Potato Protein Fining of Phenolic Compounds in Red Wine: A Study of the Kinetics and the Impact of Wine Matrix Components and Physical Factors
Abstract
1. Introduction
2. Results and Discussion
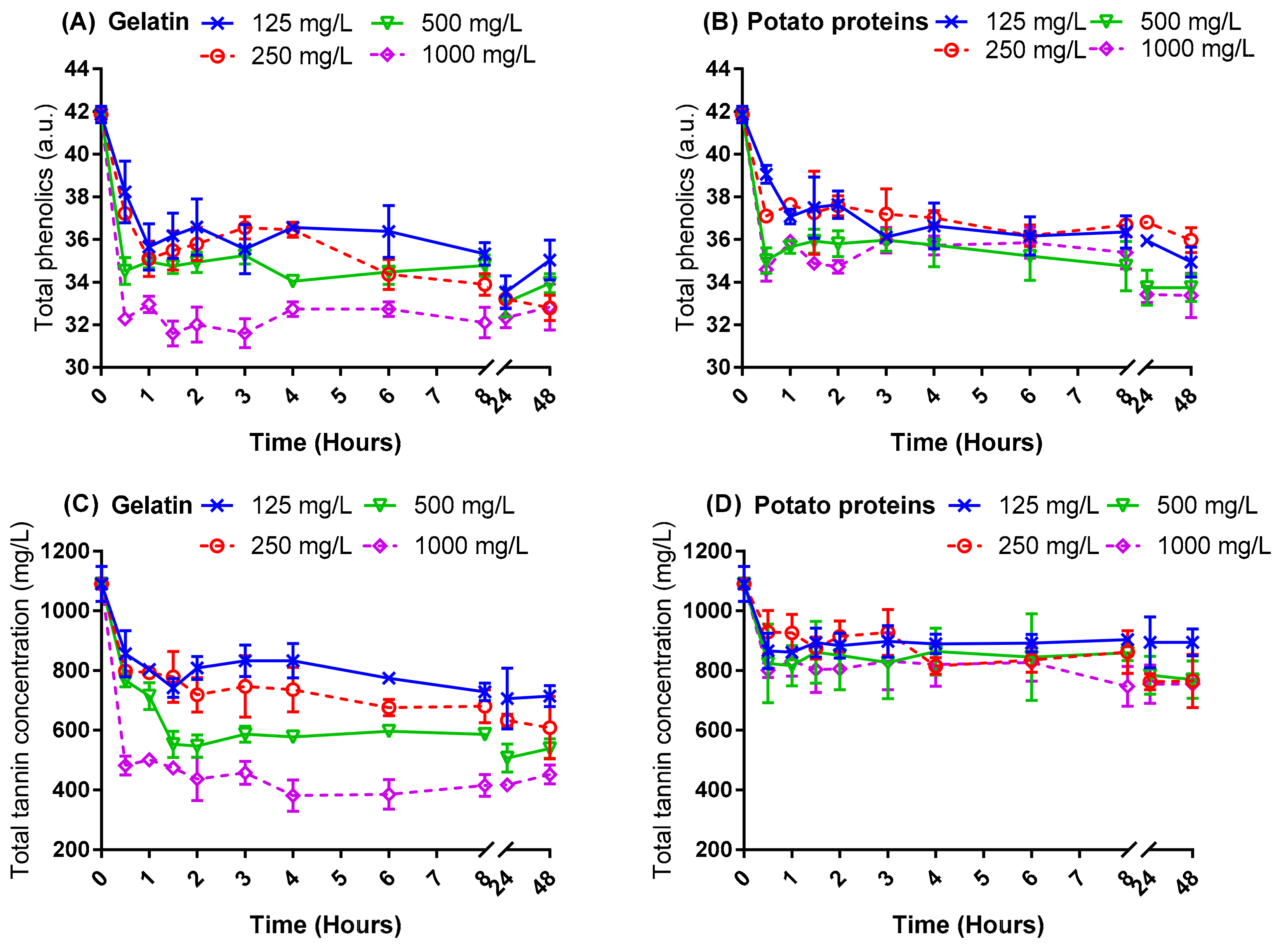
2.1. Fining Kinetics of Potato Proteins Compared with Gelatin
2.2. Relevance of the Wine Phenolic Profile in the Response to Fining Agent Addition
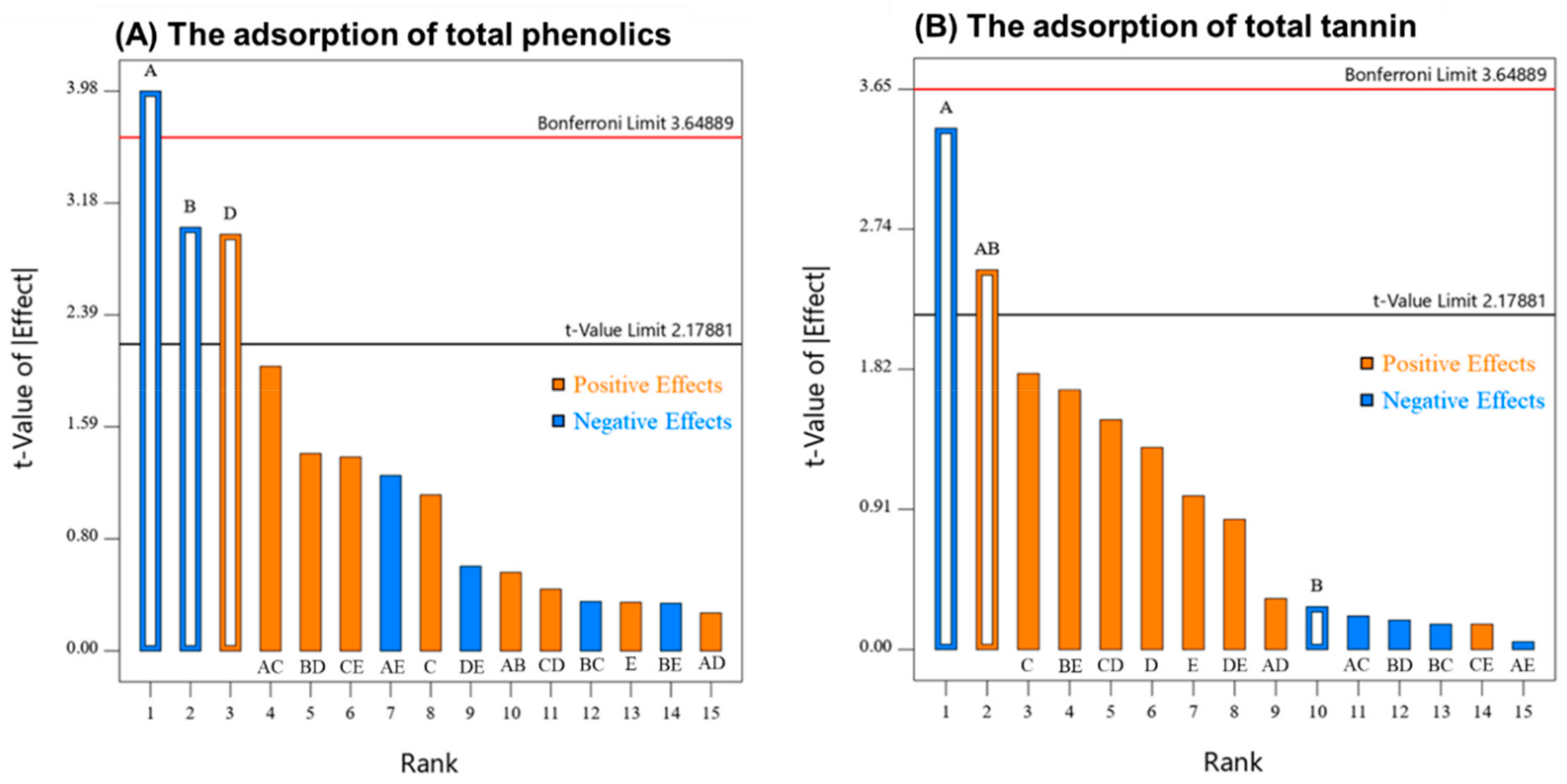
2.3. Experimental Design for Potato Proteins Fining
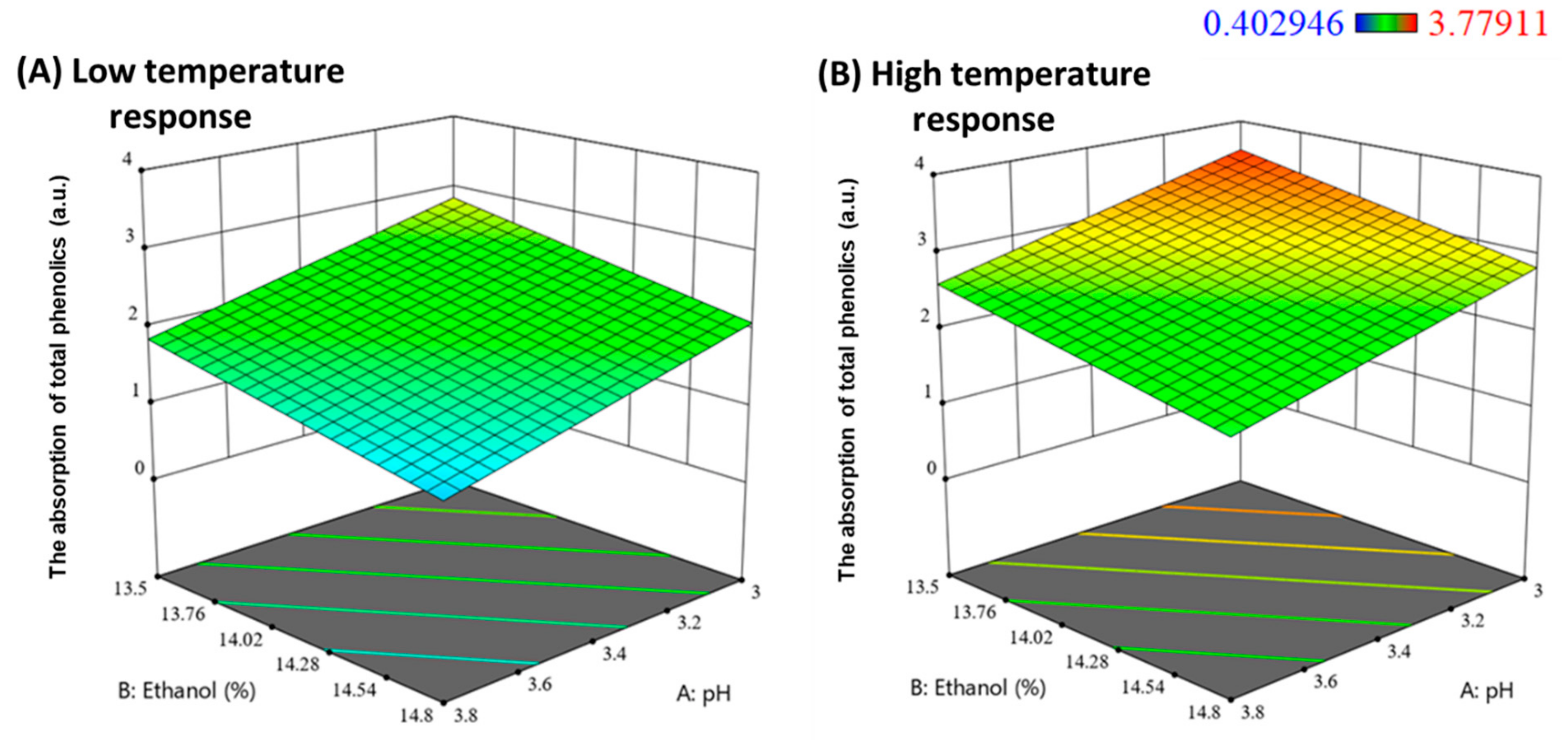
2.3.1. Modelling the Adsorption of Total Phenolics by Potato Protein Fining
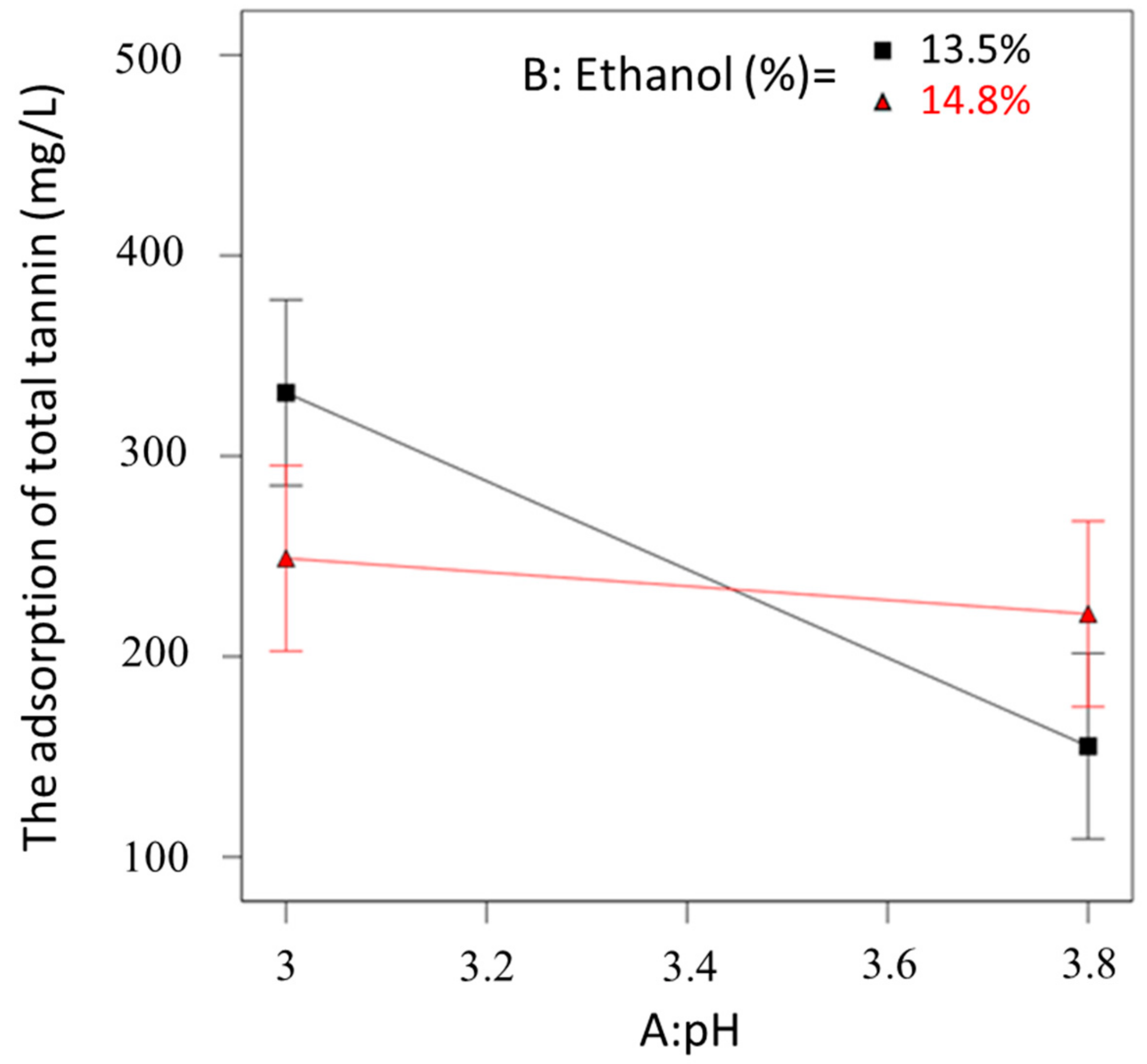
2.3.2. Modelling the Adsorption of Tannin by Potato Protein Fining
3. Materials and Methods
3.1. Wine Samples and Fining Agents
3.2. Chemicals
3.3. Fining Kinetics of Potato Proteins Compared with Gelatin
3.4. Phenolics Analyses
3.5. Experimental Design for Potato Proteins Fining
3.6. Data Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Peynaud, E.; Blouin, J. The Taste of Wine: The Art Science of Wine Appreciation; John Wiley & Sons: New Jersey, NJ, USA, 1996; pp. 1–188. [Google Scholar]
- Smith, P.A.; McRae, J.M.; Bindon, K.A. Impact of winemaking practices on the concentration and composition of tannins in red wine. Aust. J. Grape Wine Res. 2015, 21, 601–614. [Google Scholar] [CrossRef]
- Heredia, F.; Escudero-Gilete, M.; Hernanz, D.; Gordillo, B.; Meléndez-Martínez, A.; Vicario, I.; González-Miret, M. Influence of the refrigeration technique on the colour and phenolic composition of Syrah red wines obtained by pre-fermentative cold maceration. Food Chem. 2010, 118, 377–383. [Google Scholar] [CrossRef]
- Parenti, A.; Spugnoli, P.; Calamai, L.; Ferrari, S.; Gori, C. Effects of cold maceration on red wine quality from Tuscan Sangiovese grape. Eur. Food Res. Technol. 2004, 218, 360–366. [Google Scholar] [CrossRef]
- Bindon, K.; Kassara, S.; Curtin, C.; Li, S.; Hixson, J.; Teng, B.; Wilkinson, K.; Smith, P. In Cap on red wine macromolecules? Updates on how winemaking interventions influence tannin and polysaccharide composition in Shiraz wines. In Abstracts of papers of the American Chemical Society; American Chemical Society: Washington, DC, USA, 2017. [Google Scholar]
- Schmidtke, L.M.; Clark, A.C.; Scollary, G.R. Micro-oxygenation of red wine: Techniques, applications, and outcomes. Crit. Rev. Food Sci. Nutr. 2011, 51, 115–131. [Google Scholar] [CrossRef]
- Rankine, B. Making Good Wine; Macmillan Publishers: Sydney, Australia, 2007; pp. 137–144. [Google Scholar]
- Marangon, M.; Vincenzi, S.; Curioni, A. Wine Fining with Plant Proteins. Molecules 2019, 24, 2186. [Google Scholar] [CrossRef] [PubMed]
- Waglay, A.; Karboune, S.; Alli, I. Potato protein isolates: Recovery and characterization of their properties. Food Chem. 2014, 142, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Park, W.D.; Blackwood, C.; Mignery, G.A.; Hermodson, M.A.; Lister, R.M. Analysis of the heterogeneity of the 40,000 molecular weight tuber glycoprotein of potatoes by immunological methods and by NH2-terminal sequence analysis. Plant Physiol. 1983, 71, 156–160. [Google Scholar] [CrossRef]
- Løkra, S.; Helland, M.H.; Claussen, I.C.; Strætkvern, K.O.; Egelandsdal, B. Chemical characterization and functional properties of a potato protein concentrate prepared by large-scale expanded bed adsorption chromatography. LWT-Food Sci. Technol. 2008, 41, 1089–1099. [Google Scholar]
- Gambuti, A.; Rinaldi, A.; Moio, L. Use of patatin, a protein extracted from potato, as alternative to animal proteins in fining of red wine. Eur. Food Res. Technol. 2012, 235, 753–765. [Google Scholar] [CrossRef]
- Gambuti, A.; Rinaldi, A.; Romano, R.; Manzo, N.; Moio, L. Performance of a protein extracted from potatoes for fining of white musts. Food Chem. 2016, 190, 237–243. [Google Scholar] [CrossRef]
- Iturmendi, N.; Moine, V.; O’Kennedy, K. Potato, a new source of vegetal protein for allergen-free fining of juice and wine. Aust. N.Z. Grapegrow. Winemak. 2013, 598, 67–70. [Google Scholar]
- Tschiersch, C.; Nikfardjam, M.P.; Schmidt, O.; Schwack, W. Degree of hydrolysis of some vegetable proteins used as fining agents and its influence on polyphenol removal from red wine. Eur. Food Res. Technol. 2010, 231, 65–74. [Google Scholar] [CrossRef]
- Kang, W.; Niimi, J.; Bastian, S.E.P. Reduction of red wine astringency perception using vegetable protein fining agents. Am. J. Enol. Viticult. 2018, 69, 22–31. [Google Scholar] [CrossRef]
- Zoecklein, B.W.; Fugelsang, K.C.; Gump, B.H.; Nury, F.S. Production Wine Analysis; Van Nostrand Reinhold: New York, NY, USA, 1995; pp. 249–263. [Google Scholar]
- Montgomery, D.C. Design and Analysis of Experiments; John Wiley Sons: Hoboken, NJ, USA, 2001; Volume 52, pp. 28–286. [Google Scholar]
- Vila, D.H.; Mira, F.J.H.; Lucena, R.B.; Recamales, M.F. Optimization of an extraction method of aroma compounds in white wine using ultrasound. Talanta 1999, 50, 413–421. [Google Scholar] [CrossRef]
- Chassagne, D.; Guilloux-Benatier, M.; Alexandre, H.; Voilley, A. Sorption of wine volatile phenols by yeast lees. Food Chem. 2005, 91, 39–44. [Google Scholar] [CrossRef]
- Mané, C.; Souquet, J.; Olle, D.; Verries, C.; Veran, F.; Mazerolles, G.; Cheynier, V.; Fulcrand, H. Optimization of simultaneous flavanol, phenolic acid, and anthocyanin extraction from grapes using an experimental design: Application to the characterization of champagne grape varieties. J. Agr. Food Chem. 2007, 55, 7224–7233. [Google Scholar] [CrossRef]
- Muhlack, R.A.; O’Neill, B.K.; Waters, E.J.; Colby, C.B. Optimal conditions for controlling haze-forming wine protein with bentonite treatment: Investigation of matrix effects and interactions using a factorial design. Food Bioprocess Tech. 2016, 9, 936–943. [Google Scholar] [CrossRef]
- Setford, P.C.; Jeffery, D.W.; Grbin, P.R.; Muhlack, R.A. Mathematical modelling of anthocyanin mass transfer to predict extraction in simulated red wine fermentation scenarios. Food Res. Int. 2019, 121, 705–713. [Google Scholar] [CrossRef]
- Girden, E.R. ANOVA: Repeated Measures; Sage Publications: Thousand Oaks, CA, USA, 1992. [Google Scholar]
- Kennedy, J.A.; Taylor, A.W. Analysis of proanthocyanidins by high-performance gel permeation chromatography. J. Chromatogr. A 2003, 995, 99–107. [Google Scholar] [CrossRef]
- Guerrero, R.L.F.; Smith, P.; Bindon, K.A. Application of insoluble fibers in the fining of wine phenolics. J. Agr. Food Chem. 2013, 61, 4424–4432. [Google Scholar] [CrossRef] [PubMed]
- Hagerman, A.E.; Butler, L.G. Protein precipitation method for the quantitative determination of tannins. J. Agr. Food Chem. 1978, 26, 809–812. [Google Scholar] [CrossRef]
- Hagerman, A.E. Fifty years of polyphenol-protein complexes. In Recent Advances in Polyphenol Research; John Wiley & Sons Publisher: Hoboken, NJ, USA, 2012; pp. 71–97. [Google Scholar]
- Cala, O.; Pinaud, N.; Simon, C.; Fouquet, E.; Laguerre, M.; Dufourc, E.J.; Pianet, I. NMR and molecular modeling of wine tannins binding to saliva proteins: Revisiting astringency from molecular and colloidal prospects. FASEB J. 2010, 24, 4281–4290. [Google Scholar] [CrossRef] [PubMed]
- Murray, N.J.; Williamson, M.P.; Lilley, T.H.; Haslam, E. Study of the interaction between salivary proline-rich proteins and a polyphenol by 1H-NMR spectroscopy. Eur. J. Biochem. 1994, 219, 923–935. [Google Scholar] [CrossRef]
- McRae, J.M.; Ziora, Z.M.; Kassara, S.; Cooper, M.A.; Smith, P.A. Ethanol concentration influences the mechanisms of wine tannin interactions with poly (L-proline) in model wine. J. Agr. Food Chem. 2015, 63, 4345–4352. [Google Scholar] [CrossRef]
- Yokotsuka, K.; Singleton, V.L. Interactive precipitation between phenolic fractions and peptides in wine-like model solutions: Turbidity, particle size, and residual content as influenced by pH, temperature and peptide concentration. Am. J. Enol. Viticult. 1995, 46, 329–338. [Google Scholar]
- Petrie, P.R.; Teng, B.; Smith, P.A.; Bindon, K.A. Sugar reduction: Managing high Baume juice using dilution. Wine Vitic. J. 2019, 34, 36–37. [Google Scholar]
- Schelezki, O.J.; Šuklje, K.; Boss, P.K.; Jeffery, D.W. Comparison of consecutive harvests versus blending treatments to produce lower alcohol wines from Cabernet Sauvignon grapes: Impact on wine volatile composition and sensory properties. Food Chem. 2018, 259, 196–206. [Google Scholar] [CrossRef]
- Longo, R.; Blackman, J.W.; Torley, P.J.; Rogiers, S.Y.; Schmidtke, L.M. Changes in volatile composition and sensory attributes of wines during alcohol content reduction. J. Sci. Food Agric. 2017, 97, 8–16. [Google Scholar] [CrossRef]
- Maury, C.; Sarni-Manchado, P.; Poinsaut, P.; Cheynier, V.; Moutounet, M. Influence of polysaccharides and glycerol on proanthocyanidin precipitation by protein fining agents. Food Hydrocoll. 2016, 60, 598–605. [Google Scholar] [CrossRef]
- Berg, H.; Raul, T.; Akiyoshi, M. The effect of refrigeration, bentonite clarification and ion exchange on potassium behavior in wines. Am. J. Enol. Viticult. 1968, 19, 208–212. [Google Scholar]
- Simonato, B.; Mainente, F.; Selvatico, E.; Violoni, M.; Pasini, G. Assessment of the fining efficiency of zeins extracted from commercial corn gluten and sensory analysis of the treated wine. LWT-Food Sci. Technol. 2013, 54, 549–556. [Google Scholar] [CrossRef]
- Mercurio, M.D.; Dambergs, R.G.; Herderich, M.J.; Smith, P.A. High throughput analysis of red wine and grape phenolics adaptation and validation of methyl cellulose precipitable tannin assay and modified somers color assay to a rapid 96 well plate format. J. Agr. Food Chem. 2007, 55, 4651–4657. [Google Scholar] [CrossRef] [PubMed]
- Kassara, S.; Kennedy, J.A. Relationship between red wine grade and phenolics. 2. Tannin composition and size. J. Agr. Food Chem. 2011, 59, 8409–8412. [Google Scholar] [CrossRef]
- Kennedy, J.A.; Jones, G.P. Analysis of proanthocyanidin cleavage products following acid-catalysis in the presence of excess phloroglucinol. J. Agr. Food Chem. 2001, 49, 1740–1746. [Google Scholar] [CrossRef]
Sample Availability: Not available. |

| Concentration (mg/L) | ||||||
|---|---|---|---|---|---|---|
| 125 | 250 | 500 | 1000 | |||
| Wine 1 | Gelatin | Total phenolics | * | *** | *** | *** |
| Total tannin | ** | ** | *** | *** | ||
| Potato proteins | Total phenolics | *** | * | *** | *** | |
| Total tannin | ns a | ns | ns | * | ||
| Wine 2 | Gelatin | Total phenolics | ** | ** | *** | *** |
| Total tannin | ns | ns | * | *** | ||
| Potato proteins | Total phenolics | ns | ns | * | ** | |
| Total tannin | ns | ns | ns | * | ||
| mDP a | Epigallocatechin (%) a | Epicatechin Gallate (%) a | Mass Conversion (%) of Phloroglucinolysis a | MM (phloro) (g/mol) a | MM (GPC) (g/mol) b | |
|---|---|---|---|---|---|---|
| Wine 1 | 8.32 ± 0.06 | 38.7 ± 0.0 | 2.3 ± 0.0 | 45.5 ± 0.6 | 2495 ± 19 | 1628 ± 0 |
| Wine 2 | 8.76 ± 0.25 | 36.4 ± 0.0 | 2.8 ± 0.0 | 44.0 ± 0.9 | 2631 ± 77 | 1935 ± 3 |
| Wine 1 | Wine 2 | |
|---|---|---|
| Grape Source | Limestone Coast, Australia | McLaren Vale, Australia |
| Yeast strain | Maurivin AWRI 796 | Enartis Ferm red fruit |
| Malo-lactic fermentation Strain | CHR Hansen CH16 | LALLEMAND VP41 |
| Oak influence | No | No |
| pH a | 3.51 | 3.77 |
| Tartaric acidity (g/L) a | 6.67 | 5.90 |
| Malic acid (g/L) a | <0.40 | <0.40 |
| Volatile acidity (g/L) a | 0.44 | 0.76 |
| Alcohol (%) a | 13.73 | 15.60 |
| Residual sugar (g/L) a | 0.17 | 3.30 |
| Free sulfur dioxide (mg/L) b | 29 | 30 |
| Total sulfur dioxide (mg/L) b | 48 | 59 |
| Factor | Description | Low Level | High Level |
|---|---|---|---|
| A | pH a | 3.00 | 3.80 |
| B | Ethanol (%) b | 13.5 | 14.8 |
| C | Sugar (g/L) c | 0.16 | 8.00 |
| D | Temperature (°C) | 10 | 20 |
| E | Agitation | No | Yes |
| Treatments * | Treatments | ||
|---|---|---|---|
| 1 | AbcDE | 9 | abCDE |
| 2 | AbCDe | 10 | ABcDe |
| 3 | ABCDE | 11 | aBCDe |
| 4 | Abcde | 12 | aBcDE |
| 5 | aBCdE | 13 | AbCdE |
| 6 | aBcde | 14 | ABCde |
| 7 | abCde | 15 | abcdE |
| 8 | ABcdE | 16 | abcDe |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, W.; Muhlack, R.A.; Bindon, K.A.; Smith, P.A.; Niimi, J.; Bastian, S.E.P. Potato Protein Fining of Phenolic Compounds in Red Wine: A Study of the Kinetics and the Impact of Wine Matrix Components and Physical Factors. Molecules 2019, 24, 4578. https://doi.org/10.3390/molecules24244578
Kang W, Muhlack RA, Bindon KA, Smith PA, Niimi J, Bastian SEP. Potato Protein Fining of Phenolic Compounds in Red Wine: A Study of the Kinetics and the Impact of Wine Matrix Components and Physical Factors. Molecules. 2019; 24(24):4578. https://doi.org/10.3390/molecules24244578
Chicago/Turabian StyleKang, Wenyu, Richard A. Muhlack, Keren A. Bindon, Paul A. Smith, Jun Niimi, and Susan E.P. Bastian. 2019. "Potato Protein Fining of Phenolic Compounds in Red Wine: A Study of the Kinetics and the Impact of Wine Matrix Components and Physical Factors" Molecules 24, no. 24: 4578. https://doi.org/10.3390/molecules24244578
APA StyleKang, W., Muhlack, R. A., Bindon, K. A., Smith, P. A., Niimi, J., & Bastian, S. E. P. (2019). Potato Protein Fining of Phenolic Compounds in Red Wine: A Study of the Kinetics and the Impact of Wine Matrix Components and Physical Factors. Molecules, 24(24), 4578. https://doi.org/10.3390/molecules24244578








