Solution-Based Synthesis and Characterization of Cu2ZnSnS4 (CZTS) Thin Films
Abstract
1. Introduction
2. Results and Discussion
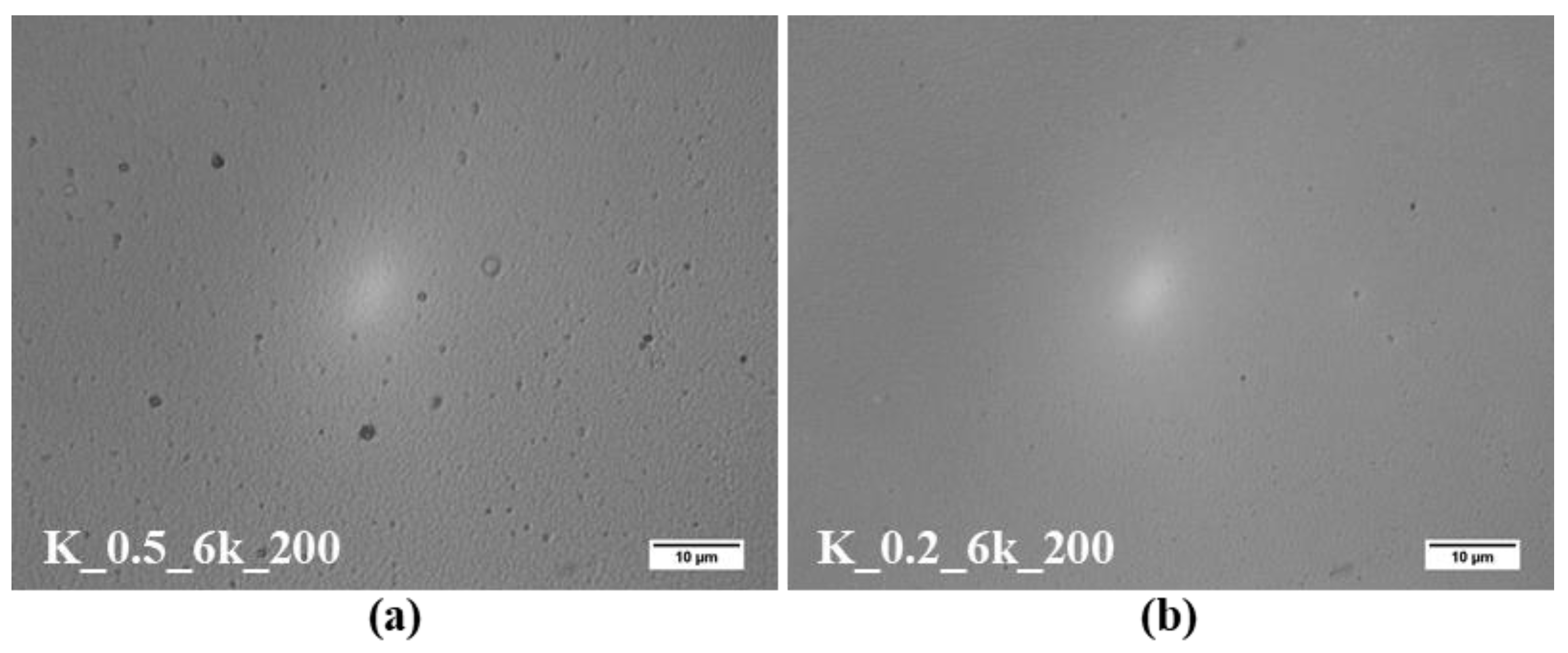
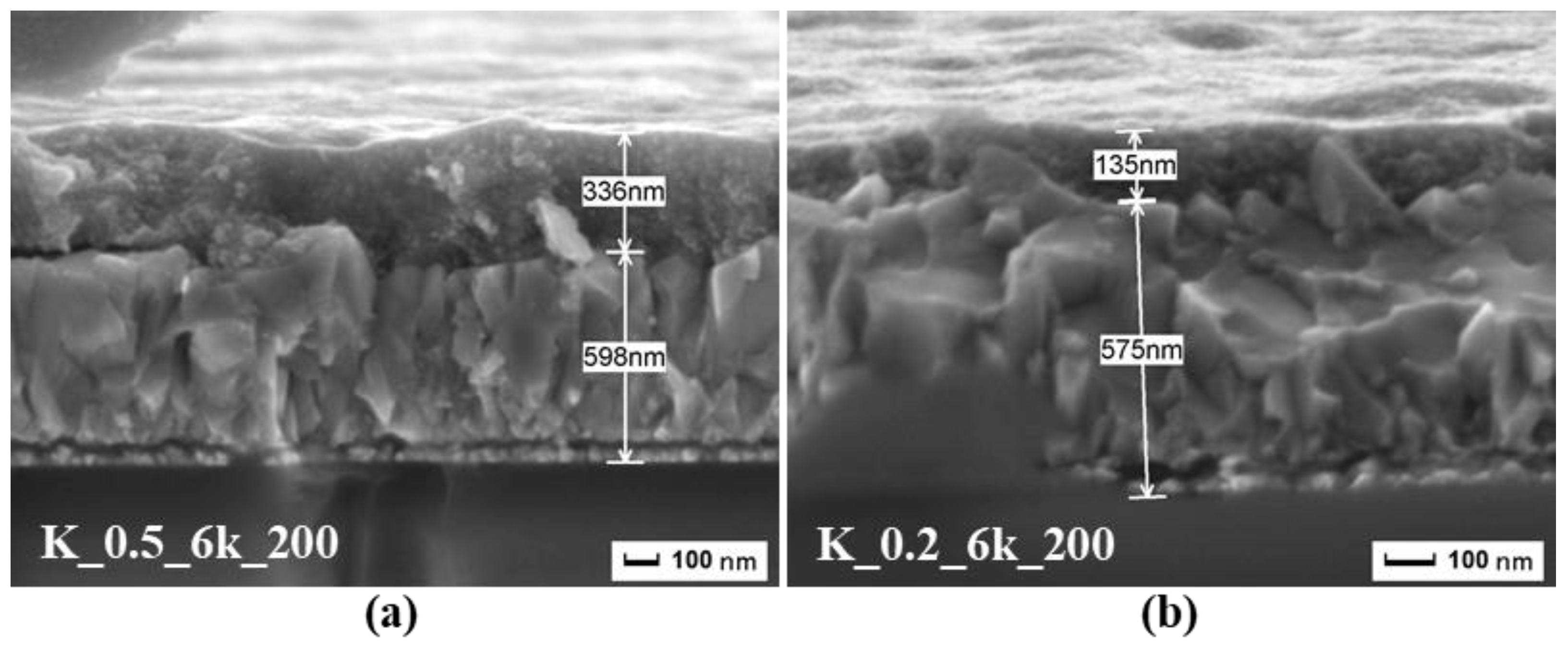
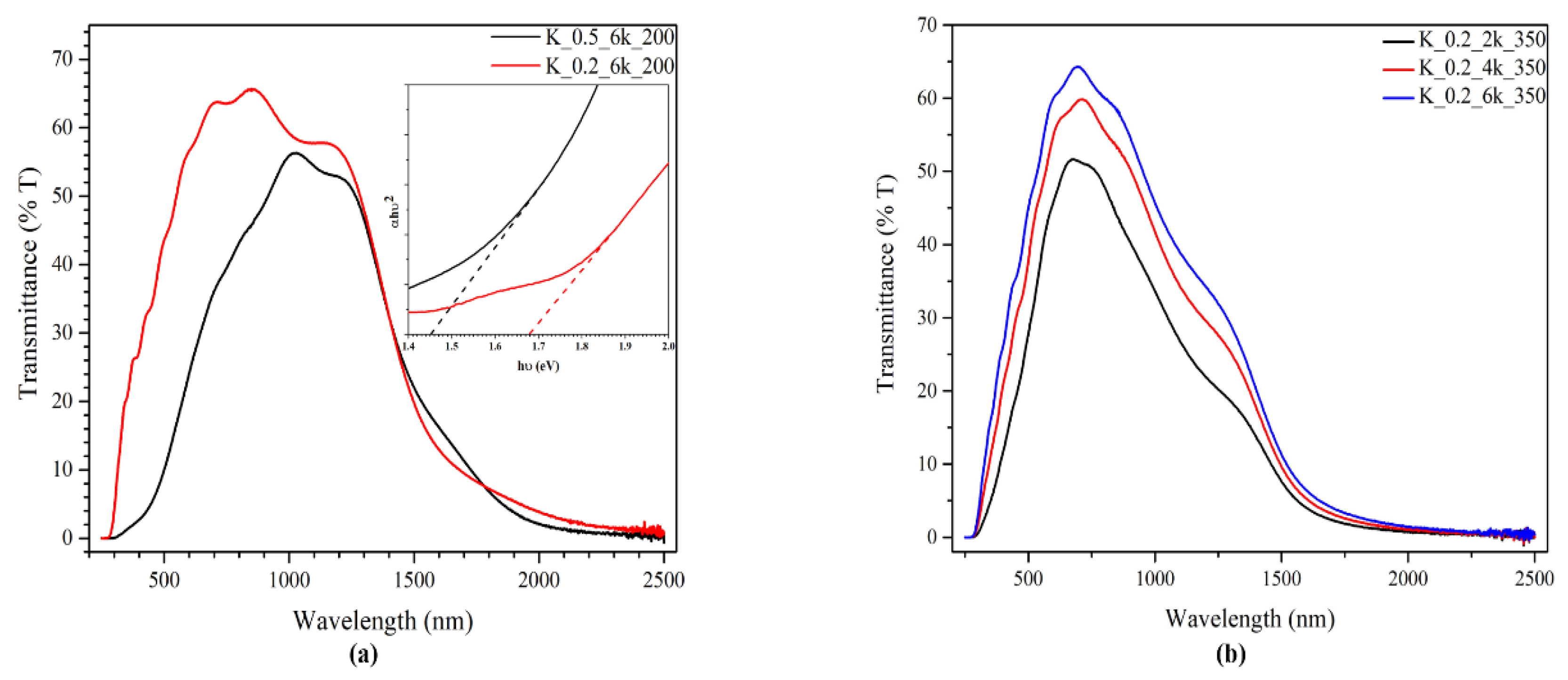
2.1. CZTS Ink Concentration and Spin Rate
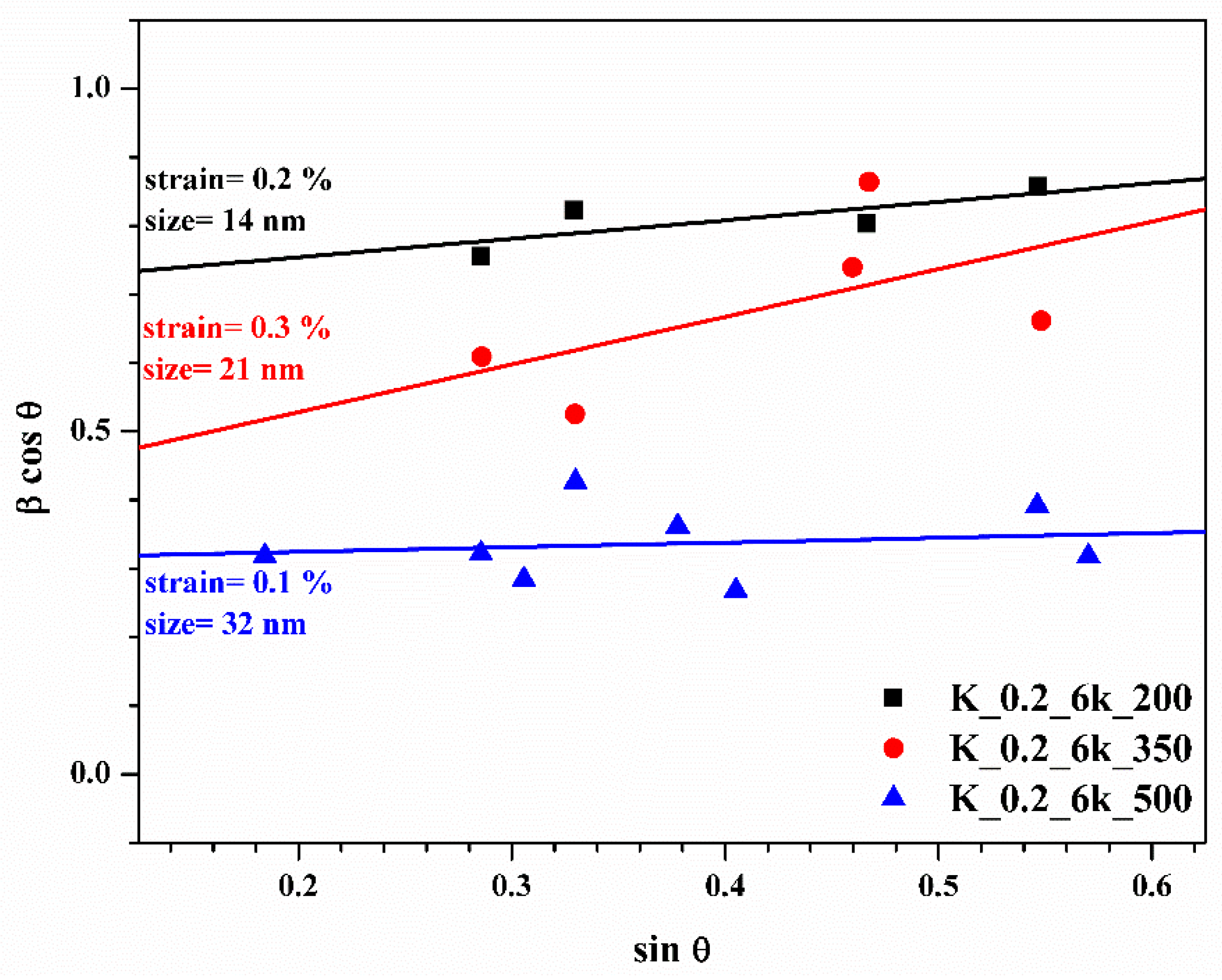
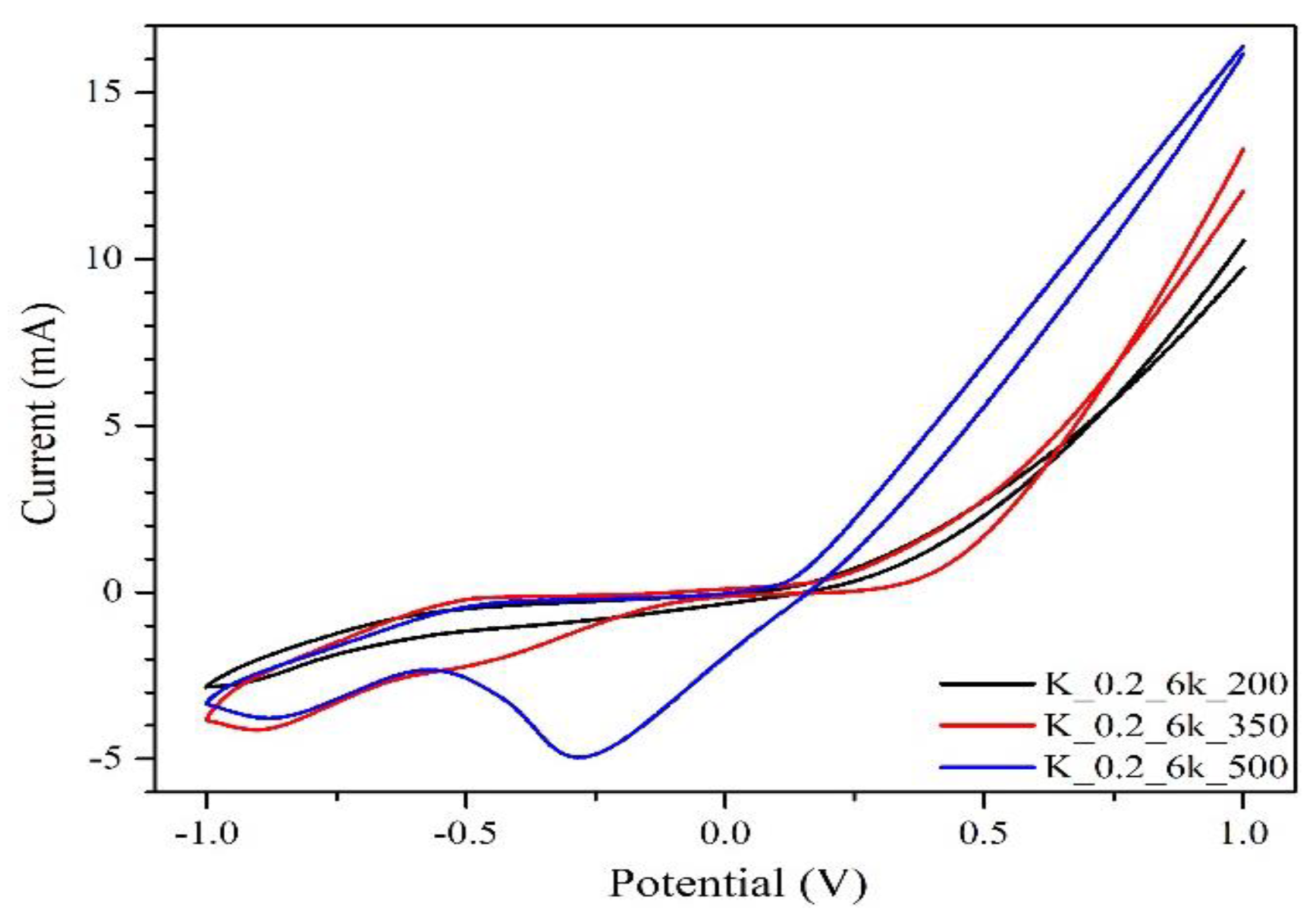
2.2. TT Temperature
3. Materials and Methods
3.1. Synthesis of CZTS Nanoparticles Ink
3.2. Deposition of CZTS Thin Film
3.3. CZTS Thin Film Characterization
- α = absorption coefficient,
- d = film thickness,
- = normal incidence transmittance, and
- = normal incidence reflectance.
- ρ = sample resistivity (Ω∙cm),
- σ = sample conductivity (Ω−1∙cm−1),
- = charge constant (1.6 × 10−19 C),
- μ = carrier mobility (cm2/Vs), and
- = carrier concentration (cm−3).
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nakayama, N.; Ito, K. Sprayed films of stannite Cu2ZnSnS4. Appl. Surf. Sci. 1996, 92, 171–175. [Google Scholar] [CrossRef]
- Kattan, N.; Hou, B.; Fermín, D.J.; Cherns, D. Crystal structure and defects visualization of Cu2ZnSnS4 nanoparticles employing transmission electron microscopy and electron diffraction. Appl. Mater. Today 2015, 1, 52–59. [Google Scholar] [CrossRef]
- Gonce, M.K.; Dogru, M.; Aslan, E.; Ozel, F.; Patir, I.H.; Kus, M.; Ersoz, M. Photocatalytic Hydrogen Evolution Based on Cu2ZnSnS4, Cu2ZnSnSe4, Cu2ZnSnSe4-xSx Nanofibers. RSC Adv. 2015, 5, 94025–94028. [Google Scholar] [CrossRef]
- Ricardo, C.L.A.; Girardi, F.; Cappelletto, E.; D’Angelo, R.; Ciancio, R.; Carlino, E.; Ricci, P.C.; Malerba, C.; Mittiga, A.; Di Maggio, R.; et al. Chloride-based route for monodisperse Cu2ZnSnS4 nanoparticles preparation Chloride-based route for monodisperse Cu 2 ZnSnS 4 nanoparticles preparation. J. Renew. Sustain. Energy 2015, 7. [Google Scholar]
- Huang, S.; Luo, W.; Zou, Z. Band positions and photoelectrochemical properties of Cu 2 ZnSnS 4 thin films by the ultrasonic spray pyrolysis method. J. Phys. D. Appl. Phys. 2013, 46, 235108. [Google Scholar] [CrossRef]
- Murata, M.; Chantana, J.; Ashida, N.; Hironiwa, D.; Minemoto, T. Influence of conduction band minimum difference between transparent conductive oxide and absorber on photovoltaic performance of thin-film solar cell In fl uence of conduction band minimum difference between transparent conductive oxide and absorber on ph. Jpn. J. Appl. Phys. 2015, 54, 032301. [Google Scholar] [CrossRef]
- Park, S.-N.; Sung, S.-J.; Sim, J.-H.; Yang, K.-J.; Hwang, D.-K.; Kim, J.; Kim, G.Y.; Jo, W.; Kim, D.-H.; Kang, J.-K. Nanostructured p-type CZTS thin films prepared by facile solution process for 3D p-n junction solar cells. Nanoscale 2015, 7, 11182–11189. [Google Scholar] [CrossRef]
- Habas, S.E.; Platt, H.A.S.; van Hest, M.F.A.M.; Ginley, D.S. Low-Cost Inorganic Solar Cells: From Ink To Printed Device. Chem. Rev. 2010, 110, 6571–6594. [Google Scholar] [CrossRef]
- Abermann, S. Non-vacuum processed next generation thin film photovoltaics: Towards marketable efficiency and production of CZTS based solar cells. Sol. Energy 2013, 94, 37–70. [Google Scholar] [CrossRef]
- Jiang, M.; Yan, X. Cu2ZnSnS4 thin film solar cells: present status and future prospects. Sol. Cells Res. Appl. Perspect. 2013, 107–143. [Google Scholar] [CrossRef]
- Yin, Y.; Alivisatos, A.P. Colloidal nanocrystal synthesis and the organic–inorganic interface. Nature 2005, 437, 664–670. [Google Scholar] [CrossRef]
- Ananthakumar, S.; Kumar, R. Influence of co-ordinating and non-coordinating solvents in structural and morphological properties of Cu2ZnSnS4 (CZTS) nanoparticles. Optik (Stuttg). 2017, 130, 99–105. [Google Scholar]
- Huang, T.J.; Yin, X.; Tang, C.; Qi, G.; Gong, H. Influence of Ligands on the Formation of Kesterite Thin Films for Solar Cells: A Comparative Study. 2016, 9, 1032–1041. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, G.; Ji, C.; Huang, K.; Zha, C.; Wang, Y.; Shen, L.; Gupta, A.; Bao, N. Efficient Thermolysis Route to Monodisperse Cu2ZnSnS4 Nanocrystals with Controlled Shape and Structure. Sci. Rep. 2015, 4, 5086. [Google Scholar] [CrossRef]
- Ataollahi, N.; Malerba, C.; Cappelletto, E.; Ciancio, R.; Edla, R.; Di Maggio, R.; Scardi, P. Control of composition and grain growth in Cu 2 ZnSnS 4 thin films from nanoparticle inks. Thin Solid Films 2019, 674, 12–21. [Google Scholar] [CrossRef]
- Hadke, S.H.; Levcenko, S.; Lie, S.; Hages, C.J.; Márquez, J.A.; Unold, T.; Wong, L.H. Synergistic Effects of Double Cation Substitution in Solution-Processed CZTS Solar Cells with over 10% Efficiency. Adv. Energy Mater. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Datta, S.K.; Chaudhuri, A.K. On the mechanism of photoconductivity in polycrystalline lead selenide films. Semicond. Sci. Technol. 1989, 4, 376–381. [Google Scholar] [CrossRef]
- Pal, U. Dark- and photoconductivity in doped and undoped zinc telluride films. Semicond. Sci. Technol. 1993, 8, 1331–1336. [Google Scholar] [CrossRef]
- Pal, R.; Chattopadhyay, K.K.; Chaudhuri, S.; Pal, A.K. Photoconductivity in CuInSe2 films. Sol. Energy Mater. Sol. Cells 1994, 33, 241–251. [Google Scholar] [CrossRef]
- Kosyak, V.; Karmarkar, M.A.; Scarpulla, M.A. Temperature dependent conductivity of polycrystalline Cu2ZnSnS4 thin films. Appl. Phys. Lett. 2012, 100, 263903. [Google Scholar] [CrossRef]
- Ghediya, P.R.; Chaudhuri, T.K.; Vankhade, D. Electrical conduction of CZTS films in dark and under light from molecular solution ink. J. Alloys Compd. 2016, 685, 498–506. [Google Scholar] [CrossRef]
- González, J.C.; Ribeiro, G.M.; Viana, E.R.; Fernandes, P.A.; Salomé, P.M.P.; Gutiérrez, K.; Abelenda, A.; Matinaga, F.M.; Leitão, J.P.; da Cunha, A.F. Hopping conduction and persistent photoconductivity in Cu2ZnSnS4 thin films. J. Phys. D. Appl. Phys. 2013, 46, 155107. [Google Scholar] [CrossRef]
- Moholkar, A.V.; Shinde, S.S.; Babar, A.R.; Sim, K.U.; bin Kwon, Y.; Rajpure, K.Y.; Patil, P.S.; Bhosale, C.H.; Kim, J.H. Development of CZTS thin films solar cells by pulsed laser deposition: Influence of pulse repetition rate. Sol. Energy 2011, 85, 1354–1363. [Google Scholar] [CrossRef]
- Park, H.; Hwang, Y.H.; Bae, B.S. Sol-gel processed Cu2ZnSnS4 thin films for a photovoltaic absorber layer without sulfurization. J. Sol-Gel Sci. Technol. 2013, 65, 23–27. [Google Scholar] [CrossRef]
- Muslih, E.Y.; Kim, K.H. Characteristics of Cu2ZnSnS4 thin film prepared by calcination and sulfurizing of metal (Cu,Zn,Sn)-ethanolamine precursor complexed from metal (Cu,Zn,Sn)-hydrate. Chalcogenide Lett. 2015, 12, 349–355. [Google Scholar]
- Tao, J.; Chen, L.; Cao, H.; Zhang, C.; Liu, J.; Zhang, Y.; Huang, L.; Jiang, J.; Yang, P.; Chu, J. Co-electrodeposited Cu2ZnSnS4 thin-film solar cells with over 7% efficiency fabricated via fine-tuning of the Zn content in absorber layers. J. Mater. Chem. A 2016, 4, 3798–3805. [Google Scholar] [CrossRef]
- Khanzada, L.S.; Levchuk, I.; Hou, Y.; Azimi, H.; Osvet, A.; Ahmad, R.; Brandl, M.; Herre, P.; Distaso, M.; Hock, R.; et al. Effective Ligand Engineering of the Cu2ZnSnS4 Nanocrystal Surface for Increasing Hole Transport Efficiency in Perovskite Solar Cells. Adv. Funct. Mater. 2016, 26, 8300–8306. [Google Scholar] [CrossRef]
- Zuo, Y.; Chen, L.; Jiang, W.; Liu, B.; Zeng, C.; Li, M.; Shi, X. High-efficiency perovskite solar cells improved with low-cost orthorhombic Cu 2 Znsns 4 as the hole-transporting layer. Mater. Tehnol. 2018, 52, 483–486. [Google Scholar] [CrossRef]
- Wu, Q.; Xue, C.; Li, Y.; Zhou, P.; Liu, W.; Zhu, J.; Dai, S.; Zhu, C.-F.; Yang, S. Kesterite Cu2ZnSnS4 as a Low Cost Inorganic Hole Transporting Material for High Efficiency Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2015, 7, 28466–28473. [Google Scholar] [CrossRef]
- Deganello, D. Printing Techniques for the Fabrication of OLEDs; Woodhead Publishing Limited: Sawston/Cambridge, UK, 2013; ISBN 9780857094254. [Google Scholar]
- Tsuda, A.; Venkata, N.K. The role of natural processes and surface energy of inhaled engineered nanoparticles on aggregation and corona formation. NanoImpact 2016, 2, 38–44. [Google Scholar] [CrossRef]
- Prasad, P.S.R.K.; Reddy, A.V.; Rajesh, P.K.; Ponnambalam, P.; Prakasan, K. Studies on rheology of ceramic inks and spread of ink droplets for direct ceramic ink jet printing. J. Mater. Process. Technol. 2006, 176, 222–229. [Google Scholar] [CrossRef]
- Soares, J.A.N.T. Introduction to Optical Characterization of Materials. In Practical Materials Characterization; Sardela, M., Ed.; Springer: New York, NY, USA, 2014; pp. 43–92. ISBN 9781461492818. [Google Scholar]
- Ingle, J.D.J.; Crouch, S.R. Spectrochemical analysis; Prentice Hall: Upper Saddle River, NJ, USA, 1988. [Google Scholar]
- Akaltun, Y.; Yildirim, M.A.; Ateş, A.; Yildirim, M. The relationship between refractive index-energy gap and the film thickness effect on the characteristic parameters of CdSe thin films. Opt. Commun. 2011, 284, 2307–2311. [Google Scholar] [CrossRef]
- Li, W.; Tan, J.M.R.; Leow, S.W.; Lie, S.; Magdassi, S.; Wong, L.H. Recent Progress in Solution-Processed Copper-Chalcogenide Thin-Film Solar Cells. Energy Technol. 2018, 6, 46–59. [Google Scholar] [CrossRef]
- Yu, N.; Zhong, R.; Zhong, W.; Chen, X.; Luo, J.; Gu, X.; Hu, X.; Zhang, L.; Hu, J.; Chen, Z. Synthesis of Cu2ZnSnS4film by air-stable molecular-precursor ink for constructing thin film solar cells. RSC Adv. 2014, 4, 36046–36052. [Google Scholar] [CrossRef]
- Kim, H.T.; Kim, D.; Park, C. Temperature effects on Cu 2ZnSnS 4 (CZTS) films deposited by spraying method. Mol. Cryst. Liq. Cryst. 2012, 564, 155–161. [Google Scholar] [CrossRef]
- Singh, S.; Brandon, M.; Liu, P.; Laffir, F.; Redington, W.; Ryan, K.M. Selective Phase Transformation of Wurtzite Cu2ZnSn ( SSe ) 4 Nanocrystals into Zinc Blende and Kesterite by Solution and Solid State Transformations Selective Phase Transformation of Wurtzite Cu 2 ZnSn ( SSe ) 4 Nano- crystals into Zinc Blende and Kesteri. Chem. Mater. 2016, 28, 5055–5062. [Google Scholar] [CrossRef]
- Isotta, E.; Pugno, N.M.; Scardi, P. Nanostructured kesterite (Cu2ZnSnS4) for applications in thermoelectric devices. Powder Diffr. 2019, 1–6. [Google Scholar] [CrossRef]
- Kapusta, K.; Drygas, M.; Janik, J.F.; Jelen, P.; Bucko, M.M.; Olejniczak, Z. From magnetic cubic pre-kesterite to semiconducting tetragonal kesterite Cu2ZnSnS4 nanopowders via the mechanochemically assisted route. J. Alloys Compd. 2019, 770, 981–988. [Google Scholar] [CrossRef]
- Indubala, E.; Sarveshvaran, S.; Sudha, V.; Mamajiwala, A.Y.; Harinipriya, S. Secondary phases and temperature effect on the synthesis and sulfurization of CZTS. Sol. Energy 2018, 173, 215–224. [Google Scholar] [CrossRef]
- Williamson, G.; Hall, W. X-ray line broadening from filed aluminium and wolfram. Acta Metall. 1953, 1, 22–31. [Google Scholar] [CrossRef]
- Scardi, P.; Leoni, M.; Delhez, R. IUCr Line broadening analysis using integral breadth methods: A critical review. J. Appl. Crystallogr. 2004, 37, 381–390. [Google Scholar] [CrossRef]
- Scardi, P. Chapter 13. Microstructural Properties: Lattice Defects and Domain Size Effects. In Powder Diffraction; Royal Society of Chemistry: Cambridge, 2008; pp. 376–413. [Google Scholar]
- Ahmad, R.; Brandl, M.; Distaso, M.; Herre, P.; Spiecker, E.; Hock, R.; Peukert, W. A comprehensive study on the mechanism behind formation and depletion of Cu2ZnSnS4 (CZTS) phases. CrystEngComm 2015, 17, 6972–6984. [Google Scholar] [CrossRef]
- Malerba, C.; Biccari, F.; Leonor, C.; Ricardo, A.; Valentini, M.; Chierchia, R.; Müller, M.; Santoni, A.; Esposito, E.; Mangiapane, P.; et al. CZTS stoichiometry effects on the band gap energy. J. Alloys Compd. 2014, 582, 528–534. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, T.; Lin, S.; Zhang, Y.; Tesfamichael, T.; Bell, J.; Wang, H. Effect of different thermo-treatment at relatively low temperatures on the properties of indium-tin-oxide thin films. Thin Solid Films 2017, 636, 702–709. [Google Scholar] [CrossRef]
- Safdar, A.; Islam, M.; Akram, M.A.; Mujahid, M.; Khalid, Y.; Shah, S.I. Reaction Time and Film Thickness Effects on Phase Formation and Optical Properties of Solution Processed Cu2ZnSnS4 Thin Films. J. Mater. Eng. Perform. 2016, 25, 457–465. [Google Scholar] [CrossRef]
- Ghorpadea, U.V.; Suryawanshi, M.P.; Shin, S.W.; woo Hong, C.; Kim, I.; Moon, J.H.; Yun, J.H.; Kima, J.H.; Kolekar, S.S. Wurtzite CZTS Nanocrystals and Phase Evolution to Kesterite Thin Film for Solar Energy Harvesting. Phys. Chem. Chem. Phys. 2015, 17, 19777–19788. [Google Scholar] [CrossRef]
- Swami, S.K.; Kumar, A.; Dutta, V. Deposition of kesterite Cu 2 ZnSnS 4 (CZTS) thin films by spin coating technique for solar cell application. Energy Procedia 2013, 33, 198–202. [Google Scholar] [CrossRef]
- Shin, S.W.; Pawar, S.M.; Park, C.Y.; Yun, J.H.; Moon, J.-H.; Kim, J.H.; Lee, J.Y. Studies on Cu2ZnSnS4 (CZTS) absorber layer using different stacking orders in precursor thin films. Sol. Energy Mater. Sol. Cells 2011, 95, 3202–3206. [Google Scholar] [CrossRef]
- Al-Shakban, M.; Matthews, P.D.; Savjani, N.; Zhong, X.L.; Wang, Y.; Missous, M.; O’Brien, P. The synthesis and characterization of Cu2ZnSnS4 thin films from melt reactions using xanthate precursors. J. Mater. Sci. 2017, 52, 12761–12771. [Google Scholar] [CrossRef]
- Muhunthan, N.; Singh, O.P.; Singh, S.; Singh, V.N. Growth of CZTS thin films by co-sputtering of metal targets and sulfurization in H 2 S N. Muhunthan, Om Pal Singh, Son Singh, and V.N. Singh* CSIR-National Physical Laboratory, Dr. K. S. Krishnan Marg, New Delhi-110012. Int. J. Photoenergy 2013, 2013, 1–23. [Google Scholar] [CrossRef]
- Islam, M.A.; Aziz, A.; Witjaksono, G.; Amin, N. Structural, Electrical and Optical Properties of Zn Rich CZTS Thin Film. IEEE Student Conf. Res. Dev. 2013, 16–17. [Google Scholar]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications; Wiley: Hoboken, NJ, USA, 2001; ISBN 9780471043720. [Google Scholar]
- Yue, G.; Ma, X.; Zhang, W.; Li, F.; Wu, J.; Li, G. A highly efficient flexible dye-sensitized solar cell based on nickel sulfide/platinum/titanium counter electrode. Nanoscale Res. Lett. 2015, 10, 1. [Google Scholar] [CrossRef]
Sample Availability: Not available. |




| Sample | Thickness (nm) | Resistivity, (Ω·cm) | Carrier Concentration, n (cm−3) | Carrier Mobility, μ (cm2/Vs) |
|---|---|---|---|---|
| TT 200 °C | ~135 | 4.0 × 104 | 2 × 1013 | 13 |
| TT 350 °C | ~100 | 4.6 × 104 | 5 × 1012 | 27 |
| TT 500 °C | ~90 | 4.1 × 10−1 | 1 × 1021 | 0.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Syafiq, U.; Ataollahi, N.; Maggio, R.D.; Scardi, P. Solution-Based Synthesis and Characterization of Cu2ZnSnS4 (CZTS) Thin Films. Molecules 2019, 24, 3454. https://doi.org/10.3390/molecules24193454
Syafiq U, Ataollahi N, Maggio RD, Scardi P. Solution-Based Synthesis and Characterization of Cu2ZnSnS4 (CZTS) Thin Films. Molecules. 2019; 24(19):3454. https://doi.org/10.3390/molecules24193454
Chicago/Turabian StyleSyafiq, Ubaidah, Narges Ataollahi, Rosa Di Maggio, and Paolo Scardi. 2019. "Solution-Based Synthesis and Characterization of Cu2ZnSnS4 (CZTS) Thin Films" Molecules 24, no. 19: 3454. https://doi.org/10.3390/molecules24193454
APA StyleSyafiq, U., Ataollahi, N., Maggio, R. D., & Scardi, P. (2019). Solution-Based Synthesis and Characterization of Cu2ZnSnS4 (CZTS) Thin Films. Molecules, 24(19), 3454. https://doi.org/10.3390/molecules24193454









