Parathyroid Autofluorescence—How Does It Affect Parathyroid and Thyroid Surgery? A 5 Year Experience
Abstract
1. Introduction
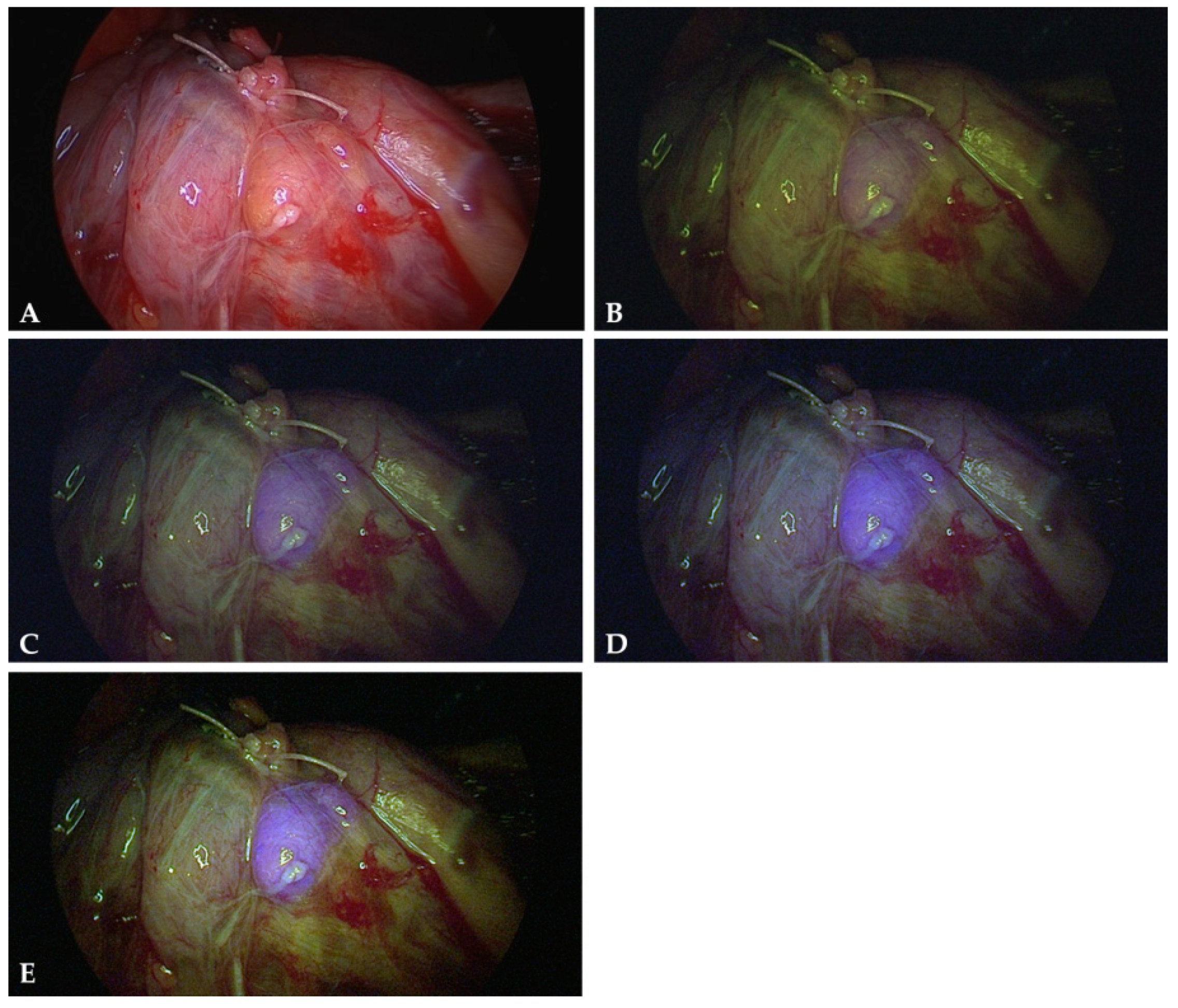
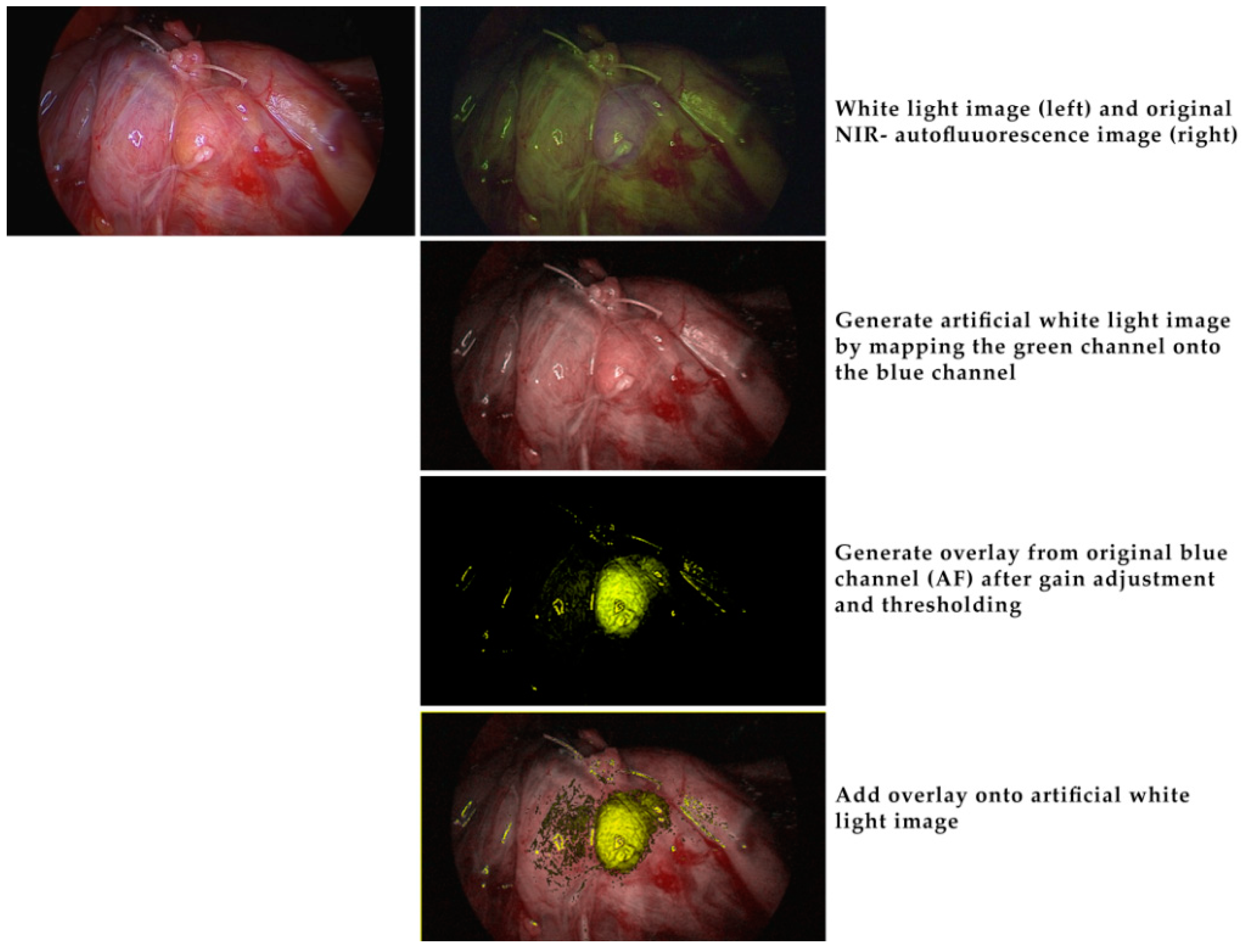
2. Results
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Imaging System
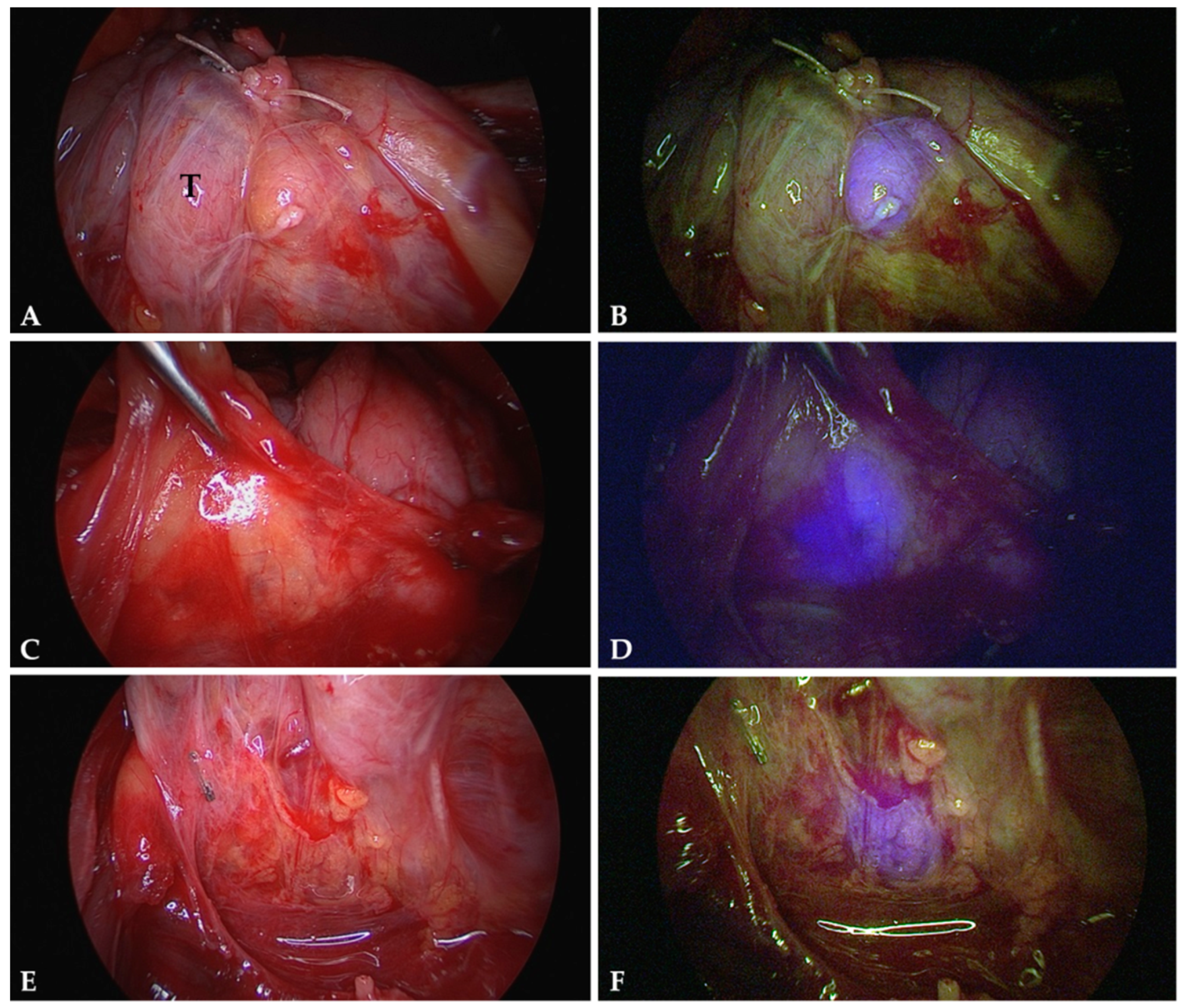
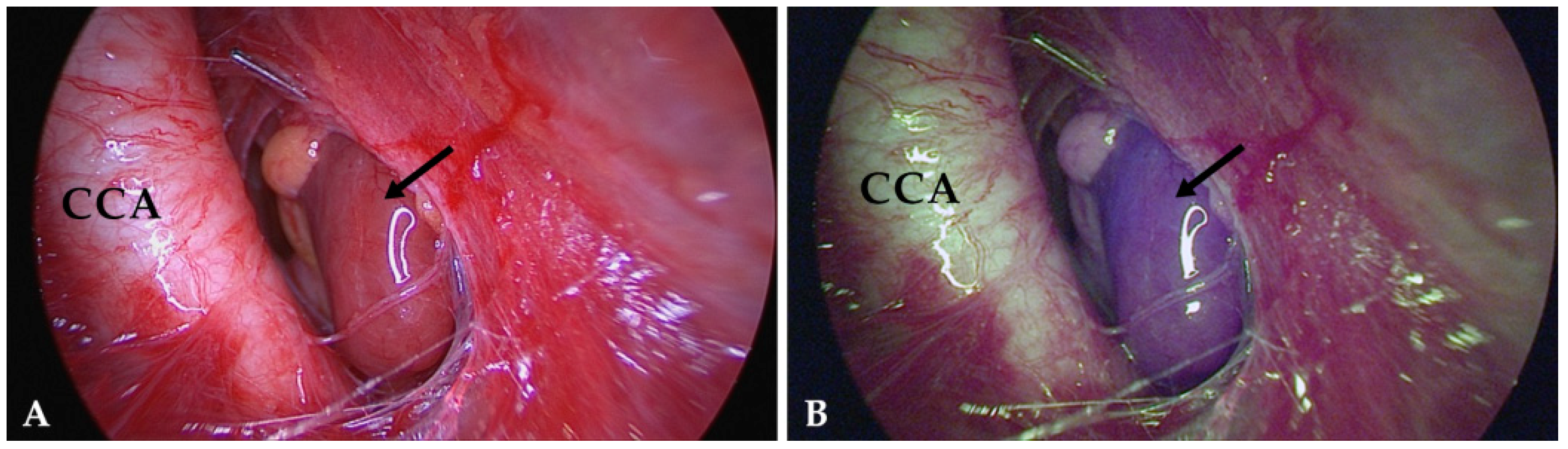
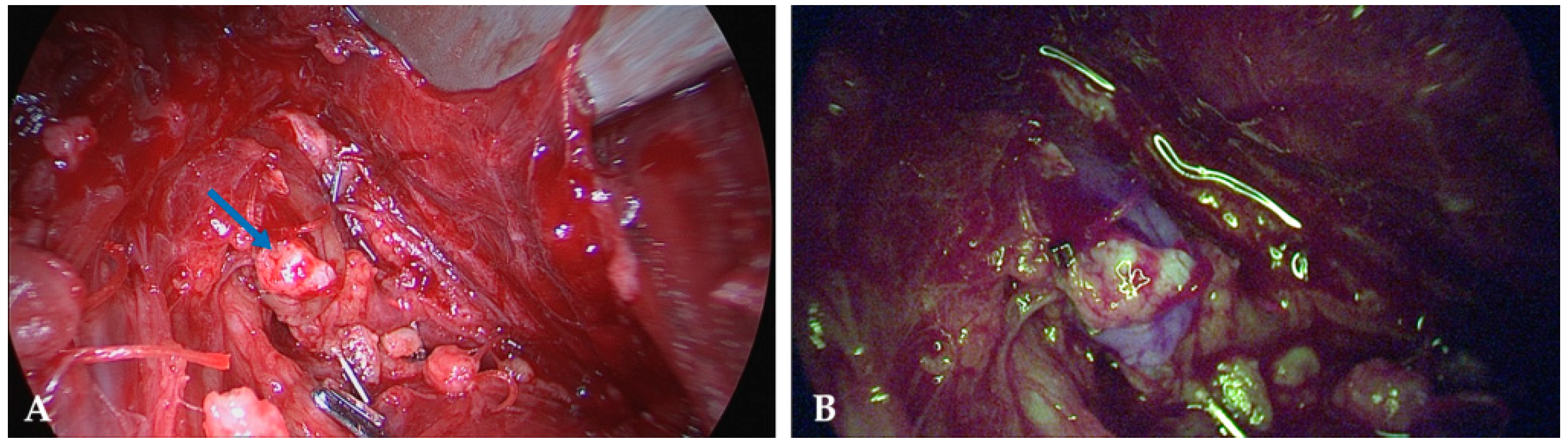
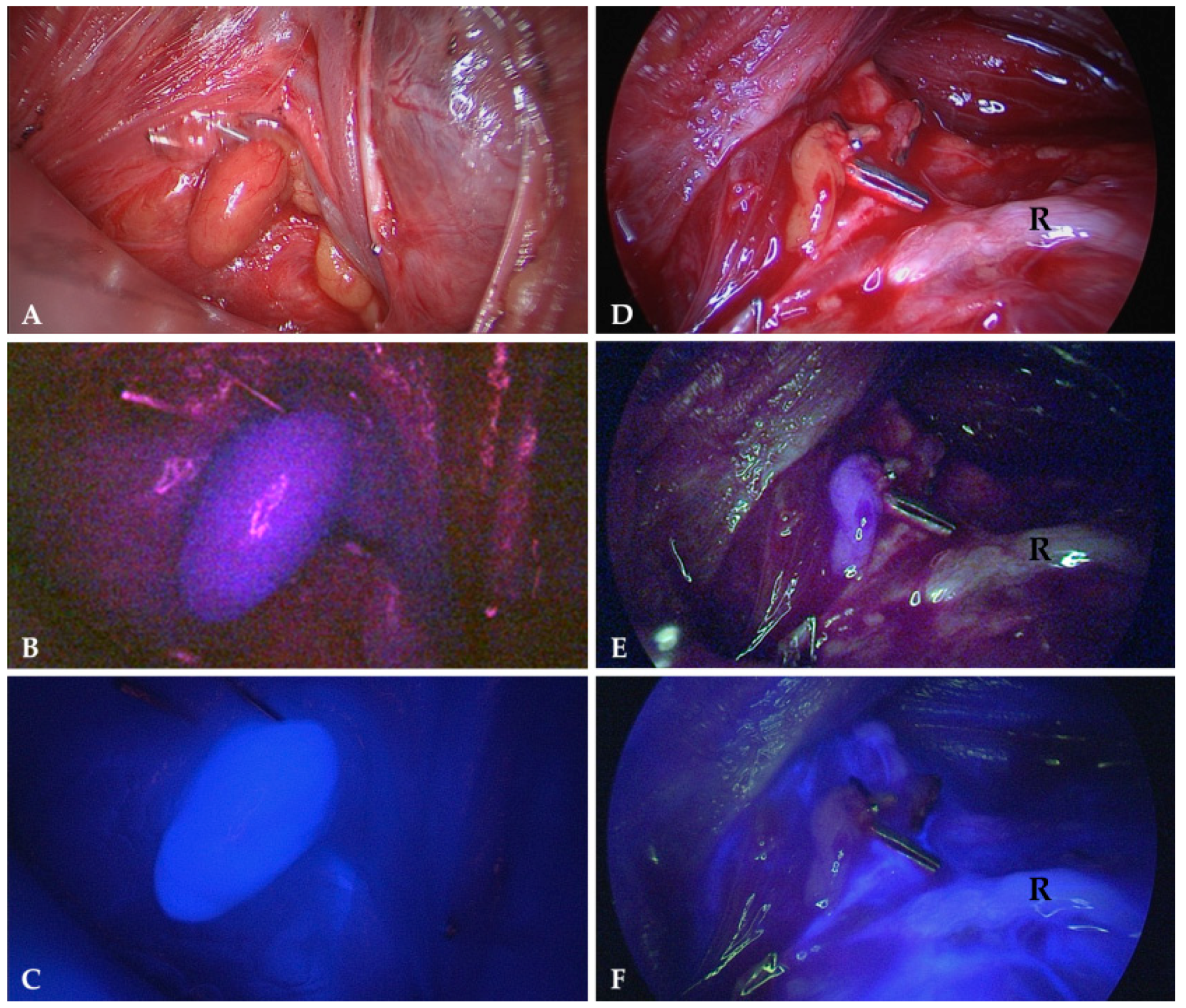
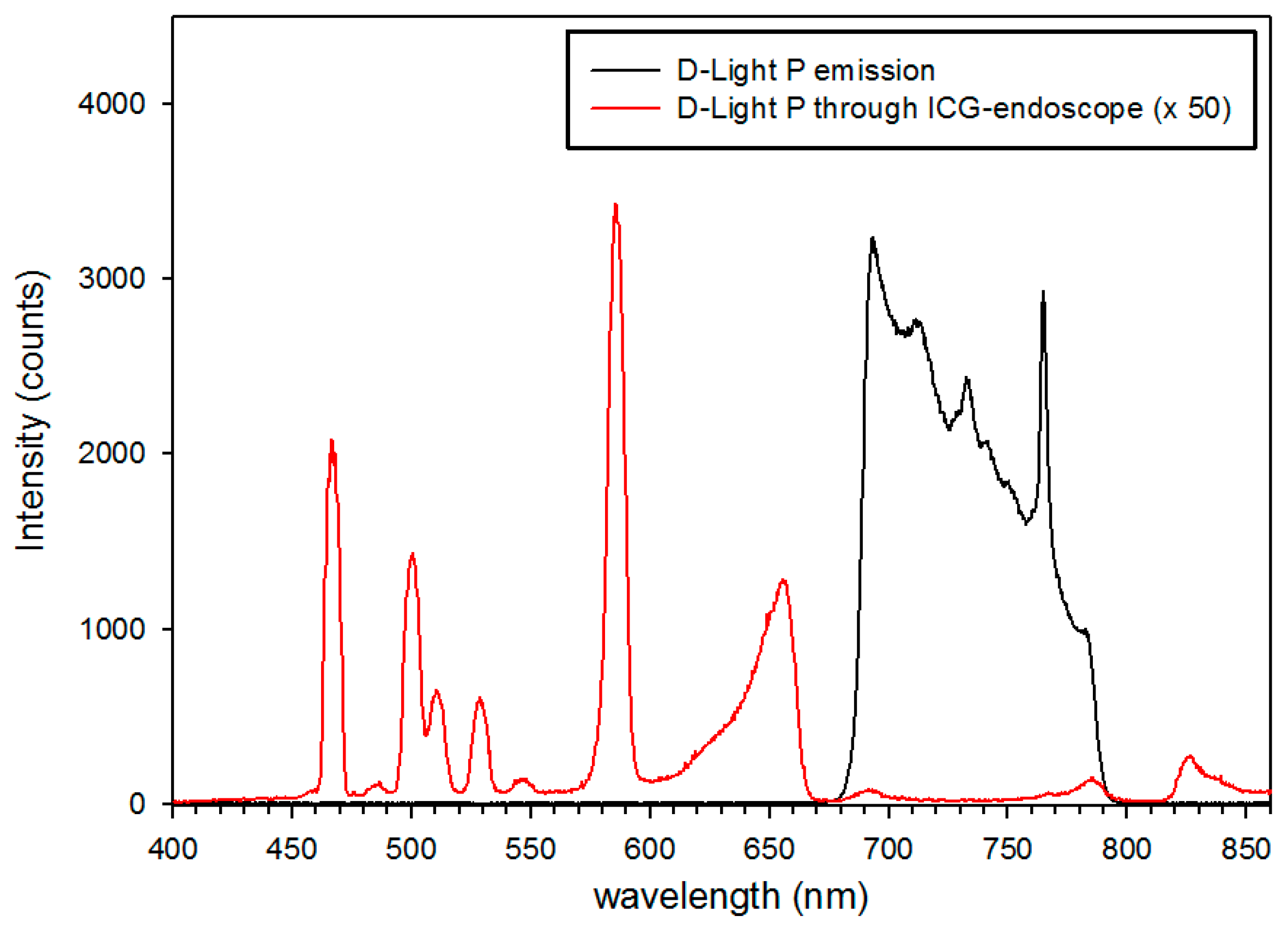
4.3. Intraoperative Autofluorescence Imaging
4.4. Intraoperative ICG Imaging
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lin, D.T.; Patel, S.G.; Shaha, A.R.; Singh, B.; Shah, J.P. Incidence of Inadvertent Parathyroid Removal During Thyroidectomy. Laryngoscope 2002, 112, 608–611. [Google Scholar] [CrossRef] [PubMed]
- Thomusch, O.; Machens, A.; Sekulla, C.; Ukkat, J.; Brauckhoff, M.; Dralle, H. The impact of surgical technique on postoperative hypoparathyroidism in bilateral thyroid surgery: A multivariate analysis of 5846 consecutive patients. Surgery 2003, 133, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Bergenfelz, A.; Jansson, S.; Kristoffersson, A.; Mårtensson, H.; Reihnér, E.; Wallin, G.; Lausen, I. Complications to thyroid surgery: Results as reported in a database from a multicenter audit comprising 3,660 patients. Langenbecks Arch. Surg. 2008, 393, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Bilezikian, J.P.; Khan, A.; Potts, J.T., Jr.; Brandi, M.L.; Clarke, B.L.; Shoback, D.; Jüppner, H.; D’Amour, P.; Fox, J.; Rejnmark, L.; et al. Hypoparathyroidism in the adult: Epidemiology, diagnosis, pathophysiology, target-organ involvement, treatment, and challenges for future research. J. Bone Miner. Res. 2011, 26, 2317–2337. [Google Scholar] [CrossRef] [PubMed]
- Dudley, N.E. Methylene blue for rapid identification of the parathyroids. Br. Med. J. 1971, 3, 680–681. [Google Scholar] [CrossRef]
- Patel, H.P.; Chadwick, D.R.; Harrison, B.J.; Balasubramanian, S.P. Systematic review of intravenous methylene blue in parathyroid surgery. Br. J. Surg. 2012, 99, 1345–1351. [Google Scholar] [CrossRef]
- Patel, A.S.; Singh-Ranger, D.; Lowery, K.A.; Crinnion, J.N. Adverse neurologic effect of methylene blue used during parathyroidectomy. Head Neck 2006, 28, 567–568. [Google Scholar] [CrossRef]
- Sweet, G.; Standiford, S.B. Methylene-Blue–Associated Encephalopathy. J. Am. Coll. Surg. 2007, 204, 454–458. [Google Scholar] [CrossRef]
- Han, N.; Bumpous, J.M.; Goldstein, R.E.; Fleming, M.M.; Flynn, M.B. Intra-operative parathyroid identification using methylene blue in parathyroid surgery. Am. Surg. 2007, 73, 820–823. [Google Scholar]
- Khavandi, A.; Whitaker, J.; Gonna, H. Serotonin toxicity precipitated by concomitant use of citalopram and methylene blue. Med. J. Aust. 2008, 189, 534–535. [Google Scholar]
- Rowley, M.; Riutort, K.; Shapiro, D.; Casler, J.; Festic, E.; Freeman, W.D. Methylene Blue-Associated Serotonin Syndrome: A ‘Green’ Encephalopathy After Parathyroidectomy. Neurocritical Care 2009, 11, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Van der Vorst, J.R.; Schaafsma, B.E.; Verbeek, F.P.; Swijnenburg, R.J.; Tummers, Q.R.; Hutteman, M.; Hamming, J.F.; Kievit, J.; Frangioni, J.V.; van de Velde, C.J.; et al. Intraoperative near-infrared fluorescence imaging of parathyroid adenomas with use of low-dose methylene blue. Head Neck 2014, 36, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Hillary, S.L.; Guillermet, S.; Brown, N.J.; Balasubramanian, S.P. Use of methylene blue and near-infrared fluorescence in thyroid and parathyroid surgery. Langenbecks Arch. Surg. 2018, 403, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Prosst, R.L.; Weiss, J.; Hupp, L.; Willeke, F.; Post, S. Fluorescence-guided minimally invasive parathyroidectomy: Clinical experience with a novel intraoperative detection technique for parathyroid glands. World J. Surg. 2010, 34, 2217–2222. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Numata, T.; Shibuya, M. Intraoperative photodynamic detection of normal parathyroid glands using 5-aminolevulinic acid. Laryngoscope 2011, 121, 1462–1466. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, S.; Shimizu, K., Jr.; Akasu, H.; Okamura, R. Identification of Pathological and Normal Parathyroid Tissue by Fluorescent Labeling with 5-aminolevulinic Acid during Endocrine Neck Surgery. J. Nippon. Med Sch. 2014, 81, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Adams, K.E.; Ke, S.; Kwon, S.; Liang, F.; Fan, Z.; Lu, Y.; Hirschi, K.; Mawad, M.E.; Barry, M.A.; Sevick-Muraca, E.M. Comparison of visible and near-infrared wavelength-excitable fluorescent dyes for molecular imaging of cancer. J. Biomed. Opt. 2007, 12, 024017. [Google Scholar] [CrossRef] [PubMed]
- Sevick-Muraca, E.M.; Rasmussen, J.C. Molecular imaging with optics: Primer and case for near-infrared fluorescence techniques in personalized medicine. J. Biomed. Opt. 2008, 13, 041303. [Google Scholar] [CrossRef] [PubMed]
- Lavazza, M.; Liu, X.; Wu, C.; Anuwong, A.; Kim, H.Y.; Liu, R.; Randolph, G.W.; Inversini, D.; Boni, L.; Rausei, S.; et al. Indocyanine green-enhanced fluorescence for assessing parathyroid perfusion during thyroidectomy. Gland. Surg. 2016, 5, 512–521. [Google Scholar] [CrossRef]
- Zaidi, N.; Bucak, E.; Yazici, P.; Soundararajan, S.; Okoh, A.; Yigitbas, H.; Dural, C.; Berber, E. The feasibility of indocyanine green fluorescence imaging for identifying and assessing the perfusion of parathyroid glands during total thyroidectomy. J. Surg. Oncol. 2016, 113, 775–778. [Google Scholar] [CrossRef]
- Zaidi, N.; Bucak, E.; Okoh, A.; Yazici, P.; Yigitbas, H.; Berber, E. The utility of indocyanine green near-infrared fluorescent imaging in the identification of parathyroid glands during surgery for primary hyperparathyroidism. J. Surg. Oncol. 2016, 113, 771–774. [Google Scholar] [CrossRef] [PubMed]
- Vidal Fortuny, J.; Belfontali, V.; Sadowski, S.M.; Karenovics, W.; Guigard, S.; Triponez, F. Parathyroid gland angiography with indocyanine green fluorescence to predict parathyroid function after thyroid surgery. Br. J. Surg. 2016, 103, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Jitpratoom, P.; Anuwong, A. The use of ICG enhanced fluorescence for the evaluation of parathyroid gland preservation. Gland Surg. 2017, 6, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Vidal Fortuny, J.; Sadowski, S.M.; Belfontali, V.; Guigard, S.; Poncet, A.; Ris, F.; Karenovics, W.; Triponez, F. Randomized clinical trial of intraoperative parathyroid gland angiography with indocyanine green fluorescence predicting parathyroid function after thyroid surgery. Br. J. Surg. 2018, 105, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Paras, C.; Keller, M.; White, L.; Phay, J.; Mahadevan-Jansen, A. Near-infrared autofluorescence for the detection of parathyroid glands. J. Biomed. Opt. 2011, 16, 067012. [Google Scholar] [CrossRef] [PubMed]
- McWade, M.A.; Paras, C.; White, L.M.; Phay, J.E.; Mahadevan-Jansen, A.; Broome, J.T. A novel optical approach to intraoperative detection of parathyroid glands. Surgery 2013, 154, 1371–1377. [Google Scholar] [CrossRef]
- McWade, M.A.; Paras, C.; White, L.M.; Phay, J.E.; Solorzano, C.C.; Broome, J.T.; Mahadevan-Jansen, A. Label-free Intraoperative Parathyroid Localization With Near-Infrared Autofluorescence Imaging. J. Clin. Endocrinol. Metab. 2014, 99, 4574–4580. [Google Scholar] [CrossRef]
- McWade, M.A.; Sanders, M.E.; Broome, J.T.; Solórzano, C.C.; Mahadevan-Jansen, A. Establishing the clinical utility of autofluorescence spectroscopy for parathyroid detection. Surgery 2016, 159, 193–202. [Google Scholar] [CrossRef]
- De Leeuw, F.; Breuskin, I.; Abbaci, M.; Casiraghi, O.; Mirghani, H.; Ben Lakhdar, A.; Laplace-Builhé, C.; Hartl, D. Intraoperative Near-infrared Imaging for Parathyroid Gland Identification by Auto-fluorescence: A Feasibility Study. World J. Surg. 2016, 40, 2131–2138. [Google Scholar] [CrossRef]
- Falco, J.; Dip, F.; Quadri, P.; De La Fuente, M.; Rosenthal, R. Cutting Edge in Thyroid Surgery: Autofluorescence of Parathyroid Glands. J. Am. Coll. Surg. 2016, 223, 374–380. [Google Scholar] [CrossRef]
- Ladurner, R.; Sommerey, S.; Arabi, N.A.; Hallfeldt, K.K.J.; Stepp, H.; Gallwas, J.K.S. Intraoperative near-infrared autofluorescence imaging of parathyroid glands. Surg. Endosc. 2017, 31, 3140–3145. [Google Scholar] [CrossRef] [PubMed]
- Ladurner, R.; Al Arabi, N.; Guendogar, U.; Hallfeldt, K.; Stepp, H.; Gallwas, J. Near-infrared autofluorescence imaging to detect parathyroid glands in thyroid surgery. Ann. R. Coll. Surg. Engl. 2018, 100, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Song, S.H.; Lee, H.S.; Noh, W.J.; Oak, C.; Lee, K.D.; Kim, S.W.; Ahn, Y.-C. Intraoperative Real-Time Localization of Normal Parathyroid Glands with Autofluorescence Imaging. J. Clin. Endocrinol. Metab. 2016, 101, 4646–4652. [Google Scholar] [CrossRef]
- Benmiloud, F.; Rebaude, S.; Varoquaux, A.; Penaranda, G.; Bannier, M.; Denizot, A. Impact of autofluorescence-based identification of parathyroids during total thyroidectomy on postoperative hypocalcemia: A before and after controlled study. Surgery 2018, 163, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Falco, J.; Dip, F.; Quadri, P.; de la Fuente, M.; Prunello, M.; Rosenthal, R.J. Increased identification of parathyroid glands using near-infrared light during thyroid and parathyroid surgery. Surg. Endosc. 2017, 31, 3737–3742. [Google Scholar] [CrossRef]
- Kahramangil, B.; Dip, F.; Benmiloud, F.; Falco, J.; De La Fuente, M.; Verna, S.; Rosenthal, R.; Berber, E. Detection of Parathyroid Autofluorescence Using Near-Infrared Imaging: A Multicenter Analysis of Concordance Between Different Surgeons. Ann. Surg. Oncol. 2018, 25, 957–962. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Lee, H.S.; Ahn, Y.-C.; Park, C.W.; Jeon, S.W.; Kim, C.H.; Ko, J.B.; Oak, C.; Kim, Y.; Lee, K.D. Near-Infrared Autofluorescence Image-Guided Parathyroid Gland Mapping in Thyroidectomy. J. Am. Coll. Surg. 2018, 226, 165–172. [Google Scholar] [CrossRef]
- Dip, F.; Falco, J.; Verna, S.; Prunello, M.; Loccisano, M.; Quadri, P.; White, K.; Rosenthal, R. Randomized Controlled Trial Comparing White Light with Near-Infrared Autofluorescence for Parathyroid Gland Identification During Total Thyroidectomy. J. Am. Coll. Surg. 2019, 228, 744–751. [Google Scholar] [CrossRef]
- McWade, M. Development of an intraoperative tool to detect parathyroid gland autofluorescence. 2016. Available online: https://etd.library.vanderbilt.edu/available/etd-04152016-131447/unrestricted/McWade.pdf (accessed on 26 January 2019).
- Thomas, G.; McWade, M.A.; Sanders, M.E.; Solórzano, C.C.; McDonald, W.H.; Mahadevan-Jansen, A. Identifying the novel endogenous near-infrared fluorophore within parathyroid and other endocrine tissues. Biomed. Opt. 2016. [Google Scholar] [CrossRef]
- Ritter, C.S.; Haughey, B.H.; Miller, B.; Brown, A.J. Differential Gene Expression by Oxyphil and Chief Cells of Human Parathyroid Glands. J. Clin. Endocrinol. Metab. 2012, 97, E1499–E1505. [Google Scholar] [CrossRef]
- Portela-Gomes, G.M.; Grimelius, L.; Stridsberg, M. Secretogranin III in human neuroendocrine tumours: A comparative immunohistochemical study with chromogranins A and B and secretogranin II. Regul. Pept. 2010, 165, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Weiler, R.; Fischer-Colbrie, R.; Schmid, K.W.; Feichtinger, H.; Bussolati, G.; Grimelius, L.; Krisch, K.; Kerl, H.; Oʼconnor, D.; Winkler, H. Immunological Studies on the Occurrence and Properties of Chromogranin A and B and Secretogranin II in Endocrine Tumors. Am. J. Surg. Pathol. 1988, 12, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Göbel, W.; Brucker, D.; Kienast, Y.; Johansson, A.; Kniebühler, G.; Rühm, A.; Eigenbrod, S.; Fischer, S.; Goetz, M.; Kreth, F.W.; et al. Optical needle endoscope for safe and precise stereotactically guided biopsy sampling in neurosurgery. Opt. Express 2012, 20, 26117–26126. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |
| Number of Patients | 117 |
| Mean age | 49.9 (19–81) years |
| Gender (female/male) | 76/41 |
| Type of operation | (n) |
| Open parathyroidectomies | 42 |
| Thyroidectomies | 75 |
| Goiter | 36 |
| Grave’s disease | 14 |
| Carcinoma | 25 |
| Authors | Study Design | Surgery | (n) | Device | Detected PG (%) |
|---|---|---|---|---|---|
| Paras 2011 [25] | Case series | P/T | 21 | 785 nm diode laser/spectrometer | 100% |
| Mac Wade 2013 [26] | Case series | P/T | 45 | 785 nm diode laser/spectrometer | 100% |
| Mac Wade 2014 [27] | Case series | P/T | 110 | 785 nm diode laser/spectrometer | 100% |
| Mac Wade 2016 [28] | Case series | P/T | 264 | 785 nm diode laser/spectrometer | 100% |
| De Leeuw 2016 [29] | Case series | P/T | 35 | Fluobeam® 800 clinical system | 98.8% |
| Falco 2016 [30] | Case series | P/T | 28 | Fluobeam® 800 clinical system | 100% |
| Kim 2016 [33] | Case series | T | 8 | 780 nm LED/single lens reflex camera | 100% |
| Falco 2017 [35] | Multi center series | P/T | 74 | 750 nm laser/imaging system | 100% |
| Ladurner 2017 [31] | Case series | P/T | 30 | Storz® ICG/NIR imaging system | 80.9% |
| Kahramangil 2018 [36] | Multi center series | P/T | 210 | Fluobeam® 800 clinical system | 98% |
| Kim 2018 [37] | Case series | T | 38 | 780 nm LED/single lens reflex camera | 98.5% |
| Ladurner 2018 [32] | Case series | T | 20 | Storz® ICG/NIR imaging system | 90.2% |
| Benmiloud 2018 [34] | Controlled study | T | 93 | Fluobeam® 800 clinical system | 76.3% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ladurner, R.; Lerchenberger, M.; Al Arabi, N.; Gallwas, J.K.S.; Stepp, H.; Hallfeldt, K.K.J. Parathyroid Autofluorescence—How Does It Affect Parathyroid and Thyroid Surgery? A 5 Year Experience. Molecules 2019, 24, 2560. https://doi.org/10.3390/molecules24142560
Ladurner R, Lerchenberger M, Al Arabi N, Gallwas JKS, Stepp H, Hallfeldt KKJ. Parathyroid Autofluorescence—How Does It Affect Parathyroid and Thyroid Surgery? A 5 Year Experience. Molecules. 2019; 24(14):2560. https://doi.org/10.3390/molecules24142560
Chicago/Turabian StyleLadurner, Roland, Maximilian Lerchenberger, Norah Al Arabi, Julia K. S. Gallwas, Herbert Stepp, and Klaus K. J. Hallfeldt. 2019. "Parathyroid Autofluorescence—How Does It Affect Parathyroid and Thyroid Surgery? A 5 Year Experience" Molecules 24, no. 14: 2560. https://doi.org/10.3390/molecules24142560
APA StyleLadurner, R., Lerchenberger, M., Al Arabi, N., Gallwas, J. K. S., Stepp, H., & Hallfeldt, K. K. J. (2019). Parathyroid Autofluorescence—How Does It Affect Parathyroid and Thyroid Surgery? A 5 Year Experience. Molecules, 24(14), 2560. https://doi.org/10.3390/molecules24142560






