A Cataluminescence Sensor Based on NiO Nanoparticles for Sensitive Detection of Acetaldehyde
Abstract
1. Introduction
2. Results and Discussion
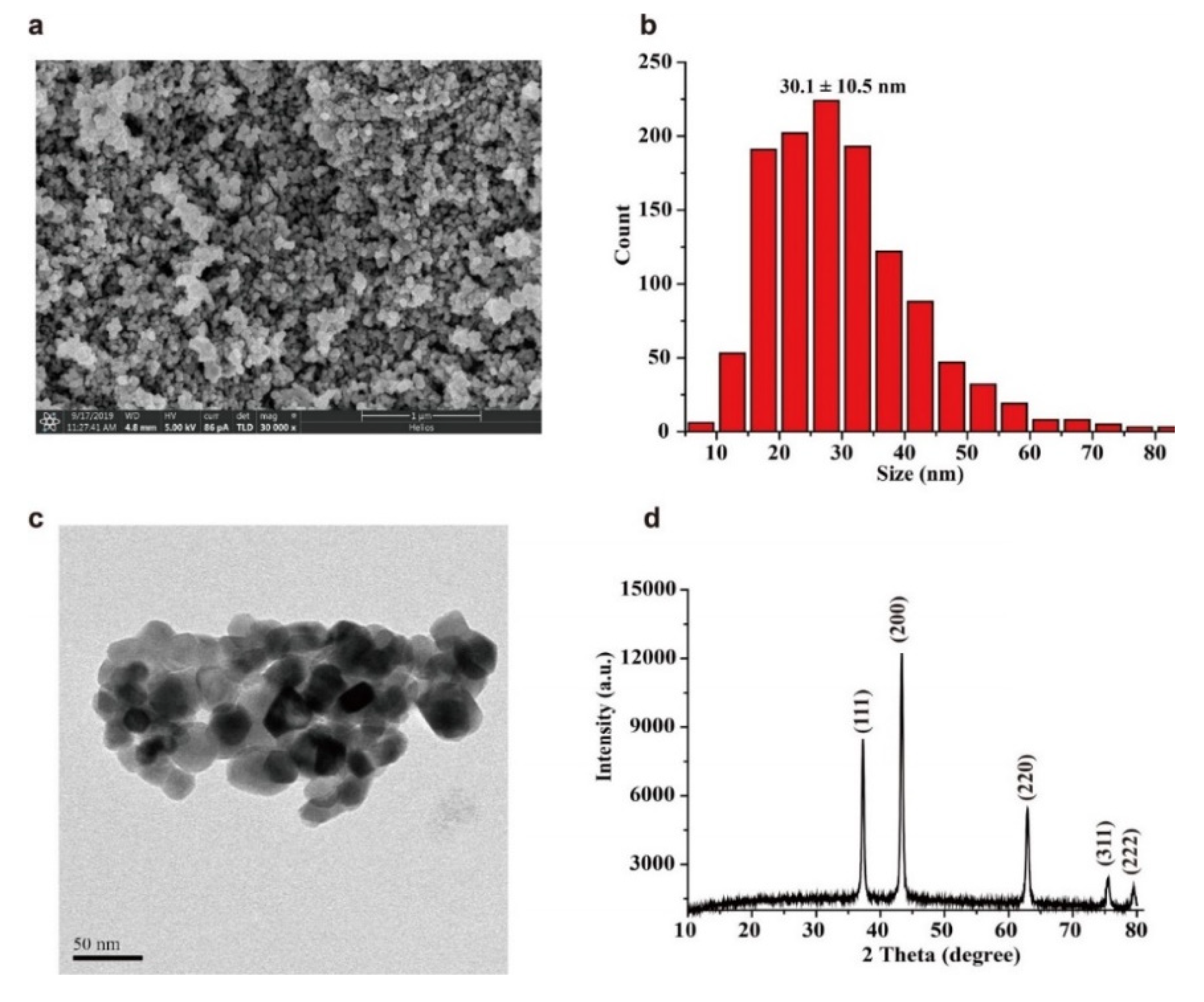
2.1. Characterization of Nickel Oxide Nanoparticles
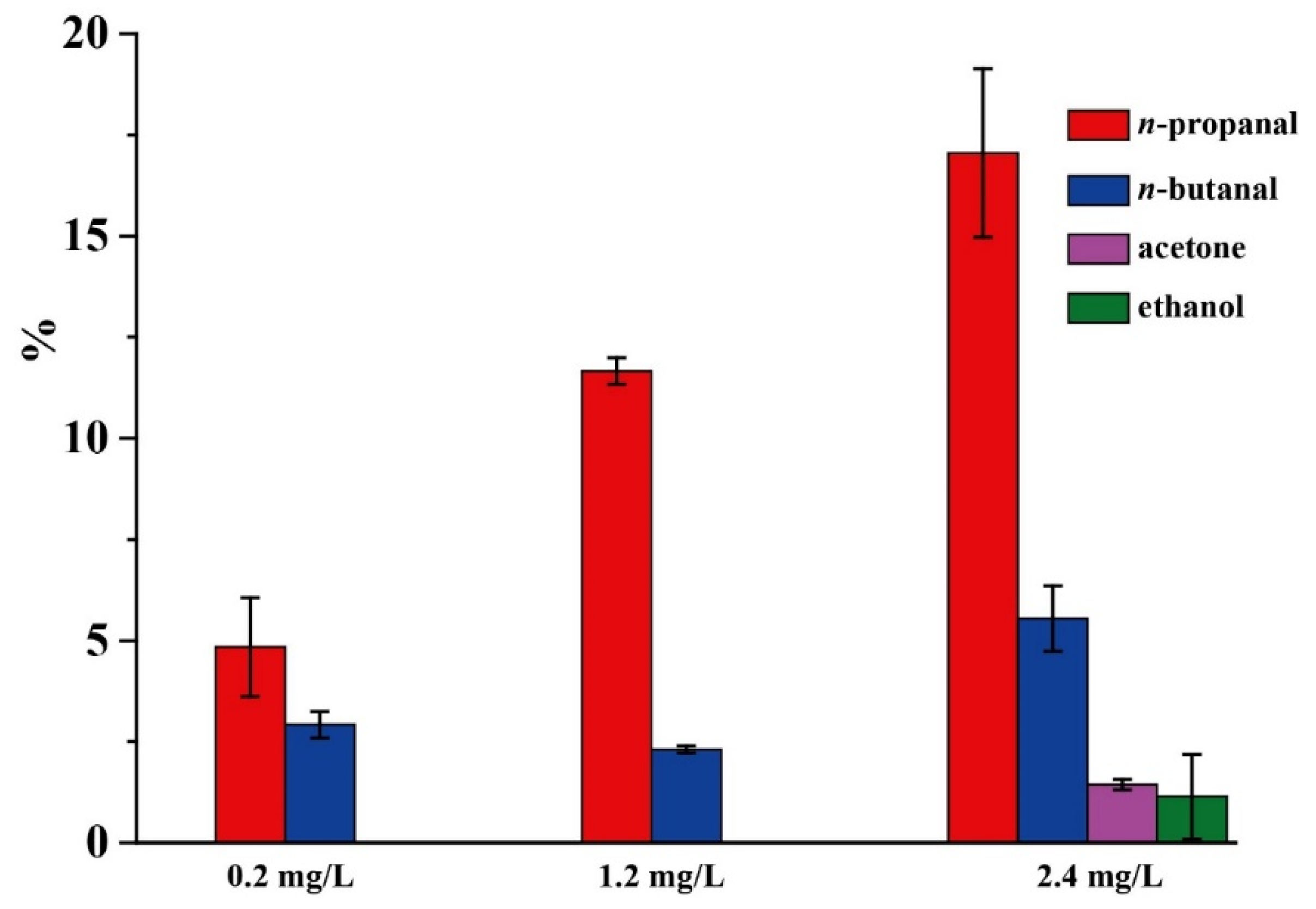
2.2. Selectivity
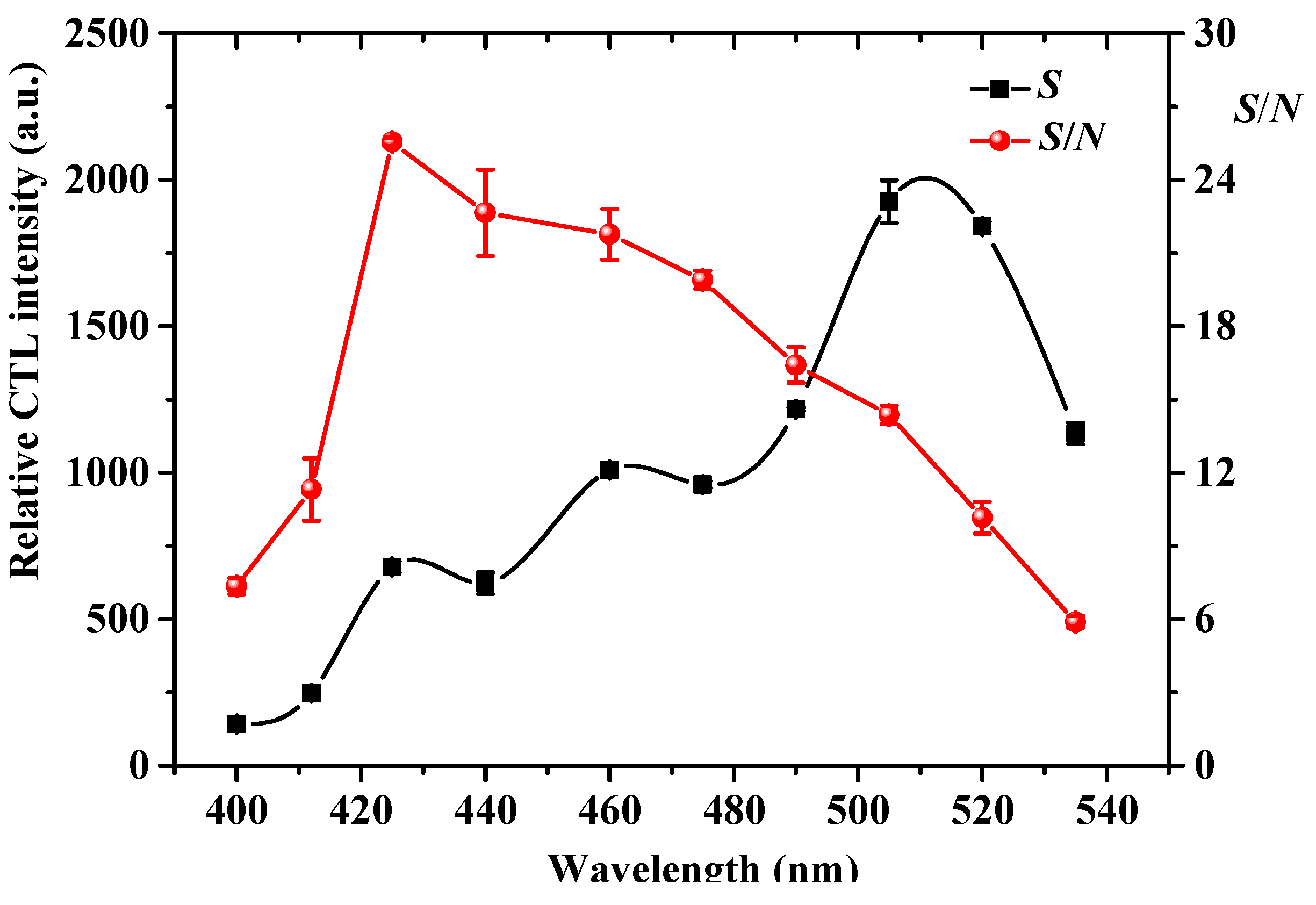
2.3. Effect of Detecting Wavelength
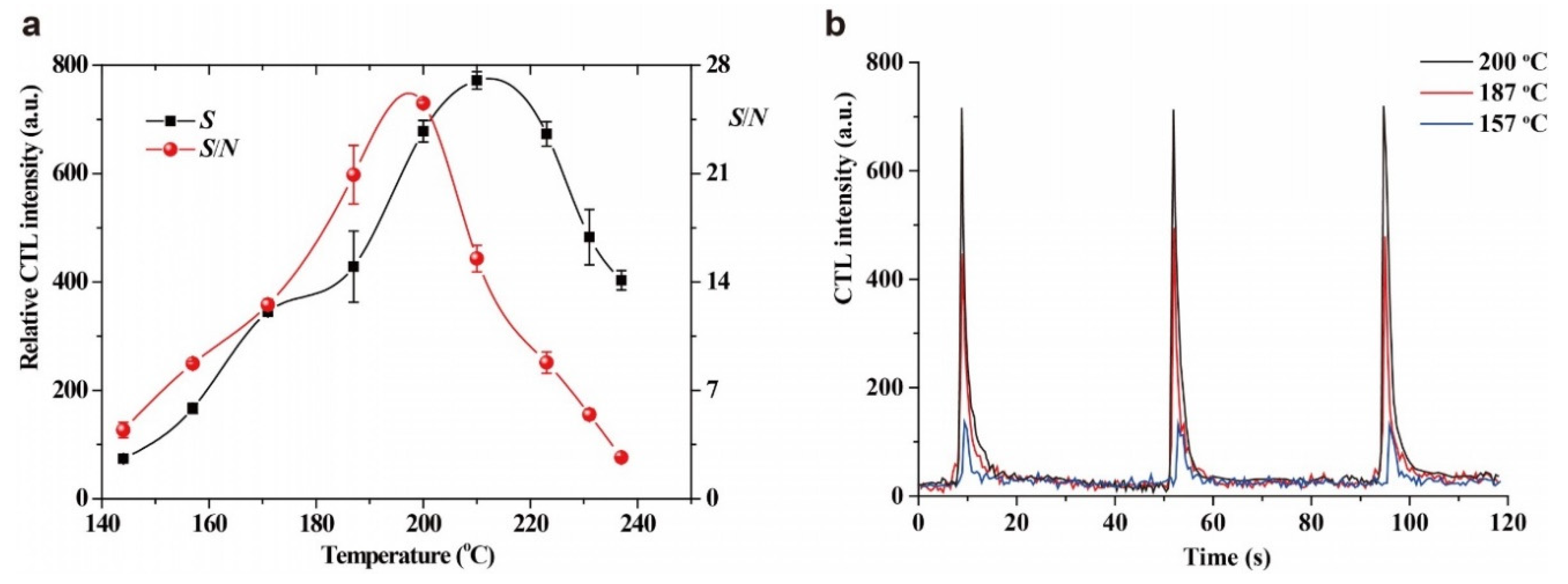
2.4. Effect of Working Temperature
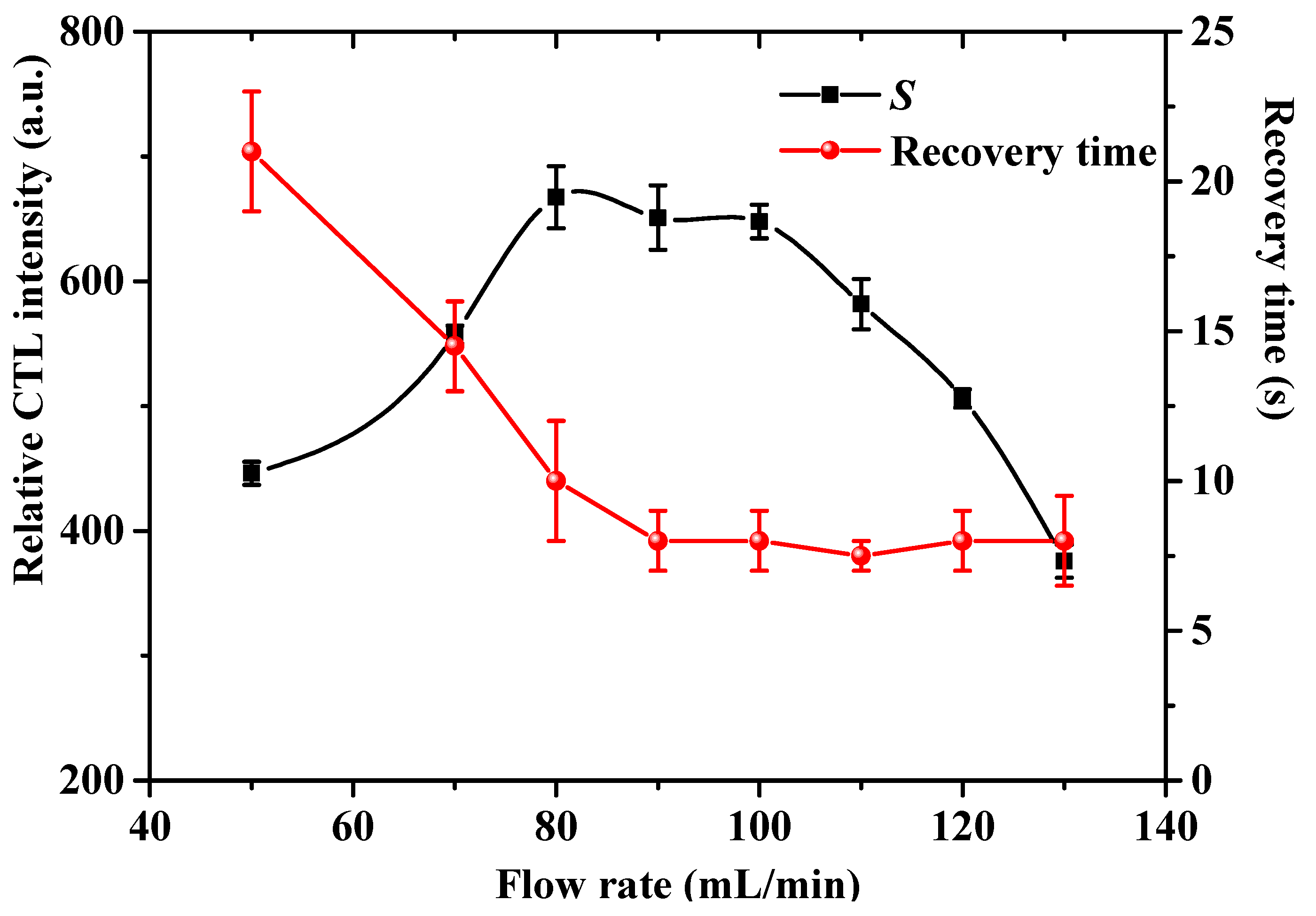
2.5. Effect of the Air Flow Rate
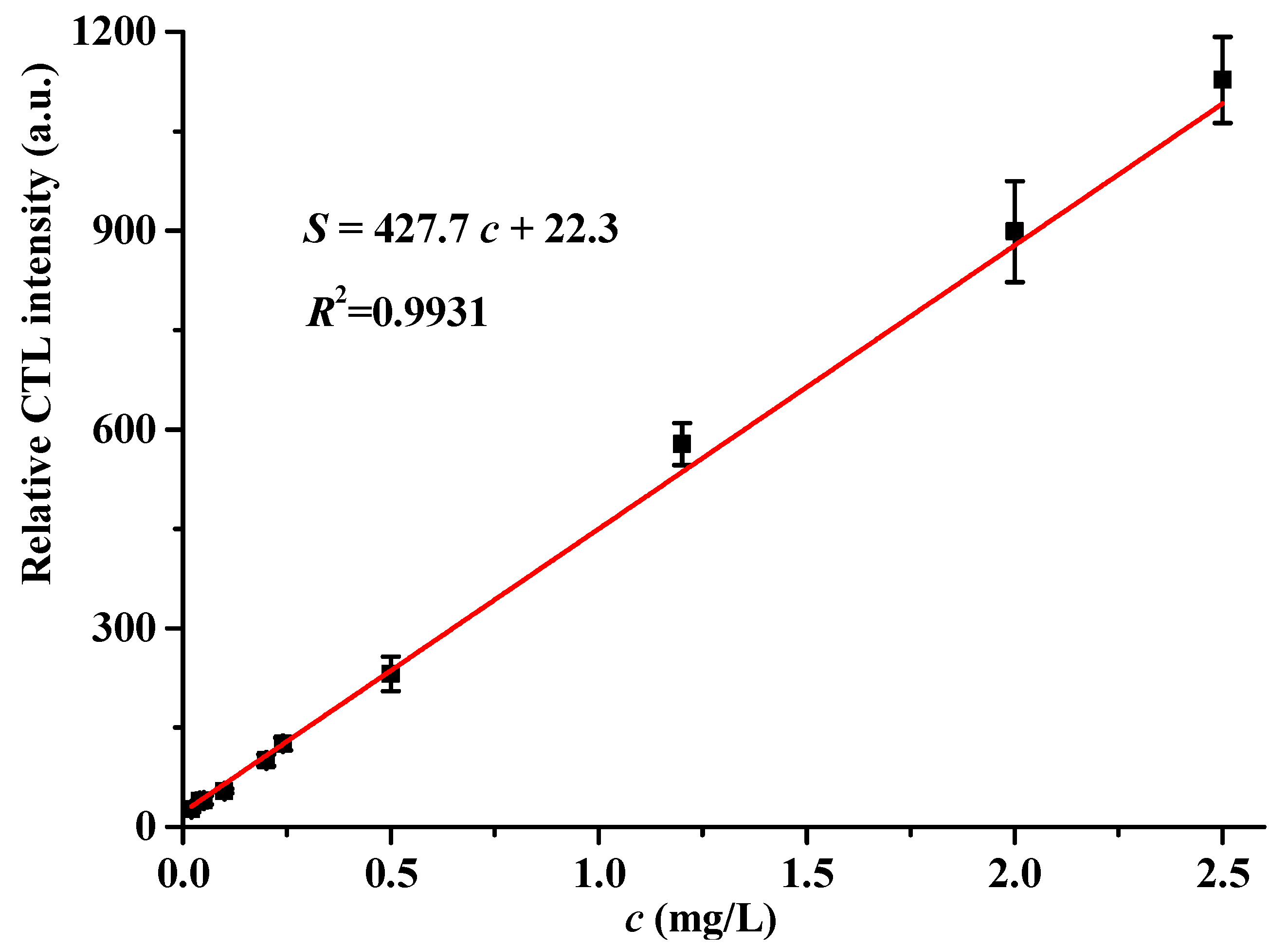
2.6. Analytical Characteristics
2.7. Sample Analysis
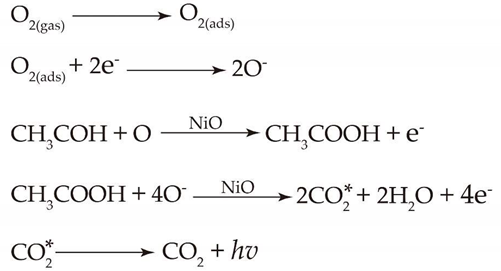
2.8. Sensing Mechanism
3. Experimental Section
3.1. Materials and Reagent
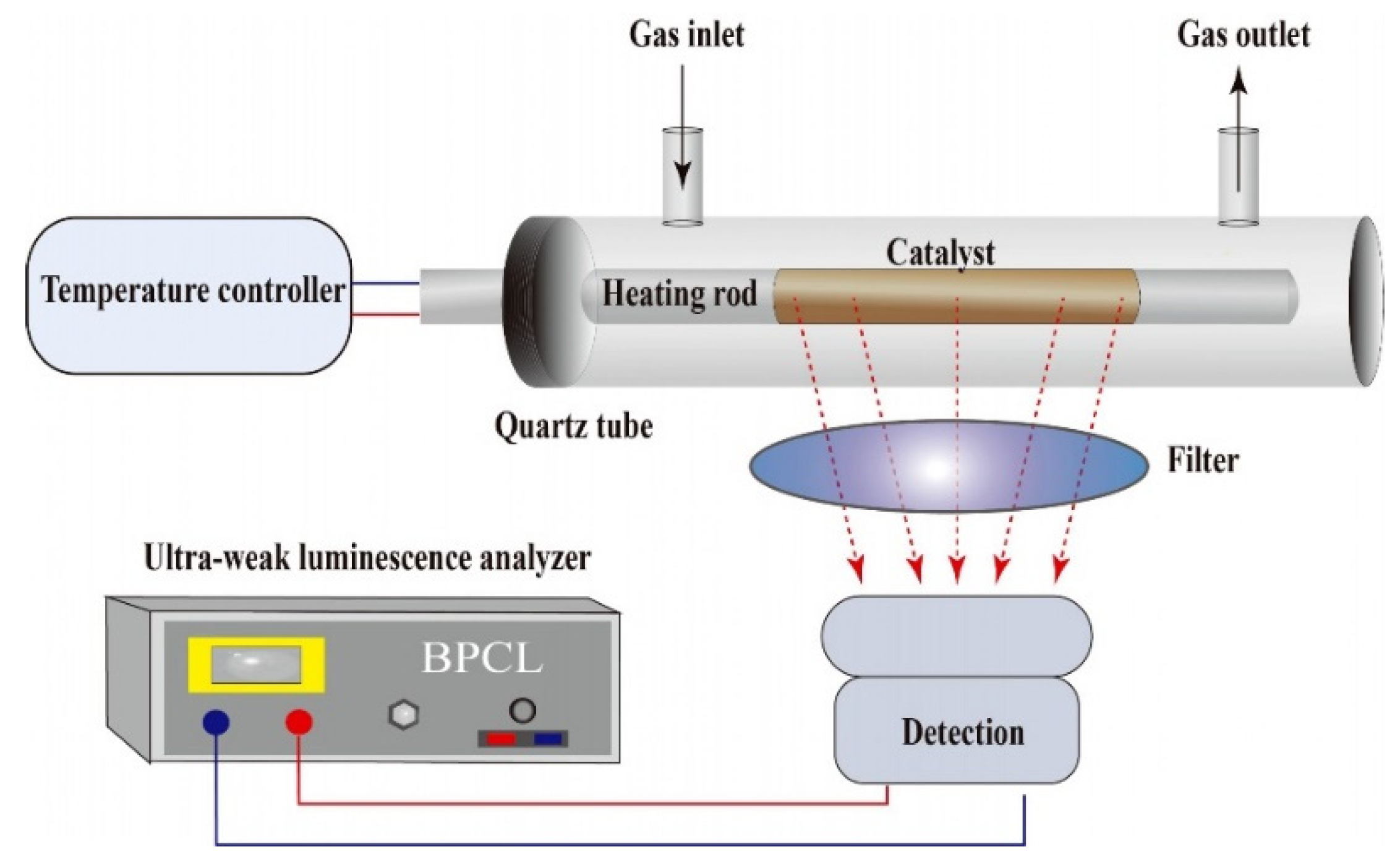
3.2. Instrumentation
3.3. Synthesis of Nickel Oxide Nanoparticles
3.4. Fabrication of the CTL Sensor
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mentese, S.; Rad, A.Y.; Arısoy, M.; Güllü, G. Multiple comparisons of organic, microbial, and fine particulate pollutants in typical indoor environments: Diurnal and seasonal variations. J. Air Waste Manage. 2012, 62, 1380–1393. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.S. Decomposition of VOCs and odorous compounds by radiolysis: A critical review. Chem. Eng. J. 2017, 316, 609–622. [Google Scholar] [CrossRef]
- Da, C.F.B.M.; Silva, G.V.; Boaventura, R.A.R.; Dias, M.M.; Lopes, J.C.B.; Vilar, V.J.P. Ozonation and ozone-enhanced photocatalysis for VOC removal from air streams: Process optimization, synergy and mechanism assessment. Sci. Total Environ. 2019, 687, 1357–1368. [Google Scholar]
- Kamal, M.S.; Razzak, S.A.; Hossain, M.M. Catalytic oxidation of volatile organic compounds (VOCs)—A review. Atmos. Environ. 2016, 140, 117–134. [Google Scholar] [CrossRef]
- Sherriff, A.; Farrow, A.; Golding, J.; the ALSPAC Study Team; Henderson, J. Frequent use of chemical household products is associated with persistent wheezing in pre-school age children. Thorax 2005, 60, 45–49. [Google Scholar] [CrossRef]
- Herbarth, O.; Fritz, G.J.; Rehwagen, M.; Richter, M.; RÖder, S.; Schlink, U. Association between indoor renovation activities and eczema in early childhood. Int. J. Hyg. Envir. Heal. 2006, 209, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Scirè, S.; Liotta, L.F. Supported gold catalysts for the total oxidation of volatile organic compounds. Appl. Catal. B-Environ. 2012, 125, 222–246. [Google Scholar]
- Dumanoglu, Y.; Kara, M.; Altiok, H.; Odabasi, M.; Elbir, T.; Bayram, A. Spatial and seasonal variation and source apportionment of volatile organic compounds (VOCs) in a heavily industrialized region. Atmos. Environ. 2014, 98, 168–178. [Google Scholar] [CrossRef]
- Bari, M.A.; Kindzierski, W.B.; Wheeler, A.J.; Héroux, M.; Wallace, L.A. Source apportionment of indoor and outdoor volatile organic compounds at homes in Edmonton, Canada. Build. Environ. 2015, 90, 114–124. [Google Scholar] [CrossRef]
- Peng, C.Y.; Yang, H.H.; Lan, C.H.; Chien, S.M. Effects of the biodiesel blend fuel on aldehyde emissions from diesel engine exhaust. Atmos. Environ. 2008, 42, 906–915. [Google Scholar] [CrossRef]
- Mallya, A.N.; Ramamurthy, P.C. Organic Molecule Based Sensor for Aldehyde Detection. In Sensing Technology: Current Status and Future Trends III; Springer: Cham, Switzerland, 2015; pp. 299–325. [Google Scholar]
- Yang, B.; Xu, C.; Shu, J.N.; Li, Z.; Zhang, H.X.; Ma, P.K. Ultrasensitive detection of volatile aldehydes with chemi-ionization-coupled time-of-flight mass spectrometry. Talanta 2019, 194, 888–894. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, P.J.; Siraki, A.G.; Shangari, N. Aldehyde sources, metabolism, molecular toxicity mechanisms, and possible effects on human health. Crit. Rev. Toxicol. 2005, 35, 609–663. [Google Scholar] [CrossRef]
- Tan, W.H.; Tan, J.F.; Fan, L.R.; Yu, Z.T.; Qian, J.; Huang, X.T. Fe2O3-loaded NiO nanosheets for fast response/recovery and high response gas sensor. Sens. Actuators B 2018, 256, 282–293. [Google Scholar] [CrossRef]
- Ali, F.I.M.; Awwad, F.; Greish, Y.E.; Abu-Hani, A.F.S.; Mahmoud, S.T. Fabrication of low temperature and fast response H2S gas sensor based on organic-metal oxide hybrid nanocomposite membrane. Org. Electron. 2020, 76, 105486–105494. [Google Scholar] [CrossRef]
- Sajin, K.; Cui, L.; Murray, E.; Mainardi, D. Kinetics of Nitric Oxide and Oxygen Gases on Porous Y-Stabilized ZrO2-Based Sensors. Molecules 2013, 18, 9901–9918. [Google Scholar]
- Breysse, M.; Bernard, C.; Lyliane, F.; Michelle, G.; Roberto, J.J.W.; Theodor, W. Chemiluminescence during the catalysis of carbon monoxide oxidation on a thoria surface. J. Catal. 1976, 45, 137–144. [Google Scholar] [CrossRef]
- Na, N.; Liu, H.Y.; Han, J.Y.; Han, F.F.; Ouyang, J. Plasma-assisted cataluminescence sensor array for gaseous hydrocarbons discrimination. Anal. Chem. 2012, 84, 4830–4836. [Google Scholar] [CrossRef]
- Zhang, Q.C.; Yan, W.L.; Jiang, L.; Zheng, Y.G.; Wang, J.X.; Zhang, R.K. Synthesis of nano-praseodymium oxide for cataluminescence sensing of acetophenone in exhaled breath. Molecules 2019, 24, 4275. [Google Scholar] [CrossRef]
- Shi, G.L.; He, Y.G.; Zhang, Y.X.; Yin, B.Q.; Ali, F.H. Detection and determination of harmful gases in confined spaces for the Internet of Things based on cataluminescence sensor. Sens. Actuators B 2019, 296, 126686. [Google Scholar] [CrossRef]
- Zhu, Y.F.; Shi, J.J.; Zhang, Z.Y.; Zhang, C.; Zhang, X.R. Development of a gas sensor utilizing chemiluminescence on nanosized titanium dioxide. Anal. Chem. 2002, 74, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.F.; Lu, Z.Y.; Zhang, R.K.; Li, G.K. Cataluminescence sensor for highly sensitive and selective detection of iso-butanol. Talanta 2019, 194, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.X.; Zhang, Q.C.; Li, Y.H.; Xu, Z.M.; Long, W.R. A cataluminescence sensor for propionaldehyde based on the use of nanosized zirconium dioxide. Microchim. Acta 2014, 181, 1125–1132. [Google Scholar] [CrossRef]
- Li, Z.H.; Xi, W.; Lu, C. Hydrotalcite-assisted cataluminescence: A new approach for sensing mesityl oxide in aldol condensation of acetone. Sens. Actuators B. 2015, 207, 498–503. [Google Scholar] [CrossRef]
- Luo, M.; Shao, K.; Long, Z.; Wang, L.X.; Peng, C.H.; Ouyang, J.; Na, N. A paper-based plasma-assisted cataluminescence sensor for ethylene detection. Sens. Actuators B. 2017, 240, 132–141. [Google Scholar] [CrossRef]
- Hu, J.X.; Zhang, L.C.; Lv, Y. Recent advances in cataluminescence gas sensor: Materials and methodologies. Appl. Spectrosc. Rev. 2019, 54, 306–324. [Google Scholar] [CrossRef]
- Wang, S.; Yuan, Z.Q.; Zhang, L.J.; Lin, Y.J.; Lu, C. Recent advances in cataluminescence-based optical sensing systems. Analyst 2017, 142, 1415–1428. [Google Scholar] [CrossRef]
- Cao, X.A.; Zhang, Z.Y.; Zhang, X.R. A novel gaseous acetaldehyde sensor utilizing cataluminescence on nanosized BaCO3. Sens. Actuators B 2004, 99, 30–35. [Google Scholar] [CrossRef]
- Yang, P.; Lau, C.W.; Liang, J.Y.; Lu, J.Z.; Liu, X. Zeolite-based cataluminescence sensor for the selective detection of acetaldehyde. Luminescence 2007, 22, 473–479. [Google Scholar] [CrossRef]
- Patil, D.R.; Patil, L.A. Room temperature chlorine gas sensing using surface modified ZnO thick film resistors. Sens. Actuators B 2007, 123, 546–553. [Google Scholar] [CrossRef]
- EH40/2005, Workplace Exposure Limits. Available online: http://www.hse.gov.uk/pubns/books/eh40.htm) (accessed on 1 March 2020).
- Chava, R.K.; Cho, H.; Yoon, J.; Yu, Y. Fabrication of aggregated In2O3 nanospheres for highly sensitive acetaldehyde gas sensors. J. Alloy. Compd. 2019, 772, 834–842. [Google Scholar] [CrossRef]
- Castro-Hurtado, I.; Malagù, C.; Morandi, S.; Pérez, N.; Mandayo, G.G.; Castaño, E. Properties of NiO sputtered thin films and modeling of their sensing mechanism under formaldehyde atmospheres. Acta Mater. 2013, 61, 1146–1153. [Google Scholar] [CrossRef]
- Hoa, N.D.; El-Safty, S.A. Synthesis of mesoporous NiO nanosheets for the detection of toxic NO2 gas. Chem.–Eur. J. 2011, 17, 12896–12901. [Google Scholar] [CrossRef] [PubMed]
- Sano, T.; Negishi, N.; Uchino, K.; Tanaka, J.; Matsuzawa, S.; Takeuchi, K. Photocatalytic degradation of gaseous acetaldehyde on TiO2 with photodeposited metals and metal oxides. J. Photoch. Photobio. A 2003, 160, 93–98. [Google Scholar] [CrossRef]
- Wahab, R.; Tripathy, S.K.; Shin, H.; Mohapatra, M.; Musarrat, J.; Al-Khedhairy, A.A.; Kaushik, N.K. Photocatalytic oxidation of acetaldehyde with ZnO-quantum dots. Chem. Eng. J. 2013, 226, 154–160. [Google Scholar] [CrossRef]
- Wang, S.M.; Shi, W.Y.; Lu, C. Chemisorbed oxygen on the surface of catalyst-improved cataluminescence selectivity. Anal. Chem. 2016, 88, 4987–4994. [Google Scholar] [CrossRef]
- Kopp, M.M.; Mathieu, O.; Petersen, E.L. Rate determination of the CO2* chemiluminescence reaction CO+ O+ M ⇌ CO2*+ M. Int. J. Chem. Kinet. 2015, 47, 50–72. [Google Scholar] [CrossRef][Green Version]
Sample Availability: not available. |
| Principle | Materials | Temperature (°C) | Recovery Time (s) | LOD (mg/L) | References |
|---|---|---|---|---|---|
| CTL | NiO | 200 | 10 | 0.006 | Present work |
| CTL | BaCO3 | 225 | 50 | 0.001 | [28] |
| CTL | Zeolite | 230 | ~100 | 0.02 | [29] |
| Electrochemistry | In2O3 | 300 | 480 | 0.002 | [32] |
| Sample No. | Mixture | Spiked Values (mg/L) | Measured Values (mg/L) | Recovery (%) |
|---|---|---|---|---|
| 1 | Acetaldehyde | 0.5 | 0.57 ± 0.01 | 114.0 ± 2.0 |
| n-Propanal | 2.0 | |||
| 2 | Acetaldehyde | 0.5 | 0.58 ± 0.04 | 116.0 ± 8.0 |
| n-Butanal | 2.0 | |||
| 3 | Acetaldehyde | 0.5 | 0.48 ± 0.01 | 96.0 ± 2.0 |
| Ethanol | 2.0 | |||
| 4 | Acetaldehyde | 0.5 | 0.52 ± 0.02 | 104.0 ± 4.0 |
| n-Hexane | 2.0 | |||
| Ethyl acetate | 2.0 | |||
| 5 | Acetaldehyde | 0.5 | 0.52 ± 0.03 | 104.0 ± 6.0 |
| Formaldehyde | 2.0 | |||
| Benzene | 2.0 | |||
| Acetic acid | 2.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.-K.; Wang, D.; Wu, Y.-J.; Hu, Y.-H.; Chen, J.-Y.; He, J.-C.; Wang, J.-X. A Cataluminescence Sensor Based on NiO Nanoparticles for Sensitive Detection of Acetaldehyde. Molecules 2020, 25, 1097. https://doi.org/10.3390/molecules25051097
Zhang R-K, Wang D, Wu Y-J, Hu Y-H, Chen J-Y, He J-C, Wang J-X. A Cataluminescence Sensor Based on NiO Nanoparticles for Sensitive Detection of Acetaldehyde. Molecules. 2020; 25(5):1097. https://doi.org/10.3390/molecules25051097
Chicago/Turabian StyleZhang, Run-Kun, Die Wang, Yan-Jun Wu, Yi-Han Hu, Jian-Yu Chen, Jin-Can He, and Jing-Xin Wang. 2020. "A Cataluminescence Sensor Based on NiO Nanoparticles for Sensitive Detection of Acetaldehyde" Molecules 25, no. 5: 1097. https://doi.org/10.3390/molecules25051097
APA StyleZhang, R.-K., Wang, D., Wu, Y.-J., Hu, Y.-H., Chen, J.-Y., He, J.-C., & Wang, J.-X. (2020). A Cataluminescence Sensor Based on NiO Nanoparticles for Sensitive Detection of Acetaldehyde. Molecules, 25(5), 1097. https://doi.org/10.3390/molecules25051097





