 | 0–0.300 | 0–0.279 | 0–0.202 |
| VAL | 0–0.102 | 0–0.077 | 0–0.117 | 0–0.122 | 0–0.122 | 0–0.122 | 0–0.239 | 0–0.239 | 0–0.144 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camps, C.; Camps, Z.-N. Optimized Prediction of Reducing Sugars and Dry Matter of Potato Frying by FT-NIR Spectroscopy on Peeled Tubers. Molecules 2019, 24, 967. https://doi.org/10.3390/molecules24050967
Camps C, Camps Z-N. Optimized Prediction of Reducing Sugars and Dry Matter of Potato Frying by FT-NIR Spectroscopy on Peeled Tubers. Molecules. 2019; 24(5):967. https://doi.org/10.3390/molecules24050967
Chicago/Turabian StyleCamps, Cédric, and Zo-Norosoa Camps. 2019. "Optimized Prediction of Reducing Sugars and Dry Matter of Potato Frying by FT-NIR Spectroscopy on Peeled Tubers" Molecules 24, no. 5: 967. https://doi.org/10.3390/molecules24050967
APA StyleCamps, C., & Camps, Z.-N. (2019). Optimized Prediction of Reducing Sugars and Dry Matter of Potato Frying by FT-NIR Spectroscopy on Peeled Tubers. Molecules, 24(5), 967. https://doi.org/10.3390/molecules24050967