In vitro Fermentation of Polysaccharides from Aloe vera and the Evaluation of Antioxidant Activity and Production of Short Chain Fatty Acids
Abstract
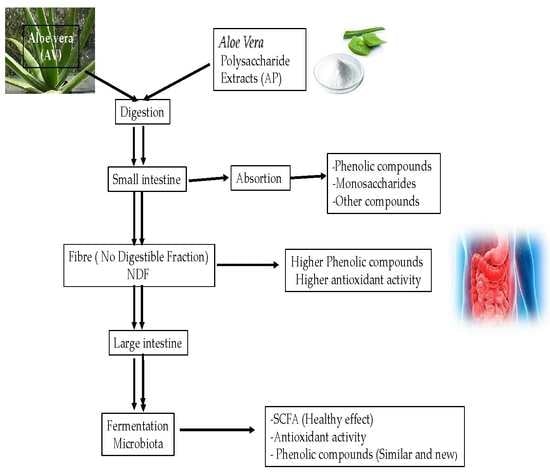
1. Introduction
2. Results and Discussion
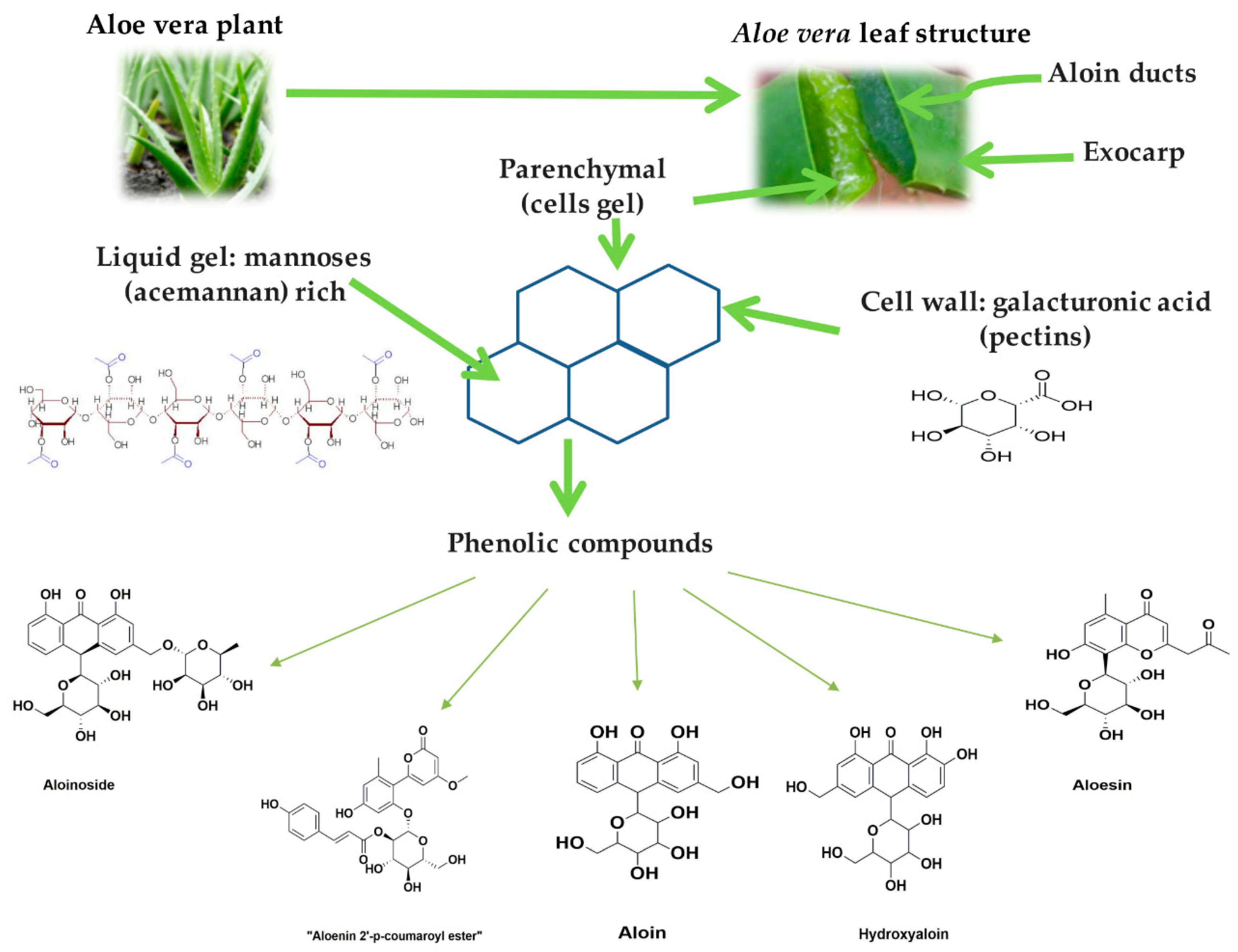
2.1. Physicochemical Characterization of Aloe Vera Gel (AV)
2.2. Identification of Phenolic Compounds in AV by UPLC-MS
2.3. Total Fibre Content of Non-Digestible Fibre Fractions AV-TNDF and AP-TNDF and of Their Soluble (AV-SNDF and AP-SNDF) and Insoluble (AV-INDF and AP-INDF) Fractions
2.4. In Vitro Fermentation
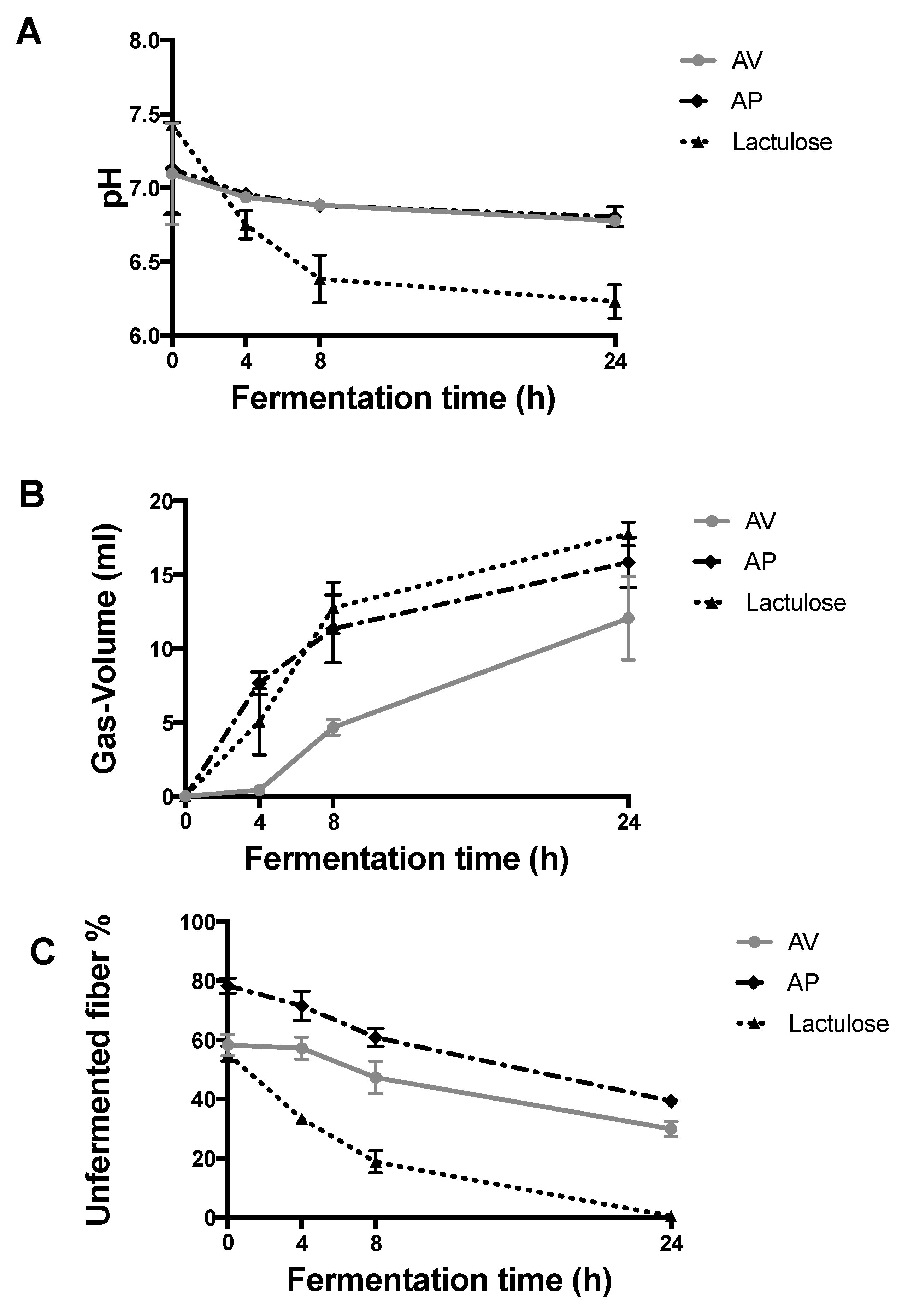
2.4.1. Changes in pH
2.4.2. Volume of gas produced
2.4.3. Unfermented fibre (UF)
2.4.4. Quantification of SCFAs
2.5. Quantification of Phenolic Compounds in AV and AP and Identification of AV, AV-TNDF, AP, AP-TNDF by HPTLC
2.5.1. Quantification of Phenolic Compounds in AV and AP
2.5.2. Identification of phenolic compounds by HPTLC
2.6. Antioxidant Activity of AV-TNDF and AP-TNDF
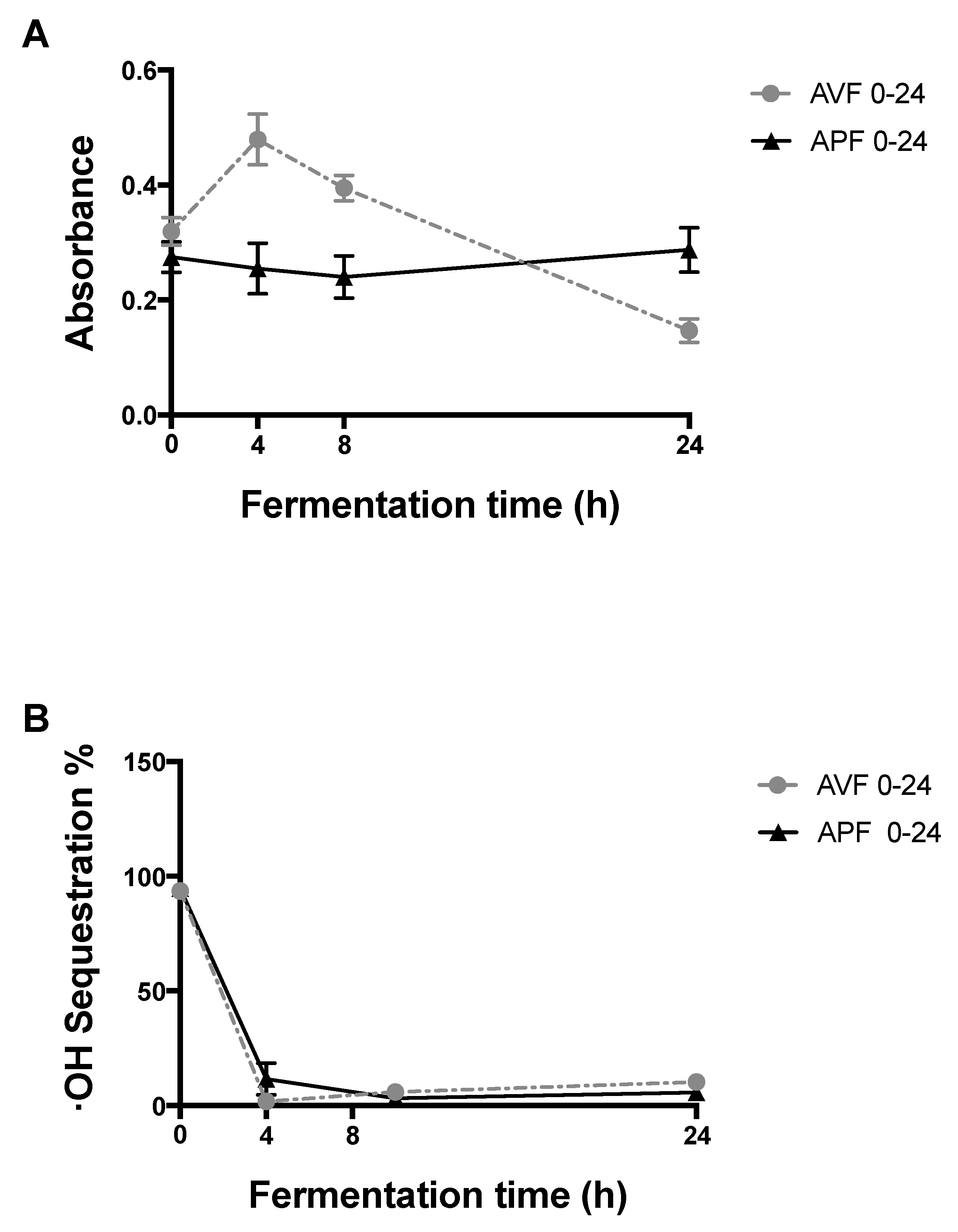
2.6.1. Reducing Power
2.6.2. OH Radical Sequestration
3. Materials and Methods
3.1. Raw Material
3.2. Physicochemical Characterization of Aloe Vera (AV)
3.3. Identification of phenolic Compounds by Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry (UPLC-MS) in Aloe Vera (AV)
3.4. Extraction of Polysaccharides (AP) from Aloe Vera Gel
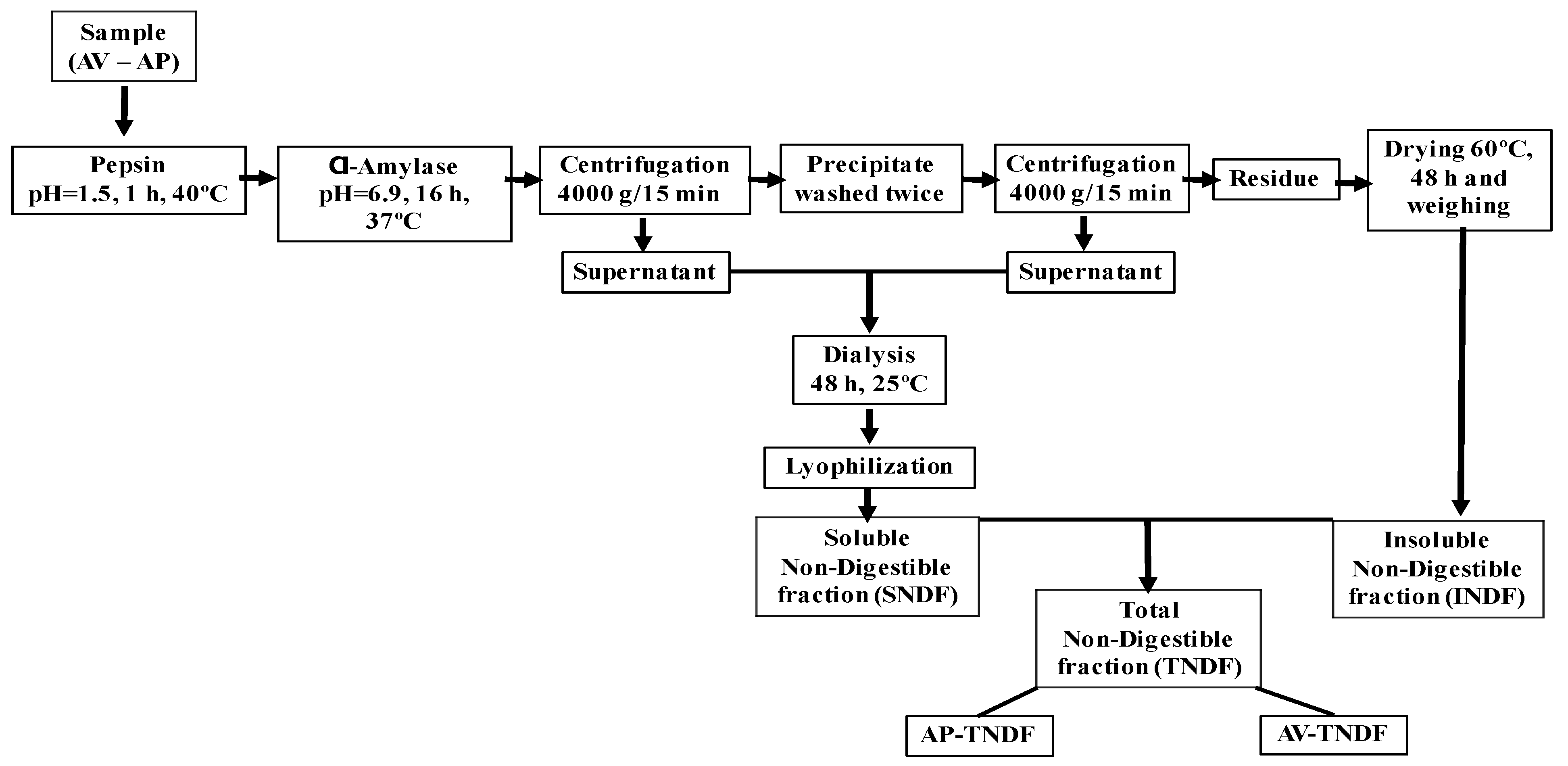
3.5. Indigestible Fraction and in Vitro Fermentation
3.5.1. Indigestible Fermentation of Aloe Vera Gel (AV) and Polysaccharides (AP)
3.5.2. In Vitro Fermentation of AV-TNDF and AP-TNDF
Inoculum
Medium
Procedure
Unfermented Fibre
3.5.3. Quantification of SCFAs in AV-TNDF and AP-TNDF
3.5.4. Quantification of Phenolic Compounds
3.5.5. Identification of Phenolic Compounds by HPTLC
3.6. Antioxidant Activity
3.6.1. Reducing Power and Hydroxyl Radical Scavenging Activity
3.6.2. ∙OH Radical Sequestration
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Durazzo, A.; D’Addezio, L.; Camilli, E.; Piccinelli, R.; Turrini, A.; Marletta, L.; Marconi, S.; Lucarini, M.; Lisciani, S.; Gabrielli, P.; et al. Plant compounds to botanicals and back: A current snapshot. Molecules 2018, 23, 18–44. [Google Scholar] [CrossRef] [PubMed]
- Nejatzadeh-Barandozi, F. Antibacterial activities and antioxidant capacity of Aloe vera. Org. Med. Chem. Lett. 2013, 3, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Kharkwal, A.C.; Kharkwal, H.; Abdin, M.Z.; Varma, A. A Review on pharmacological properties of Aloe vera. Int. J. Pharm. Sci. Rev. Res. 2014, 29, 31–37. [Google Scholar]
- Ni, Y.; Turner, D.; Yates, K.M. Isolation and characterization of structural components of Aloe vera L. leaf pulp. Int. Immunopharmacol. 2004, 4, 1745–1755. [Google Scholar] [CrossRef]
- Wang, P.G.; Zhou, W.; Wamer, W.G.; Krynitsky, A.J.; Rader, J.I. Simultaneous determination of aloin A and aloe emodin in products containing Aloe vera by ultra–performance liquid chromatography with tandem mass spectrometry. Anal. Methods 2012, 4, 3612–3619. [Google Scholar] [CrossRef]
- Hamman, J.H. Composition and applications of Aloe vera leaf gel. Molecules. 2008, 13, 1599. [Google Scholar] [CrossRef]
- Anokwuru, C.; Sigidi, M.; Boukandou, M.; Tshisikhawe, P.; Traore, A.; Potgieter, N. Antioxidant Activity and spectroscopic characteristics of extractable and non–extractable phenolics from Terminalia sericea Burch. ex DC. Molecules 2018, 23, 1303. [Google Scholar] [CrossRef]
- Rajasekaran, S.; Sivagnanam, K.; Subramanian, S. Antioxidant effect of Aloe vera gel extract in streptozotocin–induced diabetes in rats. Pharmacol. Rep. 2005, 57, 90–96. [Google Scholar]
- Ravi, S.; Kabilar, P.; Velmurugan, S.; Ashok, K.R.; Gayathiri, M. Spectroscopy studies on the status of aloin in Aloe vera and commercial samples. J. Exper. Sci. 2011, 2, 10–13. [Google Scholar]
- Patel, D.K.; Patel, K.; Dhanabal, S.P. Phytochemical standardization of Aloe vera extract by HPTLC techniques. J. Acute Dis. 2012, 1, 47–50. [Google Scholar] [CrossRef]
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef]
- Topping, D.L.; Clifton, P.M. Short–Chain fatty acids and human colonic function: Roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 2001, 81, 1031–1064. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Oliver, M.; Quiñones, P.M.A.; Pedraza-Chaverri, J. Health benefits of Aloe Vera. Rev. Esp. Cienc. Salud. 2011, 14, 53–73. [Google Scholar]
- Loots, D.T.; van der Westhuizen, F.H.; Botes, L. Aloe ferox leaf gel phytochemical content, antioxidant capacity, and possible health benefits. J. Agric. Food Chem. 2007, 55, 6891–6896. [Google Scholar]
- Minjares-Fuentes, R.; Femenia, A.; Comas-Serra, F. Compositional and structural features of the main bioactive polysaccharides present in the Aloe vera plant. Plant J. AOAC Inter. 2018, 101, 1711–1719. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Sun, Y.N.; Lee, M.J.; Kim, Y.H.; Lee, W.; Kim, K.H.; Kim, K.T.; Kang, J.S. Identification and discrimination of three common Aloe species by high performance liquid chromatography–tandem mass spectrometry coupled with multivariate analysis. J. Chromat. B. 2016, 1031, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Ding, W.; Zhong, J.; Wan, J.; Xie, Z. Simultaneous qualitative and quantitative determination of phenolic compounds in Aloe barbadensis Mill by liquid chromatography–mass spectrometry–ion trap–time–of–flight and high performance liquid chromatography–diode array detector. J. Pharm. Biomed. Anal. 2013, 80, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Yimam, M.; Brownell, L.; Jia, Q. Aloesin as a medical food ingredient for systemic oxidative stress of diabetes. World J. Diabetes 2015, 6, 1097–1107. [Google Scholar] [CrossRef]
- Reynoso-Camacho, R. Gonzalez de Mejia. Nopal (Opuntia spp.) and other traditional mexican plants. In Nutraceutical Glycemic Health & Type 2 Diabetes; Pasupulety, V.K., Anderson, J.M., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2008; Chapter 15; pp. 379–399. [Google Scholar]
- Slavin, J. Fiber and prebiotics: Mechanisms and health benefits. Nutrients. 2013, 5, 1417–1435. [Google Scholar] [CrossRef]
- Gullón, B.; Gullón, P.; Sanz, Y.; Alonso, J.L.; Parajó, J.C. Prebiotic potential of a refined product containing pectic oligosaccharides. LWT–Food Sci. Technol. 2011, 44, 1687–1696. [Google Scholar]
- Romero-López, M.R.; Osorio-Díaz, P.; Flores-Morales, A.; Robledo, N.; Mora-Escobedo, R. Chemical composition, antioxidant capacity and prebiotic effect of aguamiel (Agave atrovirens) during in vitro fermentation. Rev. Mex. Ing. Quím. 2015, 14, 281–292. [Google Scholar]
- Johnson, W.; McRorie, J.W., Jr.; Nicola, M.; McKeown. Understanding the physics of functional fibers in the gastrointestinal tract: An evidence–based approach to resolving enduring misconceptions about insoluble and soluble fiber. J. Acad. Nutr. Diet. 2017, 117, 251–264. [Google Scholar]
- Goñi, I.; Martín, N.; Saura-Calixto, F. In vitro digestibility and intestinal fermentation of grape seed and peel. Food Chem. 2005, 90, 281–286. [Google Scholar] [CrossRef][Green Version]
- Al-Madboly, L.A.; Kabbash, A.; Yassin, A.M.; Yagi, A. Dietary cancer prevention with butyrate fermented by Aloe vera gel endophytic microbiotata. J. Gastroenterol. Hepat. Res. 2017, 6, 2312–2317. [Google Scholar]
- Wong, J.M.W.; Souza, R.; Kendall, C.W.C.; Emam, A.; Jenkins, D.J.A. Colonic health: Fermentation and short chain fatty acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef]
- Mudgil, D.; Barak, S. Composition, properties and health benefits of indigestible carbohydrate polymers as dietary fiber: A review. Int. J. Biol. Macromol. 2013, 61, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Laparra, J.M.; Sanz, Y. Interactions of gut microbiota with functional food components and nutraceuticals. Pharmacol. Res. 2010, 61, 219–225. [Google Scholar] [CrossRef]
- Russell, W.R.; Hoyles, L.; Flint, H.J.; Dumas, M.E. Colonic bacterial metabolites and human health. Curr. Opin. Microbiol. 2013, 16, 246–254. [Google Scholar] [CrossRef]
- Maslowski, M.K.; Kendle, M.; Vieira, T.A.; Ng, A.; Kranich, J.; Sierro, F.; Yu, D.; Schilter, C.H.; Rolph, S.M.; Mackay, F.; et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR4. Nature 2009, 461, 1282–1286. [Google Scholar] [CrossRef]
- Fukuda, S.; Toh, H.; Hase, K.; Oshima, K.; Nakanishi, Y.; Yoshimura, K.; Tobe, T.; Clarke, M.J.; Topping, L.D.; Suzuki, T.; et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011, 469, 543–547. [Google Scholar] [CrossRef]
- Gullón, B.; Gullón, P.; Tavaria, F.; Alonso, J.L.; Pintado, M. In vitro assessment of the prebiotic potential of Aloe vera mucilage and its impact on the human microbiota. Food Funct. 2015, 6, 525. [Google Scholar]
- Verma, B.; Hucl, P.; Chibbar, R. Phenolic acid composition and antioxidant capacity of acid and alkali hydrolysed wheat bran fractions. Food Chem. 2009, 116, 947–954. [Google Scholar]
- Scaglioni, P.T.; de Souza, T.D.; Schmidt, C.G.; Badiale-Furlong, E. Availability of free and bound phenolic compounds in rice after hydrothermal treatment. J. Cereal Sci. 2014, 60, 526–532. [Google Scholar] [CrossRef]
- Cueva, C.; Sanchez-Patan, F.; Monagas, M.; Walton, G.E.; Gibson, G.R.; Martın-Alvarez, P.J.; Bartolome, B.; Moreno-Arribas, M.V. In vitro fermentation of grape seed flavan–3–ol fractions by human faecal microbiota: Changes in microbial groups and phenolic metabolites. FEMS Microbiol. Ecol. 2013, 83, 792–805. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; Jenner, A.M.; Low, C.S.; Lee, Y.K. Effect of tea phenolics and their aromatic fecal bacterial metabolites on intestinal microbiota. Res. Microbiol. 2006, 157, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Alugoju, P.; Dinesh, B.J.; Latha, P. Free radicals: Properties, sources, targets, and their implication in various diseases. Ind. J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar]
- Chun-Hui, L.; Chang-Hai, W.; Zhi-Liang, X.; Yi, W. Isolation, chemical characterization and antioxidant activities of two polysaccharides from the gel and the skin of Aloe barbadensis Miller irrigated with sea water. Process Biochem. 2007, 42, 961–970. [Google Scholar] [CrossRef]
- Ray, A.; Gupta, S.D.; Ghosh, S. Evaluation of anti–oxidative activity and UV absorption potential of the extracts of Aloe vera L. gel from different growth periods of plants. Ind. Crops Prod. 2013, 49, 712–719. [Google Scholar] [CrossRef]
- Sánchez-Machado, D.I.; López-Cervantes, J.; Sendón, R.; Sanches-Silva, A. Aloe vera: Ancient knowledge with new frontiers. Trends Food Sci. Technol. 2017, 61, 94–102. [Google Scholar] [CrossRef]
- Kunwar, A.; Priyadarsini, K.I. Free radicals, oxidative stress and importance of antioxidants in human health. J. Med. Allied Sci. 2011, 1, 53–60. [Google Scholar]
- AOAC Official Methods of Analysis (2015); AOAC International: Rockville, MD, USA, 2015.
- Cassani, J.; Ferreyra-Cruz, O.A.; Dorantes-Barrón, A.M.; Villaseñor, R.M.; Arrieta-Baez, D.; Estrada-Reyes, R. Antioxidant, hepatoprotective, and antidepression effects of Rumex tingitanus extracts and identification of a novel bioactive compound. J. Ethnopharmacol. 2015, 171, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Rosas, N.A.; García-Zebadúa, J.C.; Hernández-Delgado, N.; Torres-Castillo, S.; Figueroa-Arredondo, P.; Mora-Escobedo, R. Perfil de polifenoles, capacidad antioxidante y efecto citotóxico in vitro en líneas celulares humanas de un extracto hidroalcohólico de pétalos de Calendula officinalis L. TIP Rev. Espec. Cienc. Quím. Biol. 2018, 21, 1–11. [Google Scholar]
- Saura-Calixto, F.; García-Alonso, A.; Goñi, I.; Bravo, L. In vitro determination of the indigestible fraction in foods: An alternative to dietary fiber analysis. J. Agric. Food Chem. 2000, 48, 3342–3347. [Google Scholar] [CrossRef] [PubMed]
- Martín-Carrón, N.; Goñi, I. Prior exposure of cecal microflora to grape pomaces does not inhibit in vitro fermentation of pectin. Nutr. Res. 1998, 46, 1064–1070. [Google Scholar] [CrossRef]
- Wenzel, M.E.; Dan, M.C.T.; Cardenette, H.L.G.; Goñi, I.; Bello, L.A.; Lajolo, F.M. In vitro colonic fermentation and glycemic response of different kinds of unripe banana flour. Plant Foods Human Nutr. 2010, 63, 379–385. [Google Scholar]
- Zhao, G.; Nyman, M.; Jönsson, J.A. Orthogonal comparison of GC–MS and H NMR spectroscopy for short chain fatty acid quantitation. Biomed. Chromatogr. 2006, 20, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Blainski, A.; Lopes, G.C.; Palazo de Mello, J.C. Application and analysis of the Folin Ciocalteu method for the determination of the total phenolic content from Limonium brasiliense L. Molecules. 2013, 18, 6852–6865. [Google Scholar] [CrossRef] [PubMed]
- Flores-López, M.L.; Aloia-Romani, M.; Cerqueira, A.; Rodríguez-García, R.; Jasso de Rodríguez, D.; Vicente, A.A. Compositional features and bioactive properties of whole fraction from Aloe vera processing. Ind. Crops Prod. 2016, 91, 179–185. [Google Scholar] [CrossRef]
- Paillat, L.; Périchet, C.; Lavoine, S.; Meierhenrich, U.J.; Fernandez, J. Validated high–performance thin–layer chromatography (HPTLC) method for quantification of vanillin β–D–glucoside, and four major phenolic compounds in vanilla (Vanilla planifolia) fruits, beans, and extracts. J. Planar Chromat. 2012, 4, 295–300. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reactions: Antioxidative activities of product of browning reaction prepared from glucosamine. Jpn. J. Nutr. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, B.; Zhang, T.; Mu, W.; Liu, J. Antioxidant and free radical–scavenging activities of chickpea protein hydrolysate (CPH). Food Chem. 2008, 106, 444–450. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

| COMPONENT | AMOUNT (g/100g db) |
|---|---|
| Moisture | 97.50 ± 0.04 |
| Proteins | 2.15 ± 0.08 |
| Lipids | 3.27 ± 0.85 |
| Ashes | 12.62 ± 1.1 |
| Total Dietary Fibre (TDF) | 55.11 ± 0.55 |
| Neutral Detergent Fibre (NDF) | 15.58 ± 2.04 |
| Nitrogen-free extract | 26.85 ± 1.1 |
| PEAK | Rt a (min) | m/z [M-H]− | m [M] | Tentative Identification |
|---|---|---|---|---|
| 1 | 1.52 | 393.18 | 394.18 | Aloesin |
| 2 | 3.15 | 433.19 | 434.19 | 10-Hydroxyaloin |
| 3 | 3.15 | 561.36 | 562.36 | Aloinoside |
| 4 | 4.45 | 417.19 | 418.19 | Aloin A |
| 5 | 4.50 | 555.25 | 556.25 | Aloenin-2′-p-coumaroyl ester |
| 6 | 4.68 | 417.19 | 418.19 | Aloin B |
| Sample | SNDF (g/100g) | INDF (g/100g) | TNDF (g/100g) |
|---|---|---|---|
| AV | 20.57 a ± 0.83 | 26.57 a ± 0.02 | 47.14 |
| AP | 39.34 b ± 0.55 | 53.05 b ± 0.40 | 92.39 |
| Fermentation Time | Acetic Acid | Propionic Acid | Butyric Acid | Total SCFA | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AV | AP | Lactulose | AV | AP | Lactulose | AV | AP | Lactulose | AV | AP | Lactulose | |
| 0 | 2.16 ± 1.07 b | 0.93 ± 0.36 a | 2.16 ± 0.08 b | 0.09 ± 0.05 a | 0.14 ± 0.03 b | 0.09 ± 0.03 a | 0.09 ± 0.04 b | 0.14 ± 0.01 c | 0.05 ± 0.01 a | 2.33 ± 1.08 ab | 1.21 ± 0.39 a | 2.34 ± 0.11 b |
| 4 | 3.15 ± 0.55 a | 3.20 ± 0.64 a | 4.50 ± 0.73 a | 1.32 ± 0.26 a | 1.30 ± 0.14 a | 1.07 ± 0.06 a | 0.31 ± 0.05 a | 0.63 ± 0.04 c | 0.47 ± 0.02 b | 4.77 ± 0.84 a | 5.13 ± 0.80 a | 5.15 ± 0.93 a |
| 8 | 6.12 ± 0.66 a | 7.00 ± 0.26 a | 9.75 ± 1.92 b | 2.50 ± 0.27 ab | 2.16 ± 0.32 a | 3.42 ± 0.90 b | 0.79 ± 0.04 a | 1.02 ± 0.13 b | 2.09 ± 0.03 c | 9.41±0.96 a | 10.18 ± 0.70 a | 12.51 ± 2.79 a |
| 24 | 10.67 ± 0.3 a | 10.93 ± 1.33 a | 11.43 ± 1.57 a | 3.68 ± 0.24 b | 3.12 ± 0.14 a | 4.44 ± 0.69 b | 1.92 ± 0.10 a | 1.79 ± 0.14 a | 4.25 ± 1.12 b | 16.27 ± 0.60 a | 15.83 ± 1.47 a | 17.03 ± 1.51 a |
| Phenolic Compounds | Colour | Rf | AV | AV-TNDF | AP | AP-TNDF | FAV4 | FAV8 | FAV24 | FPA4 | FPA8 | FPA24 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vanillinic acid | Dark blue | 0.54 | - | - | - | - | - | - | - | - | - | - |
| p-Hydroxybenzaldehyde | Dark blue | 0.65 | - | - | - | - | - | - | - | - | - | - |
| p-Hydroxybenzoic acid | Dark blue | 0.45 | - | - | - | - | - | - | - | - | - | - |
| p-Cresol | Dark blue | 0.72 | - | - | - | - | - | - | - | - | - | - |
| p-Creosol | Dark blue | 0.86 | - | - | - | - | - | - | - | - | - | - |
| Ferulic acid | Light blue | 0.55 | + | + | + | + | - | - | - | - | - | - |
| Unidentified | Violet | 0.64 | - | - | - | - | ++ | ++ | ++ | - | - | - |
| Unidentified | Yellow | 0.57 | - | - | - | - | ++ | ++ | ++ | - | - | - |
| Unidentified | Yellow | 0.12 | - | - | - | - | ++ | ++ | ++ | + | + | + |
| Unidentified | Red | 0.06 | ++ | + | - | - | - | - | - | - | - | - |
| Unidentified | Light blue | 0.04 | + | + | ++ | +++ | + | + | + | + | + | + |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tornero-Martínez, A.; Cruz-Ortiz, R.; Jaramillo-Flores, M.E.; Osorio-Díaz, P.; Ávila-Reyes, S.V.; Alvarado-Jasso, G.M.; Mora-Escobedo, R. In vitro Fermentation of Polysaccharides from Aloe vera and the Evaluation of Antioxidant Activity and Production of Short Chain Fatty Acids. Molecules 2019, 24, 3605. https://doi.org/10.3390/molecules24193605
Tornero-Martínez A, Cruz-Ortiz R, Jaramillo-Flores ME, Osorio-Díaz P, Ávila-Reyes SV, Alvarado-Jasso GM, Mora-Escobedo R. In vitro Fermentation of Polysaccharides from Aloe vera and the Evaluation of Antioxidant Activity and Production of Short Chain Fatty Acids. Molecules. 2019; 24(19):3605. https://doi.org/10.3390/molecules24193605
Chicago/Turabian StyleTornero-Martínez, Antonio, Rubén Cruz-Ortiz, María Eugenia Jaramillo-Flores, Perla Osorio-Díaz, Sandra Victoria Ávila-Reyes, Guadalupe Monserrat Alvarado-Jasso, and Rosalva Mora-Escobedo. 2019. "In vitro Fermentation of Polysaccharides from Aloe vera and the Evaluation of Antioxidant Activity and Production of Short Chain Fatty Acids" Molecules 24, no. 19: 3605. https://doi.org/10.3390/molecules24193605
APA StyleTornero-Martínez, A., Cruz-Ortiz, R., Jaramillo-Flores, M. E., Osorio-Díaz, P., Ávila-Reyes, S. V., Alvarado-Jasso, G. M., & Mora-Escobedo, R. (2019). In vitro Fermentation of Polysaccharides from Aloe vera and the Evaluation of Antioxidant Activity and Production of Short Chain Fatty Acids. Molecules, 24(19), 3605. https://doi.org/10.3390/molecules24193605