Analysis of Multiclass Antibiotics in Lettuce by Liquid Chromatography–Tandem Mass Spectrometry to Monitor Their Plant Uptake
Abstract
1. Introduction
2. Results
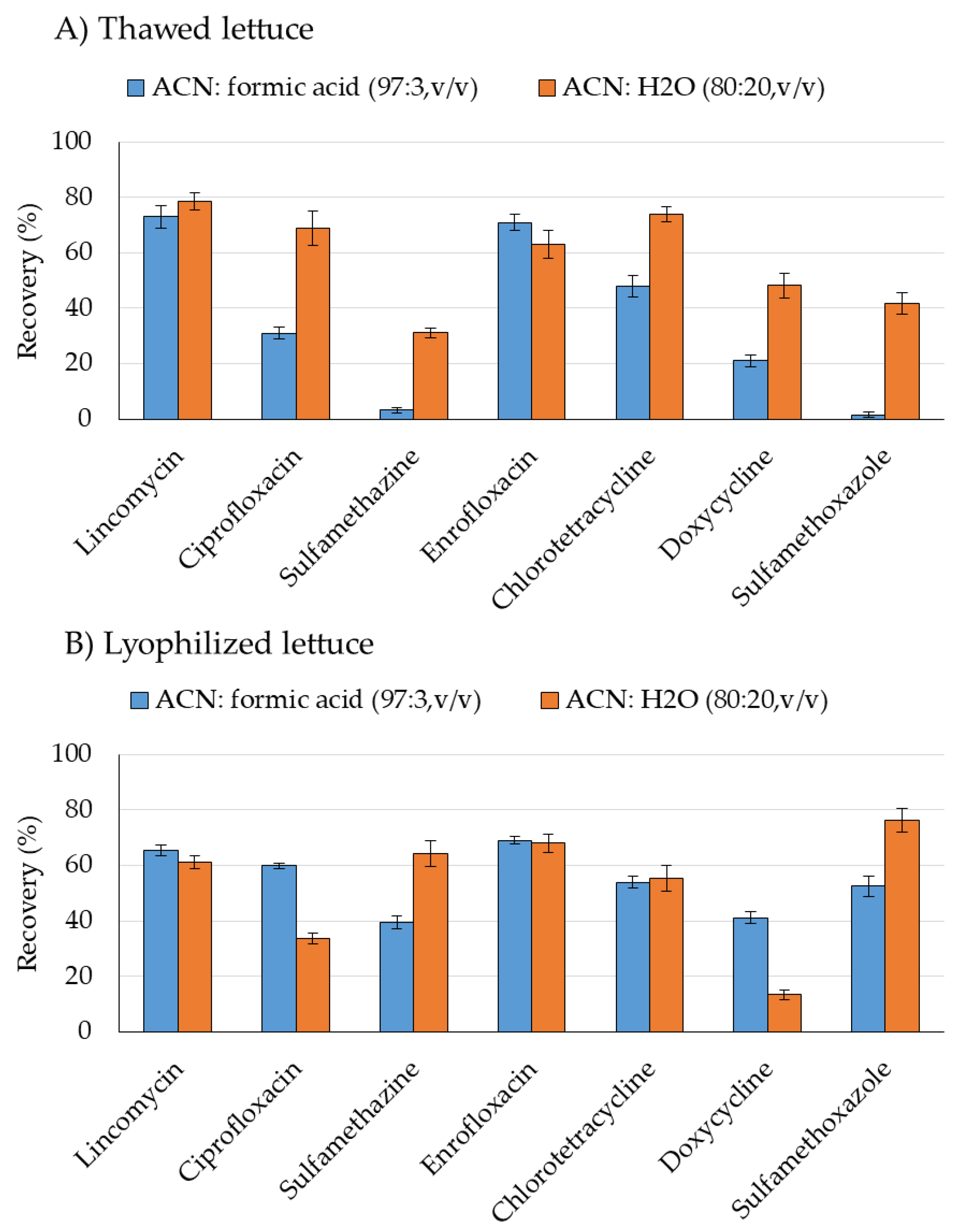
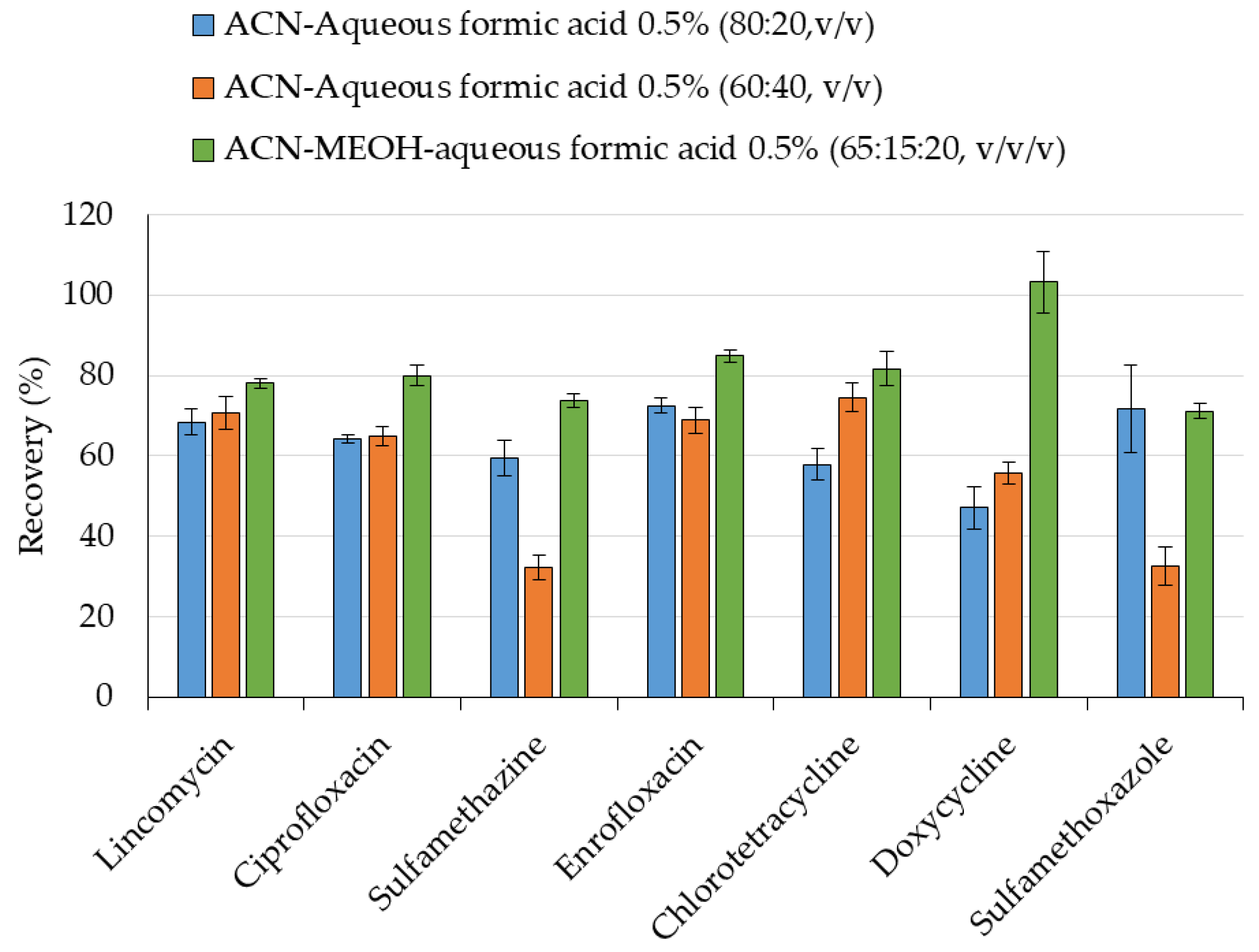
2.1. Sample Preparation
2.2. Method Validation
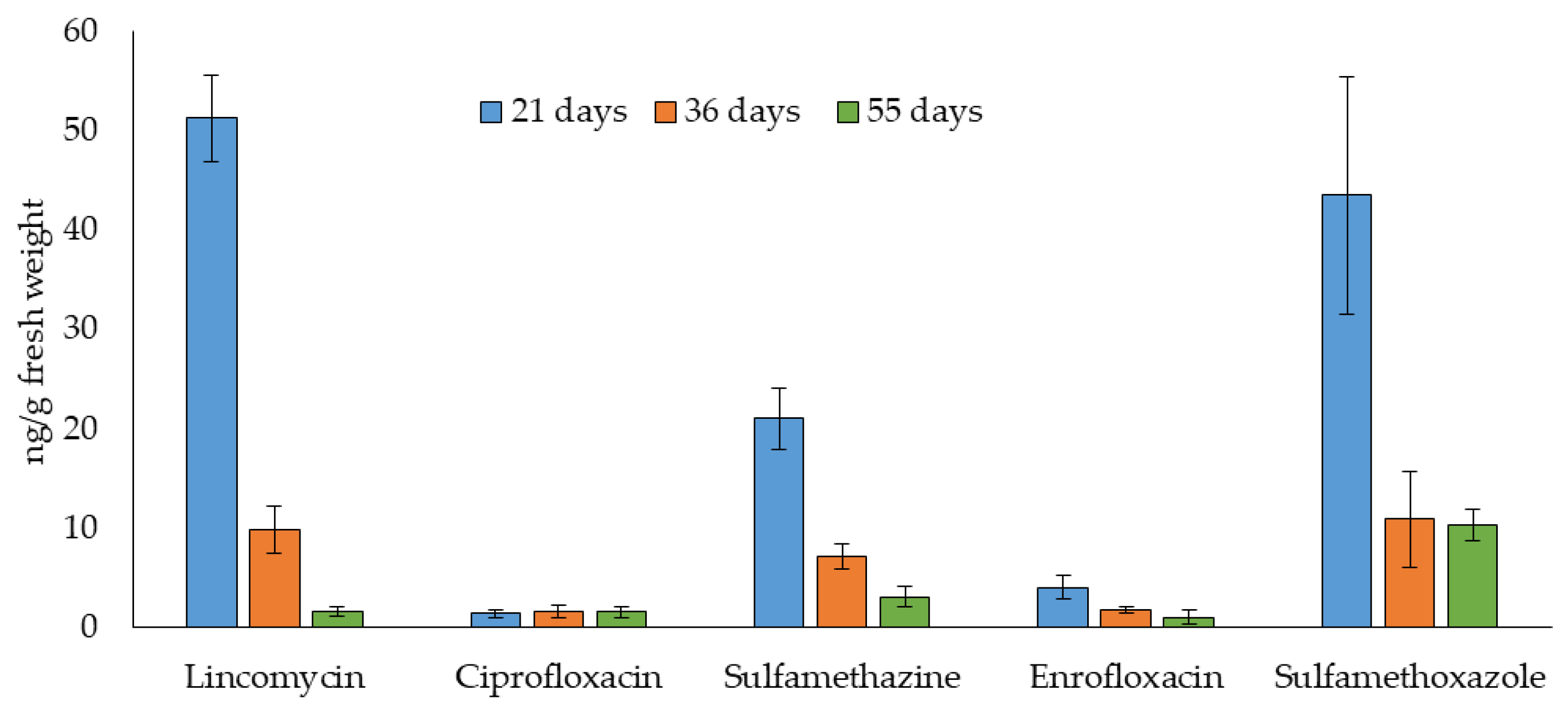
2.3. Application to the Uptake of Antibiotics in Lettuce
3. Discussion
4. Materials and Methods
4.1. Reagents and Materials
4.2. Samples
4.3. Sample Preparation
4.4. LC-MS/MS Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Carvalho, I.T.; Santos, L. Antibiotics in the aquatic environments: A review of the European scenario. Environ. Int. 2016, 94, 736–757. [Google Scholar] [CrossRef]
- Massé, D.I.; Saady, N.M.C.; Gilbert, Y. Potential of Biological Processes to Eliminate Antibiotics in Livestock Manure: An Overview. Animals 2014, 4, 146–163. [Google Scholar] [CrossRef]
- Li, W.C. Occurrence, sources, and fate of pharmaceuticals in aquatic environment and soil. Environ. Pollut. 2014, 187, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Chu, L.M. Fate of antibiotics in soil and their uptake by edible crops. Sci. Total Environ. 2017, 599–600, 500–512. [Google Scholar] [CrossRef] [PubMed]
- Boxall, A.B.A.; Johnson, P.; Smith, E.J.; Sinclair, C.J.; Stutt, E.; Levy, L.S. Uptake of veterinary medicines from soils into plants. J. Agric. Food Chem. 2006, 54, 2288–2297. [Google Scholar] [CrossRef] [PubMed]
- Chitescu, C.L.; Oosterink, E.; De Jong, J.; Stolker, A.A.M. Ultrasonic or accelerated solvent extraction followed by U-HPLC-high mass accuracy MS for screening of pharmaceuticals and fungicides in soil and plant samples. Talanta 2012, 88, 653–662. [Google Scholar] [CrossRef]
- Dolliver, H.; Kumar, K.; Gupta, S. Sulfamethazine Uptake by Plants from Manure-Amended Soil. J. Environ. Qual. 2007, 36, 1224. [Google Scholar] [CrossRef]
- Kang, D.H.; Gupta, S.; Rosen, C.; Fritz, V.; Singh, A.; Chander, Y.; Murray, H.; Rohwer, C. Antibiotic uptake by vegetable crops from manure-applied soils. J. Agric. Food Chem. 2013, 61, 9992–10001. [Google Scholar] [CrossRef]
- Kumar, K.; Gupta, S.C.; Baidoo, S.K.; Chander, Y.; Rosen, C.J. Antibiotic Uptake by Plants from Soil Fertilized with Animal Manure. J. Environ. Qual. 2005, 34, 2082. [Google Scholar] [CrossRef]
- Li, X.; Yu, H.; Xu, S.; Hua, R. Uptake of three sulfonamides from contaminated soil by pakchoi cabbage. Ecotoxicol. Environ. Saf. 2013, 92, 297–302. [Google Scholar] [CrossRef]
- Sabourin, L.; Duenk, P.; Bonte-Gelok, S.; Payne, M.; Lapen, D.R.; Topp, E. Uptake of pharmaceuticals, hormones and parabens into vegetables grown in soil fertilized with municipal biosolids. Sci. Total Environ. 2012, 431, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.S.; Lee, Y.J.; Rahman, M.M.; Abd El-Aty, A.M.; Lee, H.S.; Kabir, M.H.; Kim, S.W.; Park, B.J.; Kim, J.E.; Hacımüftüoğlu, F.; et al. Uptake of the veterinary antibiotics chlortetracycline, enrofloxacin, and sulphathiazole from soil by radish. Sci. Total Environ. 2017, 605–606, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Bhalsod, G.D.; Chuang, Y.-H.; Jeon, S.; Gui, W.; Li, H.; Ryser, E.T.; Guber, A.K.; Zhang, W. Uptake and Accumulation of Pharmaceuticals in Overhead- and Surface-Irrigated Greenhouse Lettuce. J. Agric. Food Chem. 2018, 66, 822–830. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.B.M.; Rajapaksha, A.U.; Lim, J.E.; Vu, N.T.; Kim, I.S.; Kang, H.M.; Lee, S.S.; Ok, Y.S. Distribution and accumulative pattern of tetracyclines and sulfonamides in edible vegetables of cucumber, tomato, and lettuce. J. Agric. Food Chem. 2015, 63, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Preciado, D.; Matamoros, V.; Bayona, J.M. Occurrence and potential crop uptake of emerging contaminants and related compounds in an agricultural irrigation network. Sci. Total Environ. 2011, 412–413, 14–19. [Google Scholar]
- Jones-Lepp, T.L.; Sanchez, C.A.; Moy, T.; Kazemi, R. Method development and application to determine potential plant uptake of antibiotics and other drugs in irrigated crop production systems. J. Agric. Food Chem. 2010, 58, 11568–11573. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Naeem, M.; Chaudhry, M.N.; Iqbal, M.A. Accumulation of Residual Antibiotics in the Vegetables Irrigated by Pharmaceutical Wastewater. Expo. Heal. 2016, 8, 107–115. [Google Scholar] [CrossRef]
- Pan, M.; Wong, C.K.C.; Chu, L.M. Distribution of antibiotics in wastewater-irrigated soils and their accumulation in vegetable crops in the Pearl River Delta, Southern China. J. Agric. Food Chem. 2014, 62, 11062–11069. [Google Scholar] [CrossRef]
- Sallach, J.B.; Zhang, Y.; Hodges, L.; Snow, D.; Li, X.; Bartelt-Hunt, S. Concomitant uptake of antimicrobials and Salmonella in soil and into lettuce following wastewater irrigation. Environ. Pollut. 2015, 197, 269–277. [Google Scholar] [CrossRef]
- Wu, X.; Dodgen, L.K.; Conkle, J.L.; Gan, J. Plant uptake of pharmaceutical and personal care products from recycled water and biosolids: A review. Sci. Total Environ. 2015, 536, 655–666. [Google Scholar] [CrossRef]
- Hu, F.; Bian, K.; Liu, Y.; Su, Y.; Zhou, T.; Song, X.; He, L. Development of a modified QUick, Easy, CHeap, Effective, Rugged and Safe method for the determination of multi-class antimicrobials in vegetables by liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2014, 1368, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.H.; Zhang, Y.; Zhang, W.; Boyd, S.A.; Li, H. Comparison of accelerated solvent extraction and quick, easy, cheap, effective, rugged and safe method for extraction and determination of pharmaceuticals in vegetables. J. Chromatogr. A 2015, 1404, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sallach, J.B.; Snow, D.; Hodges, L.; Li, X.; Bartelt-Hunt, S. Development and comparison of four methods for the extraction of antibiotics from a vegetative matrix. Environ. Toxicol. Chem. 2016, 35, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Tadić, Đ.; Matamoros, V.; Bayona, J.M. Simultaneous determination of multiclass antibiotics and their metabolites in four types of field-grown vegetables. Anal. Bioanal. Chem. 2019, 411, 5209–5222. [Google Scholar] [CrossRef] [PubMed]
- Albero, B.; Tadeo, J.L.; Pérez, R.A. Ultrasound-assisted extraction of organic contaminants. Trac Trends Anal. Chem. 2019, 118, 739–750. [Google Scholar] [CrossRef]
- Definition and Procedure for the Determination of the Method Detection Limit, Revision 2. Available online: https://www.epa.gov/sites/production/files/2016-12/documents/mdl-procedure_rev2_12-13-2016.pdf (accessed on 5 November 2019).
- Montemurro, N.; Postigo, C.; Lonigro, A.; Perez, S.; Barceló, D. Development and validation of an analytical method based on liquid chromatography–tandem mass spectrometry detection for the simultaneous determination of 13 relevant wastewater-derived contaminants in lettuce. Anal. Bioanal. Chem. 2017, 409, 5375–5387. [Google Scholar] [CrossRef]
- Albero, B.; Tadeo, J.L.; Escario, M.; Miguel, E.; Pérez, R.A. Persistence and availability of veterinary antibiotics in soil and soil–manure systems. Sci. Total Environ. 2018, 643, 1562–1570. [Google Scholar] [CrossRef]
- Sidhu, H.; O’Connor, G.; Kruse, J. Plant Toxicity and Accumulation of Biosolids-Borne Ciprofloxacin and Azithromycin. Sci. Total Environ. 2019, 648, 1219–1226. [Google Scholar] [CrossRef]
- Wu, X.; Conkle, J.L.; Gan, J. Multi-residue determination of pharmaceutical and personal care products in vegetables. J. Chromatogr. A 2012, 1254, 78–86. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
| 1000 ng/g dw | 50 ng/g dw | MDL | LOQ | |||
|---|---|---|---|---|---|---|
| Mean (%) | RSD (%) | Mean (%) | RSD (%) | |||
| Lincomycin | 78 | 1 | 83 | 3 | 3 | 10 |
| Ciprofloxacin | 80 | 3 | 90 | 6 | 7 | 24 |
| Sulfamethazine | 74 | 2 | 67 | 5 | 10 | 33 |
| Enrofloxacin | 85 | 2 | 70 | 9 | 3 | 10 |
| Chlorotetracycline | 82 | 4 | 93 | 8 | 13 | 45 |
| Doxycycline | 107 | 8 | 79 | 6 | 12 | 38 |
| Sulfamethoxazole | 71 | 2 | 73 | 6 | 5 | 15 |
| Days | Lincomycin | Ciprofloxacin | Sulfamethazine | Enrofloxacin | Sulfamethoxazole |
|---|---|---|---|---|---|
| 21 | 0.020 | 0.001 | 0.008 | 0.002 | 0.017 |
| 36 | 0.004 | 0.001 | 0.003 | 0.001 | 0.004 |
| 55 | 0.001 | 0.001 | 0.001 | 0.0004 | 0.004 |
| Analytes (Compounds in Common with the Present Work) | Lettuce Variety | Spiking Concentration | Antibiotic Entry Route | Harvest Time (days) | Conditions | Antibiotic Uptake by Lettuce Leaves | Ref. |
|---|---|---|---|---|---|---|---|
| 10 veterinary medicines (ENR) | All Year Round (Butterhead) | 1 mg/kg soil | Soil | 103 | 70% humidity, 20 °C light cycle 15 °C dark cycle | ENR: < LOD | [5] |
| SFZ | Not stated | 50 and 100 mg/L manure (1.25 and 2.5 mg/kg soil) | Soil–manure system | 45 | Greenhouse 18–23 °C | SFZ: 1000–1100 µg/kg dw | [7] |
| 11 pharmaceuticals (LIN, SFX) | Black Seeded Simpson | 50 and 30 μg/L | Irrigation water | 7, 14, 35 | Greenhouse, 24 °C 43% humidity | LIN: 1–6 µg/kg fw SFX: 0.05 µg/kg fw | [13] |
| 6 antibiotics (CTC, SMX, SMZ) | Not stated | 5, 10 and 20 mg/kg soil | Irrigation water | 45 | Greenhouse, 25 °C 70% humidity | TCs: 10–200 µg/kg SFs: 400–3200 µg/kg | [14] |
| 7 antibiotics (LIN, SFX) | Green Star Salad Bowl | 1 mg/L water | Irrigation water | 24, 35, 46 | Greenhouse 15–18 °C | LIN: 84–822 µg/kg fw SFX: 22–125 µg/kg fw | [19] |
| 2 antibiotics (CIP) | Buttercrunch | 0.01, 0.115, 0.371 mg/kg soil | Soil-biosolids system | 46 | Greenhouse 20–25 °C | CIP: 4–5 µg/kg dw | [29] |
| 19 PPCPs (SFX) | Iceberg | 500 ng/L | Nutrient solution | 21 | Greenhouse, 12–32 °C 40–90% humidity | SFX: <LOD | [30] |
| 7 antibiotics (CIP, CTC, DOX, ENR, LIN, SMX, SMZ) | Batavia | 2.5 mg/kg soil | Soil-poultry manure system | 21, 36, 55 | Greenhouse ambient conditions | FQs: 1–4 µg/kg fw TCs: < LODs SFs: 3–43 µg/kg fw LIN: 2–51 µg/kg fw | Present work |
| Compound | MRM 1 | CE (eV) | MRM 2 | CE (eV) | Fragmentor (V) |
|---|---|---|---|---|---|
| Lincomycin | 407 > 359 | 15 | 407 > 126 | 30 | 150 |
| Ciprofloxacin | 332 > 314 | 18 | 332 > 231 | 42 | 130 |
| Enrofloxacin | 360 > 342 | 18 | 360 > 316 | 18 | 130 |
| Chlortetracycline | 479 > 462 | 15 | 479 > 444 | 20 | 120 |
| Doxycycline | 445 > 428 | 25 | 445 > 154 | 30 | 120 |
| Sulfamethazine | 279 > 186 | 15 | 279 > 124 | 20 | 130 |
| Sulfamethoxazole | 254 > 156 | 15 | 254 > 92 | 25 | 100 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albero, B.; Tadeo, J.L.; Delgado, M.d.M.; Miguel, E.; Pérez, R.A. Analysis of Multiclass Antibiotics in Lettuce by Liquid Chromatography–Tandem Mass Spectrometry to Monitor Their Plant Uptake. Molecules 2019, 24, 4066. https://doi.org/10.3390/molecules24224066
Albero B, Tadeo JL, Delgado MdM, Miguel E, Pérez RA. Analysis of Multiclass Antibiotics in Lettuce by Liquid Chromatography–Tandem Mass Spectrometry to Monitor Their Plant Uptake. Molecules. 2019; 24(22):4066. https://doi.org/10.3390/molecules24224066
Chicago/Turabian StyleAlbero, Beatriz, José L. Tadeo, María del Mar Delgado, Esther Miguel, and Rosa Ana Pérez. 2019. "Analysis of Multiclass Antibiotics in Lettuce by Liquid Chromatography–Tandem Mass Spectrometry to Monitor Their Plant Uptake" Molecules 24, no. 22: 4066. https://doi.org/10.3390/molecules24224066
APA StyleAlbero, B., Tadeo, J. L., Delgado, M. d. M., Miguel, E., & Pérez, R. A. (2019). Analysis of Multiclass Antibiotics in Lettuce by Liquid Chromatography–Tandem Mass Spectrometry to Monitor Their Plant Uptake. Molecules, 24(22), 4066. https://doi.org/10.3390/molecules24224066








