Polyphenols, Antioxidant, Antibacterial, and Biofilm Inhibitory Activities of Peel and Pulp of Citrus medica L., Citrus bergamia, and Citrus medica cv. Salò Cultivated in Southern Italy
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Composition of the Extracts
2.1.1. Total Polyphenol Content
2.1.2. Antioxidant Activity
2.1.3. UPLC Profile
2.2. Biological Activity
2.2.1. Antibacterial Activity
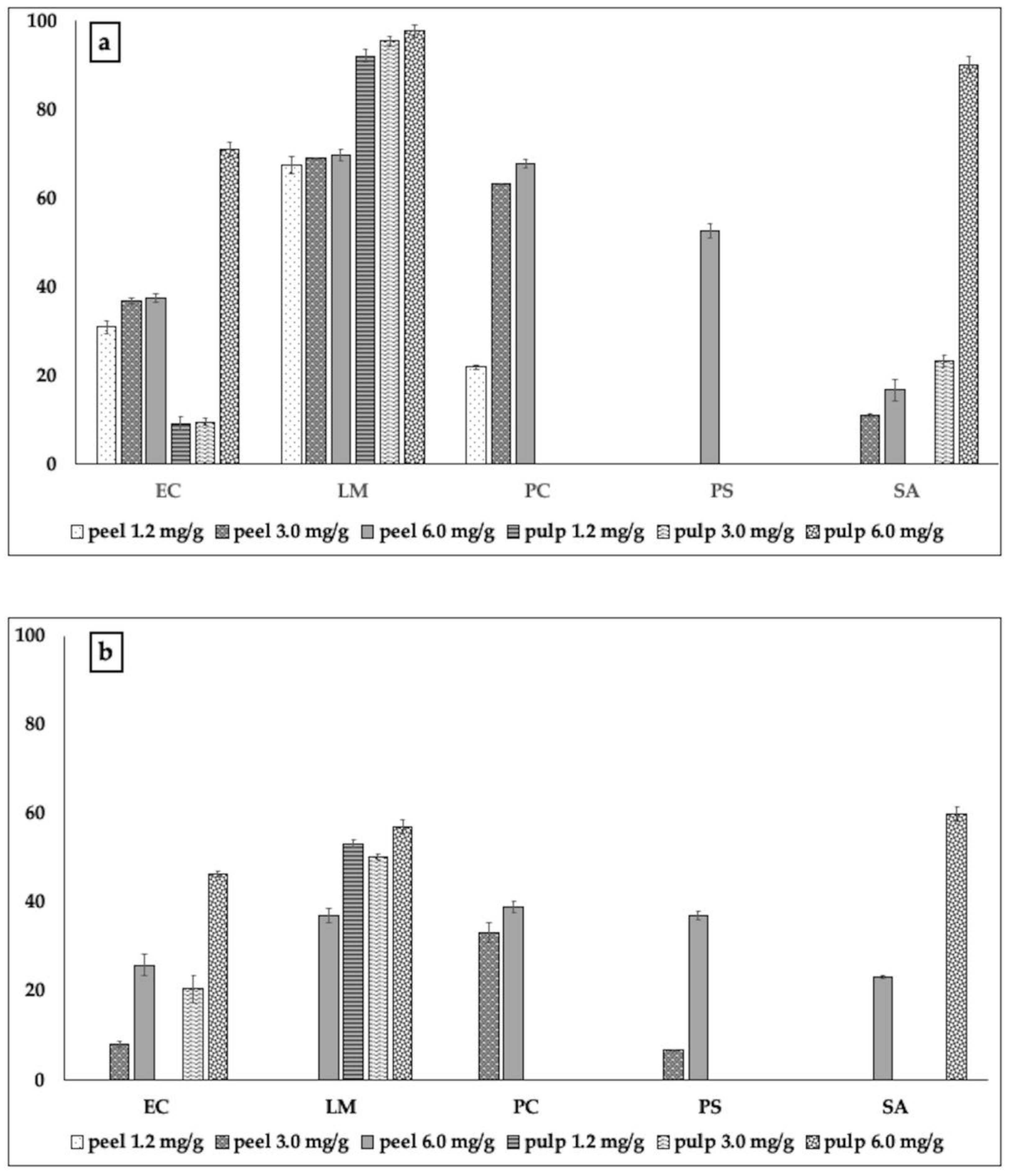
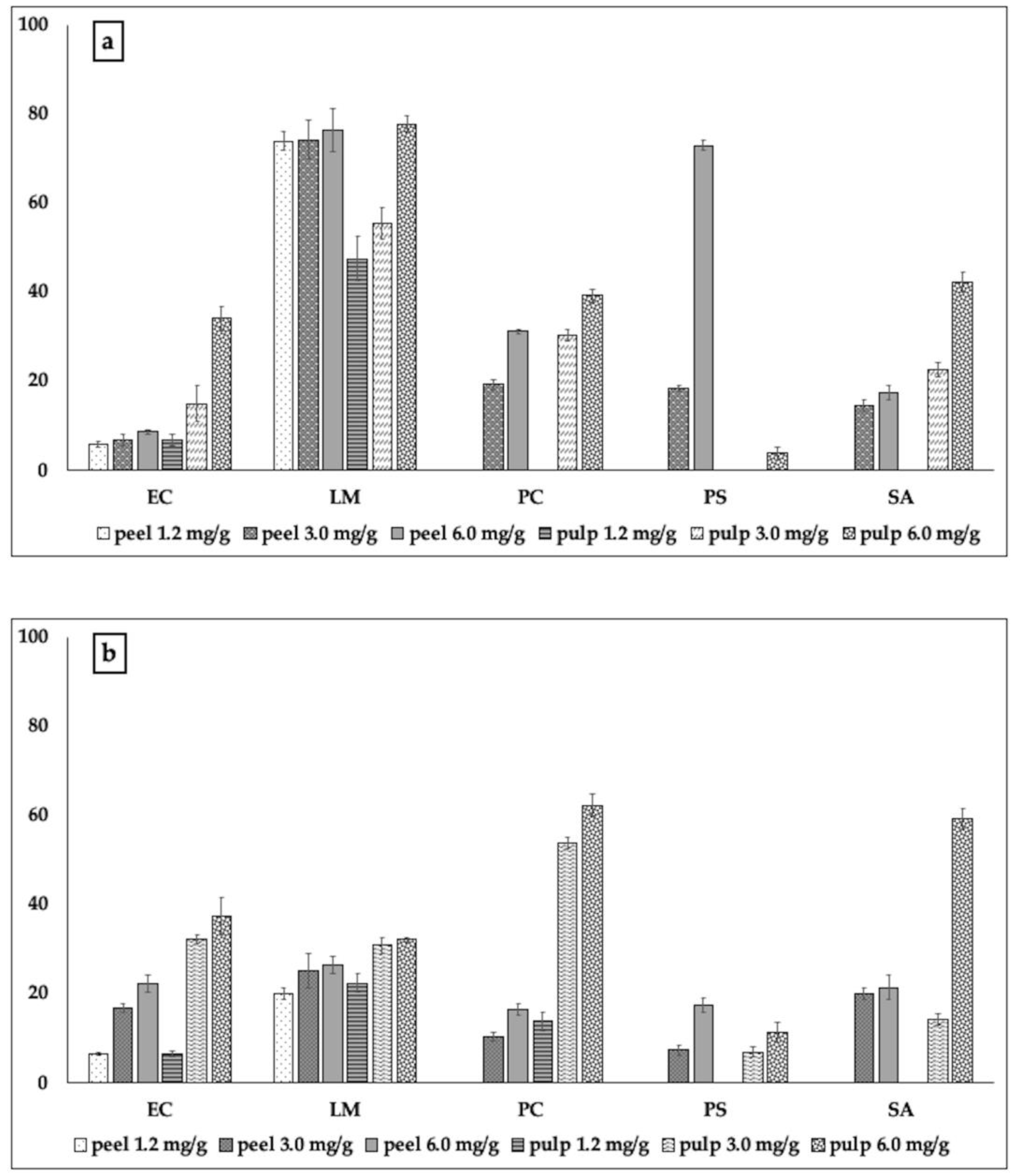
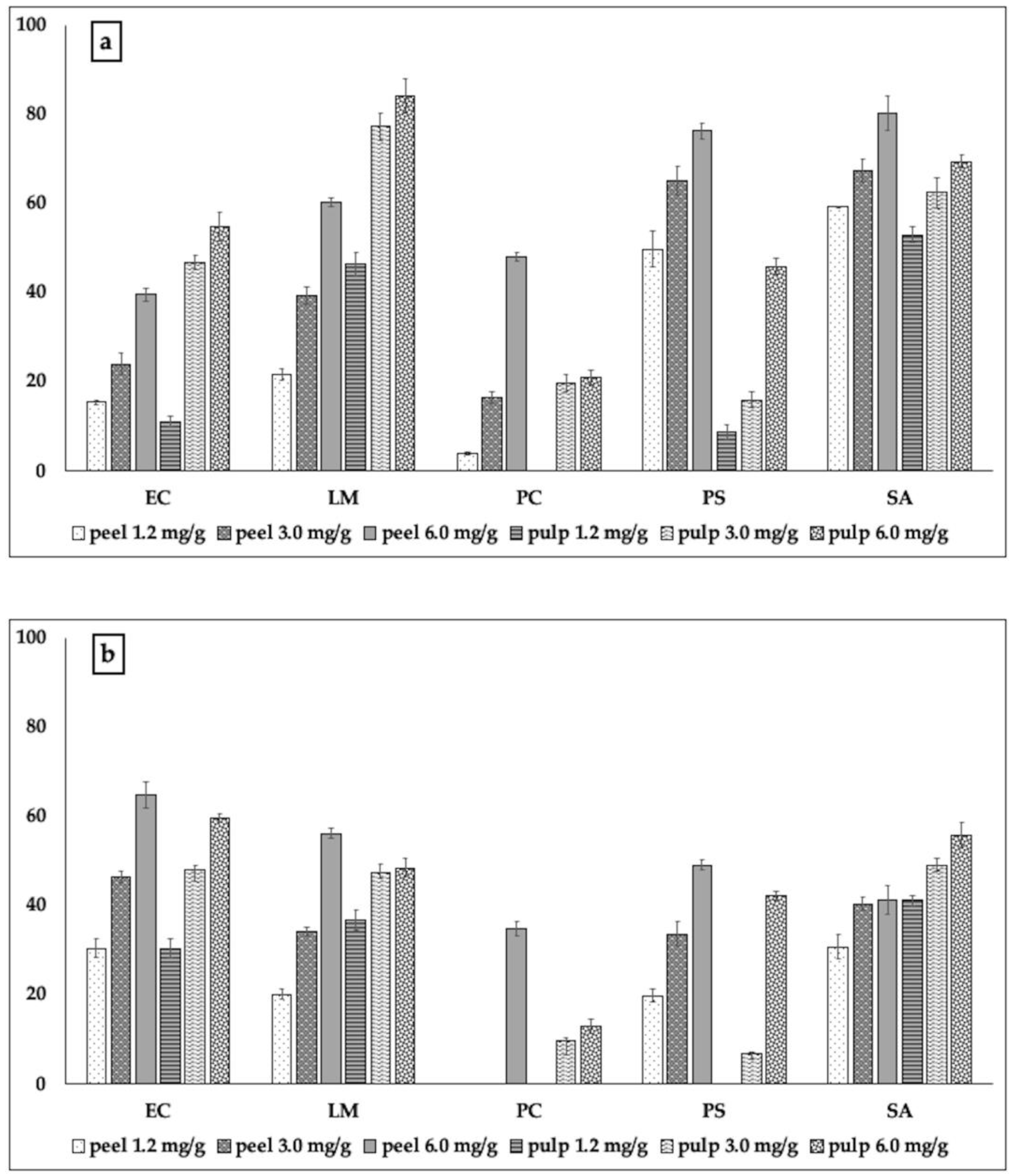
2.2.2. Biofilm Formation and Metabolic Activity of Biofilm Cells
3. Materials and Methods
3.1. Materials
Plant Material
3.2. Biochemical Characterization
3.2.1. Polyphenol Extraction
3.2.2. Total Polyphenol Content
3.2.3. Antioxidant Activity
3.2.4. Polyphenol profile
3.3. Antibacterial Activity
3.3.1. Microorganisms and Culture Conditions
3.3.2. Minimal Inhibitory Concentration (MIC)
3.3.3. Biofilm Inhibitory Activity
3.3.4. Metabolic Activity of Biofilm Cells
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nazzaro, F.; Fratianni, F.; Coppola, R. Quorum sensing and phytochemicals. Int. J. Mol. Sci. 2013, 14, 12607–12619. [Google Scholar] [CrossRef] [PubMed]
- Noumi, E.; Snoussi, M.; Merghni, A.; Nazzaro, F.; Quindós, G.; Akdamar, G.; Mastouri, M.; Al-Sieni, A.; Ceylan, O. Phytochemical composition, anti-biofilm and anti-quorum sensing potential of fruit, stem and leaves of Salvadora persica L. methanolic extracts. Microb. Pathog. 2017, 109, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Uzun, A.; Yesiloglu, T. Genetic diversity in Citrus. In Genetic Diversity in Plants; Caliskan, M., Ed.; InTech: Rijeka, Croatia, 2012; pp. 213–230. [Google Scholar]
- Gabriele, B.; Fazio, A.; Dugo, P.; Costa, R.; Mondello, L. Essential oil composition of Citrus medica var. liscia L. cv. Diamante (Diamante citron) determined after using different extraction methods. J. Sep. Sci. 2009, 32, 99–108. [Google Scholar]
- Savo, V.; Caneva, G.; Guarrera, P.M.; Reedy, D. Folk phytotherapy of the Amalfi Coast (Campania, Southern Italy). J. Ethnopharmacol. 2011, 135, 376–392. [Google Scholar] [CrossRef] [PubMed]
- Pernice, R.; Borriello, G.; Ferranca, R.; Borrelli, R.C.; Cennamo, F.; Ritieni, A. Bergamot: A source of natural antioxidants for functionalized fruit juices. Food Chem. 2009, 112, 545–550. [Google Scholar] [CrossRef]
- Li, W.Q.; Kuriyama, S.; Li, Q.; Nagai, M.; Hozawa, A.; Nishino, Y.; Tsuji, I. Citrus consumption and cancer incidence: The Ohsaki cohort study. Int. J. Cancer. 2010, 127, 1913–1922. [Google Scholar] [CrossRef]
- Xu, J.J.; Wu, X.; Li, M.M.; Li, G.Q.; Yang, Y.T.; Luo, H.J.; Huang, W.H.; Chung, H.Y.; Ye, W.C.; Wang, G.C.; et al. Antiviral Activity of Polymethoxylated Flavones from “Guangchenpi”, the Edible and Medicinal Pericarps of Citrus reticulata ‘Chachi’. J. Agric. Food Chem. 2014, 62, 2182–2189. [Google Scholar] [CrossRef]
- Kim, H.K.; Jeon, W.K.; Ko, B.S. Flavanone Glycosides from Citrus junos and their Anti-Influenza Virus Activity. Planta Med. 2001, 67, 548–549. [Google Scholar] [CrossRef]
- Tripoli, E.; La Guardia, M.; Giammanco, S.; Di Majo, D.; Giammanco, M. Citrus flavonoids: Molecular structure, biological activity and nutritional properties: A review. Food Chem. 2007, 104, 466–479. [Google Scholar]
- Ting, S.V.; Roussef, R.L. Citrus Fruits and Their Products: Analysis and Technology; Marcel Dekker: New York, NY, USA, 1986. [Google Scholar]
- Mandalari, G.; Bennett, R.N.; Bisignano, G.; Saija, A.; Dugo, G.; Lo Curto, R.B.; Faulds, C.B.; Waldron, K.W. Characterization of flavonoids and pectins from bergamot (Citrus bergamia Risso) peel, a major by-product of essential oil extraction. J. Agric. Food Chem. 2006, 54, 197–203. [Google Scholar] [CrossRef]
- Benavente-García, O.; Castillo, J. Update on uses and properties of Citrus flavonoids: New findings in anticancer, cardiovascular, and anti-inflammatory activity. J. Agric. Food Chem. 2008, 56, 6185–6205. [Google Scholar] [CrossRef] [PubMed]
- Fisher, K.; Phillips, C.A. The effect of lemon, orange and bergamot essential oils and their components on the survival of Campylobacter jejuni, Escherichia coli O157, Listeria monocytogenes, Bacillus cereus and Staphylococcus aureus in vitro and in food systems. J. Appl. Microbiol. 2006, 101, 1232–1240. [Google Scholar] [CrossRef] [PubMed]
- Aliberti, L.; Caputo, L.; De Feo, V.; De Martino, L.; Nazzaro, F.; Souza, L. Chemical Composition and in Vitro Antimicrobial, Cytotoxic, and Central Nervous System Activities of the Essential Oils of Citrus medica L. cv. ‘Liscia’ and C. medica cv. ‘Rugosa’ Cultivated in Southern Italy. Molecules 2016, 21, 1244. [Google Scholar] [CrossRef] [PubMed]
- Mandalari, G.; Bennett, R.N.; Bisignano, G.; Trombetta, D.; Saija, A.; Faulds, C.B.; Gasson, M.J.; Narbad, A. Antimicrobial activity of flavonoids extracted from bergamot (Citrus bergamia Risso) peel, a byproduct of the essential oil industry. J. Appl. Microbiol. 2007, 103, 2056–2064. [Google Scholar] [CrossRef]
- Filocamo, A.; Bisignano, C.; Ferlazzo, N.; Cirmi, S.; Mandalari, G.; Navarra, M. In vitro effect of bergamot (Citrus bergamia) juice against cagA-positive and-negative clinical isolates of Helicobacter pylori. BMC Complement. Altern. Med. 2015, 15, 256. [Google Scholar] [CrossRef]
- Ghasemi, K.; Ghasemi, Y.; Ebrahimzadeh, M.A. Antioxidant activity, phenol and flavonoid contents of 13 Citrus species peels and tissues. Pak. J. Pharmacol. Sci. 2009, 22, 277–281. [Google Scholar]
- Menichini, F.; Loizzo, M.R.; Bonesi, M.; Conforti, F.; De Luca, D.; Statti, G.A.; de Cindio, B.; Menichini, F.; Tundis, R. Phytochemical profile, antioxidant, anti-inflammatory and hypoglycemicpotential of hydroalcoholic extracts from Citrus medica L. cv. Diamante flowers, leaves and fruits at two maturity stages. Food Chem. Toxicol. 2011, 49, 1549–1555. [Google Scholar] [CrossRef]
- Gabriele, M.; Frassinetti, S.; Caltavuturo, L.; Montero, L.; Dinelli, G.; Longo, V.; Di Gioia, D.; Pucci, L. Citrus bergamia powder: Antioxidant, antimicrobial and anti-inflammatory properties. J. Funct. Foods 2017, 31, 255–265. [Google Scholar] [CrossRef]
- Tundis, R.; Loizzo, M.R.; Bonesi, M.; Menichini, F.; Mastellone, V.; Colica, C.; Menichini, F. Comparative study on the antioxidant capacity and cholinesterase inhibitory activity of Citrus aurantifolia Swingle, C. aurantium L., and C. bergamia Risso and Poit. Peel Essential Oils. J. Food Sci. 2012, 77, H40–H46. [Google Scholar] [CrossRef]
- Munwar, S.; Roy, H.; Rahaman, S.A. Antioxidant and free radical scavenging activity of Citrus medica. Int. J. Pharma Res. Health Sci. 2015, 3, 810–816. [Google Scholar]
- Umar, S.; Mishra, N.K.; Pal, K.; Sajad, M.; Neha; Ansari, M.M.; Ahmad, S.; Katiyar, C.K.; Khan, H.A. Protective effect of rutin in attenuation of collagen-induced arthritis in Wistar rat by inhibiting inflammation and oxidative stress. Indian J. Rheumatol. 2012, 7, 191–198. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Machado, D.G.; Bettio, L.E.B.; Cunha, M.P.; Santos, A.R.S.; Pizzolatti, M.G.; Brighente, I.M.C.; Rodrigues, A.L.S. Antidepressant-like effect of rutin isolated from the ethanolic extract from Schinus molle L. in mice: Evidence for the involvement of the serotonergic and noradrenergic systems. Eur. J. Pharmacol. 2008, 587, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Araújo, J.R.; Gonçalves, P.; Martel, F. Chemopreventive effect of dietary polyphenols in colorectal cancer cell lines. Nutr. Res. 2011, 31, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhang, X.; Wang, H.; Lin, B.; Wang, S.; Hu, G. Determination of rutin in rat plasma by ultra performance liquid chromatography tandem mass spectrometry and application to pharmacokinetic study. J. Chromatogr. Sci. 2015, 53, 519–525. [Google Scholar] [CrossRef]
- Morling, J.R.; Yeoh, S.E.; Kolbach, D.N. Rutosides for treatment of post-thrombotic syndrome. Cochrane Database Syst. Rev. 2015, 9, CD005625. [Google Scholar] [CrossRef]
- Faggian, M.; Sut, S.; Perissutti, B.; Baldan, V.; Grabnar, I.; Dall’Acqua, S. Natural deep eutectic solvents (NADES) as a tool for bioavailability improvement: Pharmacokinetics of rutin dissolved in proline/glycine after oral administration in rats: Possible application in nutraceuticals. Molecules 2016, 21, 1531. [Google Scholar] [CrossRef]
- Karoui, I.J.; Marzouk, B. Characterization of bioactive compounds in tunisian bitter orange (Citrus aurantium L.) peel and juice and determination of their antioxidant activities. BioMed Res. Int. 2013, 2013, 1–12. [Google Scholar] [CrossRef]
- Robbins, R.J. Phenolic acids in foods: An overview of analytical methodology. J Agric Food Chem. 2003, 51, 2866–2887. [Google Scholar] [CrossRef]
- Available online: https://www.kegg.jp/kegg-bin/search_pathway_text?map=map&keyword=epicatechin&mode=1&viewImage=true (accessed on 9 December 2019).
- Available online: https://www.kegg.jp/kegg-bin/search_pathway_text?map=map&keyword=ferulic&mode=1&viewImage=true (accessed on 9 December 2019).
- Hervert-Hernández, D.; Pintado, C.; Rotger, R.; Goñi, I. Stimulatory role of grape pomace polyphenols on Lactobacillus acidophilus growth. Int. J. Food Microbiol. 2009, 136, 119–122. [Google Scholar] [CrossRef]
- Dueñas, M.; Cueva, C.; Muñoz-González, I.; Jiménez-Girón, A.; Sánchez-Patán, F.; Santos-Buelga, C.; Moreno-Arribas, M.V.; Bartolomé, B. Studies on modulation of gut microbiota by wine polyphenols: From isolated cultures to omic approaches. Antioxidants 2015, 4, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Nazzaro, F.; Fratianni, F.; d’Acierno, A.; Coppola, R. Gut microbiota and polyphenols: A strict connection enhancing human health. In Advances in Food Biotechnology; Ravishankar Rai, V., Ed.; Wiley-Blackwell: Chichester, UK, 2015; pp. 335–350. [Google Scholar]
- Castillo, S.; Heredia, N.; Arechiga-Carvajal, E.; García, S. Citrus Extracts as Inhibitors of Quorum Sensing, Biofilm Formation and Motility of Campylobacter jejuni. Food Biotechnol. 2014, 28, 106–122. [Google Scholar] [CrossRef]
- Vikram, A.; Jayaprakasha, G.K.; Jesudhasan, P.T.; Pillai, S.D.; Patil, B.S. Suppression of bacterial cell-cell signalling, biofilm formation and type III secretion system by citrus flavonoids. J. Appl. Microbiol. 2010, 109, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadehh, D.; Fang Fang, X.; Modarresi-Ghazanij, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biofilm prevention and control by dietary phytochemicals. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.; Abreu, A.C.; Malheiro, J.; Saavedra, M.J.; Simões, M. Biofilm prevention and control by dietary phytochemical. In Microbial Pathogens and Strategies for Combating Them: Science, Technology and Education; Méndez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2013; pp. 33–42. [Google Scholar]
- Deepika, M.S.; Thangam, R.; Sakthidhasan, P.; Arun, S.; Sivasubramanian, S.; Thirumurugan, S. Combined effect of a natural flavonoid rutin from Citrus sinensis and conventional antibiotic gentamicin on Pseudomonas aeruginosa biofilm formation. Food Control 2018, 90, 282–294. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Viticult. 1965, 16, 144–158. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Fratianni, F.; Nazzaro, F.; Marandino, A.; Fusco, M.R.; Coppola, R.; De Feo, V.; De Martino, L. Biochemical composition, antimicrobial activities, and anti–quorum-sensing activities of ethanol and ethyl acetate extracts from Hypericum connatum Lam. (Guttiferae). J. Med. Food 2013, 16, 454–459. [Google Scholar] [CrossRef]
- Sarker, S.D.; Nahar, L.; Kumarasamy, Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 2007, 42, 321–324. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Cozzolino, R.; Martignetti, A.; Malorni, L.; De Feo, V.; Cruz, A.G.; d’Acierno, A. Antibacterial Activity of Three Extra Virgin Olive Oils of the Campania Region, Southern Italy, Related to Their Polyphenol Content and Composition. Microorganisms 2019, 7, 321. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, G.A.; Kolter, R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol. Microbiol. 1998, 30, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Kairo, S.K.; Bedwell, J.; Tyler, P.C.; Carter, A.; Corbel, M.J. Development of a tetrazolium salt assay for rapid determination of viability of BCG vaccines. Vaccine 1999, 17, 2423–2438. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
| Total Polyphenols (µg GAE/g of Fresh Product ± SD) | Antioxidant Activity (DPPH Test, EC50) | |||||
|---|---|---|---|---|---|---|
| Bergamot | Cedar | Cedar of Salò | Bergamot | Cedar | Cedar of Salò | |
| peel | 748.08 ± 43.67 | 291.97 ± 20.81 | 1002.3 ± 54.41 | 6.47 ± 0.48 | 19.40 ± 1.15 | 3.96 ± 0.09 |
| pulp | 208.02 ± 14.55 | 148.98 ± 14.51 | 242.73 ± 15.17 | 10.99 ± 0.58 | 24.22 ± 1.62 | 10.46 ± 1.03 |
| Bergamot | Cedar | Cedar of Salò | ||||
|---|---|---|---|---|---|---|
| Peel | Pulp | Peel | Pulp | Peel | Pulp | |
| Acidic phenols | ||||||
| Gallic acid | 24.68 ± 1.03 | 54.186 ± 2.34 | 39.02 ± 2.10 | 22.16 ± 1.55 | 33.49 ± 1.37 | 16.84 ± 1.67 |
| Chlorogenic acid | 71.98 ± 1.67 | 28.91 ± 0.57 | nd | nd | 116.39 ± 6.32 | 45.3 ± 2.14 |
| Caffeic acid | nd | nd | nd | nd | 7.11 ± 0.57 | 6.97 ± 0.57 |
| Coumaric acid | nd | nd | nd | nd | 21.05 ± 0.57 | 11.61 ± 0.57 |
| Ferulic acid | 181.58 ± 6.32 | 48.30 ± 1.67 | nd | 113.91 ± 1.67 | 295.97 ± 10.2 | 106.36 ± 6.32 |
| Flavonoids | ||||||
| Rutin | nd | nd | 115.47 ± 5.42 | 19.39 ± 1.67 | nd | nd |
| Epicatechin | 73.40 ± 1.67 | 26.87 ± 1.67 | nd | 9.85 ± 0.57 | 105.1 ± 1.67 | 44.4 ± 1.67 |
| Quercetin | 97.32 ± 10.2 | nd | nd | nd | 150.89 ± 6.32 | nd |
| Apigenin | nd | nd | nd | nd | 24.26 ± 1.67 | nd |
| Catechin | 20.56 ± 2.14 | 7.03 ± 0.57 | 4.34 ± 0.57 | nd | 68.78 ± 1.37 | 11.84 ± 1.37 |
| Total phenolic acids | 278.24 | 131.40 | 39.02 | 136.07 | 474.01 | 187.08 |
| Total flavonoids | 191.28 | 33.9 | 119.81 | 29.24 | 349.03 | 56.24 |
| Total phenolic compounds | 469.52 | 165.3 | 158.83 | 169.31 | 823.04 | 243.32 |
| MIC (mg/mL) | Bergamot | Cedar | Cedar of Salò | |||
|---|---|---|---|---|---|---|
| Peel | Pulp | Peel | Pulp | Peel | Pulp | |
| E. coli | 9.00 | 8.00 | 10.00 | 9.00 | 9.00 | 8.00 |
| L. monocytogenes | 8.00 | 7.00 | 7.00 | 8.00 | 8.00 | 7.00 |
| P. carotovorum | 8.00 | >10.00 | 9.00 | 9.00 | 9.00 | 10.00 |
| Ps. aeruginosa | 8.00 | >10.00 | 7.00 | 10.00 | 8.00 | 9.00 |
| Staph. aureus | 10.00 | 7.00 | 10.00 | 9.00 | 7.00 | 8.00 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fratianni, F.; Cozzolino, A.; De Feo, V.; Coppola, R.; Ombra, M.N.; Nazzaro, F. Polyphenols, Antioxidant, Antibacterial, and Biofilm Inhibitory Activities of Peel and Pulp of Citrus medica L., Citrus bergamia, and Citrus medica cv. Salò Cultivated in Southern Italy. Molecules 2019, 24, 4577. https://doi.org/10.3390/molecules24244577
Fratianni F, Cozzolino A, De Feo V, Coppola R, Ombra MN, Nazzaro F. Polyphenols, Antioxidant, Antibacterial, and Biofilm Inhibitory Activities of Peel and Pulp of Citrus medica L., Citrus bergamia, and Citrus medica cv. Salò Cultivated in Southern Italy. Molecules. 2019; 24(24):4577. https://doi.org/10.3390/molecules24244577
Chicago/Turabian StyleFratianni, Florinda, Autilia Cozzolino, Vincenzo De Feo, Raffaele Coppola, Maria Neve Ombra, and Filomena Nazzaro. 2019. "Polyphenols, Antioxidant, Antibacterial, and Biofilm Inhibitory Activities of Peel and Pulp of Citrus medica L., Citrus bergamia, and Citrus medica cv. Salò Cultivated in Southern Italy" Molecules 24, no. 24: 4577. https://doi.org/10.3390/molecules24244577
APA StyleFratianni, F., Cozzolino, A., De Feo, V., Coppola, R., Ombra, M. N., & Nazzaro, F. (2019). Polyphenols, Antioxidant, Antibacterial, and Biofilm Inhibitory Activities of Peel and Pulp of Citrus medica L., Citrus bergamia, and Citrus medica cv. Salò Cultivated in Southern Italy. Molecules, 24(24), 4577. https://doi.org/10.3390/molecules24244577









