Germacrone Derivatives as new Insecticidal and Acaricidal Compounds: A Structure-Activity Relationship
Abstract
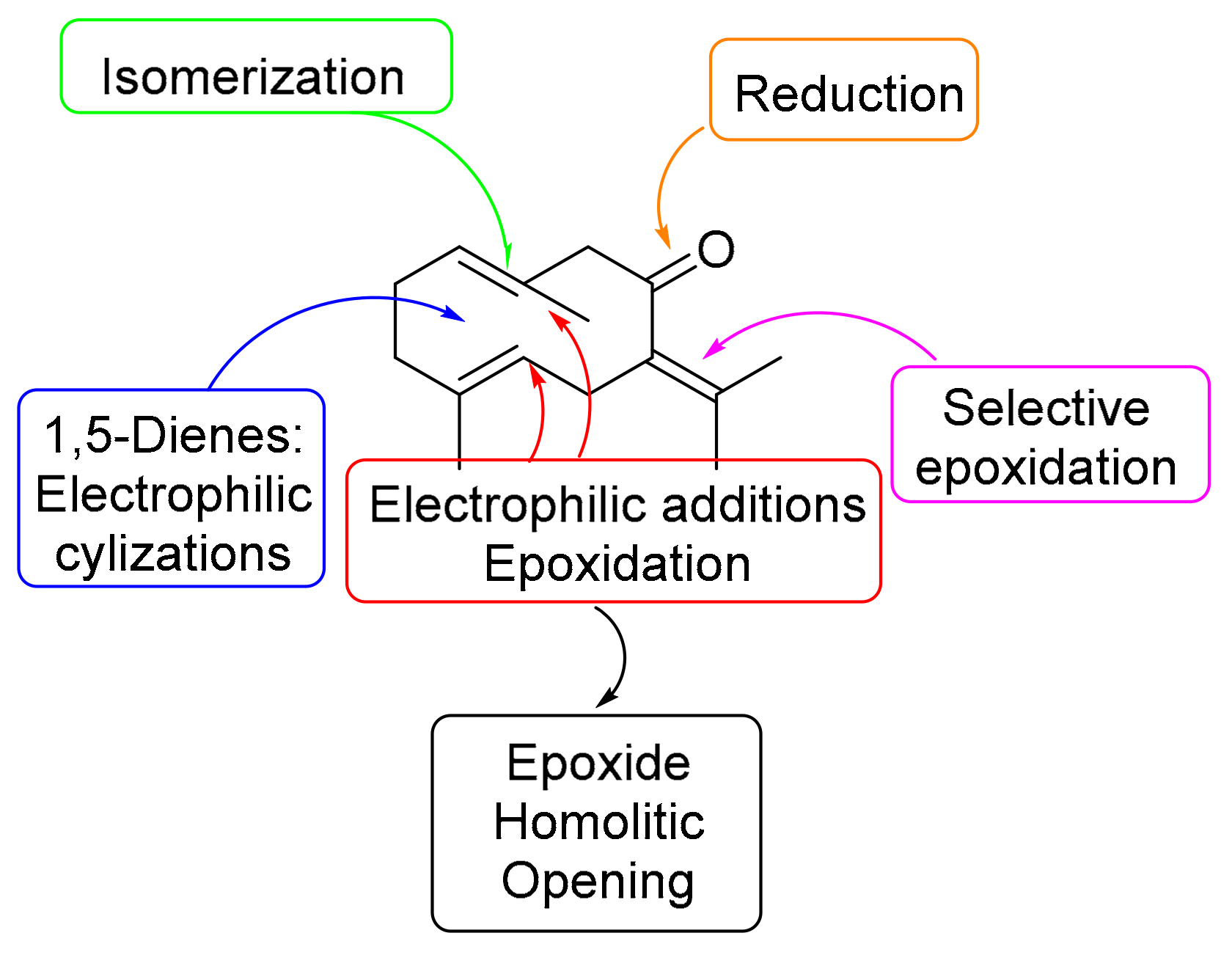
1. Introduction
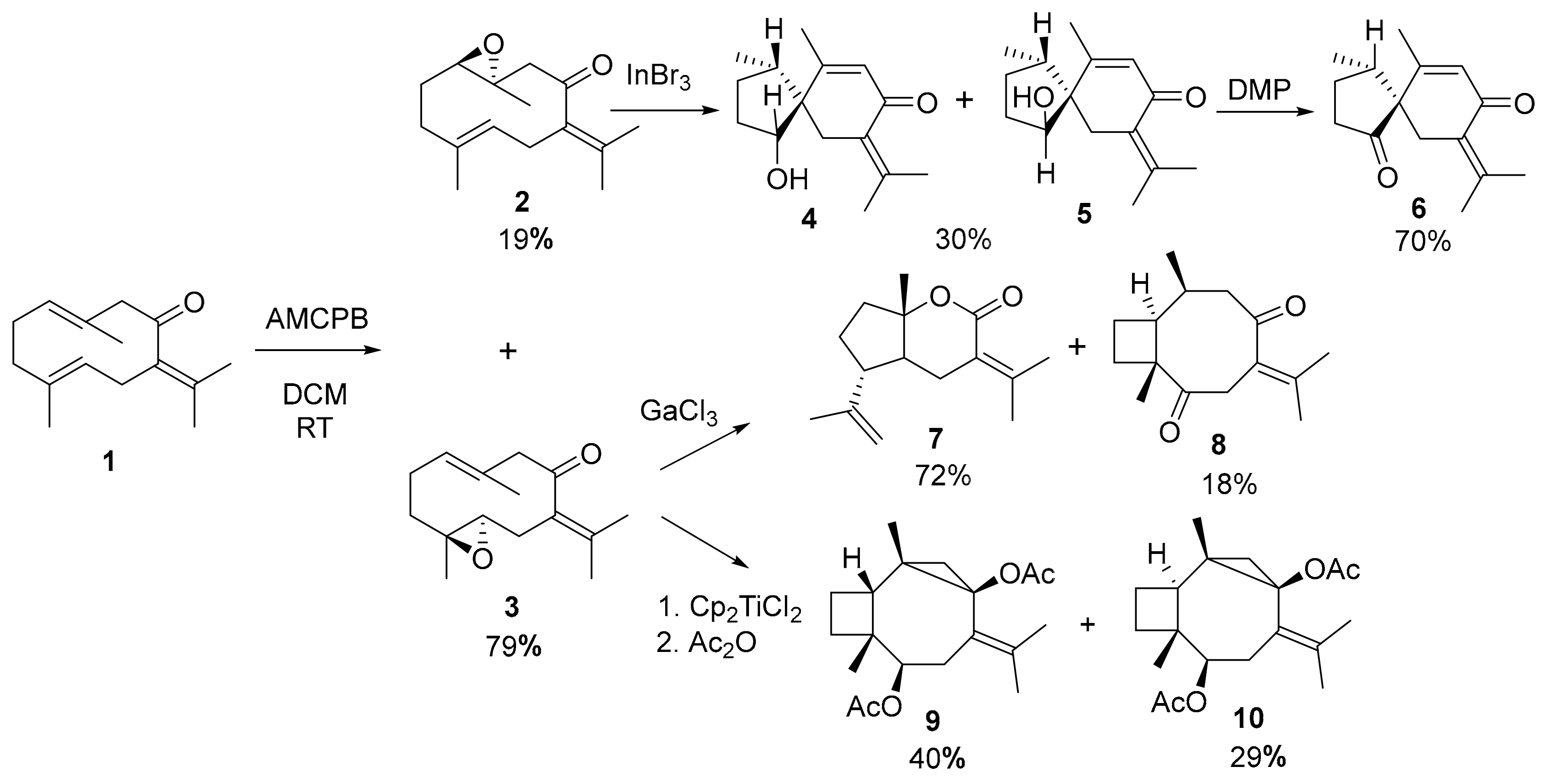
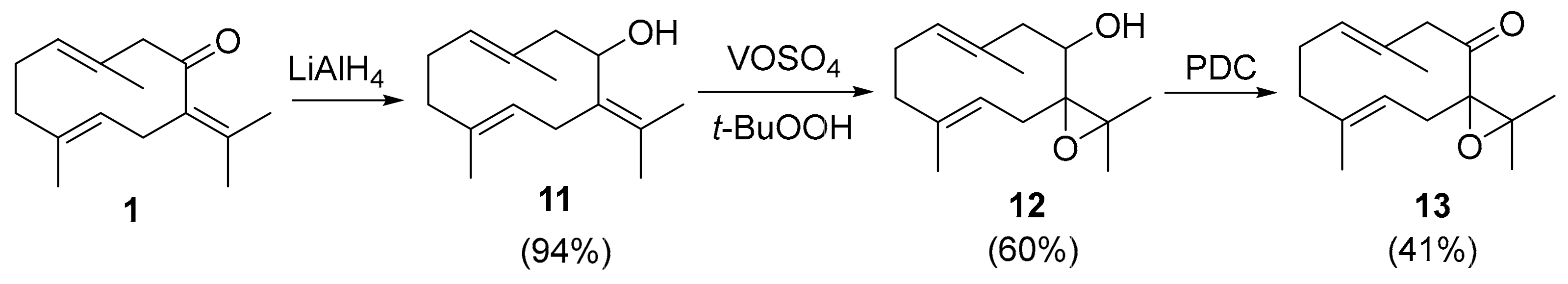
2. Results and Discussion
3. Materials and Methods
3.1. Instruments and Chemicals
3.2. Synthesis
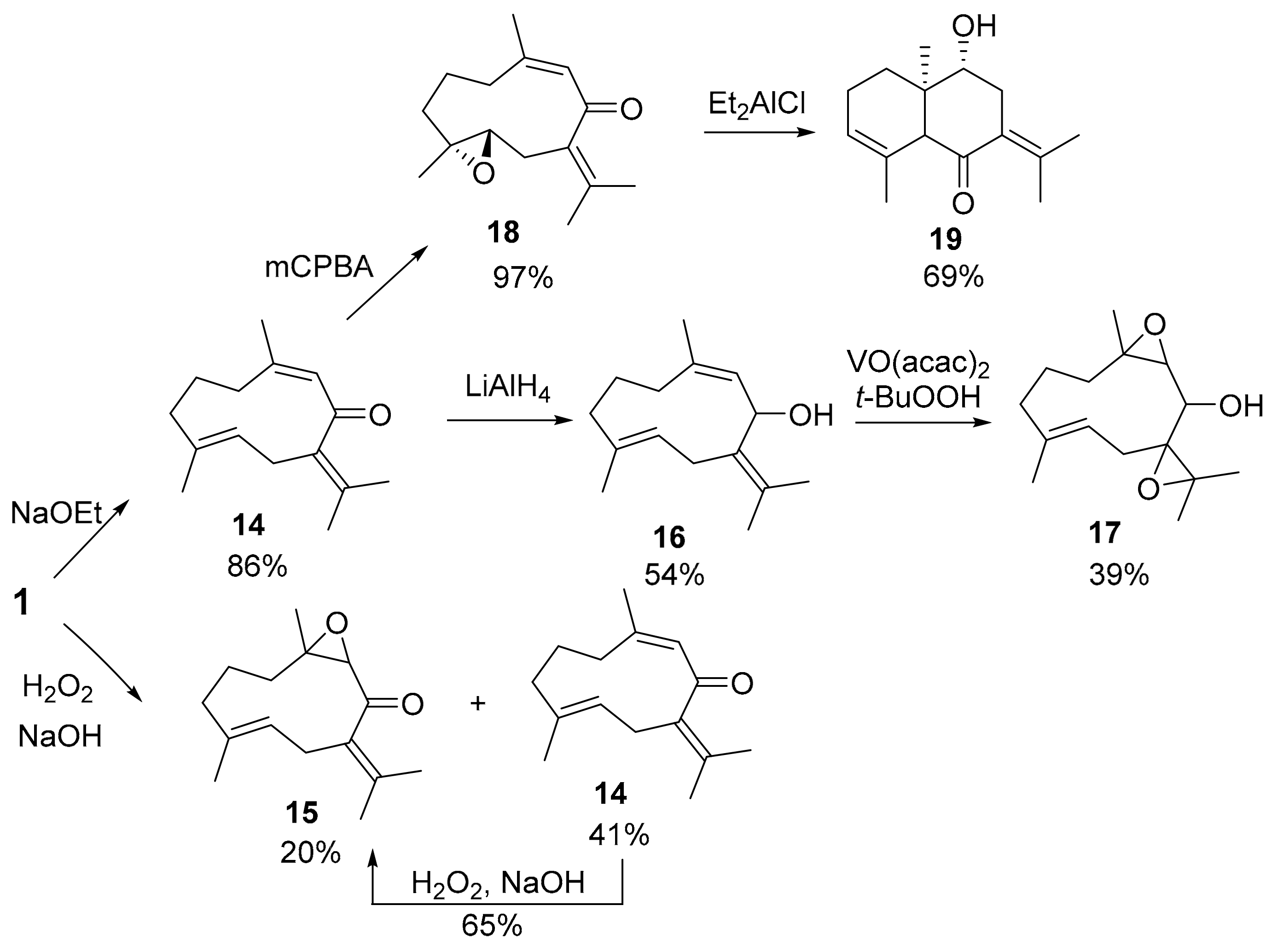
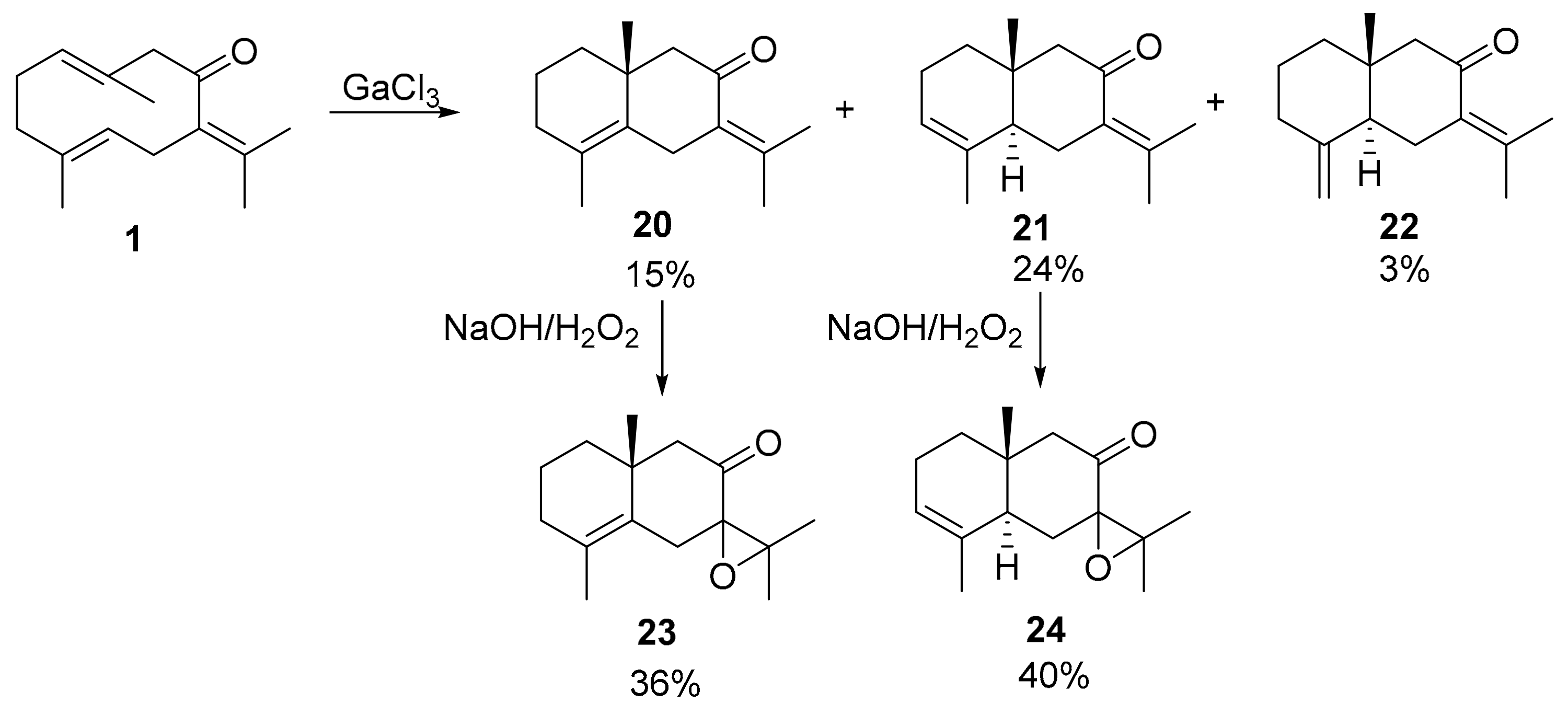
3.2.1. Cyclization of 4,5-epoxygermacrone (3) with GaCl3
3.2.2. Synthesis of 7,11-epoxygermacrone (13)
3.2.3. Synthesis of 9,10-epoxyisogermacrone (15)
3.2.4. Synthesis of 7,11-9,10-diepoxygermacr-4,5-en-8-ol (17)
3.2.5. Cyclization of germacrone (1) with GaCl3
3.2.6. Epoxidation of eudesma-4,7(11)-dien-8-one (20) with NaOH and H2O2
3.2.7. Epoxidation of eudesma-3,7(11)-dien-8-one (21) with NaOH and H2O2
3.3. Computational Analysis
3.4. Biological Evaluation
3.4.1. Antifeedant Activity
3.4.2. Ixodicidal Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Navarro-Rocha, J.; Barrero, A.F.; Burillo, J.; Olmeda, A.S.; González-Coloma, A. Valorization of essential oils from two populations (wild and commercial) of Geranium macrorrhizum L. Ind. Crops Prod. 2018, 116, 41–45. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Iannarelli, R.; Petrelli, R.; Cappellacci, L.; Cianfaglione, K.; Afshar, F.H.; Nicoletti, M.; Canale, A.; Maggi, F. Synergized mixtures of Apiaceae essential oils and related plant-borne compounds: Larvicidal effectiveness on the filariasis vector Culex quinquefasciatus Say. Ind. Crops Prod. 2017, 96, 186–195. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Canale, A.; Nicoletti, M.; Petrelli, R.; Cappellacci, L.; Galassi, R.; Maggi, F. Isofuranodiene and germacrone from Smyrnium olusatrum essential oil as acaricides and oviposition inhibitors against Tetranychus urticae: Impact of chemical stabilization of isofuranodiene by interaction with silver triflate. J. Pest Sci. 2017, 90, 693–699. [Google Scholar] [CrossRef]
- García, M.; Donadel, O.J.; Ardanaz, C.E.; Tonn, C.E.; Sosa, M.E. Toxic and repellent effects of Baccharis salicifolia essential oil on Tribolium castaneum. Pest Manag. Sci. 2005, 61, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.Y.; Liu, X.T.; Gu, J.; Liu, Y.; Ma, X.Y.; Lv, N.; Guo, S.S.; Wang, J.L.; Du, S.S.; Zhang, J. Chemical constituents and insecticidal activity of the essential oils extracted from Artemisia giraldii and Artemisia rubripes against two stored product insects. Med. Chem. 2016, 6, 396/1–396/5. [Google Scholar] [CrossRef]
- Hamilton, J.G.C.; Krishnakumari, B. Mistura racêmica, composto, uso de uma mistura racêmica ou de seu isômero constituinte, métodos de controle ou monitoração de mosquitos-pólvora, de prevenção de infecção e de sintetização de (±)-9-metilgermacreno, e, coleira de animal. BR PI0500799 (A) 2016. Available online: https://worldwide.espacenet.com/publicationDetails/biblio?DB=EPODOC&II=0&ND=3&adjacent=true&locale=en_EP&FT=D&date=20060912&CC=BR&NR=PI0500799A&KC=A (accessed on 8 August 2019).
- Pérez Morales, M.C.; Catalán, J.V.; Domingo, V.; Jaraíz, M.; Herrador, M.M.; Quílez del Moral, J.F.; López-Pérez, J.-L.; Barrero, A.F. Structural diversity from the transannular cyclizations of natural germacrone and epoxy derivatives: A theoretical–experimental study. Chem. Eur. J. 2013, 19, 6598–6612. [Google Scholar] [CrossRef] [PubMed]
- Catalán, J.V. Síntesis de terpenoides bioactivos usando sintones terpénicos y ciclaciones mediadas por Ti(III). Ph.D. Dissertation, University of Granada, Granada, Spain, 20 December 2007. [Google Scholar]
- Ulubelen, A.; Gören, N.; Jakupovic, J. Germacrane derivatives from the fruits of Smyrnium creticum. Phytochemistry 1986, 26, 312–313. [Google Scholar] [CrossRef]
- Radulović, N.S.; Zlatković, D.; Dekić, M.; Stojanović-Radić, Z. Further antibacterial geranium macrorrhizum l. metabolites and synthesis of epoxygermacrones. Chem. Biodivers. 2014, 11, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, M.; Shibuya, H.; Kitano, E.; Yanagi, K.; Kitagawa, I. The absolute stereostructure of (4s, 5s)-(+)-germacrone 4,5-epoxide from Zedoariae Rhizoma cultivated in Yakushima Island. Chem. Pharm. Bull. 1984, 32, 2059–2062. [Google Scholar] [CrossRef][Green Version]
- Matsuda, H.; Morikawa, T.; Toguchida, I.; Ninomiya, K.; Yoshikawa, M. Medicinal Foodstuffs. XXVIII. Inhibitors of nitric oxide production and new sesquiterpenes, zedoarofuran, 4-epicurcumenol, neocurcumenol, gajutsulactones A and B, and zedoarolides A and B, from Zedoariae Rhizoma. Chem. Pharm. Bull. 2001, 49, 1558–1566. [Google Scholar] [CrossRef]
- St. Enev, V.; Tsankova, E.T. Lewis acid catalysed rearrangement of 7,11-epoxyisogermacrone. Formation of a new carbon skeleton. Tetrahedron 1991, 47, 6399–6406. [Google Scholar] [CrossRef]
- Maggio, A.M.; Barone, G.; Bruno, M.; Duca, D.; Rosselli, S. Conformational analysis and DFT calculations of 8α-hydroxy-germacradiene-6,12-olide derivatives. J. Phys. Org. Chem. 2005, 18, 1116–1122. [Google Scholar] [CrossRef]
- Jimeno, M.L.; del Carmen Apreda-Rojas, M.; Cano, F.H.; Rodríguez, B. NMR and x-ray conformational study of artemisiifolin and three other related germacranolides. Magn. Reson. Chem. 2004, 42, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Milosavljević, S.; Juranić, I.; Aljančić, I.; Vajs, V.; Todorović, N. Conformational analysis of three germacranolides by the PM3 semi-empirical method. J. Serb. Chem. Soc. 2003, 68, 281–289. [Google Scholar] [CrossRef]
- Wong, H.-F.; Brown, G.D. Germacranolides from Artemisia myriantha and their conformation. Phytochemistry 2002, 59, 529–536. [Google Scholar] [CrossRef]
- Kulyyasov, A.T.; Bagryanskaya, I.Y.; Gatilov, Y.V.; Shakirov, M.M.; Raldugin, V.A.; Adekenov, S.M.; Seitembetov, T.S. Crystal and molecular structure of subchrysine (3-o-acetylridentine), a new germacranolide from Artemisia subchrysolepis. Russ. Chem. Bull. 1998, 47, 1390–1394. [Google Scholar] [CrossRef]
- Watson, W.H.; Kashyap, R.P. Conformations of germacra-1(10),4-dien-6,12-olides and -8,12-olides. A comparison of x-ray diffraction, NMR, and molecular mechanics derived conformations. J. Org. Chem. 1986, 51, 2521–2524. [Google Scholar] [CrossRef]
- Minnaard, A.J.; Wijnberg, J.B.P.A.; de Groot, A. The synthesis of germacrane sesquiterpenes and related compounds. Tetrahedron 1999, 55, 2115–2146. [Google Scholar] [CrossRef]
- Barquera-Lozada, J.E.; Cuevas, G. Biogenesis of sesquiterpene lactones pseudoguaianolides from germacranolides: Theoretical study on the reaction mechanism of terminal biogenesis of 8-epiconfertin. J. Org. Chem. 2009, 74, 874–883. [Google Scholar] [CrossRef]
- Marco, J.A.; Sanz-Cervera, J.F.; García-Lliso, V.; Domingo, L.R.; Carda, M.; Rodríguez, S.; López-Ortiz, F.; Lex, J. Influence of conformational factors on acid-catalyzed cyclizations of germacranolides. Molecular structure of the cyclization products of gallicin and 8α-hydroxygallicin (Shonachalin A). Liebigs Ann. 1995, 1837–1841. [Google Scholar] [CrossRef]
- Tashkhodzhaev, B.; Abduazimov, B.K. Stereochemistry of sesquiterpenes of the germacrane type. Chem. Nat. Compd. 1997, 33, 382–388. [Google Scholar] [CrossRef]
- Kolossváry, I.; Guida, W.C. Low Mode Search. An efficient, automated computational method for conformational analysis: Application to cyclic and acyclic alkanes and cyclic peptides. J. Am. Chem. Soc. 1996, 118, 5011–5019. [Google Scholar] [CrossRef]
- Mohamadi, F.; Richards, N.G.J.; Guida, W.C.; Liskamp, R.; Lipton, M.; Caufield, C.; Chang, G.; Hendrickson, T.; Still, W.C. Macromodel—an integrated software system for modeling organic and bioorganic molecules using molecular mechanics. J. Comput. Chem. 1990, 11, 440–467. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. B.01 2016. Available online: https://gaussian.com/gaussian16/ (accessed on 5 August 2019).
- Becke, A.D. A new mixing of Hartree–Fock and local density-functional theories. J. Chem. Phys. 1993, 98, 1372–1377. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Bally, T.; Rablen, P.R. Quantum-chemical simulation of 1H-NMR spectra. 2. Comparison of DFT-based procedures for computing proton–proton coupling constants in organic molecules. J. Org. Chem. 2011, 76, 4818–4830. [Google Scholar] [CrossRef] [PubMed]
- Bozhkova, N.V.; Stoev, G.; Orahovats, A.S.; Rizov, N.A. Sesquiterpene ketones from Geranium macrorrhizum. Phytochemistry 1984, 23, 917–918. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, J.; Wang, Y.; Donkor, P.O.; Li, Q.; Gao, S.; Hou, Y.; Xu, Y.; Cui, J.; Ding, L.; et al. Eudesmane-type sesquiterpenes from Curcuma phaeocaulis and their inhibitory activities on nitric oxide production in RAW 264.7 Cells. European, J. Org. Chem. 2014, 2014, 5540–5548. [Google Scholar] [CrossRef]
- Coll, J.C.; Bowden, B.F.; Tapiolas, D.M.; Willis, R.H.; Djura, P.; Streamer, M.; Trott, L. Studies of australian soft corals—XXXV: The terpenoid chemistry of soft corals and its implications. Tetrahedron 1985, 41, 1085–1092. [Google Scholar] [CrossRef]
- Nazaruk, J.; Kalemba, D.; Nazaruk, J.; Kalemba, D. Chemical composition of the essential oils from the roots of Erigeron acris L. and Erigeron annuus (L.) Pers. Molecules 2009, 14, 2458–2465. [Google Scholar] [CrossRef]
- Endo, K.; Taguchi, T.; Taguchi, F.; Hikino, H.; Yamahara, J.; Fujimura, H. Antiinflammatory principles of atractylodes Rhizomes. Chem. Pharm. Bull. 1979, 27, 2954–2958. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Vásquez, L.; Olmeda, A.S.; Zúñiga, G.; Villarroel, L.; Echeverri, L.F.; González-Coloma, A.; Reina, M. Insect antifeedant and ixodicidal compounds from Senecio adenotrichius. Chem. Biodivers. 2017, 14, e1600155. [Google Scholar] [CrossRef] [PubMed]
- González-Coloma, A.; Gutiérrez, C.; Hübner, H.; Achenbach, H.; Terrero, D.; Fraga, B.M. Selective insect antifeedant and toxic action of ryanoid diterpenes. J. Agric. Food Chem. 1999, 47, 4419–4424. [Google Scholar] [CrossRef] [PubMed]
- Santana, O.; Reina, M.; Fraga, B.M.; Sanz, J.; González-Coloma, A. Insect antifeedant fatty acid esters and phytosterols from Echium wildpretii. Chem. Biodiv. 2012, 9, 567–576. [Google Scholar]
- Lynch, B.J.; Zhao, Y.; Truhlar, D.G. Effectiveness of diffuse basis functions for calculating relative energies by density functional theory. J. Phys. Chem. A 2003, 107, 1384–1388. [Google Scholar] [CrossRef]
- Koch, W.; Holthausen, M.C. A Chemist’s Guide to Density Functional Theory, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2000. [Google Scholar]
- Parr, R.G.; Yang, W. Density Functional Theory of Atoms and Molecules; Claredon Press: Oxford, UK, 1989. [Google Scholar]
- Hratchian, H.P.; Schlegel, H.B. Using Hessian updating to increase the efficiency of a Hessian based predictor-corrector reaction path following method. J. Chem. Theory Comput. 2005, 1, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Hratchian, H.P.; Schlegel, H.B. Accurate reaction paths using a Hessian based predictor–corrector integrator. J. Chem. Phys. 2004, 120, 9918–9924. [Google Scholar] [CrossRef]
- Gonzalez, C.; Schlegel, H.B. Improved algorithms for reaction path following: Higher-order implicit algorithms. J. Chem. Phys. 1991, 95, 5853–5860. [Google Scholar] [CrossRef]
- Poitout, S.; Bues, S. Elevage de plusieurs espèces de lépidotéres Noctundiae sur milieu artificiel riche et sur milieu artificiel simplifiè. Ann. Zool. Ecol. Anim. 1970, 2, 79–91. [Google Scholar]
- Burgueño-Tapia, E.; Castillo, L.; González-Coloma, A.; Joseph-Nathan, P. Antifeedant and phytotoxic activity of the sesquiterpene p-benzoquinone perezone and some of its derivatives. J. Chem. Ecol. 2008, 34, 766–771. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
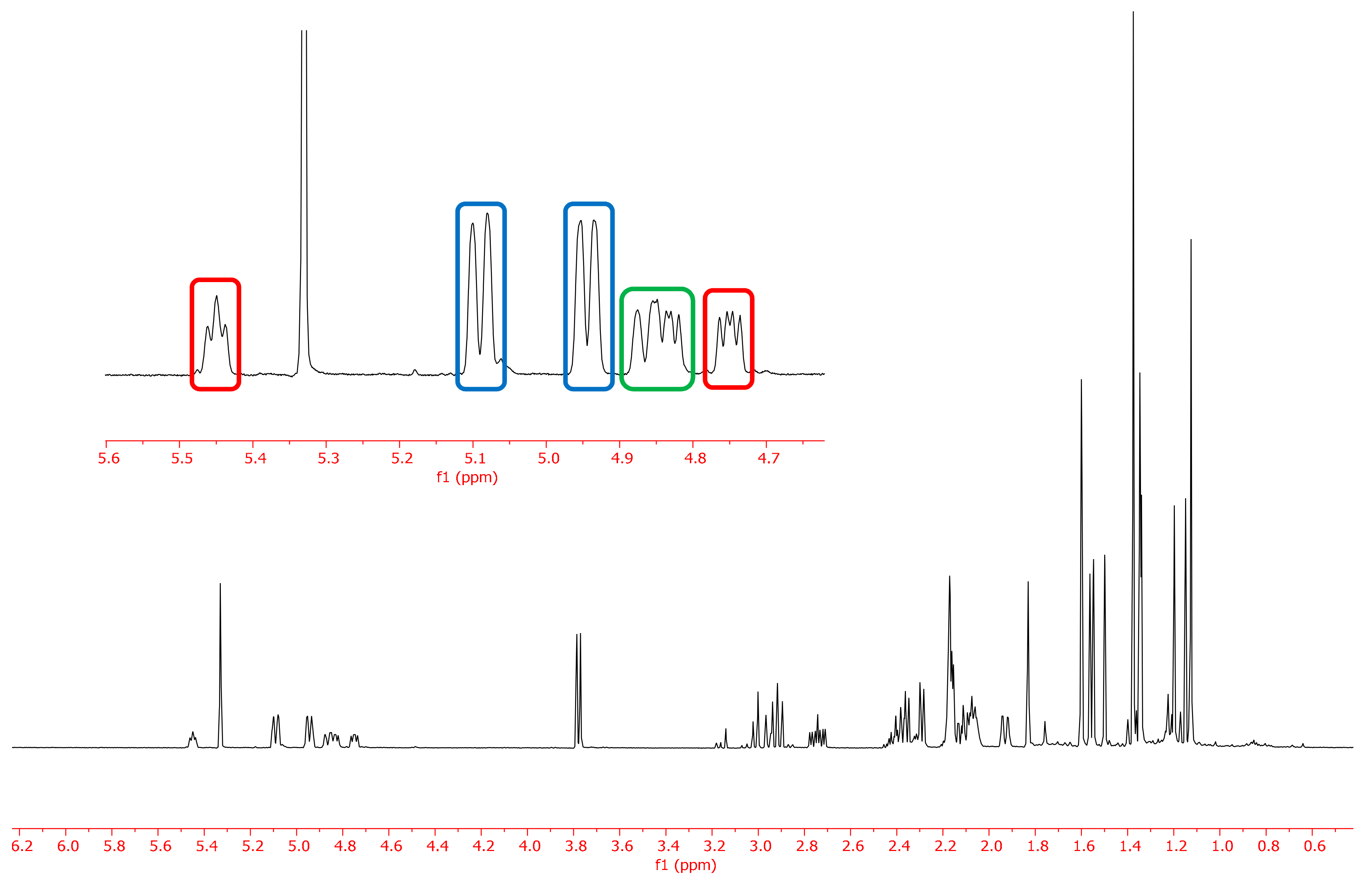
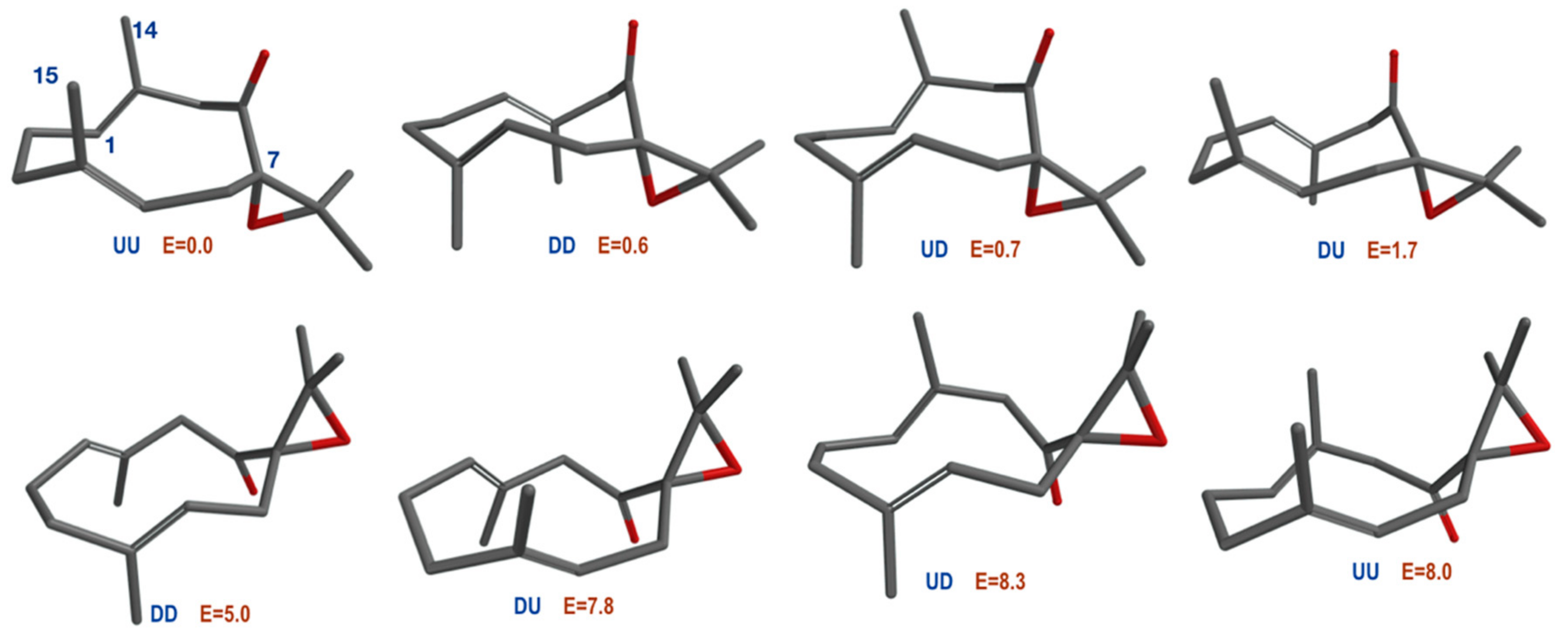


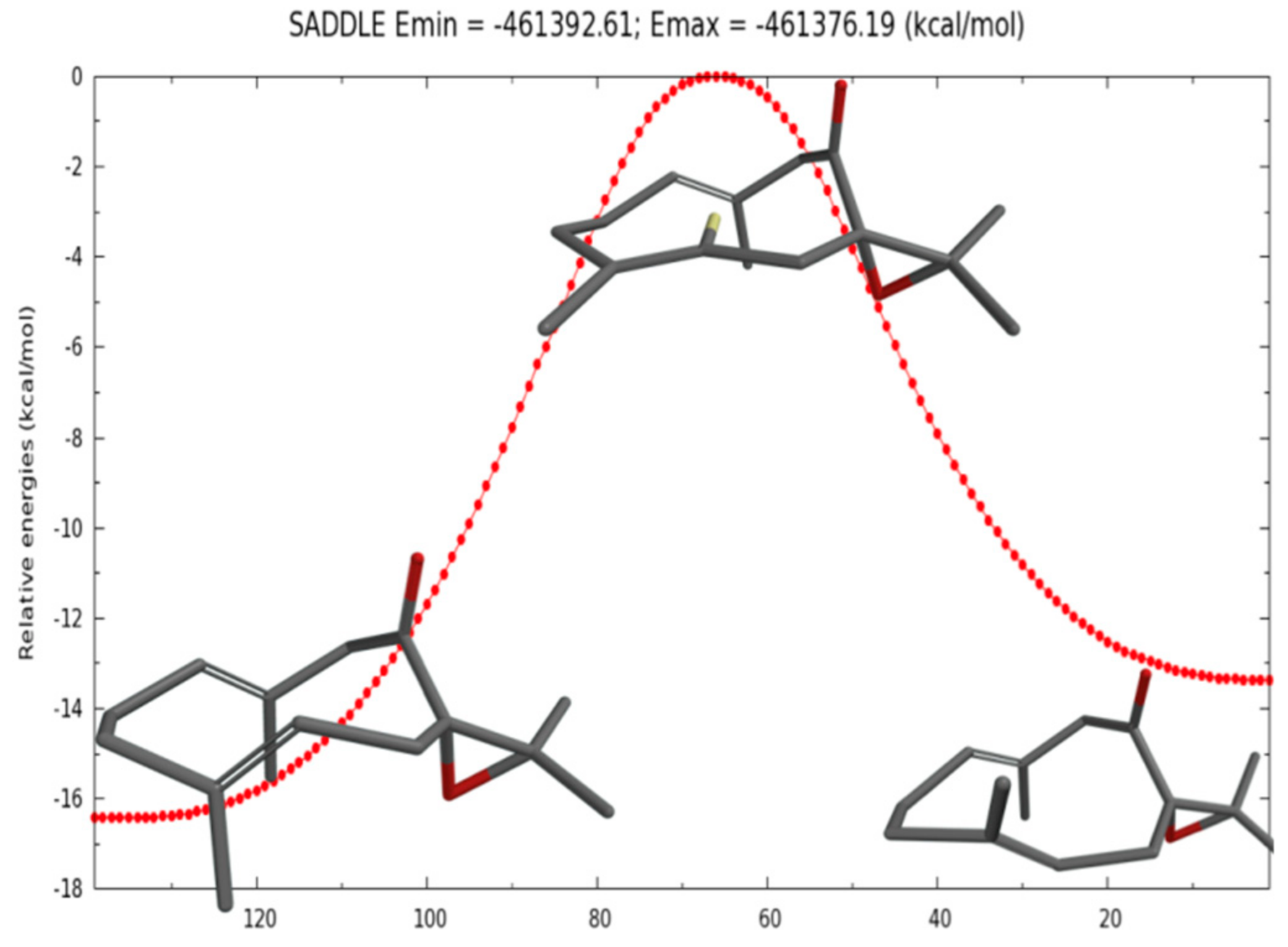
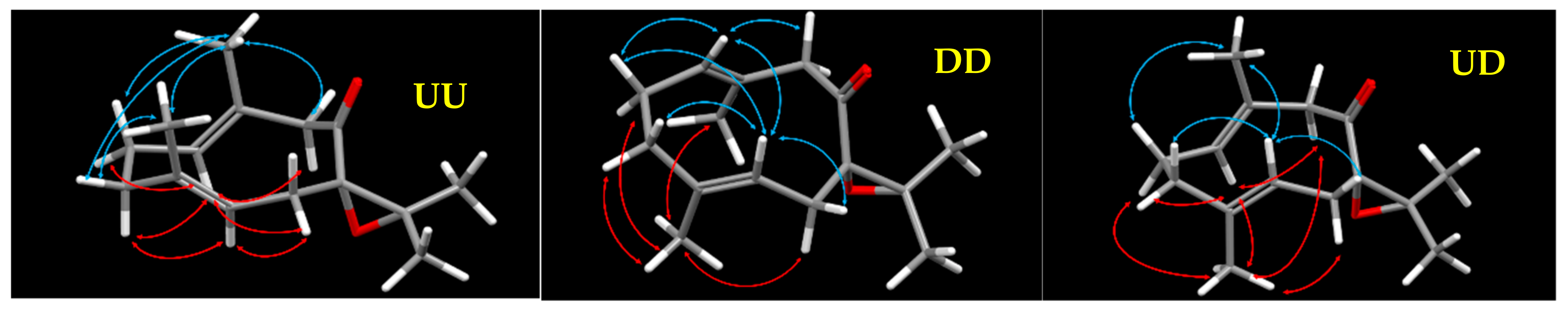
| JUU | JExp | JDD | JExp | JUD | JExp | |
|---|---|---|---|---|---|---|
| H1-2a | 3.6 | a | 12.3 | 12.5 | 7.6 | 7.5 |
| H1-2b | 12.3 | 12.3 | 3.9 | a | 9.9 | 7.5 |
| H5-6a | 3.6 | a | 10.0 | 10.7 | 10.4 | 10.4 |
| H5-6b | 12.1 | 11.9 | 6.7 | 7.0 | 6.3 | 5.9 |
| Compound | % Mortality a | Effective Lethal Doses (mg/mL) | |
|---|---|---|---|
| 10(mg/mL) | LD50 b | LD90 b | |
| 1 | 100 ± 0 | 1.08(0.7–1.38) | 3.13(2.66–3.93) |
| 2 | 100 ± 0 | 2.7(2.43–3.01) | 4.1(3.67–4.77) |
| 3 | 98.3 ± 1.7 | 1.47(1.16–1.76) | 3.45(2.48–4.22) |
| 4 | 45.3 ± 24.9 | - | - |
| 5 | 8.6 ± 12.1 | - | - |
| 6 | 42.6 ± 2.5 | - | - |
| 7 | 100 ± 0 | 2.44(2.08–2.79) | 4.47(3.95–5.27) |
| 8 | 100 ± 0 | <1.25 | <1.25 |
| 9 | - | - | - |
| 10 | 0 ± 0 | - | - |
| 11 | 24.6 ± 0.6 | - | - |
| 13 | 100 ± 0 | 1.21(1.04–1.4) | 2.43(2.12–2.89) |
| 14 | 100 ± 0 | 0.96(0.88–1.05) | 1.39(1.25–1.56) |
| 15 | 100 ± 0 | ~2.32 | ~2.98 |
| 17 | 32.1 ± 17.3 | - | - |
| 19 | 0 ± 0 | - | - |
| 20 | 0 | - | - |
| 21 | 0 | - | - |
| 23 | 100 ± 0 | 4.14(3.85–4.48) | 6.33(5.82–7.04) |
| 24 | 91.9 ± 0.6 | 0.23(0.12–0.47) | 1.91(1.57–2.54) |
| Thymol c | 100 | 0.18(0.16–0.20) | 0.27(0.24–0.31) |
| Compound | S. littoralis | M. persiscae | R. padi | |||
|---|---|---|---|---|---|---|
| %FI a | EC50 b | %SI a | EC50 b | %SI a | EC50 b | |
| 1 | 98.6 ± 0.6 | 1.9(0.1–3.6) | 82.2 ± 5.0 | 2.8(2.2–3.4) | 69.6 ± 6.5 | <5 |
| 2 | 76.93 ± 6.7 | <5 | 69.0 ± 7.8 | <5 | 97.8 ± 1.0 | 0.006 (0.27–0.2) |
| 3 | 69.9 ± 11.3 | <5 | 64.0 ± 8.9 | <5 | 94.5 ± 1.7 | 0.2 (-0.1–0.4) |
| 6 | - | - | 60.6 ± 6.8 | <5 | - | - |
| 7 | 61.1 ± 11.7 | <5 | 61.6 ± 9.1 | <5 | 97.3 ± 1.3 | 0.02 (0.006–0.07) |
| 8 | 90.8 ± 3.3 | ~2.5 | 75.6 ± 7.2 | <5 | 98.3 ± 1.2 | 0.8 (0.6–1.0) |
| 9 | 73.8 ± 15.4 | <5 | 91.0 ± 2.6 | <5 | - | - |
| 10 | 78.6 ± 12.7 | <5 | 88.5 ± 3.1 | <5 | - | - |
| 11 | 54.3 ± 8.71 | - | 45.0 ± 9.1 | - | 38.44 ± 8.8 | - |
| 13 | 54.6 ± 9.4 | - | 66.1 ± 7.4 | <5 | 92.7 ± 2.2 | 2.3 (2.1–2.5) |
| 14 | 49.8 ± 11.3 | - | 71.4 ± 6.1 | <5 | 84.4 ± 6.5 | <5 |
| 15 | 54.0 ± 11.0 | - | 75.0 ± 5.1 | 1.0(0.01–1.4) | 69.3 ± 7.2 | 3.69 (3.3–4.0) |
| 17 | 79.7 ± 7.1 | <5 | 50.4 ± 9.3 | - | 86.0 ± 4.5 | 1.8 (1.4–2.1) |
| 23 | 53.9 ± 9.6 | - | 55.8 ± 9.8 | - | 87.0 ± 4.5 | 1.5 (1.1–1.9) |
| 24 | 33.9 ± 15.6 | - | 48.5 ± 9.2 | - | 52.5 ± 8.8 | - |
| AZA c RY | 0.5 × 10−6 (0.2 × 10−8–0.7 × 10−4) | - | - | |||
| FAR d | - | 14.9 (12.1–18.3) | 1.7 (1.2–2.7) | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galisteo Pretel, A.; Pérez del Pulgar, H.; Guerrero de León, E.; López-Pérez, J.L.; Olmeda, A.S.; Gonzalez-Coloma, A.; F. Barrero, A.; Quílez del Moral, J.F. Germacrone Derivatives as new Insecticidal and Acaricidal Compounds: A Structure-Activity Relationship. Molecules 2019, 24, 2898. https://doi.org/10.3390/molecules24162898
Galisteo Pretel A, Pérez del Pulgar H, Guerrero de León E, López-Pérez JL, Olmeda AS, Gonzalez-Coloma A, F. Barrero A, Quílez del Moral JF. Germacrone Derivatives as new Insecticidal and Acaricidal Compounds: A Structure-Activity Relationship. Molecules. 2019; 24(16):2898. https://doi.org/10.3390/molecules24162898
Chicago/Turabian StyleGalisteo Pretel, Alberto, Helena Pérez del Pulgar, Estela Guerrero de León, José Luis López-Pérez, A. Sonia Olmeda, Azucena Gonzalez-Coloma, Alejandro F. Barrero, and José Francisco Quílez del Moral. 2019. "Germacrone Derivatives as new Insecticidal and Acaricidal Compounds: A Structure-Activity Relationship" Molecules 24, no. 16: 2898. https://doi.org/10.3390/molecules24162898
APA StyleGalisteo Pretel, A., Pérez del Pulgar, H., Guerrero de León, E., López-Pérez, J. L., Olmeda, A. S., Gonzalez-Coloma, A., F. Barrero, A., & Quílez del Moral, J. F. (2019). Germacrone Derivatives as new Insecticidal and Acaricidal Compounds: A Structure-Activity Relationship. Molecules, 24(16), 2898. https://doi.org/10.3390/molecules24162898








