The Effects of Vinegar Processing on the Changes in the Physical Properties of Frankincense Related to the Absorption of the Main Boswellic Acids
Abstract
1. Introduction
2. Results and Discussion
2.1. Differences in Physical Property Indices Between Frankincense and Processed Frankincense
2.1.1. Surface Morphology by Scanning Electron Microscopy (SEM)
2.1.2. Particle Size, PDI, and ZP
2.1.3. Specific Surface Area and Porosity
2.1.4. Viscosity of the Simulated Gastrointestinal Fluid
2.2. Establishment of the Content Determination Method of the Six Main BAs
2.2.1. Optimization of Sample Preparation
2.2.2. Optimization of the LC Conditions
2.2.3. Optimization of the MS Conditions
2.3. Method Validation
2.3.1. Specificity
2.3.2. Linearity, Limit of Detection (LOD), and Lower Limit of Quantitation (LLOQ)
2.3.3. Precision and Accuracy
2.3.4. Matrix Effect
2.3.5. Recovery
2.3.6. Stability
2.3.7. Carryover
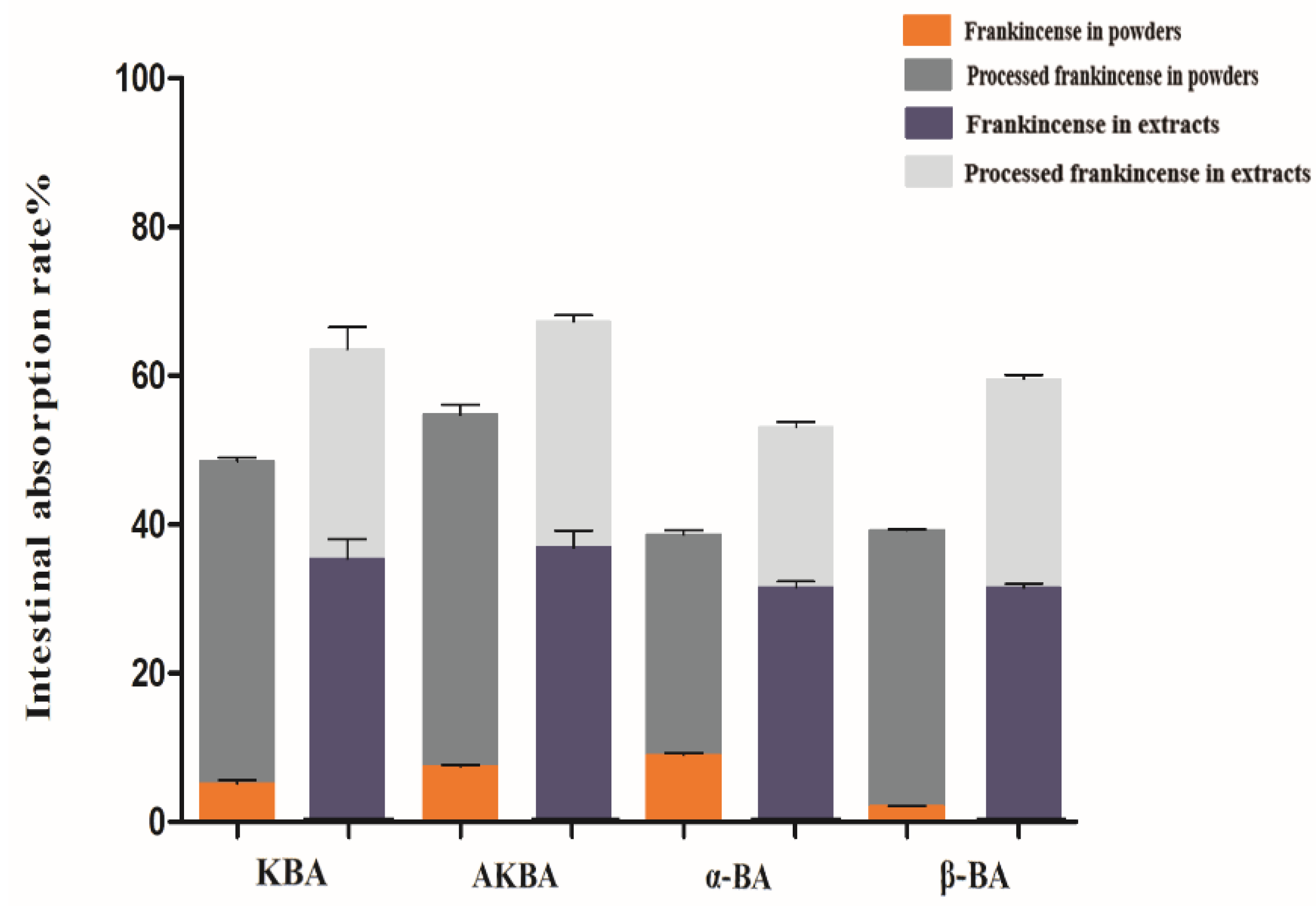
2.4. Determination of the Intestinal Absorption Rate
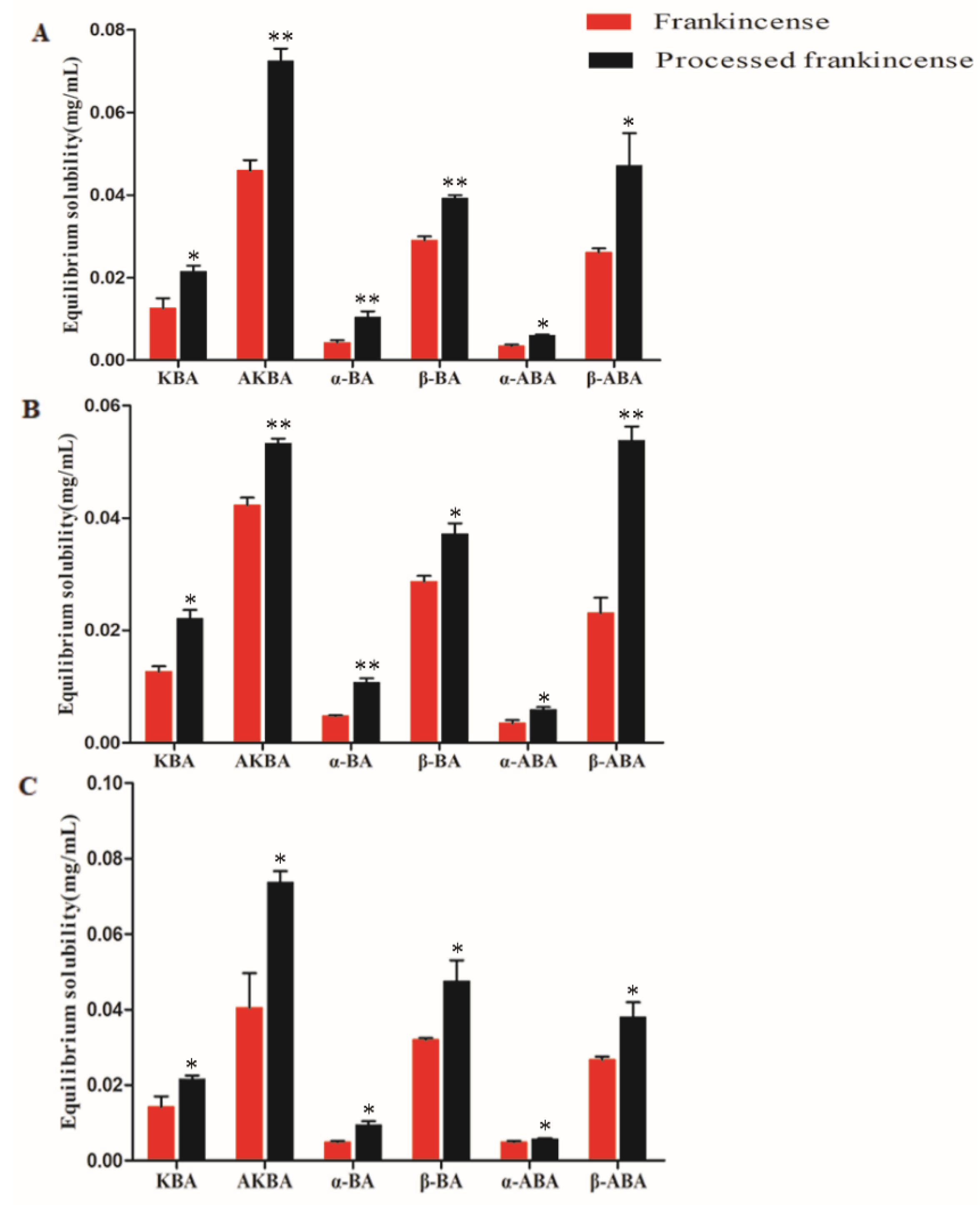
2.5. Measurement of the Equilibrium Solubility
3. Materials and Methods
3.1. Instruments, Chemicals and Animals
3.2. Measurement of the Physical Property Indices of Frankincense and Processed Frankincense
3.2.1. Measurement of the Surface Morphology
3.2.2. Measurement of the Particle Size, PDI, and ZP
3.2.3. Measurement of the Specific Surface Area and Porosity
3.2.4. Measurement of the Viscosity [31]
3.3. UHPLC-TQ-MS Conditions
3.4. Preparation of Standard Solutions
3.5. Preparation of QC Samples
3.6. Method Validation
3.6.1. Specificity
3.6.2. Linearity, LOD, and LLOQ
3.6.3. Precision and Accuracy
3.6.4. Matrix Effect
3.6.5. Recovery
3.6.6. Stability
3.6.7. Carryover
3.7. Procedure of Intestinal Absorption
3.7.1. Preparation of Frankincense and Processed Frankincense Solutions
3.7.2. Preparation of the Everted Rat Gut Sacs and Intestinal Absorption Solution
3.7.3. Sample Preparation
3.7.4. Measurement of the Rate of Intestinal Absorption
3.8. Measurement of the Equilibrium Solubility
3.8.1. Preparation of Samples
3.8.2. Determination of the Equilibrium Solubility
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Frank, A.; Unger, M. Analysis of frankincense from various Boswellia species with inhibitory activity on human drug metabolising cytochrome P450 enzymes using liquid chromatography mass spectrometry after automated on-line extraction. J. Chromatogr. A 2006, 1112, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Mathe, C.; Culioli, G.; Archier, P.; Vieillescazes, C. High-Performance Liquid Chromatographic Analysis of Triterpenoids in Commercial Frankincense. Chromatographia 2004, 60, 493–499. [Google Scholar] [CrossRef]
- Boden, S.E.; Schweizer, S.; Ammon, H.P.T.; Safayhi, H. Concentration-Dependent Potentiating and Inhibitory Effects of Boswellia Extracts on 5-Lipoxygenase Product Formation inStimulated PMNL. Planta Med. 2000, 66, 110–113. [Google Scholar]
- Notarnicola, A.; Tafuri, S.; Fusaro, L.; Moretti, L.; Pesce, V.; Moretti, B. The “MESACA” study: Methylsulfonylmethane and boswellic acids in the treatment of gonarthrosis. Adv. Ther. 2011, 28, 894–906. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, K.; Alluri, K.V.; Satish, A.R.; Mishra, S.; Golakoti, T.; Sarma, K.V.; Dey, D.; Raychaudhuri, S.P. A double blind, randomized, placebo controlled study of the efficacy and safety of 5-Loxin® for treatment of osteoarthritis of the knee. Arthritis Res. Ther. 2008, 10, R85. [Google Scholar] [CrossRef] [PubMed]
- Hamm, S.; Bleton, J.; Connan, J.; Tchapla, A. A chemical investigation by headspace SPME and GC–MS of volatile and semi-volatile terpenes in various olibanum samples. Phytochemistry 2005, 66, 1499–1514. [Google Scholar] [CrossRef]
- Zhang, C.; Sun, L.; Tian, R.-T.; Jin, H.-Y.; Ma, S.-C.; Gu, B.-R. Combination of quantitative analysis and chemometric analysis for the quality evaluation of three different frankincenses by ultra high performance liquid chromatography and quadrupole time of flight mass spectrometry. J. Sep. Sci. 2015, 38, 3324–3330. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Singh, G.; Khajuria, A.; Sidiq, T.; Singh, S.; Chashoo, G.; Pagoch, S.; Kaul, A.; Saxena, A.; Johri, R.; et al. In vivo genotoxicity evaluation of a plant based antiarthritic and anticancer therapeutic agent Boswelic acids in rodents. Phytomedicine 2009, 16, 1112–1118. [Google Scholar] [CrossRef]
- Du, Z.; Liu, Z.; Ning, Z.; Liu, Y.; Song, Z.; Wang, C.; Lu, A. Prospects of Boswellic Acids as Potential Pharmaceutics. Planta Med. 2015, 81, 259–271. [Google Scholar] [CrossRef]
- Gerbeth, K.; Hüsch, J.; Fricker, G.; Werz, O.; Schubert-Zsilavecz, M.; Abdel-Tawab, M. In vitro metabolism, permeation, and brain availability of six major boswellic acids from Boswellia serrata gum resins. Fitoterapia 2013, 84, 99–106. [Google Scholar] [CrossRef]
- Büchele, B.; Simmet, T. Analysis of 12 different pentacyclic triterpenic acids from frankincense in human plasma by high-performance liquid chromatography and photodiode array detection. J. Chromatogr. B 2003, 795, 355–362. [Google Scholar] [CrossRef]
- Pan, Y.-N.; Liang, X.-X.; Niu, L.-Y.; Wang, Y.-N.; Tong, X.; Hua, H.-M.; Zheng, J.; Meng, D.-Y.; Liu, X.-Q. Comparative studies of pharmacokinetics and anticoagulatory effect in rats after oral administration of Frankincense and its processed products. J. Ethnopharmacol. 2015, 172, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-L.; Verpoorte, R.; Yen, H.-R.; Peng, W.-H.; Cheng, Y.-C.; Chao, J.; Pao, L.-H.; Dai, Y. Effects of processing adjuvants on traditional Chinese herbs. J. Food Drug Anal. 2018, 26, S96–S114. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.-H.; Yan, C.-P.; Xu, Z.-S.; Gao, Q.-Q.; Chen, Z.-P.; Li, W.-D. The Effect of Salt-Processed Psoralea corylifolia on Generative Organ Targeting. J. Anal. Methods Chem. 2016, 2016, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Qian, X.; Huang, P. Simultaneous determination of ten flavonoids of crude and wine-processed Radix Scutellariae aqueous extracts in rat plasma by UPLC-ESI-MS/MS and its application to a comparative pharmacokinetic study. Biomed. Chromatogr. 2015, 29, 1112–1123. [Google Scholar] [CrossRef] [PubMed]
- Shilpi, S.; Sanjula, B.; Mushir, A.; Anil, K.; Javed, A. Solid Dispersion: An Alternative Technique for Bioavailability Enhancement of Poorly Soluble Drugs. J. Dispers. Sci. Technol. 2009, 30, 1458–1473. [Google Scholar]
- Shekhawat, P.B.; Pokharkar, V.B. Understanding peroral absorption: Regulatory aspects and contemporary approaches to tackling solubility and permeability hurdles. Acta Pharm. Sin. B 2017, 7, 260–280. [Google Scholar] [CrossRef]
- Göke, K.; Bunjes, H. Parameters influencing the course of passive drug loading into lipid nanoemulsions. Eur. J. Pharm. Biopharm. 2018, 126, 123–131. [Google Scholar] [CrossRef]
- Göke, K.; Lorenz, T.; Repanas, A.; Schneider, F.; Steiner, D.; Baumann, K.; Bunjes, H.; Dietzel, A.; Finke, J.H.; Glasmacher, B.; et al. Novel strategies for the formulation and processing of poorly water-soluble drugs. Eur. J. Pharm. Biopharm. 2018, 126, 40–56. [Google Scholar] [CrossRef]
- Kollipara, S.; Gandhi, R.K. Pharmacokinetic aspects and in vitro–in vivo correlation potential for lipid-based formulations. Acta Pharm. Sin. B 2014, 4, 333–349. [Google Scholar] [CrossRef]
- Zhang, X.; Xing, H.; Zhao, Y.; Ma, Z. Pharmaceutical Dispersion Techniques for Dissolution and Bioavailability Enhancement of Poorly Water-Soluble Drugs. Pharmaceutics 2018, 10, 74–107. [Google Scholar] [CrossRef] [PubMed]
- Carrier, R.L.; Miller, L.A.; Ahmed, I. The utility of cyclodextrins for enhancing oral bioavailability. J. Control Release 2007, 123, 78–99. [Google Scholar] [CrossRef] [PubMed]
- Curatolo, W. Physical chemical properties of oral drug candidates in the discovery and exploratory development settings. Pharm. Sci. Technol. Today 1998, 1, 387–393. [Google Scholar] [CrossRef]
- Aguiar, G.P.S.; Marcon, M.; Mocelin, R.; Herrmann, A.P.; Chaves, L.M.; Piato, A.L.; Lanza, M.; Oliveira, J. Micronization of N -acetylcysteine by supercritical fluid: Evaluation of in vitro and in vivo biological activity. J. Supercrit. Fluids 2017, 130, 282–291. [Google Scholar] [CrossRef]
- Dou, Z.; Li, K.; Wang, P.; Cao, L. Effect of Wine and Vinegar Processing of Rhizoma Corydalis on the Tissue Distribution of Tetrahydropalmatine, Protopine and Dehydrocorydaline in Rats. Molecules 2012, 17, 951–970. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Liu, L.; Zhu, X.; Wu, L.; Chen, Z.; Xu, Z.; Li, W. Evaluation of the Absorption Behavior of Main Component Compounds of Salt-Fried Herb Ingredients in Qing′e Pills by Using Caco-2 Cell Model. Molecules 2018, 23, 3321. [Google Scholar] [CrossRef]
- Wu, H.; Waldbauer, K.; Tang, L.; Xie, L.; McKinnon, R.; Zehl, M.; Yang, H.; Xu, H.; Kopp, B. Influence of Vinegar and Wine Processing on the Alkaloid Content and Composition of the Traditional Chinese Medicine Corydalis Rhizoma (Yanhusuo). Molecules 2014, 19, 11487–11504. [Google Scholar] [CrossRef] [PubMed]
- Aretz, A.; Ehle, L.; Haeusler, A.; Bobzin, K.; Öte, M.; Wiesner, S.; Schmidt, A.; Gillner, A.; Poprawe, R.; Mayer, J. In situ investigation of production processes in a large chamber scanning electron microscope. Ultramicroscopy 2018, 193, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Muller, E.; Meffert, M.; Gerthsen, D. On the Progress of Scanning Transmission Electron Microscopy (STEM) Imaging in a Scanning Electron Microscope. Microsc. Microanal. 2018, 24, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Bansal, M.; Mittal, N.; Yadav, S.K. Periodontal thermoresponsive, mucoadhesive dual antimicrobial loaded in-situ gel for the treatment of periodontal disease: Preparation, in-vitro characterization and antimicrobial study. J. Oral. Biol. Craniofac. Res. 2018, 8, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of People’s Republic of China; China Medical Science Press: Beijing, China, 2015; pp. 447–495. [Google Scholar]
- Li, Q.; Chen, F.; Liu, Y.; Yu, S.; Gai, X.; Ye, M.; Yang, X.; Pan, W. A novel albumin wrapped nanosuspension of meloxicam to improve inflammation-targeting effects. Int. J. Nanomed. 2018, 13, 4711–4725. [Google Scholar] [CrossRef]
- Badie, H.; Abbas, H. Novel Small Self-Assembled Resveratrol -Bearing Cubosomes and Hexosomes: Preparation, Charachterization and Ex Vivo Permeation. Drug Dev. Ind. Pharm. 2018, 44, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Ikonen, M.; Murtomäki, L.; Kontturi, K. Microcalorimetric and zeta potential study on binding of drugs on liposomes. Colloids Surf. B Biointerfaces 2010, 78, 275–282. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Y.; Zhang, J.; Hao, L.; Guo, H.; Lou, H.; Zhang, D. Bexarotene nanocrystal—Oral and parenteral formulation development, characterization and pharmacokinetic evaluation. Eur. J. Pharm. Biopharm. 2014, 87, 160–169. [Google Scholar] [CrossRef]
- Gigliobianco, M.R.; Casadidio, C.; Censi, R.; Di Martino, P. Nanocrystals of Poorly Soluble Drugs: Drug Bioavailability and Physicochemical Stability. Pharmaceutics 2018, 10, 134. [Google Scholar] [CrossRef] [PubMed]
- Sebe, I.; Szabo, P.; Kállai-Szabó, B.; Zelkó, R. Incorporating small molecules or biologics into nanofibers for optimized drug release: A review. Int. J. Pharm. 2015, 494, 516–530. [Google Scholar] [CrossRef]
- Kim, H.-I.; Park, S.Y.; Park, S.J.; Lee, J.; Cho, K.H.; Jee, J.-P.; Kim, H.-C.; Maeng, H.-J.; Jang, D.-J. Development and Evaluation of a Reconstitutable Dry Suspension to Improve the Dissolution and Oral Absorption of Poorly Water-Soluble Celecoxib. Pharmaceutics 2018, 10, 140. [Google Scholar] [CrossRef] [PubMed]
- Gibson, N.; Rauscher, H.; Roebben, G. Comments on the article by A. J. Lecloux (J Nanopart Res (2015) 17:447) regarding the use of volume-specific surface area (VSSA) to classify nanomaterials. J. Nanopart. Res. 2016, 18, 250–257. [Google Scholar] [CrossRef]
- Vasvári, G.; Kalmár, J.; Veres, P.; Vecsernyés, M.; Bácskay, I.; Fehér, P.; Ujhelyi, Z.; Haimhoffer, Á.; Rusznyák, Á.; Fenyvesi, F.; et al. Matrix systems for oral drug delivery: Formulations and drug release. Drug Discov. Today Technol. 2018, 27, 71–80. [Google Scholar]
- Valmikinathan, C.M.; DeFroda, S.; Yu, X. Polycaprolactone and Bovine Serum Albumin Based Nanofibers for Controlled Release of Nerve Growth Factor. Biomacromolecules 2009, 10, 1084–1089. [Google Scholar] [CrossRef]
- Mandal, S.; Ray, R.; Basu, S.K. Evaluation of a Matrix Tablet Prepared with Polyacrylamide-g-Sodium Alginate Co-polymers and Their Partially Hydrolyzed Co-polymers for Sustained Release of Diltiazem Hydrochloride. J. Biomater. Sci. Polym. Ed. 2012, 21, 1799–1814. [Google Scholar] [CrossRef] [PubMed]
- Sjögren, E.; Westergren, J.; Grant, I.; Hanisch, G.; Lindfors, L.; Lennernäs, H.; Abrahamsson, B.; Tannergren, C. In silico predictions of gastrointestinal drug absorption in pharmaceutical product development: Application of the mechanistic absorption model GI-Sim. Eur. J. Pharm. Sci. 2013, 49, 679–698. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; Zhao, Y.; Su, J. In Vitro Intestinal Absorption and Metabolism of Magnoflorine and its Potential Interaction in Coptidis Rhizoma Decoction in Rat. Eur. J. Drug Metab. Pharmacokinet. 2017, 42, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.H.; Wiseman, G. The use of sacs of everted small intestine for the study of the transference of substances from the mucosal to the serosal surface. J. Physiol. 1954, 123, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Barthe, L.; Woodley, J.; Houin, G. Gastrointestinal absorption of drugs: Methods and studies. Fundam. Clin. Pharmacol. 1999, 13, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Perrier, J.; Zhou, Z.; Dunn, C.; Khadra, I.; Wilson, C.G.; Halbert, G. Statistical investigation of the full concentration range of fasted and fed simulated intestinal fluid on the equilibrium solubility of oral drugs. Eur. J. Pharm. Sci. 2018, 111, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Baka, E.; Comer, J.E.; Takács-Novák, K. Study of equilibrium solubility measurement by saturation shake-flask method using hydrochlorothiazide as model compound. J. Pharm. Biomed. Anal. 2008, 46, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Ning, Z.; Wang, C.; Liu, Y.; Song, Z.; Ma, X.; Liang, D.; Liu, Z.; Lu, A. Integrating Strategies of Herbal Metabolomics, Network Pharmacology, and Experiment Validation to Investigate Frankincense Processing Effects. Front. Pharmacol. 2018, 9, 1482–1499. [Google Scholar] [CrossRef]
- Guo, N.; Yang, D.; Ablajan, K.; Niu, X.; Fan, B.; Wang, Z.; Dai, J.; Wu, X.; Liu, B. Simultaneous quantitation of seven alkaloids in processed Fuzi decoction by rapid resolution liquid chromatography coupled with tandem mass spectrometry. J. Sep. Sci. 2013, 36, 1953–1958. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds KBA, AKBA, α-BA, β-BA, α-ABA, β-ABA are available from the authors. |


| No. | Compound | RT (min) | [M + H]+ (m/z) | Daughter Ion (m/z) | Fragmentor (V) | CE (eV) |
|---|---|---|---|---|---|---|
| Time segments: 0–7 min | ||||||
| 1 | KBA | 5.522 | 469.4 | 391.5 * 407.4 | 160 | 30 |
| Time segments: 7–17 min | ||||||
| 2 | AKBA | 9.292 | 511 | 255.4 * | 115 | 11 |
| 3 | α-BA | 10.372 | 455 | 437.5 * 372.4 | 250 | 37 |
| 4 | β-BA | 15.589 | 455 | 377.4 * 437.4 | 110 | 35 |
| Time segments: 17–30 min | ||||||
| 5 | α-ABA | 25.6 | 497 | 437.5 * 361.1 | 120 | 12 |
| 6 | β-ABA | 27.598 | 497 | 437.5 * | 120 | 13 |
| No. | Compounds | Regression Equation | r | Linear Range (mg/mL) | LLOQ (mg/mL) | LOD (mg/mL) |
|---|---|---|---|---|---|---|
| 1 | KBA | Y = 2145.1X − 25.865 | 0.9960 | 0.104~1.04 | 0.054 | 0.021 |
| 2 | AKBA | Y = 19549X − 151.53 | 0.9925 | 0.102~1.02 | 0.039 | 0.014 |
| 3 | α-BA | Y = 1169.3X − 7.466 | 0.9997 | 0.106~1.06 | 0.087 | 0.032 |
| 4 | β-BA | Y = 170852X + 4894.1 | 0.9957 | 0.101~1.01 | 0.035 | 0.011 |
| 5 | α-ABA | Y = 300.64X − 46.221 | 0.9987 | 0.98~9.8 | 0.79 | 0.33 |
| 6 | β-ABA | Y = 98.805X − 29.213 | 0.9986 | 0.98~9.8 | 0.59 | 0.28 |
| Compounds | Precision (RSD%) | Accuracy (CV%) | |||
|---|---|---|---|---|---|
| Concentration Levels | Standards Mixture | Extraction of Frankincense | QC Samples | ||
| Intra-Day (n = 6) | Inter-Day (n = 6) | ||||
| KBA | L | 2.96 | 2.67 | 2.46 | 3.04 |
| M | 1.24 | 3.21 | 2.11 | 0.64 | |
| H | 2.46 | 4.03 | 1.68 | 1.05 | |
| AKBA | L | 5.01 | 4.96 | 3.77 | 2.13 |
| M | 3.84 | 4.18 | 3.26 | 0.78 | |
| H | 1.99 | 1.05 | 2.55 | 0.89 | |
| α-BA | L | 2.80 | 2.54 | 0.59 | 4.66 |
| M | 2.47 | 3.22 | 1.21 | 2.40 | |
| H | 4.90 | 2.01 | 0.97 | 2.85 | |
| β-BA | L | 1.09 | 3.49 | 1.91 | 4.13 |
| M | 3.56 | 4.54 | 0.68 | 3.56 | |
| H | 1.43 | 2.08 | 2.34 | 1.19 | |
| α-ABA | L | 3.88 | 1.52 | 3.85 | 2.34 |
| M | 2.54 | 2.73 | 2.44 | 2.90 | |
| H | 4.13 | 3.05 | 1.23 | 3.55 | |
| β-ABA | L | 3.29 | 4.00 | 2.70 | 3.30 |
| M | 1.17 | 2.27 | 2.79 | 4.01 | |
| H | 0.92 | 2.02 | 3.43 | 2.72 | |
| Extraction Samples | QC Samples | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Compounds | Original Amounts (g) | Spiked Amounts (mg) | Detected Amounts (mg) | Recovery (%) | Mean Recovery (%) | RSD (%) | QC Levels | Mean Recovery (%) | RSD (%) |
| KBA | 0.04918 | 0.750 | 1.521 | 102.60 | 98.44 | 4.78 | L | 104.75 | 1.93 |
| 0.04726 | 0.750 | 1.423 | 93.45 | ||||||
| 0.05012 | 0.750 | 1.556 | 105.36 | M | 101.63 | 2.21 | |||
| 0.05035 | 0.750 | 1.498 | 97.15 | ||||||
| 0.04509 | 0.750 | 1.395 | 94.14 | H | 104.38 | 1.05 | |||
| 0.04728 | 0.750 | 1.457 | 97.94 | ||||||
| AKBA | 0.04918 | 1.235 | 2.511 | 105.76 | 101.01 | 4.26 | L | 97.67 | 3.76 |
| 0.04726 | 1.235 | 1.376 | 98.63 | ||||||
| 0.05012 | 1.235 | 2.492 | 102.35 | M | 103.40 | 4.09 | |||
| 0.05035 | 1.235 | 2.390 | 93.64 | ||||||
| 0.04509 | 1.235 | 2.385 | 103.67 | H | 96.71 | 1.98 | |||
| 0.04728 | 1.235 | 2.418 | 102.00 | ||||||
| α-BA | 0.04918 | 0.920 | 1.807 | 97.95 | 99.12 | 1.73 | L | 101.24 | 2.64 |
| 0.04726 | 0.920 | 1.772 | 97.99 | ||||||
| 0.05012 | 0.920 | 1.841 | 99.76 | M | 91.68 | 3.01 | |||
| 0.05035 | 0.920 | 1.820 | 97.02 | ||||||
| 0.04509 | 0.920 | 1.762 | 101.24 | H | 105.11 | 2.47 | |||
| 0.04728 | 0.920 | 1.798 | 100.77 | ||||||
| β-BA | 0.04918 | 2.40 | 4.729 | 99.44 | 97.97 | 3.13 | L | 102.90 | 4.48 |
| 0.04726 | 2.40 | 4.598 | 97.79 | ||||||
| 0.05012 | 2.40 | 4.869 | 103.41 | M | 99.53 | 1.55 | |||
| 0.05035 | 2.40 | 4.704 | 96.08 | ||||||
| 0.04509 | 2.40 | 4.436 | 95.35 | H | 106.39 | 2.20 | |||
| 0.04728 | 2.40 | 4.550 | 102.60 | ||||||
| α-ABA | 0.04918 | 0.318 | 0.607 | 92.54 | 97.88 | 4.86 | L | 102.35 | 3.14 |
| 0.04726 | 0.318 | 0.635 | 105.18 | ||||||
| 0.05012 | 0.318 | 0.641 | 101.35 | M | 98.17 | 1.58 | |||
| 0.05035 | 0.318 | 0.621 | 94.60 | ||||||
| 0.04509 | 0.318 | 0.589 | 95.05 | H | 101.04 | 4.22 | |||
| 0.04728 | 0.318 | 0.614 | 98.54 | ||||||
| β-ABA | 0.04918 | 2.10 | 4.080 | 95.62 | 96.71 | 3.72 | L | 103.82 | 3.23 |
| 0.04726 | 2.10 | 3.891 | 90.47 | ||||||
| 0.05012 | 2.10 | 4.135 | 96.35 | M | 106.44 | 1.79 | |||
| 0.05035 | 2.10 | 4.202 | 99.08 | ||||||
| 0.04509 | 2.10 | 3.956 | 97.92 | H | 100.01 | 2.80 | |||
| 0.04728 | 2.10 | 4.11 | 100.86 | ||||||
| QC Samples | Extraction Samples | ||||
|---|---|---|---|---|---|
| Storage Condition/Temperature | Storage Condition/Period | QC Levels | RSD% | RSD% | |
| KBA | 6 h | L | 1.52 | 2.53 | |
| M | 3.40 | ||||
| H | 3.11 | ||||
| 4 °C | 9 h | L | 2.59 | ||
| M | 4.37 | ||||
| H | 4.05 | ||||
| −80 °C | 12–16 h | L | 1.08 | ||
| M | 2.76 | ||||
| H | 4.43 | ||||
| 4 °C | 30 days | L | 3.92 | ||
| M | 2.44 | ||||
| H | 1.67 | ||||
| AKBA | 25 °C | 6 h | L | 3.03 | 3.46 |
| M | 3.52 | ||||
| H | 4.56 | ||||
| 4 °C | 9 h | L | 1.91 | ||
| M | 2.33 | ||||
| H | 2.53 | ||||
| −80 °C | 12–16 h | L | 4.78 | ||
| M | 3.42 | ||||
| H | 2.03 | ||||
| 4 °C | 30 days | L | 2.72 | ||
| M | 3.30 | ||||
| H | 4.17 | ||||
| α-BA | 25 °C | 6 h | L | 3.00 | 2.72 |
| M | 1.35 | ||||
| H | 4.02 | ||||
| 4 °C | 9 h | L | 2.31 | ||
| M | 4.28 | ||||
| H | 3.96 | ||||
| −80 °C | 12–16 h | L | 4.39 | ||
| M | 2.88 | ||||
| H | 2.05 | ||||
| 4 °C | 30 days | L | 1.22 | ||
| M | 3.64 | ||||
| H | 3.35 | ||||
| β-BA | 25 °C | 6 h | L | 2.92 | 4.39 |
| M | 1.69 | ||||
| H | 4.87 | ||||
| 4 °C | 9 h | L | 1.32 | ||
| M | 3.77 | ||||
| H | 2.33 | ||||
| −80 °C | 12–16 h | L | 2.10 | ||
| M | 3.81 | ||||
| H | 1.54 | ||||
| 4 °C | 30 days | L | 4.00 | ||
| M | 1.79 | ||||
| H | 2.85 | ||||
| α-ABA | 25 °C | 6 h | L | 2.66 | 2.10 |
| M | 4.23 | ||||
| H | 2.05 | ||||
| 4 °C | 9 h | L | 3.48 | ||
| M | 1.77 | ||||
| H | 2.91 | ||||
| −80 °C | 12–16 h | L | 3.08 | ||
| M | 4.33 | ||||
| H | 1.59 | ||||
| 4 °C | 30 days | L | 2.70 | ||
| M | 4.40 | ||||
| H | 3.81 | ||||
| β-ABA | 25 °C | 6 h | L | 4.74 | 4.21 |
| M | 2.90 | ||||
| H | 3.21 | ||||
| 4 °C | 9 h | L | 1.68 | ||
| M | 2.03 | ||||
| H | 2.47 | ||||
| −80 °C | 12–16 h | L | 3.88 | ||
| M | 1.96 | ||||
| H | 4.07 | ||||
| 4 °C | 30 days | L | 2.85 | ||
| M | 2.72 | ||||
| H | 1.29 | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, D.; Ning, Z.; Song, Z.; Wang, C.; Liu, Y.; Wan, X.; Peng, S.; Liu, Z.; Lu, A. The Effects of Vinegar Processing on the Changes in the Physical Properties of Frankincense Related to the Absorption of the Main Boswellic Acids. Molecules 2019, 24, 3453. https://doi.org/10.3390/molecules24193453
Liang D, Ning Z, Song Z, Wang C, Liu Y, Wan X, Peng S, Liu Z, Lu A. The Effects of Vinegar Processing on the Changes in the Physical Properties of Frankincense Related to the Absorption of the Main Boswellic Acids. Molecules. 2019; 24(19):3453. https://doi.org/10.3390/molecules24193453
Chicago/Turabian StyleLiang, Dongrui, Zhangchi Ning, Zhiqian Song, Chun Wang, Yuanyan Liu, Xiaoying Wan, Shitao Peng, Zhenli Liu, and Aiping Lu. 2019. "The Effects of Vinegar Processing on the Changes in the Physical Properties of Frankincense Related to the Absorption of the Main Boswellic Acids" Molecules 24, no. 19: 3453. https://doi.org/10.3390/molecules24193453
APA StyleLiang, D., Ning, Z., Song, Z., Wang, C., Liu, Y., Wan, X., Peng, S., Liu, Z., & Lu, A. (2019). The Effects of Vinegar Processing on the Changes in the Physical Properties of Frankincense Related to the Absorption of the Main Boswellic Acids. Molecules, 24(19), 3453. https://doi.org/10.3390/molecules24193453





