Effect of the Use of Purified Grape Pomace as a Fining Agent on the Volatile Composition of Monastrell Wines
Abstract
:1. Introduction
2. Results and Discussion
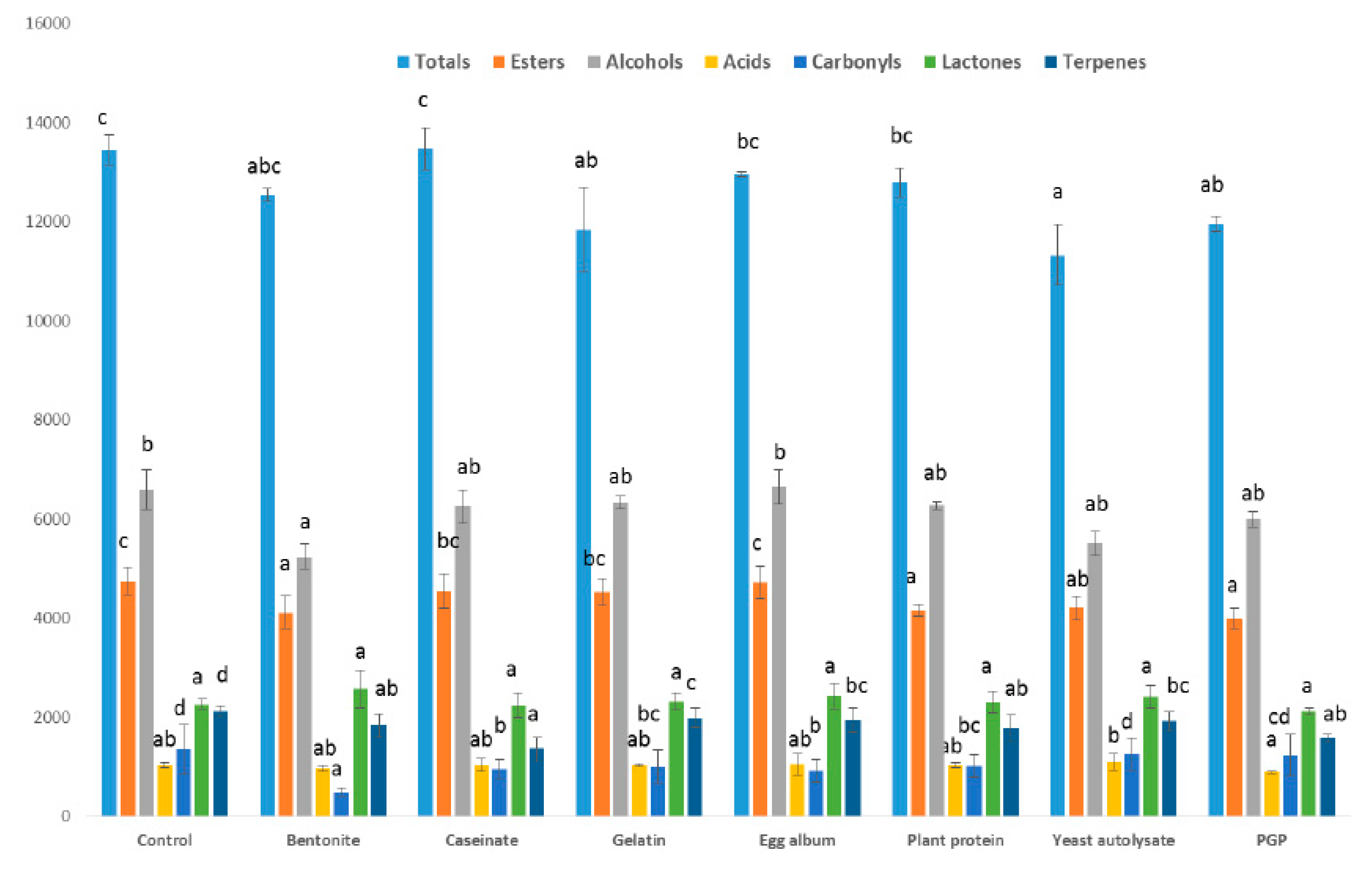
2.1. Effect of Fining Agents on Total Volatile Composition in Wine
2.2. Effect of Fining Agents on the Individual Volatile Compounds in Wine
2.2.1. Alcohols
2.2.2. Esters
2.2.3. Terpenes and Norisoprenoids
2.2.4. Carbonyls and Lactones
2.2.5. Acids
3. Material and Methods
3.1. Isolation and Purification of Pomace Grape
3.2. Wine Fining
3.3. Determination of Volatile Compounds in Wines after Fining
3.4. Statistical Analysis Treatment
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Abarghuei, M.J.; Rouzbehan, Y.; Alipour, D. The influence of the grape pomace on the ruminal parameters of sheep. Lives. Sci. 2010, 132, 73–79. [Google Scholar] [CrossRef]
- Christ, K.L.; Burrit, R.L. Critical environmental concerns in wine production: an integrative review. J. Clean. Prod. 2013, 53, 232–242. [Google Scholar] [CrossRef]
- Cuccia, P. Ethics+ economy+environment=sustainability: Gambero Rosso on the front lines with a new concept of sustainability. Wine Econ. Pol. 2015, 4, 69–70. [Google Scholar] [CrossRef]
- Beres, C.; Costa, G.N.S.; Cabezudo, I.; Da Silva-James, N.K.; Teles, A.S.C.; Cruz, A.P.G.; Mellinger-Silva, C.; Tonon, R.V.; Cabral, L.M.C.; Freitas, S.P. Towards integral utilization of grape pomace from winemaking process: A review. Waste Manag. 2017, 68, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Bindon, K.A.; Smith, P.A. Comparison of the affinity and selectivity of insoluble fibres and commercial proteins for wine proanthocyanidins. Food Chem. 2013, 136, 917–928. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, R.F.; Smith, P.; Bindon, K.A. Application of Insoluble fibres in the fining of wine phenolics. J. Agric. Food Chem. 2013, 61, 4424–4432. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Martínez, M.D.; Gil-Muñoz, R.; Gómez-Plaza, E.; Bautista-Ortín, A.B. Performance of purified grape pomace as a fining agent to reduce the levels of some contaminants from wine. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2018, 35, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Granato, T.M.; Nasi, A.; Ferranti, P.; Iametti, S.; Bonomi, F. Fining white wine with plant proteins: effects of fining on proanthocyanidins and aroma components. Eur. Food Res. Technol. 2014, 238, 265–274. [Google Scholar] [CrossRef]
- Voilley, A.; Lamer, C.; Dubois, P.; Feuillat, M. Influence of macromolecules and treatments on the behavior of aroma compounds in a model wine. J. Agric. Food Chem. 1990, 38, 248–251. [Google Scholar] [CrossRef]
- Dumitriu, G.-D.; López De Lerma, N.; Luchian, C.E.; Cotea, V.V.; Peinado, R.A. Study of the potential use of mesoporous nanomaterials as fining agent to prevent protein haze in white wines and its impact in major volatile aroma compounds and polyols. Food Chem. 2018, 240, 751–758. [Google Scholar] [CrossRef]
- Vincenzi, S.; Panighel, A.; Gazzola, D.; Flamini, R.; Curioni, A. Study of combined effect of proteins and bentonite fining on the wine aroma loss. J. Agric. Food Chem. 2015, 63, 2314–2320. [Google Scholar] [CrossRef] [PubMed]
- Dufour, C.; Bayonove, C.L. Interactions between wine polyphenols and aroma substances. An insight at the molecular level. J. Agric. Food Chem. 1999, 47, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Moio, L.; Ugliano, M.; Gambuti, A.; Genovese, A.; Piombino, P. Influence of clarification treatment on concentrations of selected free varietal aroma compounds and glycoconjugated in Falalnghina (Vitis vinifera, L.) must and wine. Am. J. Enol. Vitic. 2004, 46, 69–78. [Google Scholar]
- Sims, C.A.; Eastridge, J.S.; Bates, R.P. Changes in phenols, color, and sensory characteristics of muscadine wines by pre- and postfermentation additions of PVPP, casein, and gelatin. Am. J. Enol. Vitic. 1995, 46, 155–158. [Google Scholar]
- Lisanti, M.T.; Gambuti, A.; Genovese, A.; Piombino, P.; Moio, L. Treatment by fining agents of red wine affected by phenolic off-odour. Eur. Food Res. Technol. 2017, 243, 501–510. [Google Scholar] [CrossRef]
- Armada, L.; Falqué, E. Repercussion of the clarification treatment agents before the alcoholic fermentation on volatile composition of white wines. Eur. Food Res. Technol. 2007, 225, 553–558. [Google Scholar] [CrossRef]
- Lambri, M.; Dordoni, R.; Silva, A.; Faveri, D.M. Effect of bentonite fining on odor-active compounds in two different white wine styles. Am. J. Enol. Vitic. 2010, 61, 225–233. [Google Scholar]
- Lubbers, S.; Voilley, A.; Charpentier, C.; Feuilla, M. Mise en évidence d’interactions entre le macromolecules et les aromes du vin. Rev. Fr. Oenol. 1993, 144, 12–18. [Google Scholar]
- Lubbers, S.; Charpentier, C.; Feuillat, M.; Voilley, A. Influence of yeasts cell walls on the behavior of aroma compounds in a model wine. Am. J. Enol. Vitic. 1994, 45, 29–33. [Google Scholar]
- Geneix, C.; Lafon-Lafourcade, S.; Ribereau-Gayon, P. Effets des acides gras sur la viabilite des populations de Saccharomyces cerevisiae. CR Acad. Sci. 1983, 943–947. [Google Scholar]
- Castillo-Sánchez, J.J.; Mejuto, J.C.; Garrido, J.; García-Falcón, S. Influence of wine-making protocol and fining agents on the evolution of the anthocyanin content, colour and general organoleptic quality of Vinhão wines. Food Chem. 2006, 97, 130–136. [Google Scholar] [CrossRef]
- Armada, L.; Fernández, E.; Falqué, E. Influence of several enzymatic treatments on aromatic composition of white wines. LWT-Food Sci. Technol. 2010, 43, 1517–1525. [Google Scholar] [CrossRef]
- Salazar, F.N.; Marangon, M.; Labbé, M.; Lira, E.; Rodríguez-bencomo, J.J.; López, F. Comparative study of sodium bentonite and sodium-activated bentonite fining during white wine fermentation: Its effect on protein content, protein stability, lees volume, and volatile compounds. Eur. Food Res. Technol. 2017, 243, 2043–2054. [Google Scholar] [CrossRef]
- Kechagia, D.; Paraskevopoulos, Y.; Symeou, E.; Galiotou-Panayotou, M.; Kotseridis, Y. Influence of prefermentative treatments to the major volatile compounds of Assyrtiko wines. J. Agric. Food Chem. 2008, 56, 4555–4563. [Google Scholar] [CrossRef] [PubMed]
- Darriet, P.; Lavigne-Cruège, V.; Tominaga, T. A Paradox: The Volatile Sulphur Compounds Responsible for Both Defects and Qualities in Wines; Vigne et Vin Publications International: Bourdeaux, France, 1999; pp. 127–133. [Google Scholar]
- Cacho, J. El aroma de los vinos: retos y soluciones al análisis sensorial. ACE Enologia 2006, 66, 1–2. [Google Scholar]
- Selli, S.; Canbas, A.; Cabaroglu, T.; Erten, H.; Günata, Z. Aroma components of cv. Muscat of Bornova wines and influence of skin contact treatment. Food Chem. 2006, 94, 319–326. [Google Scholar] [CrossRef]
- Torres, C.; Schumacher, R.; Alañón, M.; Pérez-Coello, M.; Díaz-Maroto, M. Freeze-dried grape skins by-products to enhance the quality of white wines from neutral grape varieties. FRIN 2015, 69, 97–105. [Google Scholar] [CrossRef]
- Muñoz-González, C.; Rodríguez-Bencomo, J.J.; Martín-Álvarez, P.J.; Moreno-Arribas, M.V.; Pozo-Bayón, M.A. Recovery of aromatic aglycones from grape pomace winemaking by-products by using liquid-liquid and pressurized-liquid extraction. Food Anal. Method 2014, 7, 47–57. [Google Scholar] [CrossRef]
- Nieto-Rojo, R.; Ancín-Azpilicueta, C.; Garrido, J.J. Sorption of 4-ethylguaiacol and 4-ethylphenol on yeast cell walls, using a synthetic wine. Food Chem. 2014, 152, 399–406. [Google Scholar] [CrossRef]
- Milheiro, J.; Filipe-Ribeiro, L.; Cosme, F.; Nunes, F.M. A simple, cheap and reliable method for control of 4-ethylphenol and 4-ethylguaiacol in red wines. Screening of fining agents for reducing volatile phenols levels in red wines. J. Chromatogr. B 2017, 1041–1042. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.J.; Bakker, J. Wine Flavour Chemistry; Blackwell Publishing: Oxford, UK, 2004. [Google Scholar]
- Sanborn, M. The impact of fining on the chemical and sensory properties of Washington state Chardonnay and Gewürztraminer wines. Am. J. Enol. Vitic. 2010, 61, 31–41. [Google Scholar]
- Parish, K.J.; Herbst-Johnstone, M.; Bouda, F.; Klaere, S.; Fedrizzi, B. Pre-fermentation fining effects on the aroma chemistry of Marlborough Sauvignon blanc press fractions. Food Chem. 2016, 208, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Pedroza, M.A.; Carmona, M.; Pardo, F.; Salinas, M.R.; Zalacain, A. Waste grape skins thermal dehydration: potential release of colour, phenolic and aroma compounds into wine. CYTA J. Food 2012, 3, 225–234. [Google Scholar] [CrossRef]
- Noguerol-Pato, R.; González-Barreiro, C.; Cancho-Grande, B.; Santiago, J.L.; Martínez, M.C.; Simal-Gándara, J. Aroma potential of Brancellao grapes from different cluster positions. Food Chem. 2012, 132, 112–124. [Google Scholar] [CrossRef] [PubMed]
- González-Centeno, M.R.; Rosselló, C.; Simal, S.; Garau, M.C.; López, F.; Femenia, A. Physico-chemical properties of cell wall materials obtained from ten grape varieties and their byproducts: Grape pomaces and stems. LWT-Food Sci. Technol. 2010, 43, 1580–1586. [Google Scholar]
- Dwyer, K.; Hosseinian, F.; Rod, M. The market potential of grape waste alternatives. J. Food Res. 2014, 3, 91–106. [Google Scholar] [CrossRef]
- Pérez-Prieto, L.J.; López-Roca, J.M.; Martínez-Cutillas, A.; Pardo Mínguez, F.; Gómez-Plaza, E. Maturing wines in oak barrels. Effects of origin, volume, and age of the barrel on the wine volatile composition. J. Agric. Food Chem. 2002, 50, 3272–3276. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Ancín-Azpilicueta, C. Review of quality factors on wine ageing in oak barrels. Trends Food Sci. Technol. 2006, 17, 438–447. [Google Scholar] [CrossRef]
- Zea, L.; Moyano, L.; Medina, M. Changes in aroma profile of sherry wines during the oxidative ageing. Int. J. Food Sci. Technol. 2010, 45, 2425–2432. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Handbook of Enology, Volume 2: The Chemistry of Wine Stabilization and Treatments; John Wiley & Sons: Chichester, UK, 2006; Volume 2. [Google Scholar]
- Chatonnet, P.; Boidron, J.N.; Pons, M. Élevages des vins rouges en fûts de chéne: Évolution de certains composés volatils et de leur impact aromatique. Sci. Aliments 1990, 10, 565–587. [Google Scholar]
- Pérez-Coello, M.S.; Sanz, J.; Cabezudo, M.D. Determination of volatile compounds in hydro alcoholic extracts of French and American oak wood. Am. J. Enol. Vitic. 1999, 50, 162–165. [Google Scholar]
- Sauvageot, F.; Feuillat, F. The influence of oak wood (Quercus robur L., Q. petraea Liebl.) on the flavor of Burgundy Pinot noir. An examination of variation among individual trees. Am. J. Enol. Vitic. 1999, 50, 447–455. [Google Scholar]
- Vincenzi, S.; Dinnella, C.; Recchia, A.; Monteleone, E.; Gazzola, D.; Pasini, G.; Curioni, A. Grape seed proteins: A new fining agent for astringency reduction in red wine. Aust. J. Grape Wine Res. 2013, 19, 153–160. [Google Scholar] [CrossRef]
- Gómez-Plaza, E.; Mestre-Ortuño, L.; Ruiz-García, Y.; Fernández-Fernández, J.I.; López-Roca, J.M. Effect of benzothiadiazole and methyl jasmonate on the volatie compound composition of Vitis vinifera L. Monastrell grapes and wines. Am. J. Enol. Vitic. 2012, 63, 394–401. [Google Scholar] [CrossRef]

| RI | Control | PGP | Yeast Autolysate | Plant Protein | Egg Albumen | Caseinate | Bentonite | Gelatin | |
|---|---|---|---|---|---|---|---|---|---|
| 2-Methyl-propanol | 1074 | 154.60 a | 193.67 a | 181.33 a | 192.47 a | 200.00 a | 202.19 a | 207.29 a | 158.00 a |
| Butanol | 1117 | 11.50 b | 10.40 b | 11.40 b | 6.98 ab | 11.26 b | 10.09 b | 3.38 a | 10.00 b |
| 3-Methyl-butanol | 1184 | 3599.57 e | 3139.07 ab | 3.067.63 a | 3275.66 abc | 3336.55 bc | 3540.83 de | 3323.87 bcd | 3348.80 cd |
| 4-Methy-pentanol | 1281 | 2.00 a | 2.87 a | 1.95 a | 1.36 a | 2.25 a | 1.84 a | 2.20 a | 1.28 a |
| 3-Methyl-1-pentanol | 1294 | 17.80 a | 21.35 a | 21.25 a | 22.86 a | 21.87 a | 17.85 a | 23.80 a | 15.66 a |
| Hexanol | 1318 | 169.64 a | 147.37 a | 146.38 a | 153.72 a | 155.85 a | 160.93 a | 159.68 a | 158.70 a |
| cis-3-Hexenol | 1340 | 1.66 b | 3.14 c | 2.69 bc | 2.81 bc | 3.39 c | 3.05 c | 3.42 c | 0.12 a |
| 2-Ethyl-hexanol | 1448 | 15.88 abc | 24.09 d | 12.95 ab | 14.17 abc | 16.47 bc | 16.78 abc | 18.94 cd | 11.15 a |
| 2,3-Butanediol | 1488 | 37.22 a | 35.45 a | 31.26 a | 33.25 a | 35.85 a | 40.07 a | 36.43 a | 28.50 a |
| 4-Methyl-guaiacol | 1573 | 28.47 bc | 12.33 a | 17.89 ab | 16.11 a | 17.06 ab | 20.98 abc | 21.78 abc | 30.75 c |
| 3-Methyl-thiopropanol | 1650 | 21.09 d | 15.33 b | 12.56 a | 15.41 b | 15.01 b | 18.12 c | 18.59 c | 17.49 c |
| Guayacol | 1786 | 10.92 b | 10.40 b | 7.20 a | 9.52 ab | 10.35 b | 10.79 b | 9.67 ab | 10.31 b |
| 2-Phenyl-etanol | 1837 | 2369.95 a | 2243.81 a | 1459.02 a | 2344.40 a | 2356.94 a | 2460.00 a | 2293.40 a. | 1622.73 a |
| 4-Ethyl-guaiacol | 1949 | 24.39 b | 19.95 ab | 13.67 a | 21.36 b | 21.50 b | 24.03 b | 22.08 b | 23.54 b |
| 4-Propyl-guaiacol | 2027 | 7.44 a | 6.30 a | 7.92 a | 7.77 a | 9.04 a | 9.00 a | 10.30 a | 9.46 a |
| Eugenol | 2083 | 14.00 b | 21.67 c | 9.90 a | 13.29 ab | 14.20 b | 14.65 b | 13.31 ab | 15.56 b |
| 4-Ethyl-phenol | 2095 | 88.80 ab | 73.35 ab | 59.59 a | 85.66 ab | 89.23 ab | 88.82 ab | 74.53 ab | 99.66 b |
| RI | Control | PGP | Yeast Autolysate | Plant Protein | Egg Albumen | Caseinate | Bentonite | Gelatin | |
|---|---|---|---|---|---|---|---|---|---|
| Ethyl-butanoate | 1003 | 63.50 b | 45.48 ab | 42.69 ab | 54.06 ab | 52.72 ab | 46.82 ab | 53.81 ab | 36.61 a |
| Ethyl-2-methyl-butanoate | 1017 | 11.25 b | 8.11 b | 8.35 b | 11.29 b | 7.73 b | 7.56 a | 8.99 b | 4.72 a |
| Ethyl-3-methyl-butanoate | 1033 | 11.72 a | 10.93 a | 10.30 a | 12.68 b | 11.25 a | 9.88 a | 11.47 a | 7.96 a |
| 3-Methyl-butyl-acetate | 1094 | 295.14 b | 168.75 a | 214.37 ab | 258.98 ab | 238.23 ab | 235.89 ab | 269.24 ab | 171.76 a |
| 2-Methyl-butyl-acetate | 1109 | 4.21 b | 2.59 a | 3.01 ab | 8.03 b | 3.78 ab | 1.66 a | 10.29 b | 2.67 a |
| Ethyl-hexanoate | 1201 | 466.87 b | 378.33 ab | 377.00 ab | 418.86 ab | 434.00 b | 417.24 ab | 461.74 b | 179.87 a |
| Ethyl-lactate | 1303 | 242.05 b | 211.21 ab | 205.60 ab | 216.25 ab | 222.98 ab | 252.18 b | 214.91 ab | 145.99 a |
| Ethyl-octanoate | 1400 | 679.28 c | 540.36 a | 578.63 ab | 674.58 c | 645.97 bc | 641.29 bc | 607.41 abc | 667.95 c |
| Ethyl-nonanoate | 1494 | 55.15 abc | 54.65 abc | 49.29 a | 55.33 abc | 59.01 bc | 60.73 c | 52.58 ab | 55.66 abc |
| 3-Methyl-butyl-methoxy-acetate | 1520 | 32.12 abc | 34.71 bc | 33.24 abc | 31.08 ab | 33.72 c | 29.78 a | 35.29 bc | 34.66 bc |
| Ethyl-furan-2- carboxylate | 1561 | 3.27 b | 4.97 bc | 0.0 a | 0.0 a | 5.12 c | 3.33 b | 4.96 bc | 4.79 bc |
| Ethyl-decanoate | 1598 | 433.79 d | 160.64 a | 302.23 b | 371.69 c | 344.42 c | 438.01 d | 379.00 c | 374.29 c |
| 3-Methyl-butyl-octanoate | 1616 | 5.61 a | 21.39 cd | 24.87 de | 19.47 c | 25.78 de | 11.69 b | 8.00 ab | 27.61 e |
| Diethyl-succinate | 1623 | 2090.34 cde | 2025.51 bc | 1961.99 b | 2052.23 cd | 2068.65 cde | 2144.80 e | 1697.84 a | 2131.45 de |
| Methyl-salicilate | 1697 | 20.07 abc | 14.84 a | 15.51 ab | 19.50 abc | 21.60 abc | 34.64 d | 22.85 bc | 24..05 c |
| Ethyl-benzyl-acetate | 1719 | 13.78 ab | 12.58 a | 13.69 ab | 13.73 ab | 13.80 ab | 14.40 ab | 12.73 a | 15.30 b |
| 2-Phenyl-ethyl-acetate | 1747 | 91.01 bc | 70.10 ab | 84.41 a | 90.86 bc | 93.80 c | 99.15 c | 89.68 bc | 97.87 bc |
| Ethyl-hexadecanoate | 2360 | 146.47 d | 115.01 c | 58.82 a | 89.23 bc | 73.52 ab | 113.88 c | 85.57 bc | 67.83 a |
| Ethyl-hydrogen-succinate | 2440 | 131.12 c | 89.56 b | 81.99 ab | 61.14 a | 62.95 a | 82.81 ab | 115.84 c | 71.53 ab |
| RI | Control | PGP | Yeast Autolysate | Plant Protein | Egg Albumen | Caseinate | Bentonite | Gelatin | |
|---|---|---|---|---|---|---|---|---|---|
| (+) Limonene | 1345 | 3.40 c | 0.88 a | 2.15 b | 0.94 a | 0.34 a | 0.45 a | 1.17 ab | 3.30 c |
| β Ionone | 1470 | 76.97 b | 409.97 cd | 100.33 bcd | 48.12 a | 117.00 b | 82.83 b | 82.76 b | 90.66 bc |
| Linalol | 1504 | 24.21 bc | 19.06 a | 25.97 c | 23.05 ab | 23.10 ab | 22.37 ab | 22.99 ab | 23.02 ab |
| α-Terpineol | 1595 | 21.59 f | 15.13 def | 3.76 a | 9.24 abc | 6.50 ab | 20.83 ef | 17.83 ef | 12.98 ced |
| β-Citronellol | 1714 | 16.72 cd | 10.44 a | 14.26 b | 15.95 bc | 16.65 cd | 16.67 cd | 15.98 bc | 18.09 d |
| β-Damascenone | 1754 | 15.73 bc | 10.19 a | 18.91 c | 13.98 abc | 18.77 c | 12.80 ab | 15.66 bc | 15.43 bc |
| Nerolidol | 2082 | 40.10 e | 31.47d | 2.10 a | 27.31 cd | 17.66 b | 39.89 e | 24.00 c | 16.83 b |
| RI | Control | PGP | Yeast Autolysate | Plant Protein | Egg Albumen | Caseinate | Bentonite | Gelatin | |
|---|---|---|---|---|---|---|---|---|---|
| Furfural | 1404 | 13.91 b | 10.55 ab | 10.05 a | 10.24 a | 13.14 b | 12.41 ab | 12.50 ab | 11.49 ab |
| 2-Ethyl-5-methyl furan | 1444 | 4.33 abc | 7.22 c | 3.54 ab | 3.10 ab | 8.10 c | 5.55 abc | 6.98 bc | 2.20 a |
| Benzaldehyde | 1456 | 15.76 e | 4.13 b | 7.33 c | 8.50 cd | 0.00 a | 1.93 ab | 10.61 d | 0.00 a |
| 5-Methylfurfural | 1509 | 102.37 b | 102.12 b | 75.97 a | 75.05 a | 80.76 a | 72.48 a | 71.98 a | 113.64 b |
| γ-Butirolactone | 1552 | 17.77 a | 17.91 a | 18.98 a | 18.85 a | 21.48 a | 15.72 a | 19.19 a | 18.48 a |
| trans-β-Methyl γ octalactone | 1817 | 83.17 a | 82.13 a | 76.83 a | 83.94 a | 80.53 a | 84.94 a | 87.80 a | 77.60 a |
| cis- β-Methyl γ octalactone | 1881 | 126.49 c | 113.79 a | 115.46 ab | 122.11 bc | 126.61 c | 133.92 d | 124.61 c | 87.86 d |
| RI | Control | PGP | Yeast Autolysate | Plant Protein | Egg Albumen | Caseinate | Bentonite | Gelatin | |
|---|---|---|---|---|---|---|---|---|---|
| Acetic acid | 1413 | 232.09 a | 279.43 a | 242.77 a | 247.54 a | 245.17 a | 179.85 a | 206.89 a | 218.40 a |
| Butanoic acid | 1586 | 18.75 b | 18.14 ab | 16.12 a | 17.11 ab | 17.45 ab | 16.92 ab | 16.55 b | 17.37 ab |
| Hexanoic acid | 1794 | 252.48 bc | 213.95 a | 215.58 a | 229.83 ab | 229.50 abc | 268.99 c | 254.47 bc | 246.00 abc |
| Octanoic acid | 1997 | 466.67 bc | 353.50 a | 437.24 b | 474.20 cd | 474.68 cde | 507.55 de | 485.67 cde | 529.40 e |
| Nonanoic acid | 2212 | 15.07 bc | 10.66 abc | 8.25 a | 12.93 abc | 17.96 c | 14.76 bc | 15.20 bc | 19.71 c |
| Decanoic acid | 2312 | 50.95 b | 19.80 a | 54.72 b | 51.12 b | 57.39 b | 62.44 c | 56.94 b | 65.45 c |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gil-Muñoz, R.; Jiménez-Martínez, M.D.; Bautista-Ortín, A.B.; Gómez-Plaza, E. Effect of the Use of Purified Grape Pomace as a Fining Agent on the Volatile Composition of Monastrell Wines. Molecules 2019, 24, 2423. https://doi.org/10.3390/molecules24132423
Gil-Muñoz R, Jiménez-Martínez MD, Bautista-Ortín AB, Gómez-Plaza E. Effect of the Use of Purified Grape Pomace as a Fining Agent on the Volatile Composition of Monastrell Wines. Molecules. 2019; 24(13):2423. https://doi.org/10.3390/molecules24132423
Chicago/Turabian StyleGil-Muñoz, Rocio, María Dolores Jiménez-Martínez, Ana Belén Bautista-Ortín, and Encarna Gómez-Plaza. 2019. "Effect of the Use of Purified Grape Pomace as a Fining Agent on the Volatile Composition of Monastrell Wines" Molecules 24, no. 13: 2423. https://doi.org/10.3390/molecules24132423
APA StyleGil-Muñoz, R., Jiménez-Martínez, M. D., Bautista-Ortín, A. B., & Gómez-Plaza, E. (2019). Effect of the Use of Purified Grape Pomace as a Fining Agent on the Volatile Composition of Monastrell Wines. Molecules, 24(13), 2423. https://doi.org/10.3390/molecules24132423