Physicochemical Changes of Air-Dried and Salt-Processed Ulva rigida over Storage Time
Abstract
:1. Introduction
2. Results
2.1. Moisture Content
2.2. Superficial Color
2.3. Mechanical Properties
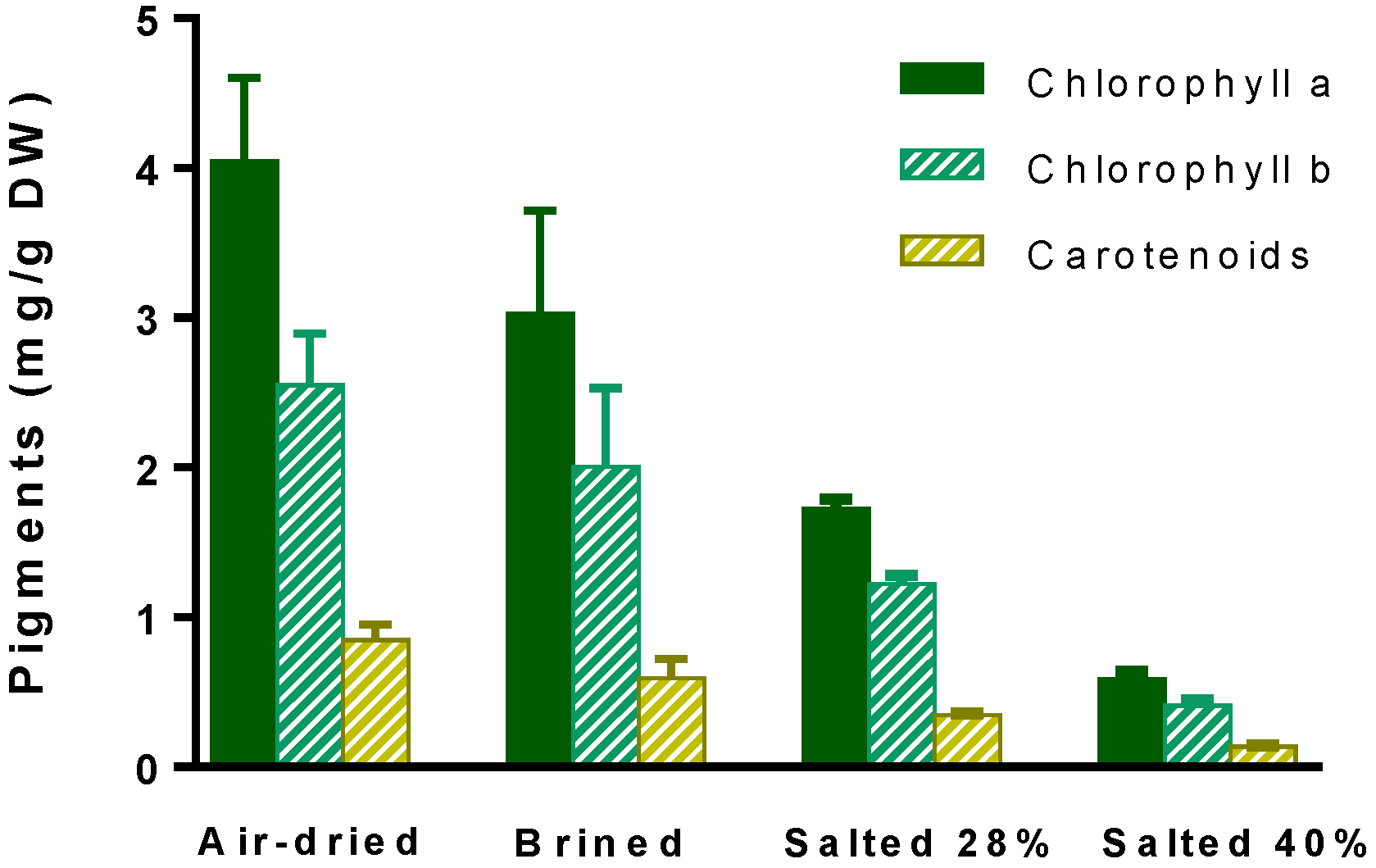
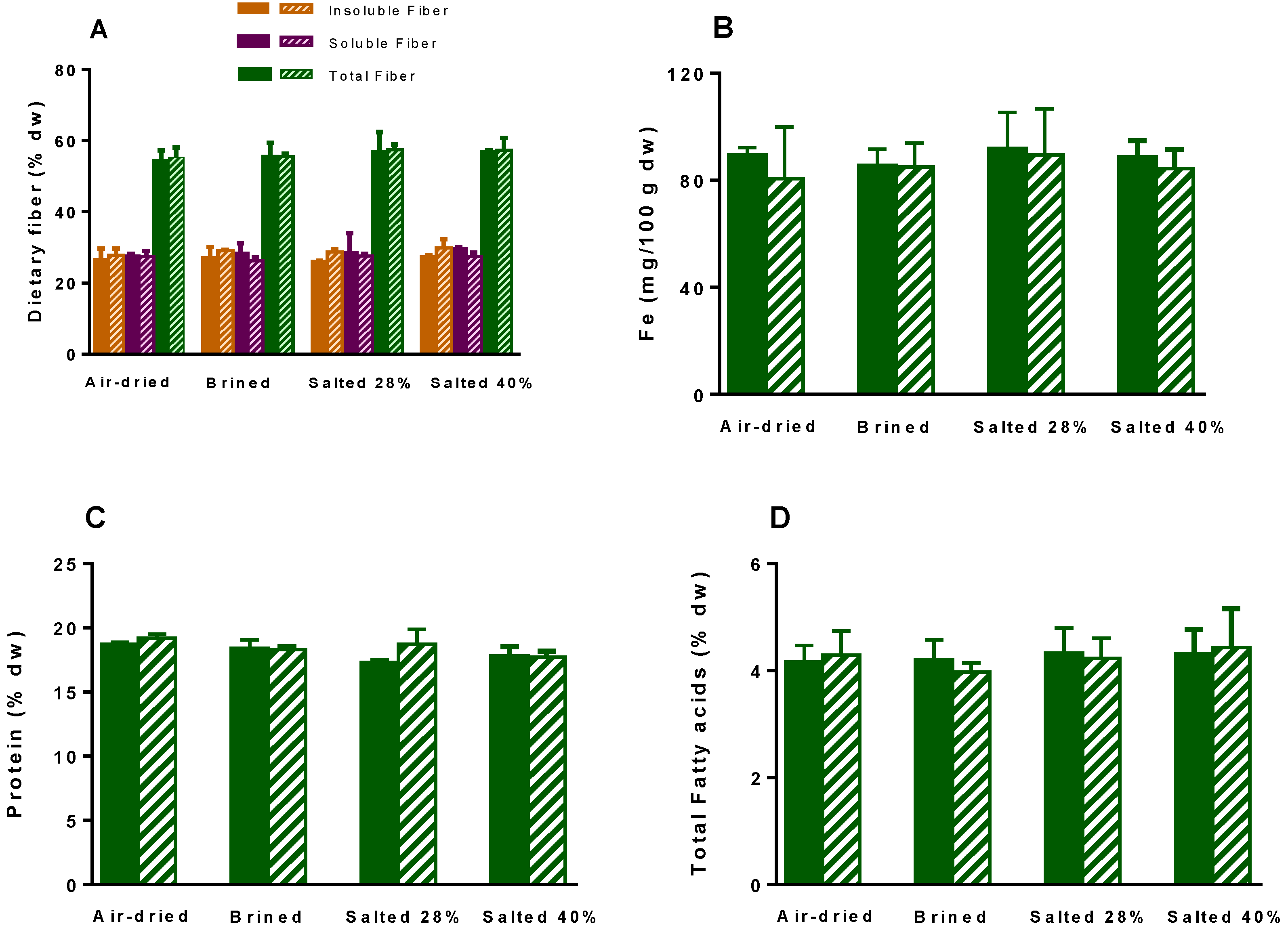
2.4. Nutritional Parameters
3. Materials and Methods
3.1. Sample Collection and Treatments
3.2. Surface Color
3.3. Texture
3.4. Chemical Composition
3.4.1. Contents of chlorophylls and carotenoids
3.4.2. Moisture Content
3.4.3. Protein Content
3.4.4. Dietary Fiber
3.4.5. Iron (Fe)
3.4.6. Fatty Acid Profile
3.5. Statistics
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ortiz, J.; Romero, N.; Robert, P.; Araya, J.; Lopez-Hernández, J.; Bozzo, C.; Navarrete, E.; Osorio, A.; Rios, A. Dietary fiber, amino acid, fatty acid and tocopherol contents of the edible seaweeds Ulva lactuca and Durvillaea antarctica. Food Chem. 2006, 99, 98–104. [Google Scholar] [CrossRef]
- Roohinejad, S.; Koubaa, M.; Barba, F.J.; Saljoughian, S.; Amid, M.; Greiner, R. Application of seaweeds to develop new food products with enhanced shelf-life, quality and health-related beneficial properties. Food Res. Int. 2017, 99, 1066–1083. [Google Scholar] [CrossRef] [PubMed]
- Bocanegra, A.; Bastida, S.; Benedí, J.; Ródenas, S.; Sánchez-Muniz, F.J. Characteristics and Nutritional and Cardiovascular-Health Properties of Seaweeds. J. Med. Food 2009, 12, 236–258. [Google Scholar] [CrossRef] [PubMed]
- Catarino, M.; Silva, A.; Cardoso, S. Phycochemical Constituents and Biological Activities of Fucus spp. Mar. Drugs 2018, 16, 249. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, S.; Carvalho, L.; Silva, P.; Rodrigues, M.; Pereira, O.; Pereira, L. Bioproducts from Seaweeds: A Review with Special Focus on the Iberian Peninsula. Curr. Org. Chem. 2014, 18, 896–917. [Google Scholar] [CrossRef]
- Cardoso, S.; Pereira, O.; Seca, A.; Pinto, D.; Silva, A. Seaweeds as Preventive Agents for Cardiovascular Diseases: From Nutrients to Functional Foods. Mar. Drugs 2015, 13, 6838–6865. [Google Scholar] [CrossRef] [Green Version]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef]
- Bogue, J.; Collins, O.; Troy, A.J. Market analysis and concept development of functional foods. In Developing New Functional Food and Nutraceutical Products; Elsevier: Amsterdam, The Netherlands, 2017; Volume 2, pp. 29–45. ISBN 978-0-12-802780-6. [Google Scholar]
- Yadav, A.K.; Singh, S.V. Osmotic dehydration of fruits and vegetables: A review. J. Food Sci. Technol. 2014, 51, 1654–1673. [Google Scholar] [CrossRef]
- Augustin, M.A.; Riley, M.; Stockmann, R.; Bennett, L.; Kahl, A.; Lockett, T.; Osmond, M.; Sanguansri, P.; Stonehouse, W.; Zajac, I.; et al. Role of food processing in food and nutrition security. Trends Food Sci. Technol. 2016, 56, 115–125. [Google Scholar] [CrossRef]
- European Commission COMMISSION RECOMMENDATION (EU) 2015/138 of 10 August 2015 on the on the monitoring of arsenic in food. Off. J. Eur. Union 2015, 7, 9–10.
- Robic, A.; Sassi, J.-F.; Lahaye, M. Impact of stabilization treatments of the green seaweed Ulva rotundata (Chlorophyta) on the extraction yield, the physico-chemical and rheological properties of ulvan. Carbohydr. Polym. 2008, 74, 344–352. [Google Scholar] [CrossRef]
- Sidónio, R.; Clélia, A.; Teresa, M.; Paulo, N. Effects of different drying conditions on the rehydration ratio and water holding capacity properties in three different species of algae Ulva lactuca, Codium vermilara and Codium tomentosum. Front. Mar. Sci. 2014, 1. [Google Scholar] [CrossRef] [Green Version]
- Uribe, E.; Vega-Gálvez, A.; García, V.; Pastén, A.; López, J.; Goñi, G. Effect of different drying methods on phytochemical content and amino acid and fatty acid profiles of the green seaweed, Ulva spp. J. Appl. Phycol. 2019, 31, 1967–1979. [Google Scholar] [CrossRef]
- Silva, A.; Abreu, H.; Silva, A.; Cardoso, S. Effect of Oven-Drying on the Recovery of Valuable Compounds from Ulva rigida, Gracilaria sp. and Fucus vesiculosus. Mar. Drugs 2019, 17, 90. [Google Scholar] [CrossRef] [PubMed]
- Perera, C. Selected Quality Attributes of Dried Foods. Dry. Technol. 2005, 23, 717–730. [Google Scholar] [CrossRef]
- Pathare, P.B.; Opara, U.L.; Al-Said, F.A.-J. Colour Measurement and Analysis in Fresh and Processed Foods: A Review. Food Bioprocess Technol. 2013, 6, 36–60. [Google Scholar] [CrossRef]
- Fontes, L.C.B.; Sarmento, S.B.S.; Spoto, M.H.F.; dos Santos Dias, C.T. Preservation of minimally processed apple using edible coatings. Ciência e Tecnol. Aliment. 2008, 28, 872–880. [Google Scholar] [CrossRef]
- Hamid, N.; Ma, Q.; Boulom, S.; Liu, T.; Zheng, Z.; Balbas, J.; Robertson, J. Seaweed minor constituents. In Seaweed Sustainability; Elsevier: Amsterdam, The Netherlands, 2015; pp. 193–242. ISBN 9780124186972. [Google Scholar]
- Kato, K.; Hayashi, M.; Umene, S.; Masunaga, H. A novel method for producing softened edible seaweed kombu. LWT-Food Sci. Technol. 2016, 65, 618–623. [Google Scholar] [CrossRef]
- Baldwin, E.A.; Nisperos-Carriedo, M.O.; Baker, R.A. Use of edible coatings to preserve quality of lightly (and slightly) processed products. Crit. Rev. Food Sci. Nutr. 1995, 35, 509–524. [Google Scholar] [CrossRef]
- Prinzivalli, C.; Brambilla, A.; Maffi, D.; Lo Scalzo, R.; Torreggiani, D. Effect of osmosis time on structure, texture and pectic composition of strawberry tissue. Eur. Food Res. Technol. 2006, 224, 119–127. [Google Scholar] [CrossRef]
- Hung, Y.-C.; Thompson, D.R. Changes in Texture of Green Peas during Freezing and Frozen Storage. J. Food Sci. 1989, 54, 96–101. [Google Scholar] [CrossRef]
- Wallenstein, F.M.; Couto, R.P.; Amaral, A.S.; Wilkinson, M.; Neto, A.I.; Rodrigues, A.S. Baseline metal concentrations in marine algae from São Miguel (Azores) under different ecological conditions—Urban proximity and shallow water hydrothermal activity. Mar. Pollut. Bull. 2009, 58, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Circuncisão, A.; Catarino, M.; Cardoso, S.; Silva, A. Minerals from Macroalgae Origin: Health Benefits and Risks for Consumers. Mar. Drugs 2018, 16, 400. [Google Scholar] [CrossRef] [PubMed]
- Yaich, H.; Garna, H.; Besbes, S.; Paquot, M.; Blecker, C.; Attia, H. Chemical composition and functional properties of Ulva lactuca seaweed collected in Tunisia. Food Chem. 2011, 128, 895–901. [Google Scholar] [CrossRef]
- Brown, E.M.; Allsopp, P.J.; Magee, P.J.; Gill, C.I.; Nitecki, S.; Strain, C.R.; McSorley, E.M. Seaweed and human health. Nutr. Rev. 2014, 72, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Paiva, L.; Lima, E.; Neto, A.I.; Marcone, M.; Baptista, J. Health-promoting ingredients from four selected Azorean macroalgae. Food Res. Int. 2016, 89, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Fleurence, J. Seaweed proteins. Trends Food Sci. Technol. 1999, 10, 25–28. [Google Scholar] [CrossRef]
- Albarracín, W.; Sánchez, I.C.; Grau, R.; Barat, J.M. Salt in food processing; usage and reduction: A review. Int. J. Food Sci. Technol. 2011, 46, 1329–1336. [Google Scholar] [CrossRef]
- Neto, R.; Marçal, C.; Queirós, A.; Abreu, H.; Silva, A.; Cardoso, S. Screening of Ulva rigida, Gracilaria sp., Fucus vesiculosus and Saccharina latissima as Functional Ingredients. Int. J. Mol. Sci. 2018, 19, 2987. [Google Scholar] [CrossRef]
- Satpati, G.G.; Pal, R. Biochemical composition and lipid characterization of marine green alga Ulva rigida—A nutritional approach. J. Algal Biomass Utln. 2011, 2, 10–13. [Google Scholar]
- Lopes, D.; Moreira, A.S.P.; Rey, F.; da Costa, E.; Melo, T.; Maciel, E.; Rego, A.; Abreu, M.H.; Domingues, P.; Calado, R.; et al. Lipidomic signature of the green macroalgae Ulva rigida farmed in a sustainable integrated multi-trophic aquaculture. J. Appl. Phycol. 2019, 31, 1369–1381. [Google Scholar] [CrossRef]
- Cefola, M.; D’Antuono, I.; Pace, B.; Calabrese, N.; Carito, A.; Linsalata, V.; Cardinali, A. Biochemical relationships and browning index for assessing the storage suitability of artichoke genotypes. Food Res. Int. 2012, 48, 397–403. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Domínguez-González, R.; Romarís-Hortas, V.; García-Sartal, C.; Moreda-Piñeiro, A.; Barciela-Alonso, M.D.C.; Bermejo-Barrera, P. Evaluation of an in vitro method to estimate trace elements bioavailability in edible seaweeds. Talanta 2010, 82, 1668–1673. [Google Scholar] [CrossRef] [PubMed]
- O’Fallon, J.V.; Busboom, J.R.; Nelson, M.L.; Gaskins, C.T. A direct method for fatty acid methyl ester synthesis: Application to wet meat tissues, oils, and feedstuffs. J. Anim. Sci. 2007, 85, 1511–1521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: Samples of the compounds not available from the authors. |
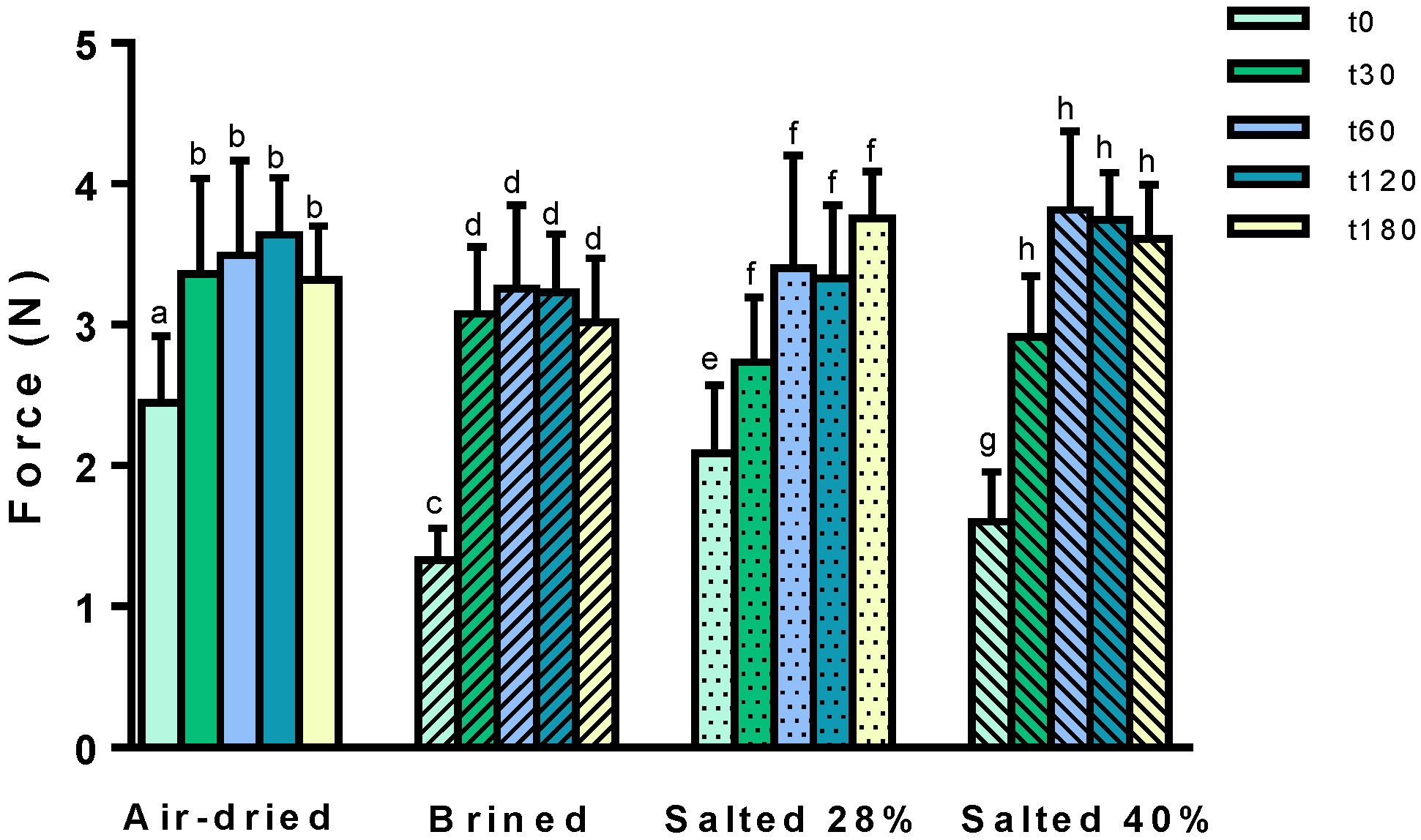
| Conditions | Moisture Content (%) | ||||
|---|---|---|---|---|---|
| t0 | t30 | t60 | t120 | t180 | |
| Air-dried | 14.22 ± 0.37 a | 14.76 ± 0.31 a | 15.50 ± 0.09 a | 15.01 ± 0.09 a | 14.47 ± 0.13 a |
| Brined | 71.37 ± 0.36 b | 70.09 ± 2.05 b | 68.17 ± 0.97 b | 69.40 ± 1.31 b | 70.12 ± 1.52 b |
| Salted 28% | 70.86 ± 5.05 c | 61.92 ± 1.03 d | 61.43 ± 2.49 d | 62.63 ± 1.58 d | 62.98 ± 1.01 d |
| Salted 40% | 60.08 ± 1.57 e | 62.54 ± 3.79 e | 57.32 ± 2.50 e | 58.62 ± 1.56 e | 58.05 ± 2.44 e |
| CIELAB | Treatment | t0 | t30 | t60 | t120 | t180 |
|---|---|---|---|---|---|---|
| a* | Air-dried | −14.78 ± 0.35 a | −13.08 ± 0.95 a | −13.35 ± 0.79 a | −13.28 ± 1.13 a | −13.16 ± 0.81 a |
| Brined | −15.42 ± 1.10 b | −14.02 ± 1.09 b | −12.63 ± 1.10 c | −10.75 ± 0.96 c | −10.35 ± 1.23 c | |
| Salted 28% | −15.19 ± 1.06 d | −14.59 ± 1.46 d | −14.26 ± 1.72 d | −11.25 ± 1.03 e | −11.20 ± 1.95 e | |
| Salted 40% | −15.14 ± 0.26 f | −14.94 ± 0.77 f | −13.35 ± 0.51 f | −12.42 ± 1.41 f | −10.69 ± 1.37 f | |
| b* | Air-dried | 41.71 ± 2.58 a | 40.84 ± 1.09 a | 43.94 ± 3.13 a | 44.34 ± 2.97 a | 47.41 ± 2.86 b |
| Brined | 38.58 ± 2.04 c | 43.80 ± 2.06 d | 43.94 ± 1.35 d | 43.12 ± 0.87 d | 46.94 ± 1.99 d | |
| Salted 28% | 39.14 ± 3.07 e | 41.59 ± 2.52 e | 44.75 ± 2.46 f | 43.28 ± 2.66 f | 45.64 ± 2.48 f | |
| Salted 40% | 38.13 ± 2.46 g | 42.52 ± 2.56 h | 43.60 ± 4.19 h | 43.95 ± 1.70 h | 46.91 ± 1.72 h | |
| L* | Air-dried | 51.33 ± 1.58 a | 49.35 ± 3.38 a | 50.34 ± 3.10 a | 50.40 ± 2.78 a | 52.63 ± 2.51 a |
| Brined | 48.78 ± 1.12 b | 50.68 ± 1.75 b | 51.02 ± 1.59 b | 49.86 ± 1.40 b | 54.50 ± 2.04 c | |
| Salted 28% | 48.85 ± 1.34 d | 48.10 ± 1.67 d | 50.76 ± 2.24 d | 50.77 ± 2.92 d | 53.46 ± 2.65 e | |
| Salted 40% | 48.65 ± 2.48 f | 49.75 ± 2.12 f | 50.78 ± 3.41 f | 51.04 ± 2.00 f | 54.61 ± 2.22 g | |
| BI | Air-dried | 115.51 ± 6.37 a | 113.17 ± 7.62 a | 137.93 ± 9.24 b | 139.36 ± 11.08 b | 146.23 ± 10.96 b |
| Brined | 105.18 ± 6.14 c | 130.46 ± 7.98 d | 138.30 ± 5.31 d | 135.27 ± 6.66 d | 138.34 ± 11.69 d | |
| Salted 28% | 105.04 ± 11.81 e | 127.25 ± 12.54 f | 136.00 ± 6.70 f | 131.87 ± 7.58 f | 133.74 ± 10.39 f | |
| Salted 40% | 108.19 ± 12.95 g | 123.27 ± 5.72 g | 136.61 ± 13.74 h | 133.58 ± 8.03 h | 136.49 ± 5.64 h | |
| ΔE* | Air-dried | _ | 3.91 a | 4.46 a | 4.90 a | 5.81 a |
| Brined | _ | 5.79 b | 6.38 b | 6.40 b | 11.45 c | |
| Salted 28% | _ | 3.77 d | 6.55 d | 6.91 d | 9.21 e | |
| Salted 40% | _ | 6.16 f | 7.74 f | 7.86 f | 12.12 g |
| Air-Dried | Brined | Salted 28% | Salted 40% | |||||
|---|---|---|---|---|---|---|---|---|
| t0 | t180 | t0 | t180 | t0 | t180 | t0 | t180 | |
| Saturated | ||||||||
| C14:0 | 6.44 ± 0.26 a | 5.37 ± 0.20 b | 7.33 ± 0.02 a | 7.33 ± 0.25 a | 6.37 ± 0.03 a | 7.21 ± 0.10 b | 6.52 ± 0.14 a | 6.07 ± 0.03 a |
| C16:0 | 29.87 ± 0.11 a | 30.44 ± 0.13 a | 32.32 ± 0.31 a | 31.87 ± 0.80 a | 29.99 ± 0.20 a | 31.20 ± 0.31 a | 30.15 ± 0.17 a | 31.19 ± 0.64 a |
| C18:0 | 4.40 ± 0.35 a | 4.99 ± 0.64 a | 5.27 ± 0.00 a | 4.82 ± 0.56 a | 5.01 ± 0.25 a | 5.54 ± 0.91 a | 4.70 ± 0.85 a | 4.81 ± 0.77 a |
| C22:0 | 10.86 ± 0.30 a | 11.11 ± 0.03 a | 11.07 ± 0.32 a | 11.87 ± 0.06 a | 10.71 ± 0.02 a | 10.74 ± 0.22 a | 10.56 ± 0.33 a | 10.02 ± 0.36 a |
| Unsaturated | ||||||||
| C16:1 (n-7) | 10.14 ± 0.45 a | 9.88 ± 0.25 a | 10.52 ± 0.08 a | 10.67 ± 0.16 a | 9.92 ± 0.04 a | 10.25 ± 0.49 a | 9.80 ± 0.40 a | 9.77 ± 0.53 a |
| C18:1 (n-9) | 10.39 ± 0.23 a | 11.07 ± 0.19 a | 11.13 ± 0.24 a | 9.81 ± 0.13 b | 11.26 ± 0.09 a | 10.85 ± 0.06 a | 10.48 ± 0.23 a | 11.65 ± 0.29 b |
| C18:2 (n-6) | 8.80 ± 0.32 a | 8.32 ± 0.34 a | 7.98 ± 0.43 a | 8.10 ± 0.25 a | 7.80 ± 0.02 a | 7.83 ± 0.91 a | 7.70 ± 0.57 a | 7.27 ± 0.41 a |
| C18:3 (n-3) | 9.21 ± 1.05 a | 9.59 ± 0.21 a | 7.23 ± 0.96 a | 7.87 ± 0.72 a | 9.73 ± 0.24 a | 8.31 ± 2.53 a | 10.75 ± 1.02a | 10.37 ± 1.10 a |
| C18:4 (n-3) | 9.88 ± 0.59 a | 9.23 ± 0.20 a | 7.15 ± 0.23 a | 7.67 ± 0.35 a | 9.21 ± 0.02 a | 8.05 ± 0.09 a | 9.32 ± 0.31 a | 8.84 ± 0.27 a |
| C20:4 (n-6) | d | d | d | d | d | d | d | d |
| C20:5 (n-3) | d | d | d | d | d | d | d | d |
| ∑ SFA | 50.58 ± 1.01 a | 51.91 ± 1.00 a | 55.99 ± 0.65 a | 55.89 ± 1.67 a | 52.07 ± 0.50 a | 54.70 ± 1.53 a | 51.94 ± 1.49 a | 52.09 ± 1.80 a |
| ∑ MUFA | 20.53 ± 0.67 a | 20.95 ± 0.44 a | 21.65 ± 0.31 a | 20.47 ± 0.20 a | 21.18 ± 0.13 a | 21.10 ± 0.55 a | 20.29 ± 0.63 a | 21.42 ± 0.82 a |
| ∑ PUFA | 28.89 ± 1.96 a | 27.14 ± 0.74 a | 22.36 ± 1.62 a | 23.64 ± 1.32 a | 26.74 ± 0.27 a | 24.20 ± 3.52 a | 27.78 ± 1.91 a | 26.48 ± 1.79 a |
| UFA/SFA | 0.94 | 0.93 | 0.79 | 0.79 | 0.92 | 0.83 | 0.93 | 0.92 |
| Ω6/Ω3 | 0.44 | 0.44 | 0.56 | 0.52 | 0.41 | 0.48 | 0.38 | 0.38 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinheiro, V.F.; Marçal, C.; Abreu, H.; Lopes da Silva, J.A.; Silva, A.M.S.; Cardoso, S.M. Physicochemical Changes of Air-Dried and Salt-Processed Ulva rigida over Storage Time. Molecules 2019, 24, 2955. https://doi.org/10.3390/molecules24162955
Pinheiro VF, Marçal C, Abreu H, Lopes da Silva JA, Silva AMS, Cardoso SM. Physicochemical Changes of Air-Dried and Salt-Processed Ulva rigida over Storage Time. Molecules. 2019; 24(16):2955. https://doi.org/10.3390/molecules24162955
Chicago/Turabian StylePinheiro, Valentina F., Catarina Marçal, Helena Abreu, José A. Lopes da Silva, Artur M. S. Silva, and Susana M. Cardoso. 2019. "Physicochemical Changes of Air-Dried and Salt-Processed Ulva rigida over Storage Time" Molecules 24, no. 16: 2955. https://doi.org/10.3390/molecules24162955
APA StylePinheiro, V. F., Marçal, C., Abreu, H., Lopes da Silva, J. A., Silva, A. M. S., & Cardoso, S. M. (2019). Physicochemical Changes of Air-Dried and Salt-Processed Ulva rigida over Storage Time. Molecules, 24(16), 2955. https://doi.org/10.3390/molecules24162955









