Insights into the Phytochemistry of the Cuban Endemic Medicinal Plant Phyllanthus orbicularis: Fideloside, a Novel Bioactive 8-C-glycosyl 2,3-Dihydroflavonol
Abstract
1. Introduction
2. Results
2.1. Phytochemical Characterization of Cuban Phyllanthus Species
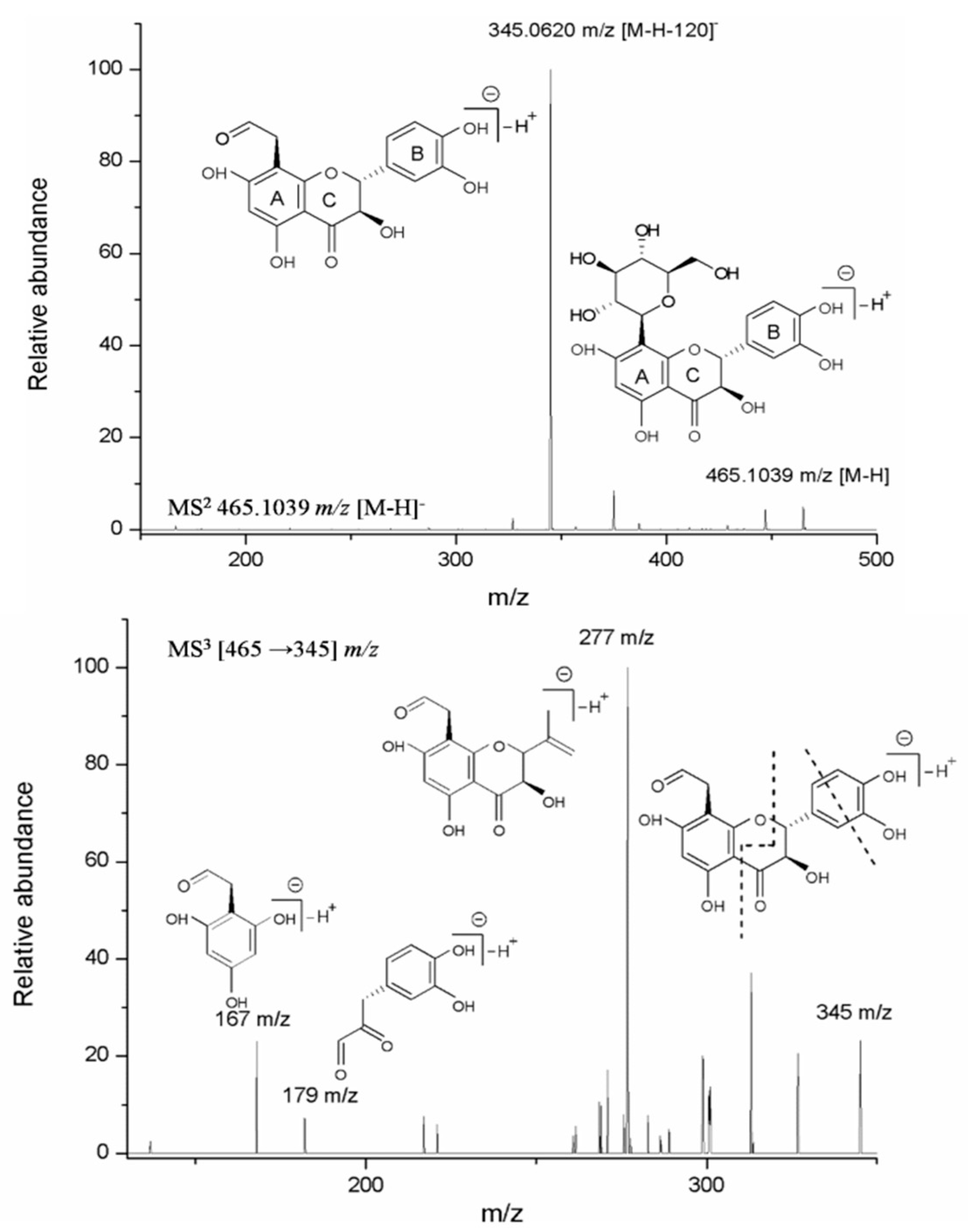
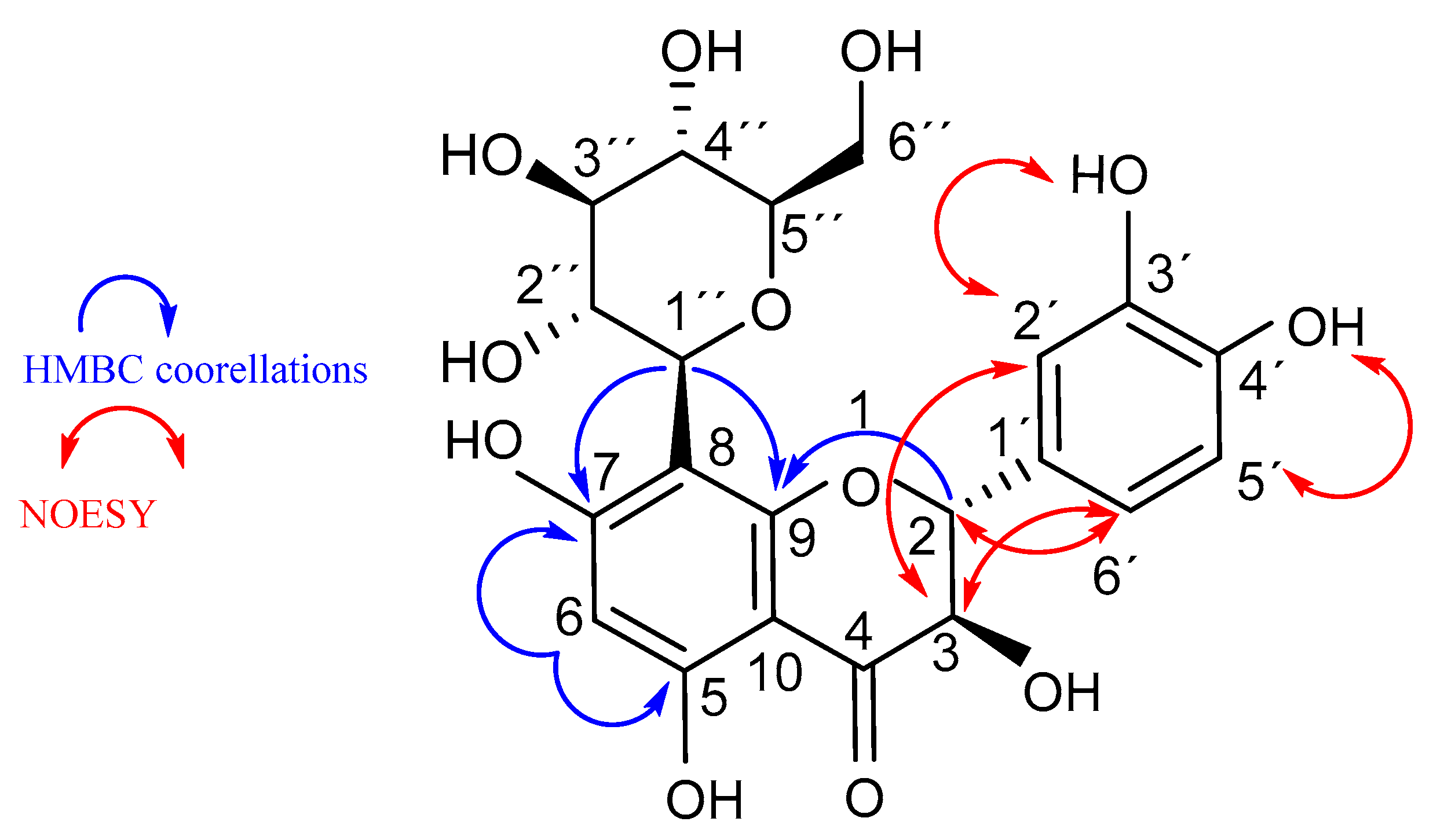
2.2. Structure Elucidation of Compound 3 (Fideloside)
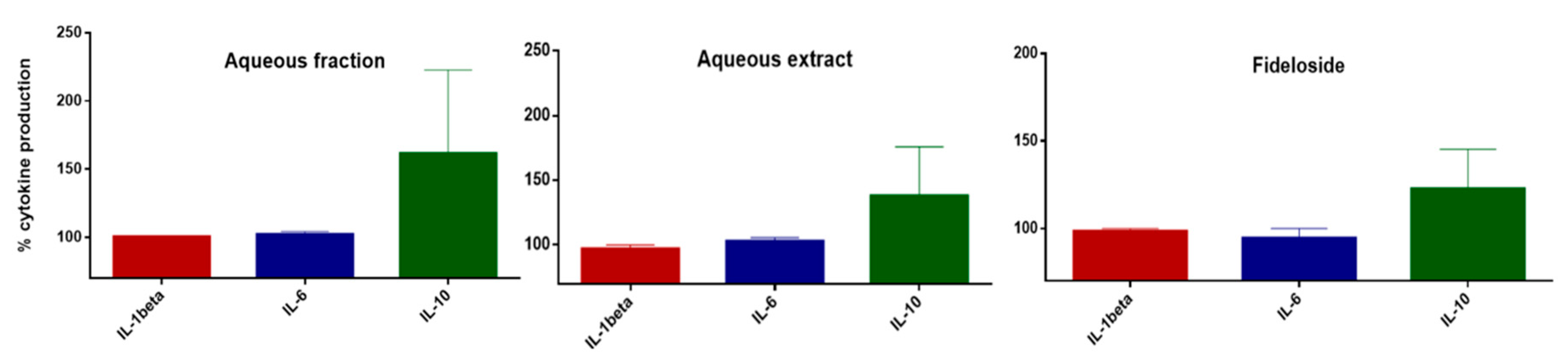
2.3. Modulation of Cytokine Production
3. Discussion
4. Materials and Methods
4.1. Chemicals, Reagents and General Procedures
4.2. Plant Material
4.3. Extraction and Compound Isolation
4.4. UPLC-DAD-MS and ESI-MS/MS
4.5. LC-High Resolution-MSn
4.6. Molecular Modeling and DFT Calculations
4.7. Human Monocyte Isolation andAssessment of Cytokine Release
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Mastromarino, P.; Capobianco, D.; Cannata, F.; Nardis, C.; Mattia, E.; De Leo, A.; Restignoli, R.; Francioso, A.; Mosca, L. Resveratrol inhibits rhinovirus replication and expression of inflammatory mediators in nasal epithelia. Antiviral Res. 2015, 123, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.-G.; Koffas, M.A.G. Bioavailability and Recent Advances in the Bioactivity of Flavonoid and Stilbene Compounds. Curr. Org. Chem. 2010, 14, 1727–1751. [Google Scholar] [CrossRef]
- Mattioli, R.; Francioso, A.; d’Erme, M.; Trovato, M.; Mancini, P.; Piacentini, L.; Casale, A.; Wessjohann, L.; Gazzino, R.; Costantino, P.; et al. Anti-Inflammatory Activity of A Polyphenolic Extract from Arabidopsis thaliana in In Vitro and In Vivo Models of Alzheimer’s Disease. Int. J. Mol. Sci. 2019, 20, 708. [Google Scholar] [CrossRef] [PubMed]
- Menes, M.D.O. Dos nuevasvariedades de Portulaca brevifolia Urb. (Portulacaceae) para la Flora de Cuba. FeddesRepert 2007. [Google Scholar] [CrossRef]
- Hammer, K.; Esquivel, M.; Fuentes, V.; Lima, H.; Knüpffer, H. Additional notes to the cheklist of cuban cultivated plants (1). Die Kult. 1990, 38, 325–343. [Google Scholar] [CrossRef]
- Riverón-Giró, F.B.; Sánchez, C. Two new species of Tectaria (Tectariaceae) from Cuba. Willdenowia 2015, 45, 189–196. [Google Scholar] [CrossRef]
- Ihantola-Vormisto, A.; Summanen, J.; Kankaanranta, H.; Vuorela, H.; Asmawi, Z.M.; Moilanen, E. Anti-inflammatory activity of extracts from leaves of Phyllanthus emblica. Planta Med. 1997, 63, 518–524. [Google Scholar] [CrossRef]
- Thyagarajan, S.P.; Thirunalasundari, T.; Subramanian, S.; Venkateswaran, P.S.; Blumberg, B.S. Effect of phyllanthusamarus on chronic carriers of hepatitis b virus. Lancet 1988, 332, 764–766. [Google Scholar] [CrossRef]
- Kuttan, R.; Harikumar, K.B. Phyllanthus Species: Scientific Evaluation and Medicinal Applications; CRC Press: Boca Raton, FL, USA, 2011; ISBN 1439821445. [Google Scholar]
- Joy, K.L.; Kuttan, R. Inhibition by Phyllanthus amarus of Hepatocarcinogenesis Induced by N-Nitrosodiethylamine. J. Clin. Biochem. Nutr. 2011, 24, 133–139. [Google Scholar] [CrossRef]
- Gowrishanker, B.; Vivekanandan, O.S. In vivo studies of a crude extract of Phyllanthus amarus L. in modifying the genotoxicity induced in Viciafaba L. by tannery effluents. Mutat. Res. Toxicol. 1994, 322, 185–192. [Google Scholar] [CrossRef]
- Gaitén, Y.I.G.; Martínez, M.M.; Alarcón, A.B.; Vãzquez, M.M.; Hernãndez, J.L.F.; Roche, L.D.; Rastrelli, L. Anti-inflammatory and antioxidant activity of a methanolic extract of phyllanthus orbicularis and its derived flavonols). J. Essent. Oil Res. 2011, 23, 50–53. [Google Scholar] [CrossRef]
- Quintero, A.R.; Gutiérrez, I.S.; Díaz, L.M.; del Barrio Alonso, G. Effect of Phyllanthus orbicularis on the cell viability and the hepatitis B surface antigen in PLC/PRF/5 cells. Rev. Cuba. Farm. 2011, 45, 536–544. [Google Scholar]
- Álvarez, Á.L.; Dalton, K.P.; Nicieza, I.; Diñeiro, Y.; Picinelli, A.; Melón, S.; Roque, A.; Suárez, B.; Parra, F. Bioactivity-guided fractionation of phyllanthus orbicularis and identification of the principal anti HSV-2 compounds. Phyther. Res. 2012, 26, 1513–1520. [Google Scholar] [CrossRef] [PubMed]
- Del Barrio, G.; Parra, F. Evaluation of the antiviral activity of an aqueous extract from Phyllanthus orbicularis. J. Ethnopharmacol. 2000, 72, 317–322. [Google Scholar] [CrossRef]
- Álvarez, Á.L.; del Barrio, G.; Kourí, V.; Martínez, P.A.; Suárez, B.; Parra, F. In vitro anti-herpetic activity of an aqueous extract from the plant Phyllanthus orbicularis. Phytomedicine 2009, 16, 960–966. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sànchez-Lamar, A.; Fiore, M.; Cundari, E.; Ricordy, R.; Cozzi, R.; De Salvia, R. Phyllanthus orbicularis aqueous extract: Cytotoxic, genotoxic, and antimutagenic effects in the CHO cell line. Toxicol. Appl. Pharmacol. 1999, 161, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, M.; Sánchez-Lamar, A.; Fuentes, J.L.; Barbé, J.; Llagostera, M. Studies on the antimutagenesis of Phyllanthus orbicularis: Mechanisms involved against aromatic amines. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2001, 498, 99–105. [Google Scholar] [CrossRef]
- Ferrer, M.; Cristófol, C.; Sánchez-Lamar, A.; Fuentes, J.L.; Barbé, J.; Llagostera, M. Modulation of rat and human cytochromes P450 involved in PhIP and 4-ABP activation by an aqueous extract of Phyllanthus orbicularis. J. Ethnopharmacol. 2004, 90, 273–277. [Google Scholar] [CrossRef]
- Vernhes, M.; González-Pumariega, M.; Alonso, A.; Baly, L.; Menck, C.F.M.; Sánchez-Lamar, A. Photoprotective effect phyllanthus orbicularis HBK in E. coli cells. Vaccimonitor 2010, 19, 233. [Google Scholar]
- Alonso, A.; Fuentes, J.L.; Sánchez-Lamar, A.; Llagostera, M. Antimutagenic Effect of Phyllanthus orbicularis against γ-radiation. Lat. Am. J. Pharm. 2010, 29, 148–152. [Google Scholar]
- Vernhes, M.; González-Pumariega, M.; Passaglia, A.; Martins, F.C.; Sánchez-Lamar, A. Aqueous extract of Phyllanthus orbicularis K protects the plasmid DNA from the damage induced by ultraviolet radiation. Ars Pharm. 2013, 54, 1. [Google Scholar]
- Tamayo, M.V.; Schuch, A.P.; Yagura, T.; Gil, L.B.; Menck, C.F.M.; Sánchez-Lamar, A. Genoprotective Effect of Phyllanthus orbicularis Extract Against UVA, UVB, and Solar Radiation. Photochem. Photobiol. 2018, 94, 1026–1031. [Google Scholar] [CrossRef] [PubMed]
- Menéndez-Perdomo, I.M.; Wong-Guerra, M.; Fuentes-León, F.; Carrazana, E.; Casadelvalle, I.; Vidal, A.; Sánchez-Lamar, A. Antioxidant, photoprotective and antimutagenic properties of Phyllanthus spp. from Cuban flora. J. Pharm. Pharmacogn. Res. 2017, 5, 251–261. [Google Scholar]
- Gutiérrez, Y.; Miranda, M.; Henriques, A.; del Barrio, G. Flavonoids analysis of a butanol fraction obtained from Phyllanthus orbicularis HBK. Univ. Revista Cubana de Farmacia 2010, 44, 367–373. [Google Scholar]
- Catarino, M.D.; Silva, A.M.S.; Saraiva, S.C.; Sobral, A.J.F.N.; Cardoso, S.M. Characterization of phenolic constituents and evaluation of antioxidant properties of leaves and stems of Eriocephalus africanus. Arab. J. Chem. 2018, 11, 62–69. [Google Scholar] [CrossRef]
- Jaiswal, R.; Halabi, E.A.; Karar, M.G.E.; Kuhnert, N. Identification and characterisation of the phenolics of Ilex glabra L. Gray (Aquifoliaceae) leaves by liquid chromatography tandem mass spectrometry. Phytochemistry 2014, 106, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Elliger, C.A.; Lundin, R.E.; Haddon, W.F. Caffeyl esters of glucaric acid in Lycopersiconesculentum leaves. Phytochemistry 1981, 20, 1133–1134. [Google Scholar] [CrossRef]
- Coutinho, I.D.; Baker, J.M.; Ward, J.L.; Beale, M.H.; Creste, S.; Cavalheiro, A.J. Metabolite profiling of sugarcane genotypes and identification of flavonoid glycosides and phenolic acids. J. Agric. Food Chem. 2016, 64, 4198–4206. [Google Scholar] [CrossRef]
- Risch, B.; Herrmann, K.; Wray, V. (E)-O-p-Coumaroyl-, (E)-O-Feruloyl-derivatives of glucaric acid in citrus. Phytochemistry 1988, 27, 3327–3329. [Google Scholar] [CrossRef]
- Lin, L.Z.; Sun, J.; Chen, P.; Monagas, M.J.; Harnly, J.M. UHPLC-PDA-ESI/HRMSnprofiling method to identify and quantify oligomeric proanthocyanidins in plant products. J. Agric. Food Chem. 2014, 62, 9387–9400. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yu, H.; Wu, H.; Pan, Y.; Wang, K.; Jin, Y.; Zhang, C. Characterization and quantification by LC-MS/MS of the chemical components of the heating products of the flavonoids extract in Pollen typhae for transformation rule exploration. Molecules 2015, 20, 18352–18366. [Google Scholar] [CrossRef] [PubMed]
- Geng, P.; Sun, J.; Zhang, M.; Li, X.; Harnly, J.M.; Chen, P. Comprehensive characterization of C-glycosyl flavones in wheat (Triticum aestivum L.) germ using UPLC-PDA-ESI/HRMSn and mass defect filtering. J. Mass Spectrom. 2016, 51, 914–930. [Google Scholar] [CrossRef] [PubMed]
- Dokkedal, A.L.; Lavarda, F.; Dos Santos, L.C.; Vilegas, W. Xeractinol—A new flavanonol C-glucoside from Paepalanthus argenteus var. argenteus (Bongard) Hensold (Eriocaulaceae). J. Braz. Chem. Soc. 2007, 18, 437–439. [Google Scholar] [CrossRef]
- Mbafor, J.T.; Fomum, Z.T.; Promsattha, R.; Sanson, D.R.; Tempesta, M.S. Isolation and characterization of taxifolin 6-c-glucoside from garcinia epunctata. J. Nat. Prod. 1989, 52, 417–419. [Google Scholar] [CrossRef] [PubMed]
- Rawat, P.; Kumar, M.; Sharan, K.; Chattopadhyay, N.; Maurya, R. Ulmosides A and B: Flavonoid 6-C-glycosides from Ulmuswallichiana, stimulating osteoblast differentiation assessed by alkaline phosphatase. Bioorg. Med. Chem. Lett. 2009, 19, 4684–4687. [Google Scholar] [CrossRef] [PubMed]
- Sokolová, R.; Nycz, J.E.; Ramešová, Š.; Fiedler, J.; Degano, I.; Szala, M.; Kolivoška, V.; Gál, M. Electrochemistry and spectroelectrochemistry of bioactive hydroxyquinolines: A mechanistic study. J. Phys. Chem. B 2015, 119, 6074–6080. [Google Scholar] [CrossRef]
- Nonaka, G.-I.; Goto, Y.; Kinjo, J.-E.; Nohara, T.; Nishioka, I. Tannins and related compounds. LII Studies on the constituents of the leaves of Thujopsisdolabrata SIEB. et ZUCC. Chem. Pharm. Bull. 2011, 35, 1105–1108. [Google Scholar] [CrossRef]
- Tofazzal Islam, M.; Tahara, S. Dihydroflavonols from Lanneacoromandelica. Phytochemistry 2000, 54, 901–907. [Google Scholar] [CrossRef]
- Xiao, J.; Capanoglu, E.; Jassbi, A.R.; Miron, A. Advance on the Flavonoid C-gly cosides and Health Benefits. Crit. Rev. Food Sci. Nutr. 2016, 56, S29–S45. [Google Scholar] [CrossRef]
- Gocer, H.; Topal, F.; Topal, M.; Küçük, M.; Teke, D.; Gülçin, I.; Alwasel, S.H.; Supuran, C.T. Acetylcholinesterase and carbonic anhydrase isoenzymes i and II inhibition profiles of taxifolin. J. Enzyme Inhib. Med. Chem. 2016, 31, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Kuang, H.; Tang, Z.; Zhang, C.; Wang, Z.; Li, W.; Yang, C.; Wang, Q.; Yang, B.; Kong, A.N. Taxifolin activates the Nrf2 anti-oxidative stress pathway in mouse skin epidermal JB6 P+ cells through epigenetic modifications. Int. J. Mol. Sci. 2017, 18, 1546. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Yamamoto, Y.; Maki, T.; Hattori, Y.; Ito, H.; Mizuno, K.; Harada-Shiba, M.; Kalaria, R.N.; Fukushima, M.; Takahashi, R.; et al. Taxifolin inhibits amyloid-β oligomer formation and fully restores vascular integrity and memory in cerebral amyloid angiopathy. Acta Neuropathol. Commun. 2017, 5, 26. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Chen, R.C.; Yang, Z.H.; Sun, G.B.; Wang, M.; Ma, X.J.; Yang, L.J.; Sun, X.B. Taxifolin prevents diabetic cardiomyopathy in vivo and in vitro by inhibition of oxidative stress and cell apoptosis. Food Chem. Toxicol. 2014, 63, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Singh, A.; Tiwari, S.; Mishra, A.; Maurya, R.; Singh, S. Ulmosides A: Flavonoid 6-C-glycosides from Ulmuswallichiana attenuates lipopolysacchride induced oxidative stress, apoptosis and neuronal death. Neurotoxicology 2019, 73, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Enzymatic C-Alkylation of Aromatic Compounds. In Biocatalysis in Organic Synthesis; hieme E-Books & E-Journals Partner: Stuttgart, Germany, 2016.
- Ruela, H.S.; Leal, I.C.R.; de Almeida, M.R.A.; dos Santos, K.R.N.; Wessjohann, L.A.; Kuster, R.M. Antibacterial and antioxidant activities and acute toxicity of Bumeliasartorum Mart., Sapotaceae, a Brazilian medicinal plant. Rev. Bras. Farmacogn. 2011, 21, 86–91. [Google Scholar] [CrossRef]
- Chemical Computing Group ULC. Molecular Operating Environment (MOE). Available online: https://www.chemcomp.com/ (accessed on 5 August 2019).
- Halgren, T.A. MMFF VI. MMFF94s option for energy minimization studies. J. Comput. Chem. 1999, 20, 720–729. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098. [Google Scholar] [CrossRef]
- Perdew, J.P. Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B 1986, 33, 8822. [Google Scholar] [CrossRef]
- Karton, A.; Tarnopolsky, A.; Lamère, J.F.; Schatz, G.C.; Martin, J.M.L. Highly accurate first-principles benchmark data sets for the parametrization and validation of density functional and other approximate methods. Derivation of a robust, generally applicable, double-hybrid functional for thermochemistry and thermochemical. J. Phys. Chem. A 2008, 112, 12868–12886. [Google Scholar] [CrossRef]
- Schäfer, A.; Horn, H.; Ahlrichs, R. Fully optimized contracted Gaussian basis sets for atoms Li to Kr. J. Chem. Phys. 1992, 97, 2571–2577. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Sinnecker, S.; Rajendran, A.; Klamt, A.; Diedenhofen, M.; Neese, F. Calculation of solvent shifts on electronic g-tensors with the conductor-like screening model (COSMO) and its self-consistent generalization to real solvents (direct COSMO-RS). J. Phys. Chem. A 2006, 110, 2235–2245. [Google Scholar] [CrossRef] [PubMed]
- Bruhn, T.; Schaumlöffel, A.; Hemberger, Y.; Bringmann, G. SpecDis: Quantifying the comparison of calculated and experimental electronic circular dichroism spectra. Chirality 2013, 25, 243–249. [Google Scholar] [CrossRef] [PubMed]
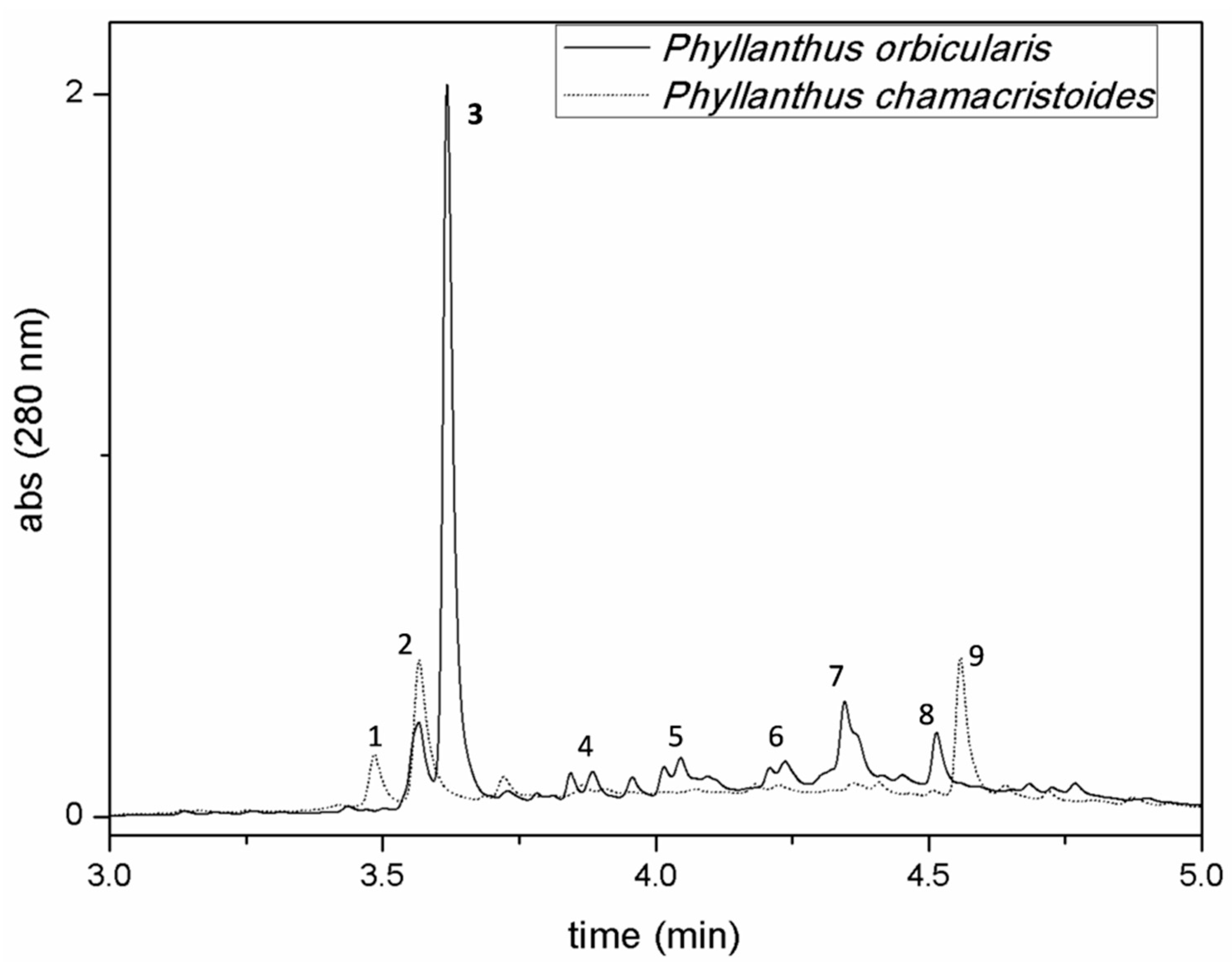

| Peak | Retention Time (min) | Compound | Molecular Formula | λmaxabs(nm) | MS1 [M − H]−(m/z) | MS2 [M − H]−(m/z) | MS3[M − H]−(m/z) |
|---|---|---|---|---|---|---|---|
| 1 | 3.49 | Protocatechuic acid glucoside | C13H16O9 | 290 | 315.0717 | 153.0196 | 109 |
| 2 | 3.52 | p-Cumaroyl-glucaric acid | C15H16O10 | 326 | 355.0668 | 191.0198 | 147; 85 |
| 3 | 3.65 | Fideloside | C21H22O12 | 290 | 465.1039 | 345.0620 | 277; 179; 167 |
| 4 * | 3.85 | Catechin | C15H14O6 | 278 | 289.0720 | 271.0620; 245.0825 | |
| 5 * | 4.07 | Procyanidin B2 | C30H26O12 | 280 | 577.1352 | 451.1036; 425.088; 289.0720 | |
| 6 * | 4.23 | Epicatechin | C15H14O6 | 278 | 289.0720 | 271.0620; 245.0825 | |
| 7 | 4.35 | Procyanidin C1 | C45H38O18 | 281 | 865.1986 | 847.1882; 739.1667; 695.1407; 577.1353 | [865 → 577] 289 |
| 8 * | 4.51 | Rutoside | C27H30O16 | 355 | 609.1460 | ||
| 9 | 4.60 | Nicotiflorin | C27H30O15 | 343 | 593.1514 | 285.0403 | 255; 227; 151 |
| Nr. | δ c | DEPT | δ H (J in Hz) | 1H-1H COSY | NOESY | HMBC |
|---|---|---|---|---|---|---|
| 2 | 82.1 | CH | 5.02 (d, 11.02) | H-3 | H-3-; H-6′; H-2′ | H-2′;H-6′; OH-3 |
| 3 | 72.1 | CH | 4.25 (m) | H-2; OH-3 | H-2′, H-6′; OH-3 | OH-3; H-2 |
| 4 | 197.9 | C | H-2; H-6;OH-3 | |||
| 5 | 162.1 | C | H-6; OH-5 | |||
| 6 | 95.6 | CH | 6.04 (s) | OH-5 | ||
| 7 | 165.7 | C | H-6; H-1″ | |||
| 8 | 105.5 | C | H-6; H-1″-H; H-2″ | |||
| 9 | 161.4 | C | H-2; H-1″ | |||
| 10 | 100.5 | C | H-6; OH-5 | |||
| 1′ | 128.4 | C | H-2; H-5′; H-2′ | |||
| 2′ | 115.0 | CH | 6.94 (brs) | H-6′ | H-3; H-2 | H-2; H-5′ |
| 3′ | 144.6 | C | H-5′ | |||
| 4′ | 145.1 | C | H-2′; H-6′ | |||
| 5′ | 115.0 | CH | 6.73 (d, 8.09) | H-6′ | H-6′ | H-2′; H-6′ |
| 6′ | 118.3 | CH | 6.84 (brd, 8.09) | H-2′; H-5′ | H-2; H-5′; H-3 | H-2′; H-2 |
| 1″ | 73.0 | CH | 4.45 (d, 9.63) | H-2″ | H-3″ | H-2″; H-6 |
| 2″ | 70.2 | CH | 3.82 (brt, 9.53) | H-1″; H-3″, OH-2″ | H-4″; H-3″; H-1″ | H-1″ |
| 3″ | 78.6 | CH | 3.11 (m) | H-2″; H-4″; OH-3″ | H-1″; H-2″ | H-1″; H-2″ |
| 4″ | 70.4 | CH | 2.95 (br) | H-3″; H-5″; OH-4″ | H-2″; OH-4; H2-6″ | H-5″; H-3″; H-1″ |
| 5″ | 81.3 | CH | 3.09 (m) | H-6″ | H-2″; Hb-6″ | H-1″ |
| 6″ | 61.7 | CH2 | Ha: 3.70 (m) Hb: 3.43 (m) | H-5″; H-6″; OH-6″ | H-6″; H-4″ | |
| 3-OH | 5.82(d, 6.13) | H-3 | H-3 | |||
| 5-OH | 12.01(s) | H-6 | ||||
| 7-OH | - | |||||
| 3′-OH | 8.87 (brs) | H-2′ | ||||
| 4″-OH | 9.00 (brs) | H-5′ | ||||
| 2″-OH | 4.62 (brs) | H-2″ | ||||
| 3″-OH | 4.83 (brs) | H-3″ | H-2″, H-4″ | |||
| 4″-OH | 4.84 (brs) | H-4″ | ||||
| 6″-OH | 4.57 (brs) | H2-6″ |
| Conformation | O-C2-C1′-C2′ (in°) | C2′-C3′-O-H (in°) | Energy (kcal/mol) | Boltzmann Weight | CD-Fit |
|---|---|---|---|---|---|
| 1 | −61.8 | −179.5 | 0 | 59.4 | 0.6757 |
| 2 | 122.8 | 2.4 | 0.66 | 19.5 | 0.5712 |
| 3 | −54.7 | 1.0 | 0.97 | 11.5 | 0.7065 |
| 4 | 121.9 | −179.0 | 1.08 | 9.6 | 0.5974 |
| Boltzmann | 0.7099 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Francioso, A.; Franke, K.; Villani, C.; Mosca, L.; D’Erme, M.; Frischbutter, S.; Brandt, W.; Sanchez-Lamar, A.; Wessjohann, L. Insights into the Phytochemistry of the Cuban Endemic Medicinal Plant Phyllanthus orbicularis: Fideloside, a Novel Bioactive 8-C-glycosyl 2,3-Dihydroflavonol. Molecules 2019, 24, 2855. https://doi.org/10.3390/molecules24152855
Francioso A, Franke K, Villani C, Mosca L, D’Erme M, Frischbutter S, Brandt W, Sanchez-Lamar A, Wessjohann L. Insights into the Phytochemistry of the Cuban Endemic Medicinal Plant Phyllanthus orbicularis: Fideloside, a Novel Bioactive 8-C-glycosyl 2,3-Dihydroflavonol. Molecules. 2019; 24(15):2855. https://doi.org/10.3390/molecules24152855
Chicago/Turabian StyleFrancioso, Antonio, Katrin Franke, Claudio Villani, Luciana Mosca, Maria D’Erme, Stefan Frischbutter, Wolfgang Brandt, Angel Sanchez-Lamar, and Ludger Wessjohann. 2019. "Insights into the Phytochemistry of the Cuban Endemic Medicinal Plant Phyllanthus orbicularis: Fideloside, a Novel Bioactive 8-C-glycosyl 2,3-Dihydroflavonol" Molecules 24, no. 15: 2855. https://doi.org/10.3390/molecules24152855
APA StyleFrancioso, A., Franke, K., Villani, C., Mosca, L., D’Erme, M., Frischbutter, S., Brandt, W., Sanchez-Lamar, A., & Wessjohann, L. (2019). Insights into the Phytochemistry of the Cuban Endemic Medicinal Plant Phyllanthus orbicularis: Fideloside, a Novel Bioactive 8-C-glycosyl 2,3-Dihydroflavonol. Molecules, 24(15), 2855. https://doi.org/10.3390/molecules24152855









