Fabrication of pH-Sensitive Tetramycin Releasing Gel and Its Antibacterial Bioactivity against Ralstonia solanacearum
Abstract
1. Introduction
2. Results and Discussion
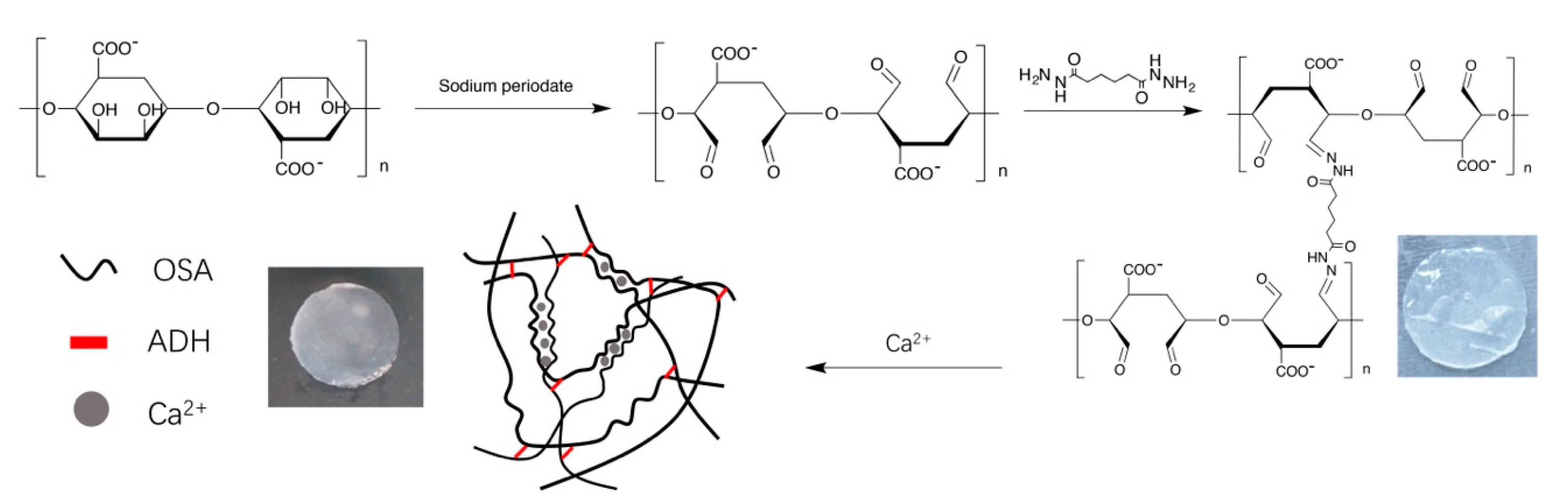
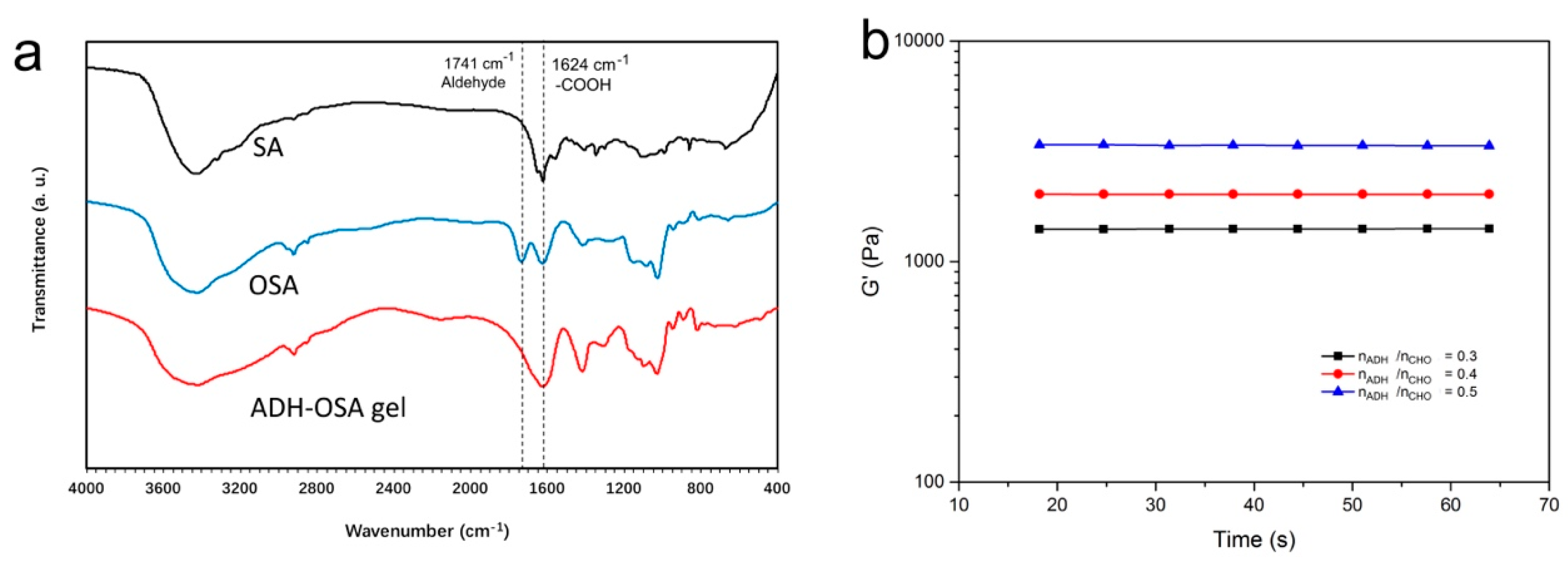
2.1. Preparation of Tetramycin-Loaded ADH-OSA Gel
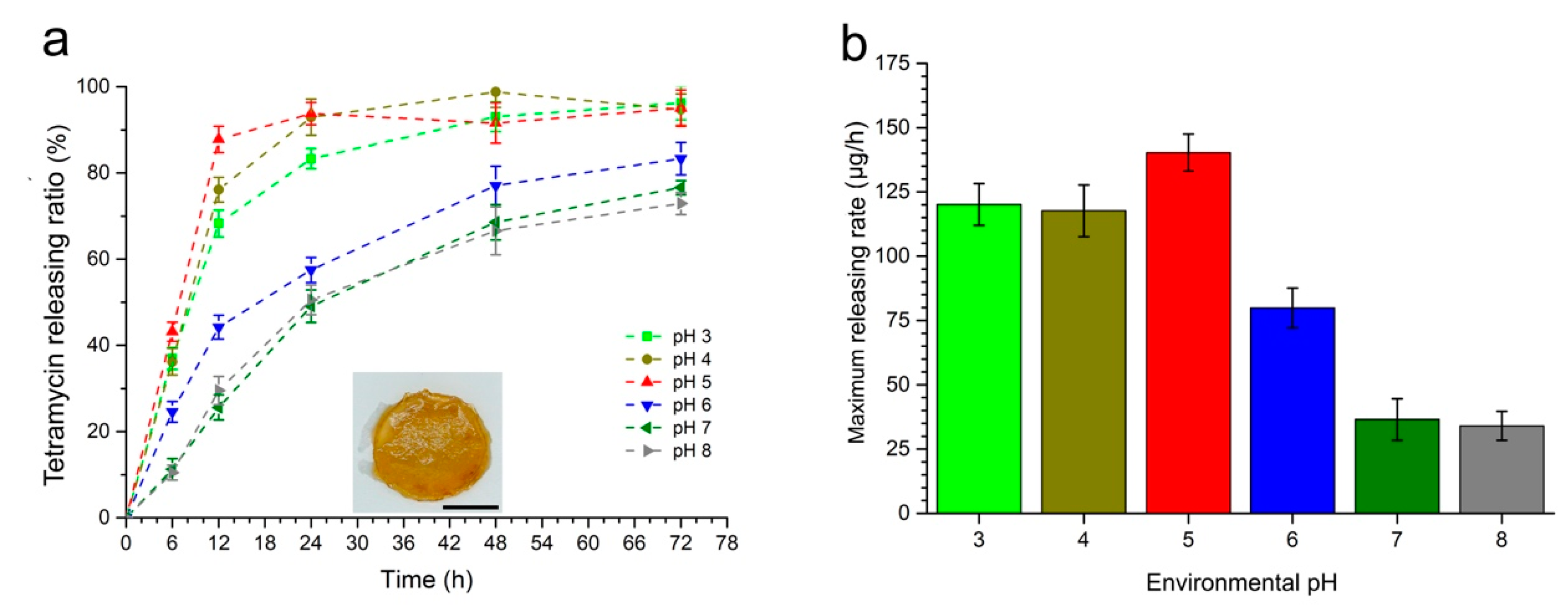
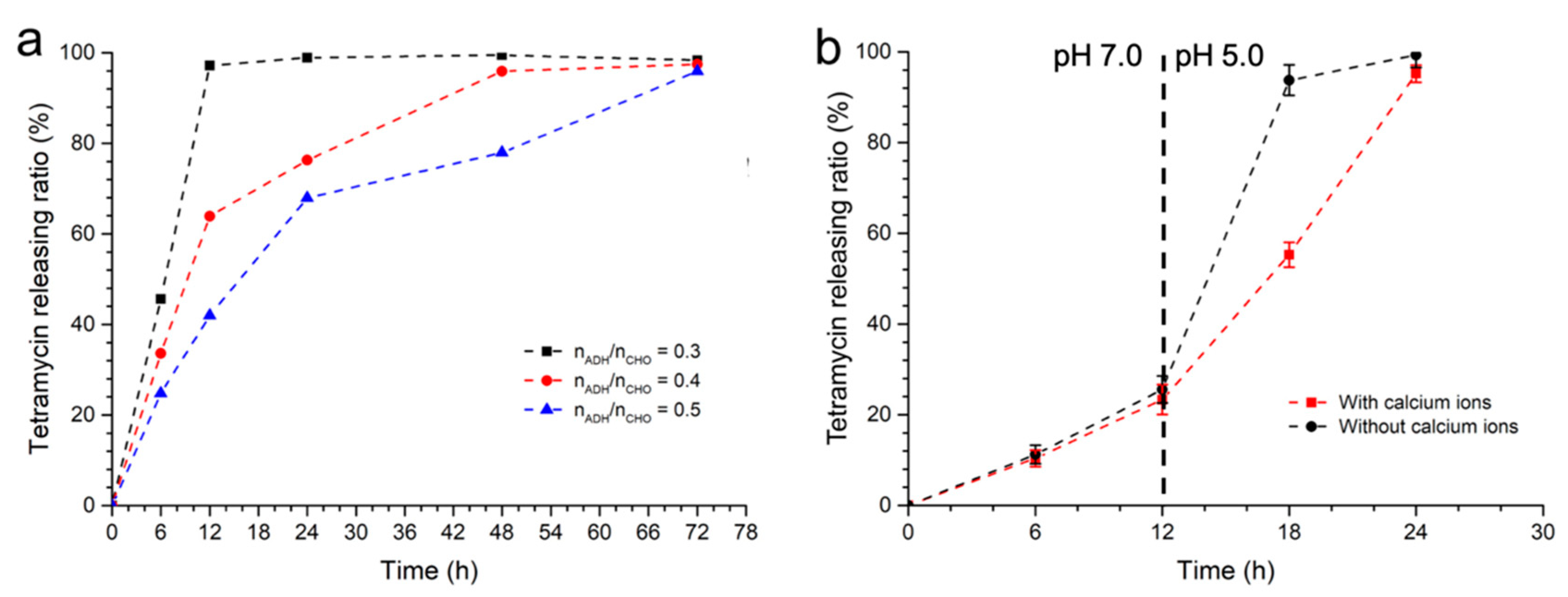
2.2. Affection of Environmental pH to the Tetramycin Releasing Rate
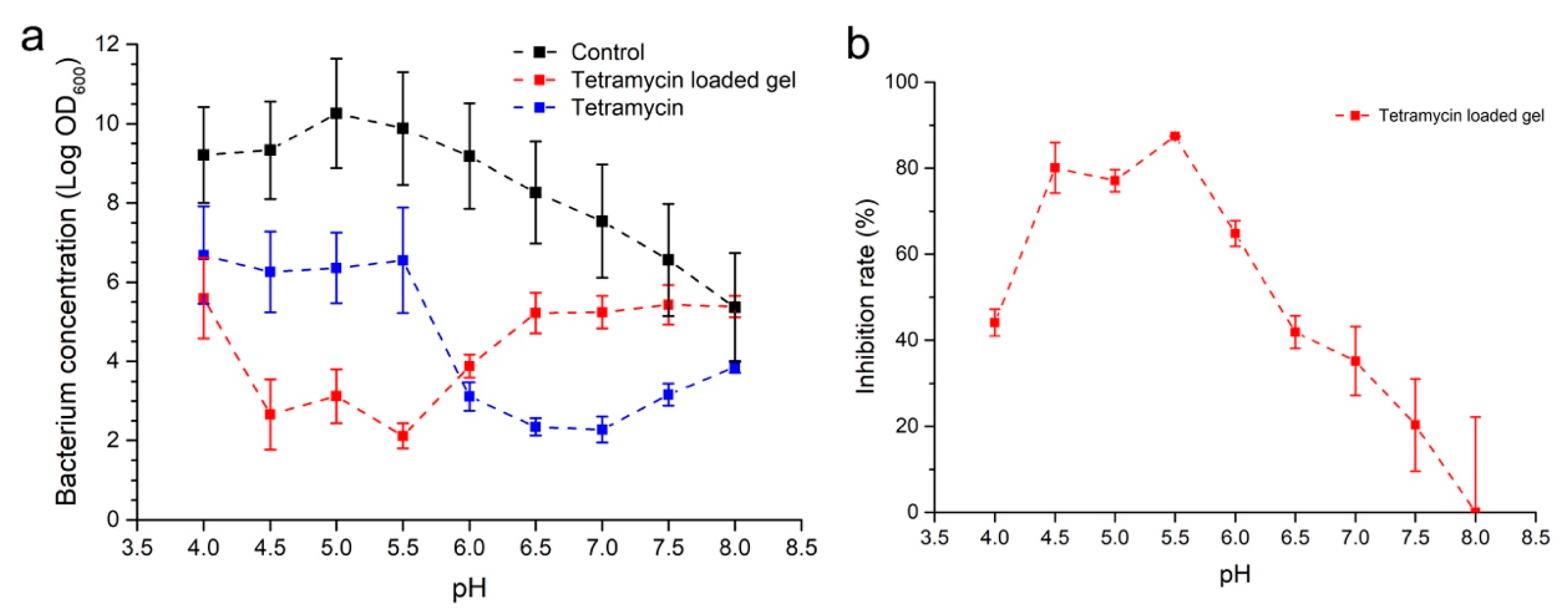
2.3. pH-Dependent Inhibition of R. solanacearum Growth of Tetramycin Loaded ADH-OSA Gel
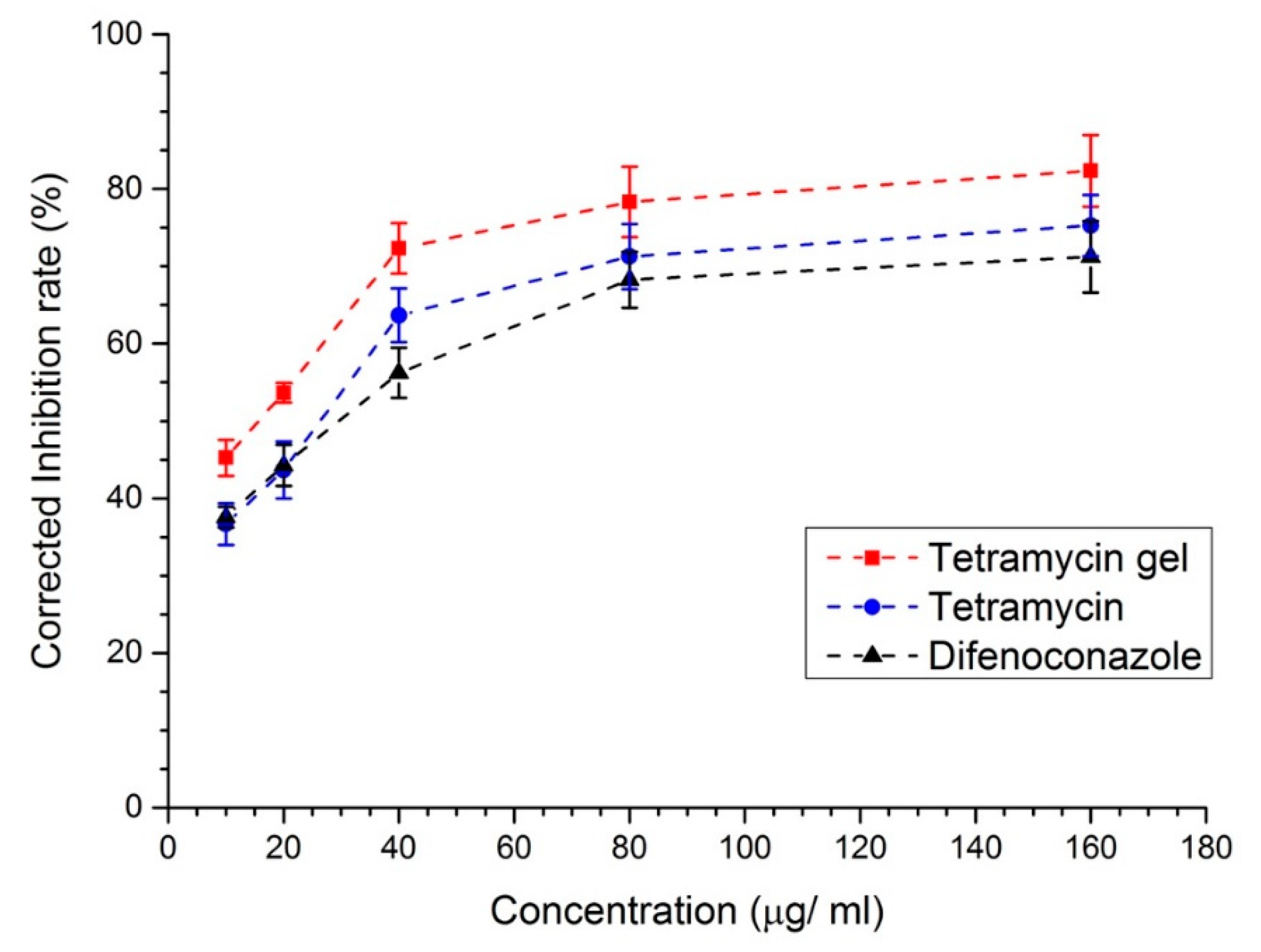
2.4. Antibacterial Activity of Tetramycin Gel, Tetramycin, and Difenoconazole
3. Methods and Experiments
3.1. Materials
3.2. Oxidation of Sodium Alginate
3.3. Preparation of Tetramycin Loaded ADH-OSA Gel
3.4. Calculation of Tetramycin Releasing Rate
3.5. Assessment of Colony Density of R. solanacearum
3.6. Antibacterial Activity of Tetramycin Gel and Difenoconazole
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Peeters, N.; Guidot, A.; Vailleau, F.; Valls, M. Ralstonia solanacearum, a widespread bacterial plant pathogen in the post-genomic era. Mol. Plant Pathol. 2013, 14, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Hikichi, Y.; Mori, Y.; Ishikawa, S.; Hayashi, K.; Ohnishi, K.; Kiba, A.; Kai, K. Regulation involved in colonization of intercellular spaces of host plants in Ralstonia solanacearum. Front. Plant Sci. 2017, 8, 967. [Google Scholar] [CrossRef] [PubMed]
- Lopes, C.A.; Rossato, M. History and status of selected hosts of the Ralstonia solanacearum species complex causing bacterial wilt in brazil. Front. Microbiol. 2018, 9, 1228. [Google Scholar] [CrossRef] [PubMed]
- Suchoff, D.H.; Louws, F.J.; Gunter, C.C. Yield and disease resistance for three bacterial wilt-resistant tomato rootstocks. Horttechnology 2019, 29, 330–337. [Google Scholar] [CrossRef]
- Lowe-Power, T.M.; Khokhani, D.; Allen, C. How Ralstonia solanacearum exploits and thrives in the flowing plant xylem environment. Trends Microbiol. 2018, 26, 929–942. [Google Scholar] [CrossRef] [PubMed]
- Genin, S. Molecular traits controlling host range and adaptation to plants in Ralstonia solanacearum. New Phytol. 2010, 187, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Plener, L.; Manfredi, P.; Valls, M.; Genin, S. Prhg, a transcriptional regulator responding to growth conditions, is involved in the control of the type iii secretion system regulon in Ralstonia solanacearum. J. Bacteriol. 2010, 192, 1011–1019. [Google Scholar] [CrossRef]
- Furusawa, A.; Uehara, T.; Ikeda, K.; Sakai, H.; Tateishi, Y.; Sakai, M.; Nakaho, K. Ralstonia solanacearum colonization of tomato roots infected by Meloidogyne incognita. J. Phytopathol. 2019, 167, 338–343. [Google Scholar] [CrossRef]
- Shi, X.; Zhan, H.; Zeng, Y.; Zhang, X.; Wu, B.; Liu, L. A case of hemophagocytic lymphohistiocytosis secondary to Ralstonia solanacearum infection. Clin. Lab. 2019, 65. [Google Scholar] [CrossRef]
- Sikirou, R.; Dossoumou, M.E.E.A.; Zocli, B.; Afari-Sefa, V.; Honfoga, J.; Azoma, K.; Chen, J.R.; Paret, M.L.; Bihon, W. First report of bacterial wilt of amaranth (Amaranthus cruentus) caused by Ralstonia solanacearum in benin. Plant Dis. 2019, 103, 578. [Google Scholar] [CrossRef]
- Yuliar; Nion, Y.A.; Toyota, K. Recent trends in control methods for bacterial wilt diseases caused by Ralstonia solanacearum. Microbes Environ. 2015, 30, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Zhang, S.; Liu, X.; Jiang, Q.; Ding, W. Soil acidification amendments change the rhizosphere bacterial community of tobacco in a bacterial wilt affected field. Appl. Microbiol. Biotechnol. 2018, 102, 9781–9791. [Google Scholar] [CrossRef] [PubMed]
- Nabgan, W.; Abdullah, T.A.T.; Mat, R.; Nabgan, B.; Gambo, Y.; Ibrahim, M.; Ahmad, A.; Jalil, A.A.; Triwahyono, S.; Saeh, I. Renewable hydrogen production from bio-oil derivative via catalytic steam reforming: An overview. Renew. Sustain. Energy Rev. 2017, 79, 347–357. [Google Scholar] [CrossRef]
- Giri, T.K.; Thakur, D.; Alexander, A.; Ajazuddin; Badwaik, H.; Tripathi, D.K. Alginate based hydrogel as a potential biopolymeric carrier for drug delivery and cell delivery systems: Present status and applications. Curr. Drug Deliv. 2012, 9, 539–555. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Lei, L.; Shi, S.; Li, X. Stimulus-responsive hydrogel for ophthalmic drug delivery. Macromol. Biosci. 2019, 19, 1900001. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, C.; Li, C.; Qin, Y.; Wang, Z.; Yang, F.; Li, Z.; Wang, J. A functional chitosan-based hydrogel as a wound dressing and drug delivery system in the treatment of wound healing. RSC Adv. 2018, 8, 7533–7549. [Google Scholar] [CrossRef]
- Wan, J. Microfluidic-based synthesis of hydrogel particles for cell microencapsulation and cell-based drug delivery. Polymers 2012, 4, 1084–1108. [Google Scholar] [CrossRef]
- Lin, G.; Chen, X.; Zhou, H.; Zhou, X.; Xu, H.; Chen, H. Elaboration of a feather keratin/carboxymethyl cellulose complex exhibiting pH sensitivity for sustained pesticide release. J. Appl. Polym. Sci. 2019, 136, 47160. [Google Scholar] [CrossRef]
- Xiang, Y.; Zhang, G.; Chen, C.; Liu, B.; Cai, D.; Wu, Z. Fabrication of a pH-responsively controlled-release pesticide using an attapulgite-based hydrogel. ACS Sustain. Chem. Eng. 2018, 6, 1192–1201. [Google Scholar] [CrossRef]
- Cui, H.; Ni, X.; Liu, S.; Wang, J.; Sun, Z.; Ren, J.; Su, J.; Chen, G.; Xia, H. Characterization of three positive regulators for tetramycin biosynthesis in streptomyces ahygroscopicus. FEMS Microbiol. Lett. 2016, 363, 109. [Google Scholar] [CrossRef][Green Version]
- Gao, Y.; He, L.; Li, X.; Lin, J.; Mu, W.; Liu, F. Toxicity and biochemical action of the antibiotic fungicide tetramycin on colletotrichum scovillei. Pestic. Biochem. Physiol. 2018, 147, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Zhu, J.; He, L.; Cui, K.; Mu, W.; Liu, F. Baseline sensitivity and control efficacy of tetramycin against phytophthora capsici isolates in china. Plant Dis. 2018, 102, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Zhu, J.; Jiang, J.; Zhao, Y.; Li, B.; Mu, W.; Liu, F. Evaluation of bioactivity and control efficacy of tetramycin against Corynespora cassiicola. Pestic. Biochem. Physiol. 2018, 152, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; He, L.; Chen, L.; Ren, Y.; Lu, H.; Geng, S.; Mu, W.; Liu, F. Baseline sensitivity and control efficacy of antibiosis fungicide tetramycin against Botrytis cinerea. Eur. J. Plant Pathol. 2016, 146, 337–347. [Google Scholar] [CrossRef]
- Zhao, X.; Zhong, L.; Zhang, Q.; Xu, C.; Zhu, H.; Lu, Z.; Shen, L.; Wang, G.; Jie, D. Effect of tetramycin on mycelia growth and spore germination of rice blast pathogen. J. Microbiol. 2010, 2, 43–45. [Google Scholar]
- Liu, L.; Kong, C.; Huo, M.; Liu, C.; Peng, L.; Zhao, T.; Wei, Y.; Qian, F.; Yuan, J. Schiff base interaction tuned mesoporous organosilica nanoplatforms with pH-responsive degradability for efficient anti-cancer drug delivery in vivo. Chem. Commun. 2018, 54, 9190–9193. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; He, C.; Wu, Y.; Chen, X. Synergistic therapeutic effects of schiffs base cross-linked injectable hydrogels for local co-delivery of metformin and 5-fluorouracil in a mouse colon carcinoma model. Biomaterials 2016, 75, 148–162. [Google Scholar] [CrossRef] [PubMed]
- Antony, R.; Arun, T.; Manickam, S.T.D. A review on applications of chitosan-based schiff bases. Int. J. Biol. Macromol. 2019, 129, 615–633. [Google Scholar] [CrossRef]
- Majid, S.A.; Mir, J.M.; Paul, S.; Akhter, M.; Parray, H.; Ayoub, R.; Shalla, A.H. Experimental and molecular topology-based biological implications of schiff base complexes: A concise review. Rev. Inorg. Chem. 2019, 39, 113–128. [Google Scholar] [CrossRef]
- Xin, Y.; Yuan, J. Schiff’s base as a stimuli-responsive linker in polymer chemistry. Polym. Chem. 2012, 3, 3045–3055. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, W.; Wang, Q.; Xu, C.; Tang, Q.; Yang, H. An injectable, dual responsive, and self-healing hydrogel based on oxidized sodium alginate and hydrazide-modified poly(ethyleneglycol). Molecules 2018, 23, 546. [Google Scholar] [CrossRef] [PubMed]
- Hozumi, T.; Kageyama, T.; Ohta, S.; Fukuda, J.; Ito, T. Injectable hydrogel with slow degradability composed of gelatin and hyaluronic acid cross-linked by schiff’s base formation. Biomacromolecules 2018, 19, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Chen, M.; Guan, Y.; Zhang, Y. Multiple responsive hydrogel films based on dynamic schiff base linkages. Polym. Chem. 2014, 5, 7081–7089. [Google Scholar] [CrossRef]
- Xiang, S.; Ma, X.; Liao, S.; Shi, H.; Liu, C.; Shen, Y.; Lv, X.; Yuan, M.; Fan, G.; Huang, J.; et al. Cellulose nanocrystal surface cationization: A new fungicide with high activity against Phycomycetes capsici. Molecules 2019, 24, 2467. [Google Scholar] [CrossRef] [PubMed]
- Ramdhan, T.; Ching, S.H.; Prakash, S.; Bhandari, B. Time dependent gelling properties of cuboid alginate gels made by external gelation method: Effects of alginate-cacl2 solution ratios and pH. Food Hydrocoll. 2019, 90, 232–240. [Google Scholar] [CrossRef]
- Hirooka, T.; Ishii, H. Chemical control of plant diseases. J. Gen. Plant Pathol. 2013, 79, 390–401. [Google Scholar] [CrossRef]
- Tang, X.; Su, S.; Chen, M.; He, J.; Xia, R.; Guo, T.; Chen, Y.; Zhang, C.; Wang, J.; Xue, W. Novel chalcone derivatives containing a 1,2,4-triazine moiety: Design, synthesis, antibacterial and antiviral activities. RSC Adv. 2019, 9, 6011–6020. [Google Scholar] [CrossRef]
- Wu, D.; Ding, W.; Zhang, Y.; Liu, X.; Yang, L. Oleanolic acid induces the type iii secretion system of Ralstonia solanacearum. Front. Microbiol. 2015, 6. [Google Scholar] [CrossRef]
- Yoshimochi, T.; Zhang, Y.; Kiba, A.; Hikichi, Y.; Ohnishi, K. Expression of HRPG and activation of response regulator HRPG are controlled by distinct signal cascades in Ralstonia solanacearum. J. Gen. Plant Pathol. 2009, 75, 196–204. [Google Scholar] [CrossRef]
- Glensk, M.; Gajda, B.; Franiczek, R.; Krzyzanowska, B.; Biskup, I.; Wlodarczyk, M. In vitro evaluation of the antioxidant and antimicrobial activity of dimboa 2,4-dihydroxy-7-methoxy-2h-1,4-benzoxazin-3(4h)-one. Nat. Prod. Res. 2016, 30, 1305–1308. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Xiang, S.; Xie, H.; He, L.; Sun, X.; Zhang, Y.; Huang, J. Fabrication of pH-Sensitive Tetramycin Releasing Gel and Its Antibacterial Bioactivity against Ralstonia solanacearum. Molecules 2019, 24, 3606. https://doi.org/10.3390/molecules24193606
Ma X, Xiang S, Xie H, He L, Sun X, Zhang Y, Huang J. Fabrication of pH-Sensitive Tetramycin Releasing Gel and Its Antibacterial Bioactivity against Ralstonia solanacearum. Molecules. 2019; 24(19):3606. https://doi.org/10.3390/molecules24193606
Chicago/Turabian StyleMa, Xiaozhou, Shunyu Xiang, Huijun Xie, Linhai He, Xianchao Sun, Yongqiang Zhang, and Jin Huang. 2019. "Fabrication of pH-Sensitive Tetramycin Releasing Gel and Its Antibacterial Bioactivity against Ralstonia solanacearum" Molecules 24, no. 19: 3606. https://doi.org/10.3390/molecules24193606
APA StyleMa, X., Xiang, S., Xie, H., He, L., Sun, X., Zhang, Y., & Huang, J. (2019). Fabrication of pH-Sensitive Tetramycin Releasing Gel and Its Antibacterial Bioactivity against Ralstonia solanacearum. Molecules, 24(19), 3606. https://doi.org/10.3390/molecules24193606





