The Contribution of Carotenoids, Phenolic Compounds, and Flavonoids to the Antioxidative Properties of Marine Microalgae Isolated from Mediterranean Morocco
Abstract
1. Introduction
2. Results and Discussion
2.1. Extraction of Polyphenolic Compounds and Carotenoids
2.2. Total Phenolics, Flavonoids, and Carotenoids and Antioxidant Activity
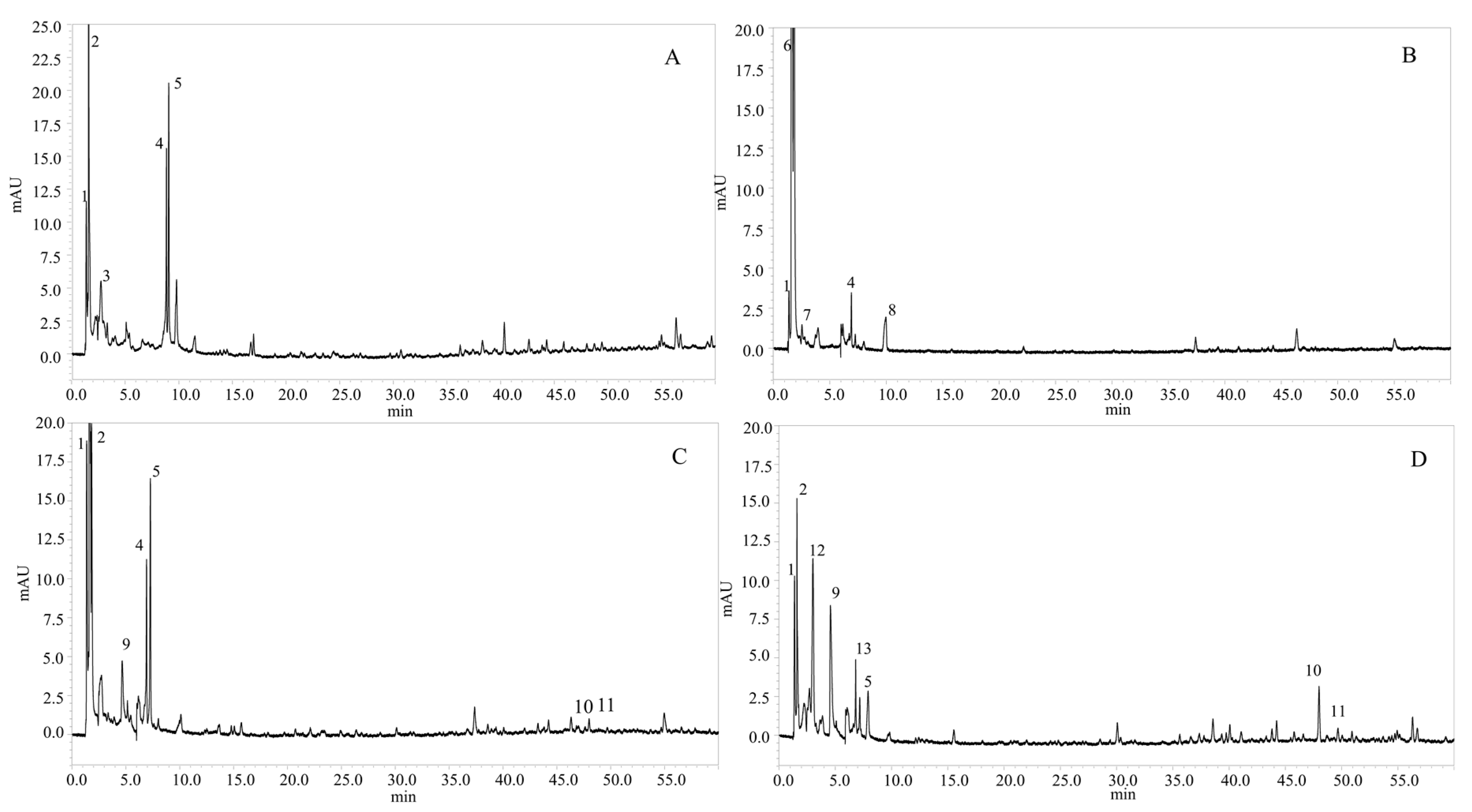
2.3. Determination of Phenolic Compounds in the Microalgae Species
2.3.1. Identification of Phenolic Compounds
2.3.2. Quantification of Phenolic Compounds
2.4. Determination of the Carotenoids in the Microalgae Species
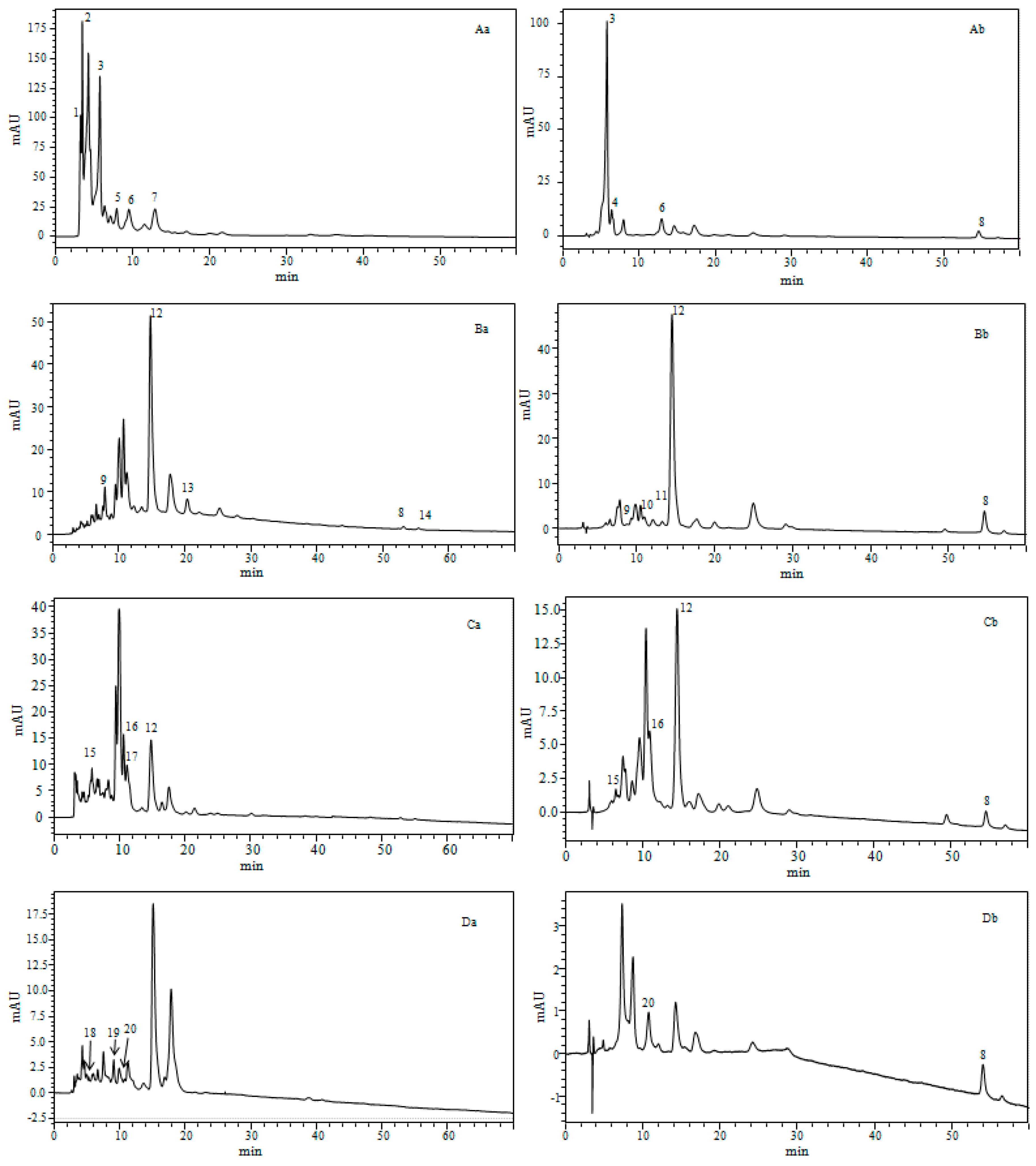
2.4.1. Identification of Carotenoids
2.4.2. Quantification of Carotenoids in Four Marine Microalgae
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Micro-Organism and Culture Conditions
3.3. Sample Preparations
3.3.1. Phenolic Compounds
3.3.2. Carotenoids Contents
3.4. Analytical Methods
3.4.1. Antioxidant Properties
DPPH Radical Scavenging Activity Assay
Ferrous Ions Reduction Power (FRAP)
Ferrous Ion-Chelating Ability
3.4.2. Phenolic Compounds Analysis
Total Phenolic Content
Total Flavonoids
Phenolic Compounds by HPL–-PDA–(ESI)–MS Analysis
3.4.3. Carotenoids Analysis
Total Carotenoids and Estimation of Chlorophyll
Chlorophyll (b) = (21.5 A646.8) − (5.10 A663.2)
Total Carotenoids = (1000 A470) − (1.82 Chlorophyll (a)) − (85.02 Chlorophyll (b)) /198
Carotenoid and Pigment Composition by HPLC–PDA–(ESI)–MS Analysis
3.4.4. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pokorný, J. Natural antioxidants for food use. Trends Food Sci. Technol. 1991, 2, 223–227. [Google Scholar] [CrossRef]
- Stengel, D.B.; Connan, S.; Popper, Z.A. Algal chemodiversity and bioactivity: Sources of natural variability and implications for commercial application. Biotechnol. Adv. 2011, 29, 483–501. [Google Scholar] [CrossRef]
- Li, H.-B.; Cheng, K.-W.; Wong, C.-C.; Fan, K.-W.; Chen, F.; Jiang, Y. Evaluation of antioxidant capacity and total phenolic content of different fractions of selected microalgae. Food Chem. 2007, 102, 771–776. [Google Scholar] [CrossRef]
- Guzman, S.; Gato, A.; Calleja, J.M. Antiinflammatory, analgesic and free radical scavenging activities of the marine microalgae Chlorella stigmatophora and Phaeodactylum tricornutum. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2001, 15, 224–230. [Google Scholar]
- Banskota, A.H.; Sperker, S.; Stefanova, R.; McGinn, P.J.; O’Leary, S.J.B. Antioxidant properties and lipid composition of selected microalgae. J. Appl. Phycol. 2019, 31, 309–318. [Google Scholar] [CrossRef]
- Sansone, C.; Galasso, C.; Orefice, I.; Nuzzo, G.; Luongo, E.; Cutignano, A.; Romano, G.; Brunet, C.; Fontana, A.; Esposito, F.; et al. The green microalga Tetraselmis suecica reduces oxidative stress and induces repairing mechanisms in human cells. Sci. Rep. 2017, 7, 41215. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Bulut, O.; Akın, D.; Sönmez, Ç.; Öktem, A.; Yücel, M.; Öktem, H.A. Phenolic compounds, carotenoids, and antioxidant capacities of a thermo-tolerant Scenedesmus sp. (Chlorophyta) extracted with different solvents. J. Appl. Phycol. 2019, 31, 1675–1683. [Google Scholar] [CrossRef]
- Goiris, K.; Muylaert, K.; Fraeye, I.; Foubert, I.; De Brabanter, J.; De Cooman, L. Antioxidant potential of microalgae in relation to their phenolic and carotenoid content. J. Appl. Phycol. 2012, 24, 1477–1486. [Google Scholar] [CrossRef]
- Jahnke, L.S. Massive carotenoid accumulation in Dunaliella bardawil induced by ultraviolet-A radiation. J. Photochem. Photobiol. B. 1999, 48, 68–74. [Google Scholar] [CrossRef]
- Goiris, K.; Muylaert, K.; Voorspoels, S.; Noten, B.; De Paepe, D.E.; Baart, G.J.; De Cooman, L. Detection of flavonoids in microalgae from different evolutionary lineages. J. Phycol. 2014, 50, 483–492. [Google Scholar] [CrossRef]
- Barredo, J.L. Microbial Carotenoids from Bacteria and Microalgae: Methods and Protocols; Springer: Leon, Spain, 2012; ISBN 1-61779-879-7. [Google Scholar]
- Hernández-Ledesma, B.; Herrero, M. Bioactive Compounds from Marine Foods: Plant and Animal Sources; John Wiley & Sons: Madrid, Spain, 2013; ISBN 1-118-41287-7. [Google Scholar]
- Landete, J.M. Dietary intake of natural antioxidants: Vitamins and polyphenols. Crit. Rev. Food Sci. Nutr. 2013, 53, 706–721. [Google Scholar] [CrossRef]
- Safafar, H.; Van Wagenen, J.; Møller, P.; Jacobsen, C. Carotenoids, phenolic compounds and tocopherols contribute to the antioxidative properties of some microalgae species grown on industrial wastewater. Mar. Drugs. 2015, 13, 7339–7356. [Google Scholar] [CrossRef]
- Craft, N.E.; Soares, J.H. Relative solubility, stability, and absorptivity of lutein and beta-carotene in organic solvents. J. Agric. Food Chem. 1992, 40, 431–434. [Google Scholar] [CrossRef]
- Miceli, N.; Buongiorno, L.P.; Celi, M.G.; Cacciola, F.; Dugo, P.; Donato, P.; Mondello, L.; Bonaccorsi, I.; Taviano, M.F. Role of the flavonoid-rich fraction in the antioxidant and cytotoxic activities of Bauhinia forficata Link. (Fabaceae) leaves extract. Nat. Prod. Res. 2016, 30, 1229–1239. [Google Scholar] [CrossRef]
- Miceli, N.; Marino, A.; Köroğlu, A.; Cacciola, F.; Dugo, P.; Mondello, L.; Taviano, M.F. Comparative study of the phenolic profile, antioxidant and antimicrobial activities of leaf extracts of five Juniperus, L. (Cupressaceae) taxa growing in Turkey. Nat. Prod. Res. 2018, 30, 1–6. [Google Scholar] [CrossRef]
- Taviano, M.F.; Filocamo, A.; Ragusa, S.; Cacciola, F.; Dugo, P.; Mondello, L.; Paterniti Mastrazzo, G.; De Rose, R.F.; Celano, M.; Lombardo, G.E. Phenolic profile, antioxidant and cytotoxic properties of polar extracts from leaves and flowers of Isatis tinctoria L.(Brassicaceae) growing in Sicily. Plant Biosyst. 2018, 152, 795–803. [Google Scholar] [CrossRef]
- Maadane, A.; Merghoub, N.; Ainane, T.; El Arroussi, H.; Benhima, R.; Amzazi, S.; Bakri, Y.; Wahby, I. Antioxidant activity of some Moroccan marine microalgae: Pufa profiles, carotenoids and phenolic content. J. Biotechnol. 2015, 215, 13–19. [Google Scholar] [CrossRef]
- López, A.; Rico, M.; Rivero, A.; Suaréz de Tanguil, M. The effects of solvents on the phenolic contents and antioxidant activity of stypocaulon scoparium algae extracts. Food Chem. 2011, 125, 1104–1109. [Google Scholar] [CrossRef]
- Duval, B.; Shetty, K.; Thomas, W.H. Phenolic compounds and antioxidantproperties in the snow alga Chlamydomonas nivalis after exposure to UV light. J. Appl. Phycol. 2000, 11, 559–566. [Google Scholar] [CrossRef]
- Kovácik, J.; Klejdus, B.; Backor, M. Physiological responses of Scenedesmus quadricauda(Chlorophyceae) to UV-A and UV-C light. Photochem. Photobiol. 2010, 86, 612–616. [Google Scholar] [CrossRef]
- Wang, S.; Liu, L.; Wang, L.; Hu, Y.; Zhang, W.; Liu, R. Structural characterization and identification of major constituents in Jitai tablets by high-performance liquid chromatography/diode-array detection coupled with electrospray ionization tandem mass spectrometry. Molecules 2012, 17, 10470–10493. [Google Scholar] [CrossRef]
- Hossain, M.B.; Rai, D.K.; Brunton, N.P.; Martin-Diana, A.B.; Barry-Ryan, C. Characterization of phenolic composition in Lamiaceae spices by LC-ESI-MS/MS. J. Agric. Food Chem. 2010, 58, 10576–10581. [Google Scholar] [CrossRef]
- Scaglioni, P.T.; Quadros, L.; de Paula, M.; Furlong, V.B.; Abreu, P.C.; Badiale-Furlong, E. Inhibition of Enzymatic and Oxidative Processes by Phenolic Extracts from Spirulina sp. and Nannochloropsis sp. Food Technol. Biotechnol. 2018, 56, 344–353. [Google Scholar] [CrossRef]
- Spínola, V.; Pinto, J.; Castilho, P.C. Identification and quantification of phenolic compounds of selected fruits from Madeira Island by HPLC-DAD-ESI-MSn and screening for their antioxidant activity. Food Chem. 2015, 173, 14–30. [Google Scholar] [CrossRef]
- Pitakpawasutthi, Y.; Palanuvej, C.; Ruangrungsi, N. Quality evaluation of Kaempferia parviflora rhizome with reference to 5, 7-dimethoxyflavone. J. Adv. Pharm. Technol. Res. 2018, 9, 26. [Google Scholar]
- PubChem (2S)-2-Amino-3-[4-[(E)-3-(4-hydroxyphenyl)prop-2-enoyl]oxyphenyl]propanoic acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/90119822 (accessed on 26 May 2019).
- Benayad, Z.; Gómez-Cordovés, C.; Es-Safi, N.E. Characterization of Flavonoid Glycosides from Fenugreek (Trigonella foenum-graecum) Crude Seeds by HPLC–DAD–ESI/MS Analysis. Int. J. Mol. Sci. 2014, 15, 20668–20685. [Google Scholar] [CrossRef]
- Bystrom, L.M.; Lewis, B.A.; Brown, D.L.; Rodriguez, E.; Obendorf, R.L. Characterisation of phenolics by LC–UV/Vis, LC–MS/MS and sugars by GC in Melicoccus bijugatus Jacq. ‘Montgomery’ fruits. Food Chem. 2008, 111, 1017–1024. [Google Scholar] [CrossRef]
- Spínola, V.; Castilho, P.C. Evaluation of Asteraceae herbal extracts in the management of diabetes and obesity. Contribution of caffeoylquinic acids on the inhibition of digestive enzymes activity and formation of advanced glycation end-products (in vitro). Phytochemistry 2017, 143, 29–35. [Google Scholar] [CrossRef]
- Ncube, E.N.; Mhlongo, M.I.; Piater, L.A.; Steenkamp, P.A.; Dubery, I.A.; Madala, N.E. Analyses of chlorogenic acids and related cinnamic acid derivatives from Nicotiana tabacum tissues with the aid of UPLC-QTOF-MS/MS based on the in-source collision-induced dissociation method. Chem. Cent. J. 2014, 8, 66. [Google Scholar] [CrossRef]
- Gouveia, S.C.; Castilho, P.C. Phenolic composition and antioxidant capacity of cultivated artichoke, Madeira cardoon and artichoke-based dietary supplements. Food Res. Int. 2012, 48, 712–724. [Google Scholar] [CrossRef]
- Ben Said, R.; Hamed, A.I.; Mahalel, U.A.; Al-Ayed, A.S.; Kowalczyk, M.; Moldoch, J.; Oleszek, W.; Stochmal, A. Tentative characterization of polyphenolic compounds in the male flowers of Phoenix dactylifera by liquid chromatography coupled with mass spectrometry and DFT. Int. J. Mol. Sci. 2017, 18, 512. [Google Scholar] [CrossRef]
- Jang, G.H.; Kim, H.W.; Lee, M.K.; Jeong, S.Y.; Bak, A.R.; Lee, D.J.; Kim, J.B. Characterization and quantification of flavonoid glycosides in the Prunus genus by UPLC-DAD-QTOF/MS. Saudi J. Biol. Sci. 2018, 25, 1622–1631. [Google Scholar] [CrossRef]
- Kim, S.M.; Jung, Y.-J.; Kwon, O.-N.; Cha, K.H.; Um, B.-H.; Chung, D.; Pan, C.-H. A potential commercial source of fucoxanthin extracted from the microalga Phaeodactylum tricornutum. Appl. Biochem. Biotechnol. 2012, 166, 1843–1855. [Google Scholar] [CrossRef]
- Soontornchaiboon, W.; Joo, S.S.; Kim, S.M. Anti-inflammatory effects of violaxanthin isolated from microalga Chlorella ellipsoidea in RAW 264.7 macrophages. Biol. Pharm. Bull. 2012, 35, 1137–1144. [Google Scholar] [CrossRef]
- Rodrigues, D.B.; Flores, É.M.; Barin, J.S.; Mercadante, A.Z.; Jacob-Lopes, E.; Zepka, L.Q. Production of carotenoids from microalgae cultivated using agroindustrial wastes. Food Res. Int. 2014, 65, 144–148. [Google Scholar] [CrossRef]
- Inbaraj, B.S.; Chien, J.T.; Chen, B.H. Improved high performance liquid chromatographic method for determination of carotenoids in the microalga Chlorella pyrenoidosa. J. Chromatogr. A. 2006, 1102, 193–199. [Google Scholar] [CrossRef]
- Jin, E.; Polle, J.E.; Lee, H.-K.; Hyun, S.-M.; Chang, M. Xanthophylls in microalgae: From biosynthesis to biotechnological mass production and application. J. Microbiol. Biotechnol. 2003, 13, 165–174. [Google Scholar]
- Veith, T.; Büchel, C. The monomeric photosystem I-complex of the diatom Phaeodactylum tricornutum binds specific fucoxanthin chlorophyll proteins (FCPs) as light-harvesting complexes. Biochim. Biophys. Acta BBA-Bioenerg. 2007, 1767, 1428–1435. [Google Scholar] [CrossRef]
- Pasquet, V.; Morisset, P.; Ihammouine, S.; Chepied, A.; Aumailley, L.; Berard, J.-B.; Serive, B.; Kaas, R.; Lanneluc, I.; Thiery, V. Antiproliferative activity of violaxanthin isolated from bioguided fractionation of Dunaliella tertiolecta extracts. Mar. Drugs. 2011, 9, 819–831. [Google Scholar] [CrossRef]
- Sachindra, N.M.; Sato, E.; Maeda, H.; Hosokawa, M.; Niwano, Y.; Kohno, M.; Miyashita, K. Radical scavenging and singlet oxygen quenching activity of marine carotenoid fucoxanthin and its metabolites. J. Agric. Food Chem. 2007, 55, 8516–8522. [Google Scholar] [CrossRef]
- Ragonese, C.; Tedone, L.; Beccaria, M.; Torre, G.; Cichello, F.; Cacciola, F.; Dugo, P.; Mondello, L. Characterisation of lipid fraction of marine macroalgae by means of chromatography techniques coupled to mass spectrometry. Food Chem. 2014, 145, 932–940. [Google Scholar] [CrossRef]
- Sakata, T.; Yoshikawa, T.; Maeda, K.; del Castillo, C.S.; Dureza, L.A. Identification of microalgae isolated from green water of tilapia culture ponds in the Philippines. Mem. Fac. Fish Kagoshima Univ. 2005, 54, 35–44. [Google Scholar]
- Díez, B.; Pedrós-Alió, C.; Marsh, T.L.; Massana, R. Application of Denaturing Gradient Gel Electrophoresis (DGGE) To Study the Diversity of Marine Picoeukaryotic Assemblages and Comparison of DGGE with Other Molecular Techniques. Appl. Environ. Microbiol. 2001, 67, 2942–2951. [Google Scholar] [CrossRef]
- Choochote, W.; Suklampoo, L.; Ochaikul, D. Evaluation of antioxidant capacities of green microalgae. J. Appl. Phycol. 2014, 26, 43–48. [Google Scholar] [CrossRef]
- Romeilah, R.M.; Fayed, S.A.; Mahmoud, G.I. Chemical compositions, antiviral and antioxidant activities of seven essential oils. J. Appl. Sci. Res. 2010, 6, 50–62. [Google Scholar]
- Nadhiya, K.; Vijayalakshmi, K. Evaluation of total phenol, flavonoid contents and invitro antioxidant activity of benincasa hispida fruit extracts. Int. J. Pharm. Chem. Biol. Sci. 2014, 4, 332–338. [Google Scholar]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. In Methods in Enzymology; Plant Cell Membranes; Academic Press: Cambridge, MA, USA, 1987; pp. 350–382. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |
| Species | Total Phenolics (mg/g GAE)* | Total Flavonoids (mg/g QE)** | Total Carotenoids (mg/g) +, (µg/mL)++ | |
|---|---|---|---|---|
| Nannochloropsis gaditana | 22.94 a ± 0.88 | 5.18 a ± 0.07 | 3.34 a ± 0.05 + | 1.24 b ± 0.01 ++ |
| Phaeodactylum tricornutum | 39.34 d ± 0.60 | 3.05 b ± 0.11 | 5.14 b ± 0.05 + | 2.09 d ± 0.01 ++ |
| Nannochloris sp | 33.23 c ± 0.76 | 4.22 c ± 0.09 | 5.63 d ± 0.11 + | 1.69 c ± 0.01 ++ |
| Tetraselmis suecica | 28.03 b ± 1.17 | 0.61 d ± 0.08 | 5.62 c ± 0.12 + | 0.09 a ± 0.01 ++ |
| Extract | DPPH Test IC50 (µg/mL)* | Reducing Power ASE/mL** | Chelating Activity IC50 (mg/mL)*** |
|---|---|---|---|
| Nannochloropsis gaditana | 400.00 d ± 0.01 | 32.71 d ± 0.02 | 3.52 b ± 0.18 |
| Phaeodactylum tricornutum | 380.00 b ± 0.01 | 23.98 a ± 0.11 | 9.69 c ± 0.32 |
| Nannochloris sp | 356.00 a ± 0.01 | 31.48 c ± 0.05 | 12.82 d ± 0.04 |
| Tetraselmis suecica | 394.40 c ± 0.01 | 28.55 b ± 0.03 | 2.81 a ± 0.01 |
| Standard | 3.70 ± 0.17 | 1.443 ± 0.02 | 0.01 ± 3.55E − 05 |
| Species | Peak (N°) | Rt (min) | λMax(nm) | [M − H]− | Fragment | Compound Identification | References |
|---|---|---|---|---|---|---|---|
| Phaeodactylum tricornutum | 1 | 1.38 | 228, 260 | 153.1 | - | Protocatechuic acid | [25] |
| 2 | 1.60 | 295, 324 | 179.2 | - | Caffeic acid | [12] | |
| 3 | 2.76 | 267, 354 | 683.4 | 341.5 | Caffeic acid hexoside dimer | [26] | |
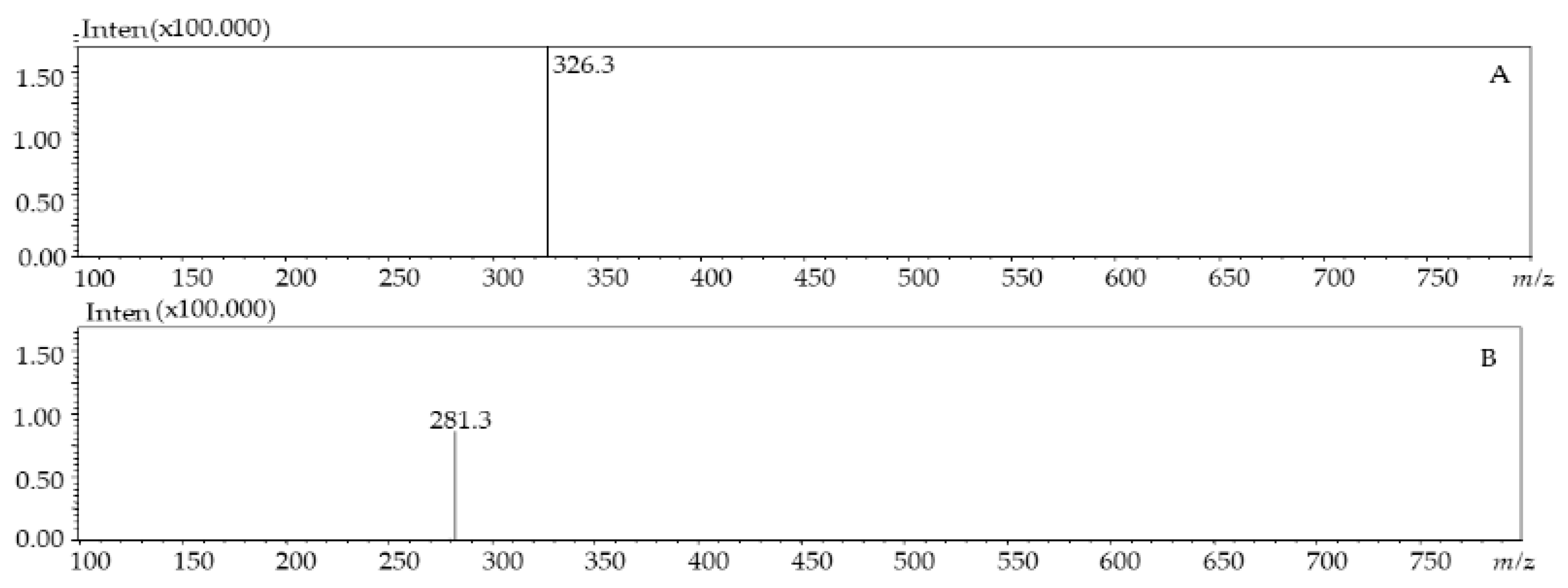
| 4 | 8.85 | 251, 380 | 281.3 | - | Dimethoxyflavone | [27] | |
| 5 | 9.06 | 257 | 326.3 | - | p-coumaroyl tyrosine | [28] | |
| Nannochloris sp | 1 | 1.38 | 228, 260 | 153.1 | - | Protocatechuic acid | [25] |
| 6 | 1.60 | 217, 269 | 277.4 | - | Caffeoyl-coumaroyl-quinic acid | [29] | |
| 7 | 2.53 | 273 | 289.3 | - | Catechin | [30] | |
| 4 | 6.92 | 251, 380 | 281.3 | - | Dimethoxyflavone | [27] | |
| 8 | 9.95 | - | 285.2 | - | Kaempferol | [31] | |
| Tetraselmis suecica | 1 | 1.36 | 228, 260 | 153.1 | - | Protocatechuic acid | [25] |
| 2 | 1.60 | 295, 324 | 179.2 | - | Caffeic acid | [11] | |
| 9 | 4.66 | 263, 339 | 341.3 | - | Caffeoyl glucoside | [32] | |
| 4 | 6.93 | 251, 380 | 281.3 | - | Dimethoxyflavone | [27] | |
| 5 | 7.28 | 257 | 326.3 | - | p-coumaroyl tyrosine | [28] | |
| 10 | 47.99 | 330 | 577.5 | 269 | Apigenin-O-rutinoside | [33] | |
| 11 | 49.67 | 249, 330, 375 | 611.6 | 594 | Rhamnosyl hexosyl-methyl-quercetin | [34] | |
| Nannochloropsis gaditana | 1 | 1.39 | 228, 260 | 153.1 | 135 | Protocatechuic acid | [25] |
| 2 | 1.60 | 295, 324 | 179.2 | 135 | Caffeic acid | [11] | |
| 12 | 3.02 | 247 | 301.2 | 227 | Quercetin | [35,36] | |
| 9 | 4.66 | 263, 339 | 341.3 | 323 | Caffeoyl glucoside | [32] | |
| 13 | 6.82 | 257, 360 | 385.4 | 348 | Feruloylglucaric acid | [26] | |
| 5 | 7.18 | 257 | 326.3 | - | p-coumaroyl tyrosine | [28] | |
| 10 | 47.99 | 330 | 577.5 | 269 | Apigenin-O-rutinoside | [33] | |
| 11 | 49.68 | 249, 330, 375 | 611.6 | 594 | Rhamnosyl hexosyl-methyl-quercetin | [34] |
| Peak | Molecules | Quantity (ppm) | Quantity (µg/g Dry Biomass) |
|---|---|---|---|
| Phaeodactylum tricornutum | |||
| 1 | Protocatechuic acid | 6.85 ± 0.90 | 22.83 ± 2.99 |
| 2 | Caffeic acid | 16.88 ± 1.14 | 56.25 ± 3.81 |
| 3 | Caffeicacidhexosidedimer | 6.32 ± 1.13 | 21.07 ± 3.82 |
| 4 | Dimethoxyflavone | 8.51 ± 0.80 | 28.38 ± 2.90 |
| 5 | p-coumaroyl tyrosine | 4.10 ± 3.78 | 13.68 ± 4.58 |
| Total | 42.66 | 113.83 | |
| Nannochloris sp | |||
| 1 | Protocatechuic acid | 2.26 ± 0.02 | 7.55 ± 0.06 |
| 6 | Caffeoyl-coumaroyl-quinic acid | 17.11 ± 0.52 | 57.04 ± 1.73 |
| 7 | Catechin | 10.04 ± 2.14 | 33.47 ± 3.14 |
| 4 | Dimethoxyflavone | 1.96 ± 0.16 | 6.53 ± 1.84 |
| 8 | kaempferol | 3.63 ± 0.21 | 12.10 ± 1.32 |
| Total | 35.00 | 116.69 | |
| Tetraselmis suecica | |||
| 1 | Protocatechuic acid | 12.16 ± 0.13 | 40.55 ± 0.44 |
| 2 | Caffeic acid | 17.86 ± 0.30 | 59.53 ± 0.98 |
| 9 | Caffeoylglucoside | 4.04 ± 0.35 | 13.46 ± 1.16 |
| 4 | Dimethoxyflavone | 5.7 ± 0.28 | 19.01 ± 1.58 |
| 5 | p-coumaroyl tyrosine | 5.2 ± 0.46 | 17.40 ± 1.55 |
| 10 | Apigenin-O-rutinoside | 10.73 ± 0.34 | 35.75 ± 1.13 |
| 11 | Rhamnosylhexosyl-methyl-quercetin | 1.38 ± 0.18 | 4.59 ± 1.36 |
| Total | 57.07 | 190.29 | |
| Nannochloropsis gaditana | |||
| 1 | Protocatechuic acid | 6.38 ± 0.96 | 21.26 ± 0.96 |
| 2 | Caffeic acid | 5.29 ± 0.21 | 17.64 ± 1.32 |
| 12 | Quercetin | 10.00 ± 0.13 | 33.34 ± 1.46 |
| 9 | Caffeoyl glucoside | 8.55 ± 0.32 | 28.49 ± 1.19 |
| 13 | Feruloylglucaricacid | 2.33 ± 0.18 | 7.78 ± 1.46 |
| 5 | p-coumaroyl tyrosine | 0.64 ± 0.07 | 2.12 ± 0.22 |
| 10 | Apigenin-O-rutinoside | 2.23 ± 0.22 | 7.43 ± 0.74 |
| 11 | Rhamnosylhexosyl-methyl-quercetin | 2.07 ± 0.16 | 6.89 ± 1.21 |
| Total | 37.49 | 124.95 |
| Species | Peaks N⁰ | Rt(min) | ʎ max(nm) | m/zAPCI+/MS | Compound Identification | Sample State |
|---|---|---|---|---|---|---|
| Phaeodactylum tricornutum | 1 | 3.26 | 423, 666 | 545.3 | Unidentified carotenoids | d.bio |
| 2 | 3.48 | 331, 361, 422 | 556.4 | Unidentified carotenoids | d.bio | |
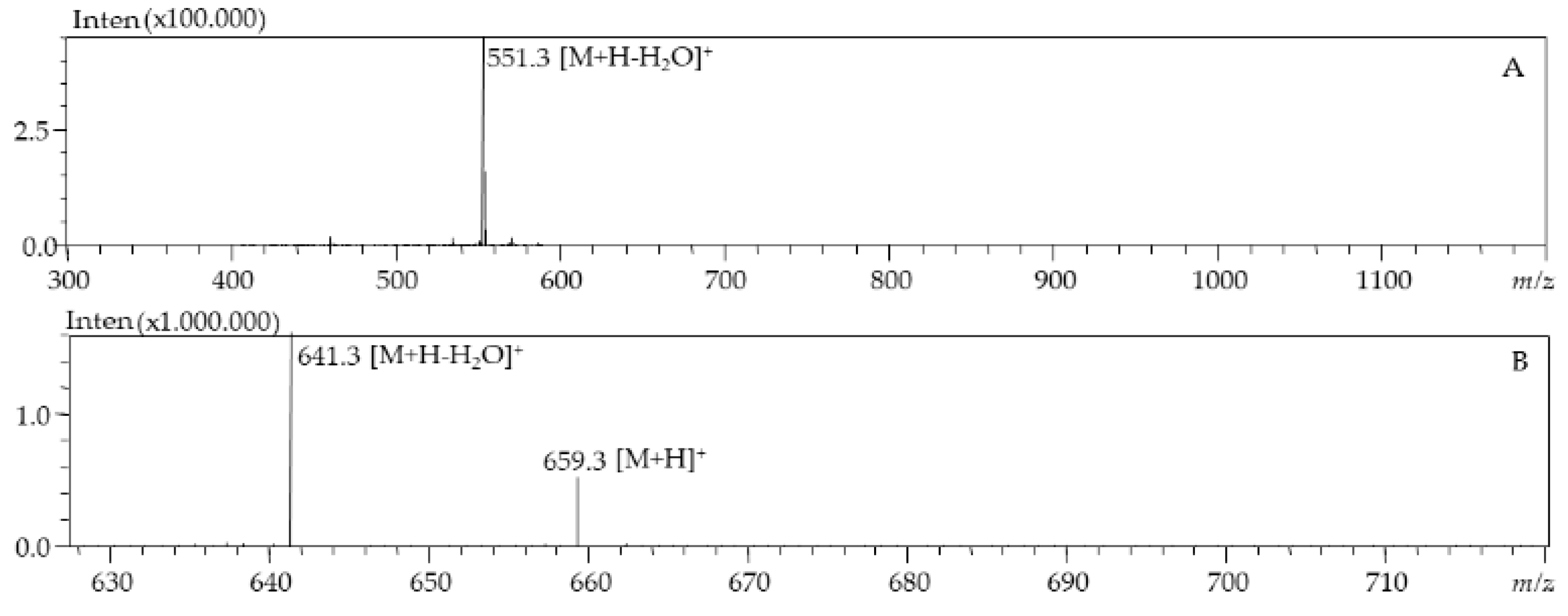
| 3 | 5.77 | 446 | 659.9 | All-E-Fucoxanthin | d.bio, c.liq | |
| 4 | 6.38 | 333, 442 | 659.9 | Fucoxanthin isomer | c.liq | |
| 5 | 7.96 | 436 | 659.9 | Fucoxanthin type | d.bio | |
| 6 | 9.54 | 441 | 659.9 | Fucoxanthin type | d.bio | |
| 7 | 12.93 | 422,446,476 | 585.9 | Diadinoxanthin | d.bio, c.liq | |
| 8 | 54.61 | 425, 450, 478 | 537.9 | beta-carotene | c.liq | |
| 9 | 7.55 | 417, 442, 471 | 601.3 | Vaucheriaxanthin | d.bio, c.liq | |
| 10 | 12.01 | 330, 436, 463 | 569.9 | cis-15-lutein | c.liq | |
| 11 | 13.21 | 331,465 | 569.9 | cis-13-lutein | c.liq | |
| 12 | 14.75 | 442, 472 | 569.9 | Lutein | d.bio, c.liq | |
| 13 | 20.33 | 461 | 551.9 | Echinone | d.bio | |
| 8 | 54,59 | 425, 450, 478 | 537.9 | beta-carotene | d.bio, c.liq | |
| 14 | 55.38 | 342, 424, 446 | 537.9 | 9-cis-beta-carotene | d.bio | |
| Tetraselmis suecica | 15 | 5.76 | 449, 467 | 659.9 | Fucoxanthin | d.bio, c.liq |
| 16 | 10.60 | 253, 345, 457, 592, 640 | 601.9 | Violaxanthin | d.bio, c.liq | |
| 17 | 11.11 | 345, 457, 592, 640 | 600.8 | Cis- Prasinoxanthine | d.bio | |
| 12 | 14.47 | 442, 472 | 569.9 | Lutein | d.bio, c.liq | |
| 8 | 54.59 | 425, 450, 478 | 537.9 | beta-carotene | c.liq | |
| Nannochloropsis gaditana | 18 | 5.27 | 418, 438, 465 | 601.9 | Neoxanthin | d.bio |
| 19 | 9.92 | 422, 444, 472 | 585.9 | Antheraxanthin | d.bio | |
| 20 | 10.75 | 427, 449, 477 | 569.9 | Zeaxanthin | d.bio, c.liq | |
| 8 | 54.59 | 425, 450, 478 | 537.9 | beta-carotene | c.liq |
| Species | Class | Phylum | Infrakingdom | Kingdom | Empire |
|---|---|---|---|---|---|
| Phaedactylum tricornitum | Bacillariophycea | Achrophyta | - | Chromista | Eukaryota |
| Nannochloropsis gaditana | Eustigmatophyceae | Achrophyta | - | Chromista | Eukaryota |
| Nannochloris sp | Trebouxiophyceae | Chlorophyta | Chlorophyta | Plantae | Eukaryota |
| Tetraselmis suecica | Chlorodendrophyceae | Chlorophyta | Chlorophyta | Plantae | Eukaryota |
| Component | Molecular formula | Concentrations (mg) |
|---|---|---|
| Zinc sulphate | ZnSO4 | 30 |
| Copper sulfate | CuSO4 | 25 |
| Cobalt sulphate | CoSO4 | 30 |
| Manganese sulphate | MnSO4 | 20 |
| Ironchloride | FeCl3 | 50 |
| Sodium molybdate | NaMoO4 | 25 |
| Ethylenediaminetetraaceticacid (EDTA) | C10H16N2O8 | 50 |
| Sodium nitrate | NaNO3 | 300 |
| Sodium dihydrogen phosphate | NaH2PO4 | 30 |
| Ammonium sulphate | (NH4)2SO4 | 20 |
| Biotin Vit. H | C10H16N2O3S | 0.1 |
| Thiamine Vit. B1 | C12H17N4OS | 10 |
| Cyanocobalamin Vit. B12 | C63H89CoN14O14P | 0.1 |
| Compounds | UV (nm) | Regression Equation | LOQ(µg/mL) | UV LOD(µg/mL) | R2 |
|---|---|---|---|---|---|
| Gallicacid | 270 | y = 3989.3x + 398.1 | 0.85 | 0.25 | 0.9989 |
| Caffeicacid | 321 | y = 5552.1x + 4136.1 | 0.76 | 0.23 | 0.9983 |
| Rutin | 355 | y = 1602.8x + 2741.9 | 2.49 | 0.75 | 0.9968 |
| Catechin | 278 | y = 807.2x + 1461.2 | 3.25 | 0.97 | 0.9983 |
| Coumarin | 277 | y = 8237.3x + 9230.6 | 0.67 | 0.20 | 0.9975 |
| Kaempferol | 365 | y = 3481.0x + 5372.4 | 1.46 | 0.44 | 0.9974 |
| Apigenin | 336 | y = 4915.8x − 105.3 | 1.16 | 0.35 | 1.0000 |
| Quercetin | 370 | y = 5993.6x + 1452.1 | 1.29 | 0.39 | 0.9999 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haoujar, I.; Cacciola, F.; Abrini, J.; Mangraviti, D.; Giuffrida, D.; Oulad El Majdoub, Y.; Kounnoun, A.; Miceli, N.; Fernanda Taviano, M.; Mondello, L.; et al. The Contribution of Carotenoids, Phenolic Compounds, and Flavonoids to the Antioxidative Properties of Marine Microalgae Isolated from Mediterranean Morocco. Molecules 2019, 24, 4037. https://doi.org/10.3390/molecules24224037
Haoujar I, Cacciola F, Abrini J, Mangraviti D, Giuffrida D, Oulad El Majdoub Y, Kounnoun A, Miceli N, Fernanda Taviano M, Mondello L, et al. The Contribution of Carotenoids, Phenolic Compounds, and Flavonoids to the Antioxidative Properties of Marine Microalgae Isolated from Mediterranean Morocco. Molecules. 2019; 24(22):4037. https://doi.org/10.3390/molecules24224037
Chicago/Turabian StyleHaoujar, Imane, Francesco Cacciola, Jamal Abrini, Domenica Mangraviti, Daniele Giuffrida, Yassine Oulad El Majdoub, Ayoub Kounnoun, Natalizia Miceli, Maria Fernanda Taviano, Luigi Mondello, and et al. 2019. "The Contribution of Carotenoids, Phenolic Compounds, and Flavonoids to the Antioxidative Properties of Marine Microalgae Isolated from Mediterranean Morocco" Molecules 24, no. 22: 4037. https://doi.org/10.3390/molecules24224037
APA StyleHaoujar, I., Cacciola, F., Abrini, J., Mangraviti, D., Giuffrida, D., Oulad El Majdoub, Y., Kounnoun, A., Miceli, N., Fernanda Taviano, M., Mondello, L., Rigano, F., & Skali Senhaji, N. (2019). The Contribution of Carotenoids, Phenolic Compounds, and Flavonoids to the Antioxidative Properties of Marine Microalgae Isolated from Mediterranean Morocco. Molecules, 24(22), 4037. https://doi.org/10.3390/molecules24224037










