A Reversible Colorimetric and Fluorescence “Turn-Off” Chemosensor for Detection of Cu2+ and Its Application in Living Cell Imaging
Abstract
:1. Introduction
2. Results
2.1. Optimum Conditions for Sensing Process
2.1.1. Effect of Solution
2.1.2. Effect of pH
2.2. UV-Vis Measurement and Visual Detection
2.3. Fluorescence Measurements
2.4. Selectivity of Detection
2.5. Reversibility
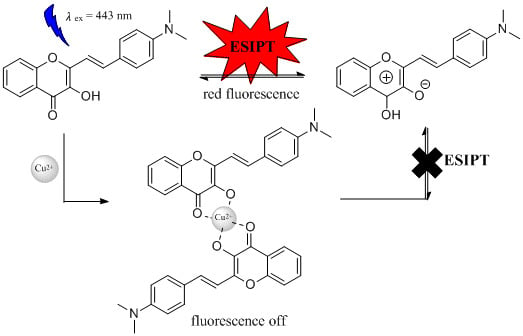
2.6. Interaction Mechanism of 4 with Cu2+
2.7. Bioimaging Experiments
3. Materials and Methods
3.1. Synthesis of (E)-2-(4-(dimethylamino)styryl)-3-hydroxy-4H-chromen-4-one (4)
3.2. Spectral Measurements
3.3. Cell Culture
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- More, P.A.; Shankarling, G.S. Reversible ‘turn off’ fluorescence response of Cu2+ ions towards 2-pyridyl quinoline based chemosensor with visible colour change. Sens. Actuators B Chem. 2017, 241, 552–559. [Google Scholar] [CrossRef]
- Mahapatra, A.K.; Hazra, G.; Das, N.K.; Goswami, S. A highly selective triphenylamine-based indolylmethane derivatives as colorimetric and turn-off fluorimetric sensor toward Cu2+ detection by deprotonation of secondary amines. Sens. Actuators B Chem. 2011, 156, 456–462. [Google Scholar] [CrossRef]
- Shahid, M.; Chawla, H.M.; Bhatia, P. A calix [4]arene based turn off/turn on molecular receptor for Cu2+ and CN− ions in aqueous medium. Sens. Actuators B Chem. 2016, 237, 470–478. [Google Scholar] [CrossRef]
- Zhao-Qi, G.; Wei-Qiang, C.; Xuan-Ming, D. Highly selective visual detection of Cu(II) utilizing intramolecular hydrogen bond-stabilized merocyanine in aqueous buffer solution. Org. Lett. 2010, 12, 2202. [Google Scholar]
- Qian, B.V.; Váradi , L.; Trinchi, A.; Reichman, S.M.; Bao, L.; Lan, M.; Wei, G.; Cole, I.S. The Design and Synthesis of Fluorescent Coumarin Derivatives and Their Study for Cu2+ Sensing with an Application for Aqueous Soil Extracts. Molecules 2019, 24, 3569. [Google Scholar] [CrossRef]
- Zhanxian, L.; Wanying, Z.; Xiaoya, L.; Yanyan, Z.; Chunmei, L.; Lina, W.; Mingming, Y.; Liuhe, W.; Mingsheng, T.; Hongyan, Z. 1,8-naphthyridine-derived Ni2+/Cu2+-selective fluorescent chemosensor with different charge transfer processses. Inorg. Chem. 2012, 51, 12444–12449. [Google Scholar]
- Porento, M.; Sutinen, V.; Julku, T.; Oikari, R. Detection of Copper in Water Using On-Line Plasma-Excited Atomic Absorption Spectroscopy (AAS). Appl. Spectrosc. 2011, 65, 678–683. [Google Scholar] [CrossRef]
- Adeleye, A.S.; Oranu, E.A.; Tao, M.; Keller, A.A. Release and detection of nanosized copper from a commercial antifouling paint. Water Res. 2016, 102, 374–382. [Google Scholar] [CrossRef]
- Lin, Q.; Chen, P.; Liu, J.; Fu, Y.P.; Zhang, Y.M.; Wei, T.B. Colorimetric chemosensor and test kit for detection copper(II) cations in aqueous solution with specific selectivity and high sensitivity. Dye. Pigment. 2013, 98, 100–105. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, L.; Wei, Y.; Chao, J.; Shuang, S.; Cai, Z.; Dong, C. A selectively rhodamine-based colorimetric probe for detecting copper(II) ion. Spectrochim. Acta. Part A Mol. Biomol. Spectrosc. 2014, 132, 191–197. [Google Scholar] [CrossRef]
- Cheng, D.; Liu, X.; Yang, H.; Zhang, T.; Han, A.; Zang, L. A Cu2+-Selective Probe Based on Phenanthro-Imidazole Derivative. Sensors 2017, 17, 35. [Google Scholar] [CrossRef] [PubMed]
- An, R.; Zhang, D.; Yan, C.; Cui, Y.Z. A novel “turn-on” fluorescent and colorimetric sensor for selective detection of Cu2+ in aqueous media and living cells. Sens. Actuators B Chem. 2015, 222, 48–54. [Google Scholar] [CrossRef]
- Li, W.Z.; Zhu, G.; Li, J.; Wang, Z.; Jin, Y. An Amidochlorin-Based Colorimetric Fluorescent Probe for Selective Cu2+ Detection. Molecules 2016, 21, 107. [Google Scholar] [CrossRef]
- Wei, C.; Wang, L.; Gang, X.; Zhou, L.; An, X.; Cao, D. A colorimetric and fluorescence “turn-off” chemosensor for the detection of silver ion based on a conjugated polymer containing 2,3-di(pyridin-2-yl)quinoxaline. Sens. Actuators B Chem. 2015, 207, 281–290. [Google Scholar]
- Lan, M.; Wu, J.; Liu, W.; Zhang, H.; Zhang, W.; Zhuang, X.; Wang, P. Highly sensitive fluorescent probe for thiols based on combination of PET and ESIPT mechanisms. Sens. Actuators B Chem. 2011, 156, 332–337. [Google Scholar] [CrossRef]
- Chen, S.; Hou, P.; Zhou, B.; Song, X.; Foley, J.W. A red fluorescent probe for thiols based on 3-hydroxyflavone and its application in living cell imaging. RSC Adv. 2013, 3, 11543–11546. [Google Scholar] [CrossRef]
- Song, C.; Peng, H.; Song, X. A red-emitting fluorescent probe for imaging hydrogen sulphide with a large Stokes shift. Sens. Actuators B Chem. 2015, 221, 951–955. [Google Scholar]
- Liu, Y.; Yu, D.; Ding, S.; Xiao, Q.; Guo, J.; Feng, G. Rapid and ratiometric fluorescent detection of cysteine with high selectivity and sensitivity by a simple and readily available probe. ACS Appl. Mater. Interfaces 2014, 6, 17543–17550. [Google Scholar] [CrossRef]
- Jin, X.; Liu, C.; Wang, X.; Hai, H.; Zhang, X.; Zhu, H. A flavone-based ESIPT fluorescent sensor for detection of N2H4 in aqueous solution and gas state and its imaging in living cells. Sens. Actuators B Chem. 2015, 216, 141–149. [Google Scholar] [CrossRef]
- Borase, P.N.; Thale, P.B.; Shankarling, G.S. Dihydroquinazolinone based “turn-off” fluorescence sensor for detection of Cu2+ ions. Dye. Pigment. 2016, 134, 276–284. [Google Scholar] [CrossRef]
- McNaught, A.D.; IUPAC. Compendium of Chemical Terminology. In The Gold Book, 2nd ed.; IUPAC: Oxford, UK, 1997. [Google Scholar]
- Long, G.L.; Winefordner, J.D. Limit of detection. A closer look at the IUPAC definition. Anal. Chem. 1983, 55, 712–724. [Google Scholar]
- Xue, W.; Li, L.; Li, Q.; Wu, A. Novel furo[2,3-d] pyrimidine derivative as fluorescent chemosensor for HSO4−. Talanta 2012, 88, 734–738. [Google Scholar] [CrossRef] [PubMed]
- Mergu, N.; Gupta, V.K. A novel colorimetric detection probe for copper (II) ions based on a Schiff base. Sens. Actuators B Chem. 2015, 210, 408–417. [Google Scholar] [CrossRef]
- Yu, D.; Zhang, Q.; Ding, S.; Feng, G. A colorimetric and near-infrared fluorescent probe for biothiols and its application in living cells. RSC Adv. 2014, 4, 46561–46567. [Google Scholar] [CrossRef]
- Hyo Sung, J.; Pil Seung, K.; Jeong Won, L.; Jae Il, K.; Seop, H.C.; Jong Wan, K.; Shihai, Y.; Yong, L.J.; Jung Hwa, L.; Taiha, J. Coumarin-derived Cu2+-selective fluorescence sensor: Synthesis, mechanisms, and applications in living cells. J. Am. Chem. Soc. 2009, 131, 2008–2012. [Google Scholar]
Sample Availability: Samples of the compounds 4 are available from the authors. |









© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Chen, A.; Kong, Z.; Sun, D. A Reversible Colorimetric and Fluorescence “Turn-Off” Chemosensor for Detection of Cu2+ and Its Application in Living Cell Imaging. Molecules 2019, 24, 4283. https://doi.org/10.3390/molecules24234283
Hu Y, Chen A, Kong Z, Sun D. A Reversible Colorimetric and Fluorescence “Turn-Off” Chemosensor for Detection of Cu2+ and Its Application in Living Cell Imaging. Molecules. 2019; 24(23):4283. https://doi.org/10.3390/molecules24234283
Chicago/Turabian StyleHu, Yun, Aiqian Chen, Zhuo Kong, and Demeng Sun. 2019. "A Reversible Colorimetric and Fluorescence “Turn-Off” Chemosensor for Detection of Cu2+ and Its Application in Living Cell Imaging" Molecules 24, no. 23: 4283. https://doi.org/10.3390/molecules24234283
APA StyleHu, Y., Chen, A., Kong, Z., & Sun, D. (2019). A Reversible Colorimetric and Fluorescence “Turn-Off” Chemosensor for Detection of Cu2+ and Its Application in Living Cell Imaging. Molecules, 24(23), 4283. https://doi.org/10.3390/molecules24234283