Enantioselective Synthesis of 8-Hydroxyquinoline Derivative, Q134 as a Hypoxic Adaptation Inducing Agent
Abstract
:1. Introduction
2. Results
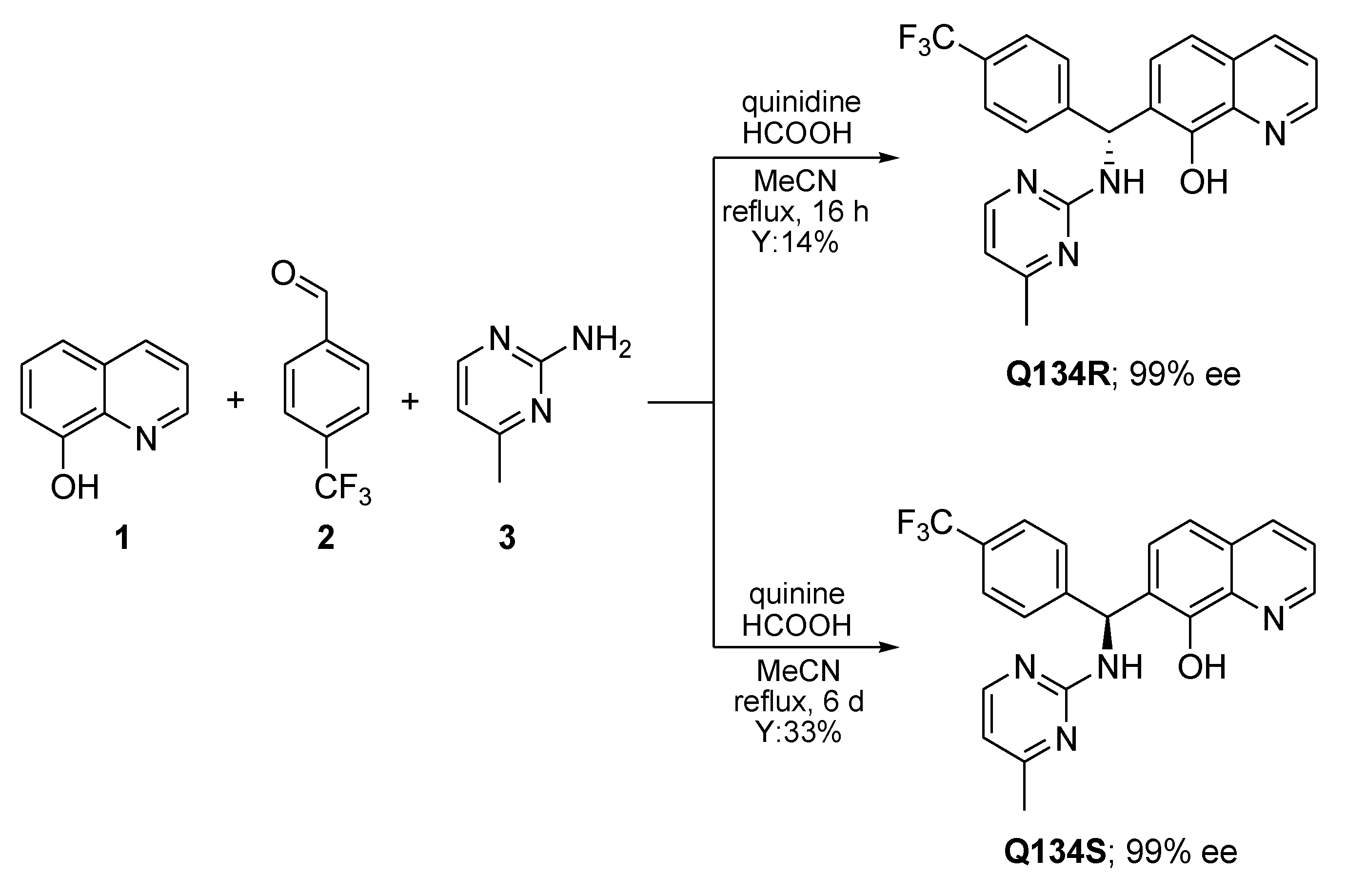
2.1. Enantioselective Synthesis of Q134R and Q134S
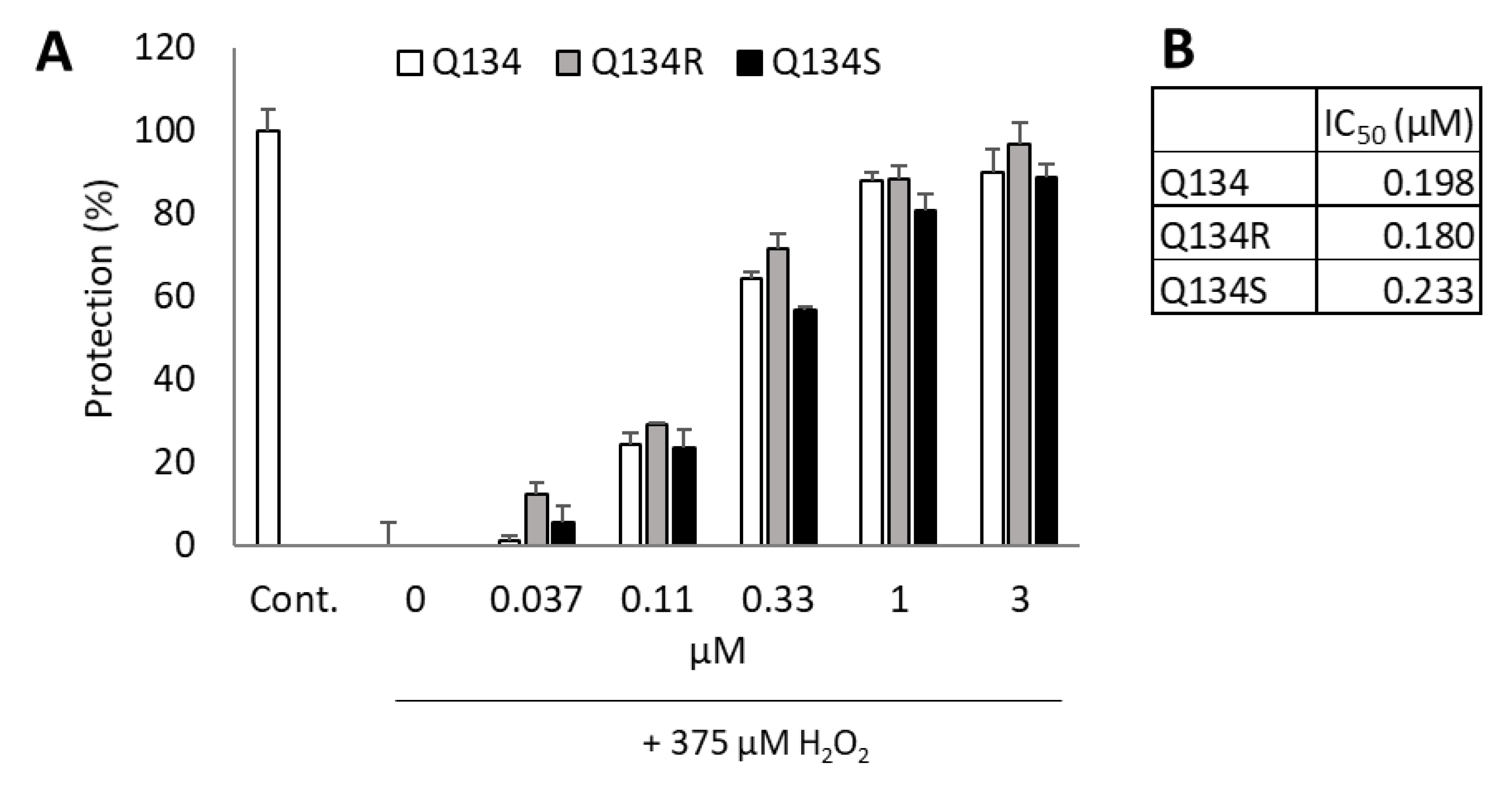
2.2. Enantiomers of Q134 Show Similar Cytoprotective Activity
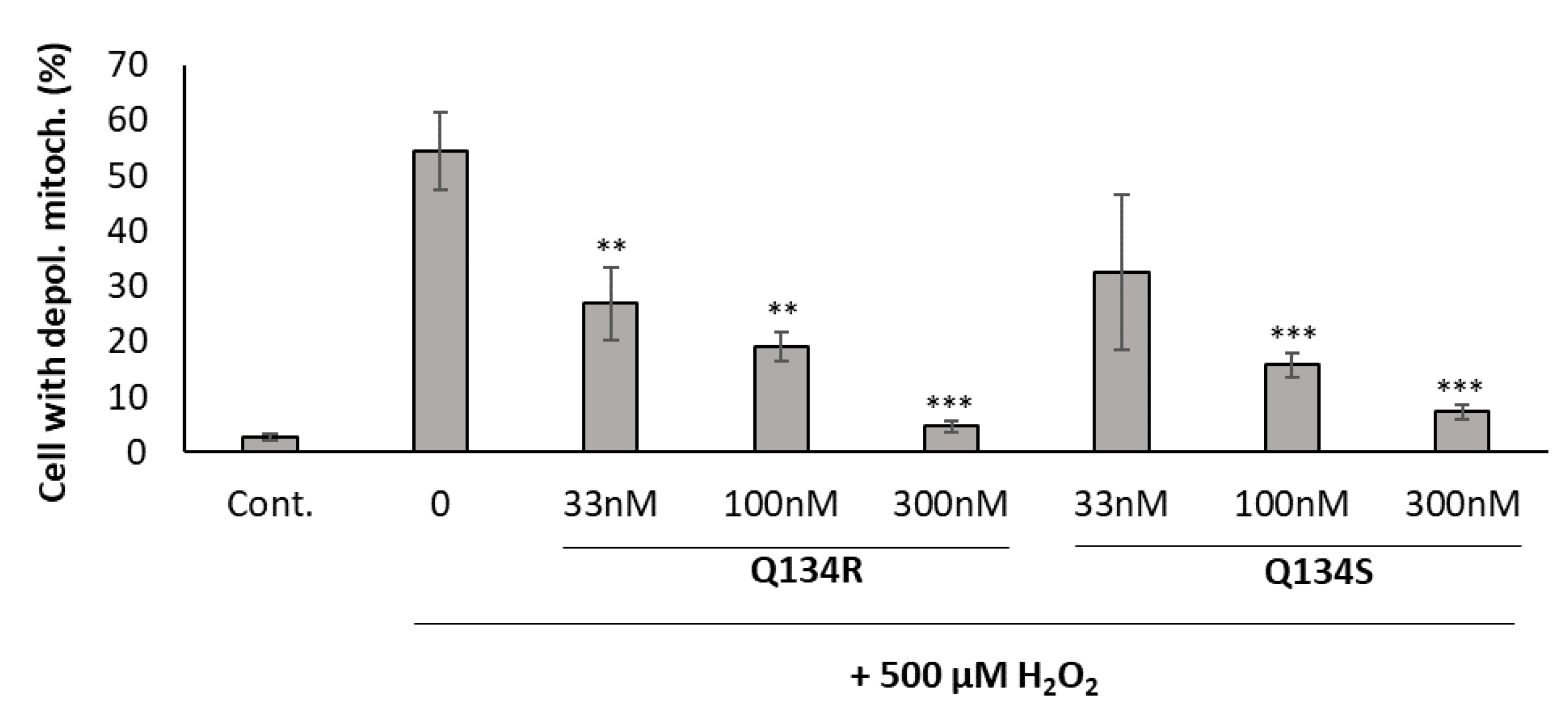
2.3. Mitochondrial Membrane Depolarization Following Oxidative Stress Is Reversed by Treatment with Q134R and Q134S
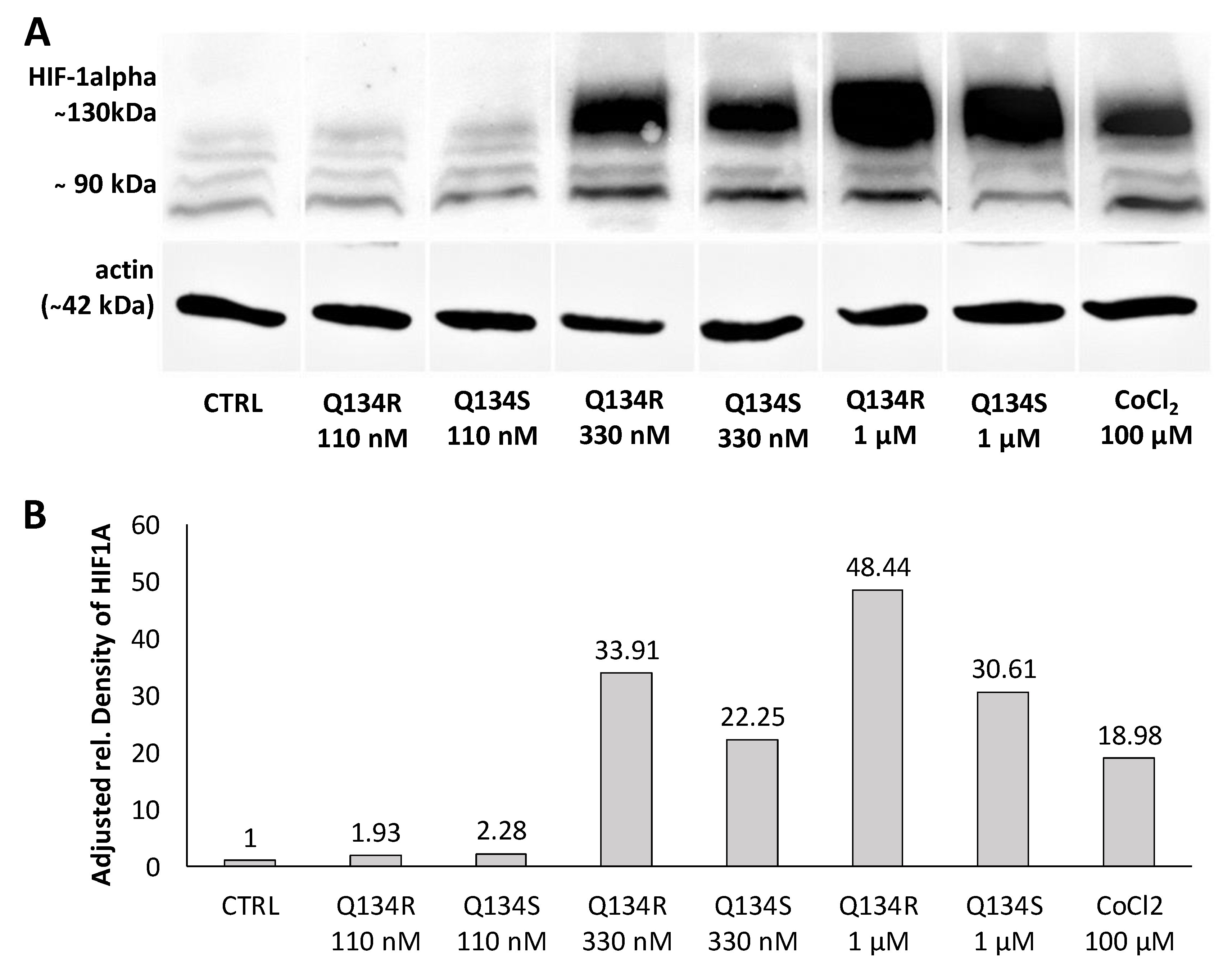
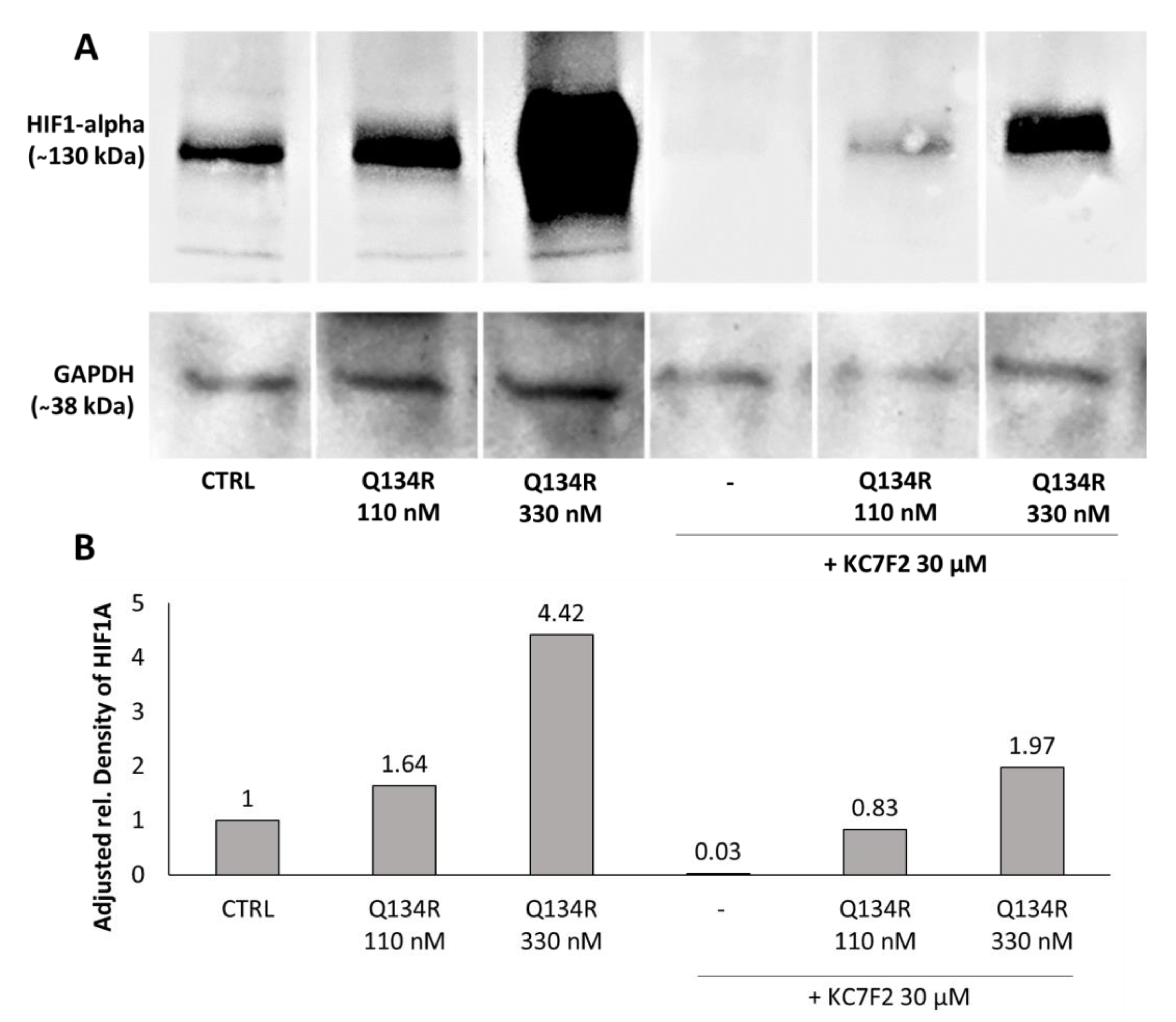
2.4. Q134R and Q134S Stabilize HIF1A Protein In Vitro
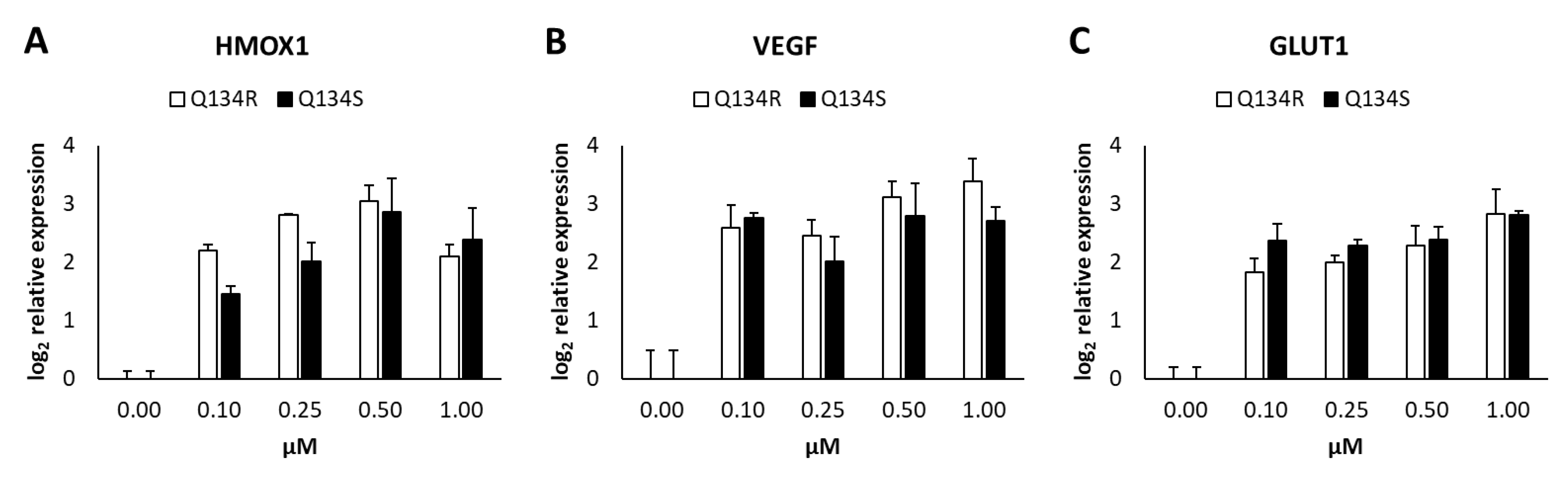
2.5. Gene Expression Analysis. Q134R and Q134S Induce Hypoxia Related Genes and Glucose Transporter Expression
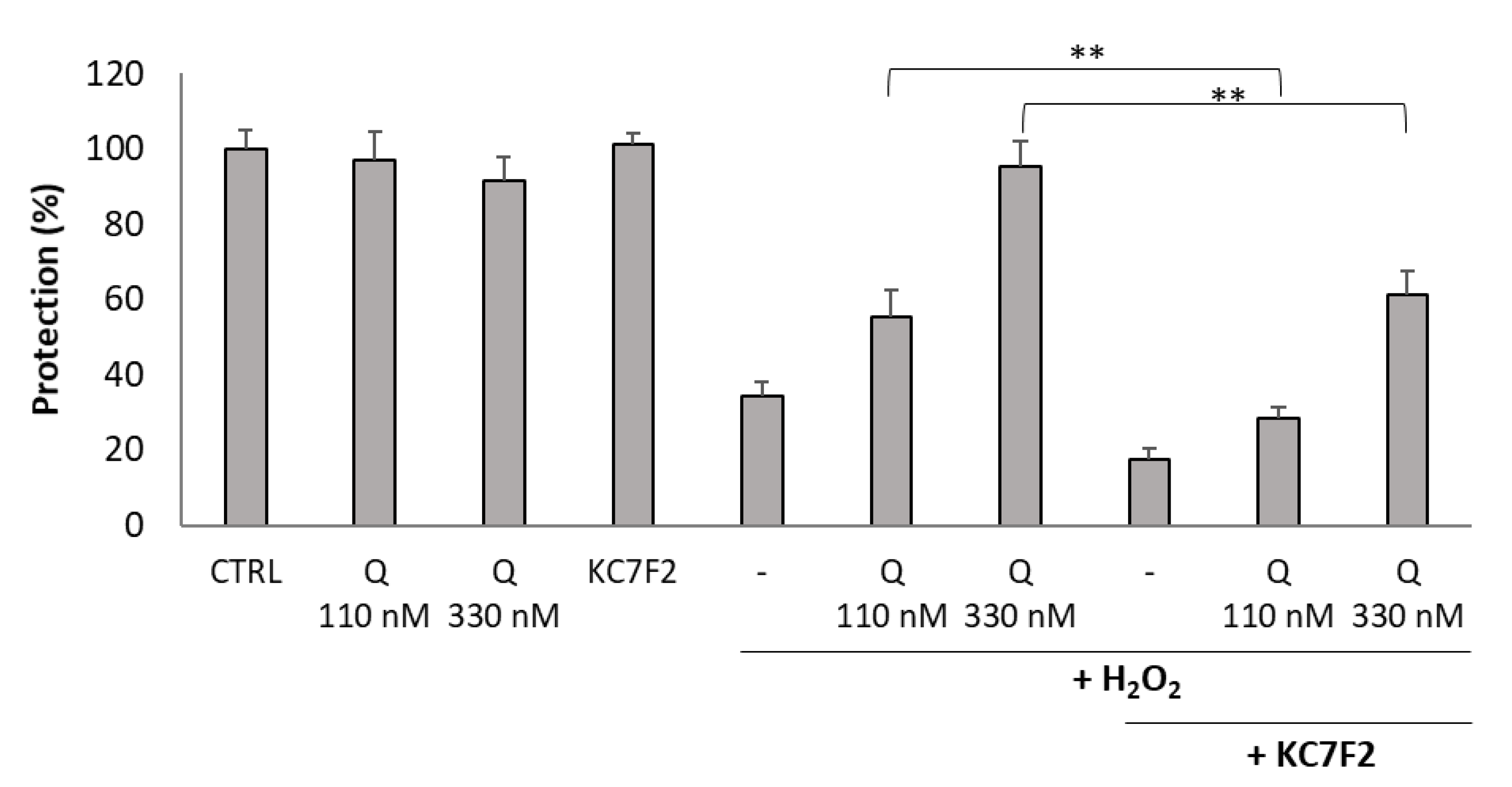
2.6. Inhibition of HIF1A Translation Abolished Cytoprotective Activity
3. Discussion
4. Materials and Methods
4.1. Enantioselective Synthesis of Q134R and Q134S
4.1.1. General Information
4.1.2. Synthesis of (R)-7-((4-methylpyrimidin-2-ylamino)(4-trifluoromethyl)phenyl)methyl)quinolin-8-ol (Q134R)
4.1.3. Synthesis of (S)-7-((4-methylpyrimidin-2-ylamino)(4-trifluoromethyl)phenyl)methyl)quinolin-8-ol (Q134S)
4.2. Cell Culture
4.3. Real-Time CellE Sensing (RT-CES) Cytoprotection Assay
4.4. Detection of Mitochondrial Membrane Potential
4.5. Gene Expression Analysis
4.6. Western Blot Analysis
4.7. Resazurin Viability Assay
4.8. Statistical Analysis
5. Conclusions
6. Patents
Author Contributions
Funding
Conflicts of Interest
References
- Serý, O.; Povová, J.; Míšek, I.; Pešák, L.; Janout, V. Molecular mechanisms of neuropathological changes in Alzheimer’s disease: A review. Folia Neuropathol. 2013, 51, 1–9. [Google Scholar] [CrossRef]
- Lane, C.A.; Hardy, J.; Schott, J.M. Alzheimer’s disease. Eur. J. Neurol. 2018, 25, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Varga, J.; Klausz, B.; Domokos, Á.; Kálmán, S.; Pákáski, M.; Szűcs, S.; Garab, D.; Zvara, Á.; Puskás, L.; Kálmán, J.; et al. Increase in Alzheimer’s related markers preceeds memory disturbances: Studies in vasopressin-deficient Brattleboro rat. Brain Res. Bull. 2014, 100, 6–13. [Google Scholar] [CrossRef]
- Ogunshola, O.O.; Antoniou, X. Contribution of hypoxia to Alzheimer’s disease: Is HF1alpha a mediator of neurodegeneration? Cell Mol. Life Sci. 2009, 66, 3555–3563. [Google Scholar] [CrossRef]
- Zhang, X.; Le, W. Pathological role of hypoxia in Alzheimer’s disease. Exp. Neurol. 2010, 223, 299–303. [Google Scholar] [CrossRef]
- Peers, C.; Pearson, H.A.; Boyle, J.P. Hypoxia and Alzheimer’s disease. Essays Biochem. 2007, 43, 153–164. [Google Scholar] [CrossRef]
- Kietzmann, T.; Knabe, W.; Schmidt-Kastner, R. Hypoxia and hypoxia-inducible factor modulated gene expression in brain: Involvement in neuroprotection and cell death. Eur. Arch. Psychiatry Clin. Neurosci. 2001, 251, 170–178. [Google Scholar] [CrossRef]
- Ashok, B.S.; Ajith, T.A.; Sivanesan, S. Hypoxia-inducible factors as neuroprotective agent in Alzheimer’s disease. Clin. Exp. Pharmacol. Physiol. 2017, 44, 327–334. [Google Scholar] [CrossRef]
- Doeppner, T.R.; Mlynarczuk-Bialy, I.; Kuckelkorn, U.; Kaltwasser, B.; Herz, J.; Hasan, M.R.; Hermann, D.M.; Bähr, M. The novel proteasome inhibitor BSc2118 protects against cerebral ischaemia through HIF1A accumulation and enhanced angioneurogenesis. Brain 2012, 135, 3282–3297. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, N.A.; Rakhman, I.; Moroz, N.; Basso, M.; Payappilly, J.; Kazakov, S.; Hernandez-Guzman, F.; Gaisina, I.N.; Kozikowski, A.P.; Ratan, R.R.; et al. Utilization of an In Vivo Reporter for High Throughput Identification of Branched Small Molecule Regulators of Hypoxic Adaptation. Chem. Biol. 2010, 17, 380–391. [Google Scholar] [CrossRef]
- Warshakoon, N.C.; Wu, S.; Boyer, A.; Kawamoto, R.; Sheville, J.; Renock, S.; Xu, K.; Pokross, M.; Zhou, S.; Winter, C.; et al. Structure-based design, synthesis, and SAR evaluation of a new series of 8-hydroxyquinolines as HF1alpha prolyl hydroxylase inhibitors. Bioorg. Med. Chem. Lett. 2006, 16, 5517–5522. [Google Scholar] [CrossRef] [PubMed]
- Neitemeier, S.; Dolga, A.M.; Honrath, B.; Karuppagounder, S.S.; Alim, I.; Ratan, R.R.; Culmsee, C. Inhibition of HIF-prolyl-4-hydroxylases prevents mitochondrial impairment and cell death in a model of neuronal oxytosis. Cell Death Dis. 2016, 7, e2214. [Google Scholar] [CrossRef] [PubMed]
- Darvas, F.; Dorman, G.; Krajcsi, P.; Puskas, L.G.; Kovari, Z.; Lõrincz, Z.; Urge, L. Recent advances in chemical genomics. Curr. Med. Chem. 2004, 11, 3119–3145. [Google Scholar] [CrossRef] [PubMed]
- Ózsvári, B.; Puskás, L.G.; Nagy, L.I.; Kanizsai, I.; Gyuris, M.; Madácsi, R.; Fehér, L.Z.; Gerő, D.; Szabó, C. A cell-microelectronic sensing technique for the screening of cytoprotective compounds. Int. J. Mol. Med. 2010, 25, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, S.; Barnucz, E.; Loganathan, S.; Li, S.; Radovits, T.; Hegedus, P.; Zubarevich, A.; Hirschberg, K.; Weymann, A.; Puskás, L.G.; et al. Q50, an Iron-Chelating and zinc-complexing agent, improves cardiac function in rat models of ischemia/reperfusion-induced myocardial injury. Circ. J. 2013, 77, 1817–1826. [Google Scholar] [CrossRef] [PubMed]
- Kanizsai, I.; Madácsi, R.; Hackler, L., Jr.; Gyuris, M.; Szebeni, G.; Huzián, O.; Puskas, L.G. Synthesis and Cytoprotective Characterization of 8-Hydroxyquinoline Betti Products. Molecules 2018, 23, 1934. [Google Scholar] [CrossRef] [PubMed]
- Puskas, L.; Kanizsai, I.; Pillot, T.; Gyuris, M.; Szabo, A.; Takacs, F.; Hackler, L. Enantiomers of 8-Hydroxyquinoline Derivatives and the Synthesis Thereof. U.S. Patent Application 2017/0197936 A1, 13 July 2017. [Google Scholar]
- Narita, T.; Yin, S.; Gelin, C.F.; Moreno, C.S.; Yepes, M.; Nicolaou, K.C.; Van Meir, E.G. Identification of a novel small molecule HF1alpha translation inhibitor. Clin. Cancer Res. 2009, 15, 6128–6136. [Google Scholar] [CrossRef]
- Semenza, G.L. Hypoxia-inducible factor 1 and the molecular physiology of oxygen homeostasis. J. Lab. Clin. Med. 1998, 131, 207–214. [Google Scholar] [CrossRef]
- Zhang, C.P.; Zhu, L.L.; Zhao, T.; Zhao, H.; Huang, X.; Ma, X.; Wang, H.; Fan, M. Characteristics of neural stem cells expanded in lowered oxygen and the potential role of hypoxia-inducible factor-1Alpha. Neurosignals 2006, 15, 259–265. [Google Scholar] [CrossRef]
- Fan, X.; Heijnen, C.J.; van der Kooij, M.A.; Groenendaal, F.; van Bel, F. The role and regulation of hypoxia-inducible factor-1alpha expression in brain development and neonatal hypoxic-ischemic brain injury. Brain Res. Rev. 2009, 62, 99–108. [Google Scholar] [CrossRef]
- Poloznikov, A.A.; Khristichenko, A.Y.; Smirnova, N.A.; Hushpulian, D.M.; Gaisina, I.N.; Osipyants, A.I.; Tishkov, V.I.; Gazaryan, I.G. Structural optimization of adaptaquin, a HIF prolyl hydroxylase inhibitor. Russ. Chem. Bull. 2019, 68, 168. [Google Scholar] [CrossRef]
- Angyal, A.; Demjén, A.; Harmat, V.; Wölfling, J.; Puskás, L.G.; Kanizsai, I. 1,3-Dipolar Cycloaddition of Isatin-Derived Azomethine Ylides with 2-Azirines: Stereoselective Synthesis of 1,3-Diazaspiro[bicyclo[3.1.0]hexane]oxindoles. J. Org. Chem. 2019, 84, 4273–4281. [Google Scholar] [CrossRef] [PubMed]
- Hackler, L., Jr.; Ózsvári, B.; Gyuris, M.; Sipos, P.; Fábián, G.; Molnár, E.; Marton, A.; Faragó, N.; Mihály, J.; Nagy, L.; et al. The curcumin analog C-150, influencing NF-κB, UPR and Akt/Notch pathways has potent anticancer activity in vitro and in vivo. PLoS ONE 2016, 11, e0149832. [Google Scholar] [CrossRef] [PubMed]
- Antal, O.; Hackler, L., Jr.; Shen, J.; Mán, I.; Hideghéty, K.; Kitajka, K.; Puskás, L.G. Combination of unsaturated fatty acids and ionizing radiation on human glioma cells: Cellular, biochemical and gene expression analysis. Lipids Health Dis. 2014, 13, 142. [Google Scholar] [CrossRef]
- Szebeni, G.J.; Balázs, Á.; Madarász, I.; Pócz, G.; Ayaydin, F.; Kanizsai, I.; Fajka-Boja, R.; Alföldi, R.; Hackler, L., Jr.; Puskás, L.G. Achiral Mannich-Base Curcumin Analogs Induce Unfolded Protein Response and Mitochondrial Membrane Depolarization in PANC-1 Cells. Int. J. Mol. Sci. 2017, 18, 2105. [Google Scholar] [CrossRef]
- Andersen, C.L.; Jensen, J.L.; Ørntoft, T.F. Normalization of real-time quantitative RT-PCR data: A model based variance estimation approach to identify genes suited for normalization—Applied to bladder- and colon-cancer data-sets. Cancer Res. 2004, 64, 5245–5250. [Google Scholar] [CrossRef]
- Gyuris, M.; Hackler, L., Jr.; Nagy, L.; Alföldi, R.; Rédei, E.; Marton, A.; Vellai, T.; Faragó, N.; Ózsvári, B.; Hetényi, A.; et al. Mannich Curcuminoids as Potent Anticancer Agents. Arch. Pharm. 2017, 350, e1700005. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
| Gene Name | Abbreviation | Forward_Sequence | Reverse_Sequence |
|---|---|---|---|
| Tubulin beta class I | TUBB | ataccttgaggcgagcaaaa | ctgatcacctcccagaacttg |
| Peptidylprolyl isomerase A | PPIA | atgctggacccaacacaaat | tctttcactttgccaaacacc |
| Heme oxygenase 1 | HMOX1 | ggcagagggtgatagaagagg | agctcctgcaactcctcaaa |
| Vascular endothelial growth factor | VEGF | gcagcttgagttaaacgaacg | ggttcccgaaaccctgag |
| Solute carrier family 2 member 1 | GLUT1 | ccccatcccatggttcatc | tgaggtccagttggagaagc |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hackler, L., Jr.; Gyuris, M.; Huzián, O.; Alföldi, R.; Szebeni, G.J.; Madácsi, R.; Knapp, L.; Kanizsai, I.; Puskás, L.G. Enantioselective Synthesis of 8-Hydroxyquinoline Derivative, Q134 as a Hypoxic Adaptation Inducing Agent. Molecules 2019, 24, 4269. https://doi.org/10.3390/molecules24234269
Hackler L Jr., Gyuris M, Huzián O, Alföldi R, Szebeni GJ, Madácsi R, Knapp L, Kanizsai I, Puskás LG. Enantioselective Synthesis of 8-Hydroxyquinoline Derivative, Q134 as a Hypoxic Adaptation Inducing Agent. Molecules. 2019; 24(23):4269. https://doi.org/10.3390/molecules24234269
Chicago/Turabian StyleHackler, László, Jr., Márió Gyuris, Orsolya Huzián, Róbert Alföldi, Gábor J. Szebeni, Ramóna Madácsi, Levente Knapp, Iván Kanizsai, and László G. Puskás. 2019. "Enantioselective Synthesis of 8-Hydroxyquinoline Derivative, Q134 as a Hypoxic Adaptation Inducing Agent" Molecules 24, no. 23: 4269. https://doi.org/10.3390/molecules24234269
APA StyleHackler, L., Jr., Gyuris, M., Huzián, O., Alföldi, R., Szebeni, G. J., Madácsi, R., Knapp, L., Kanizsai, I., & Puskás, L. G. (2019). Enantioselective Synthesis of 8-Hydroxyquinoline Derivative, Q134 as a Hypoxic Adaptation Inducing Agent. Molecules, 24(23), 4269. https://doi.org/10.3390/molecules24234269









