Fucoxanthin and Polyunsaturated Fatty Acids Co-Extraction by a Green Process
Abstract
1. Introduction
2. Results and Discussion
2.1. Fx and ω3 PUFAs Contents
2.2. Solvent Selection
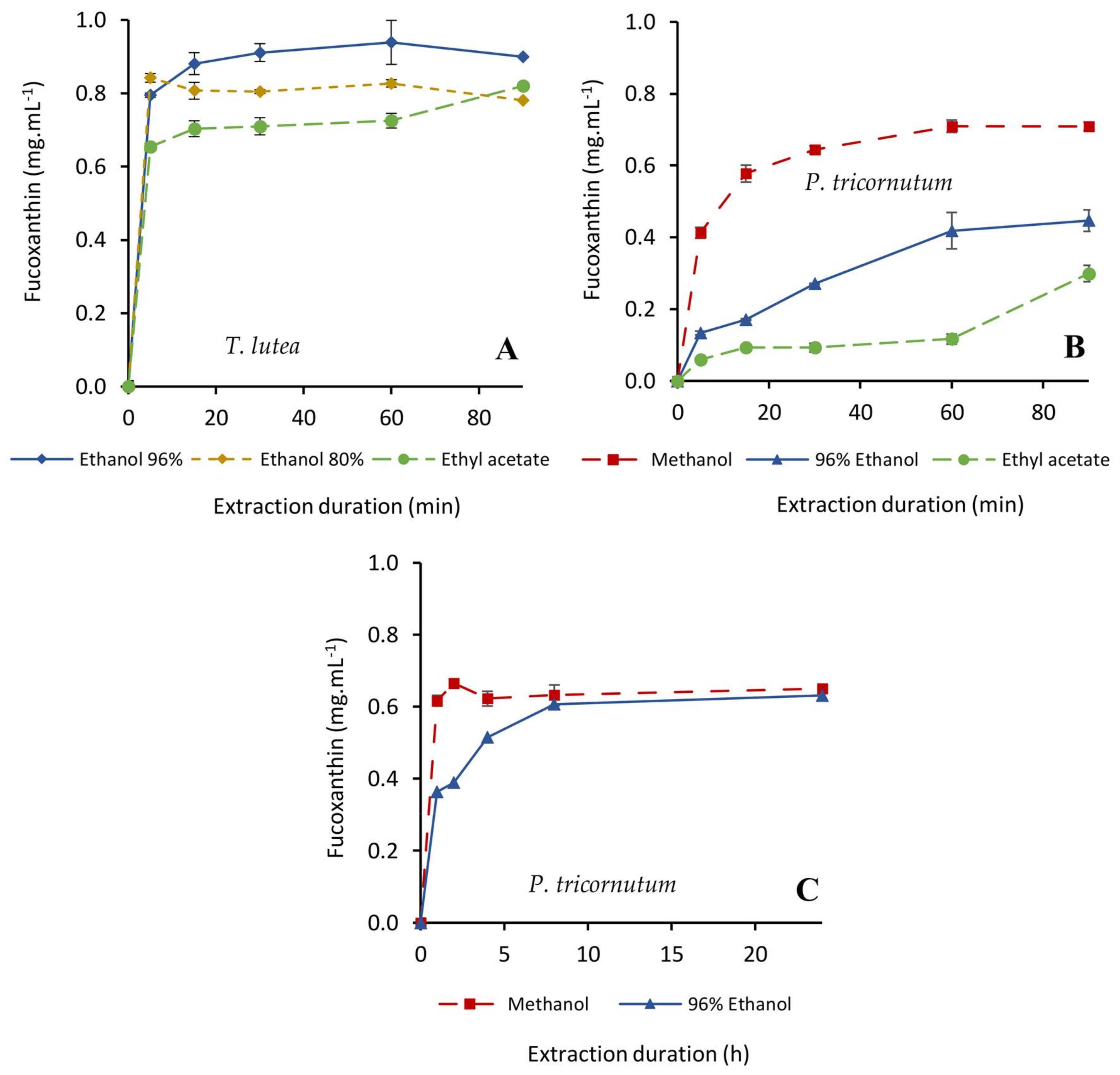
2.2.1. Fx Extraction
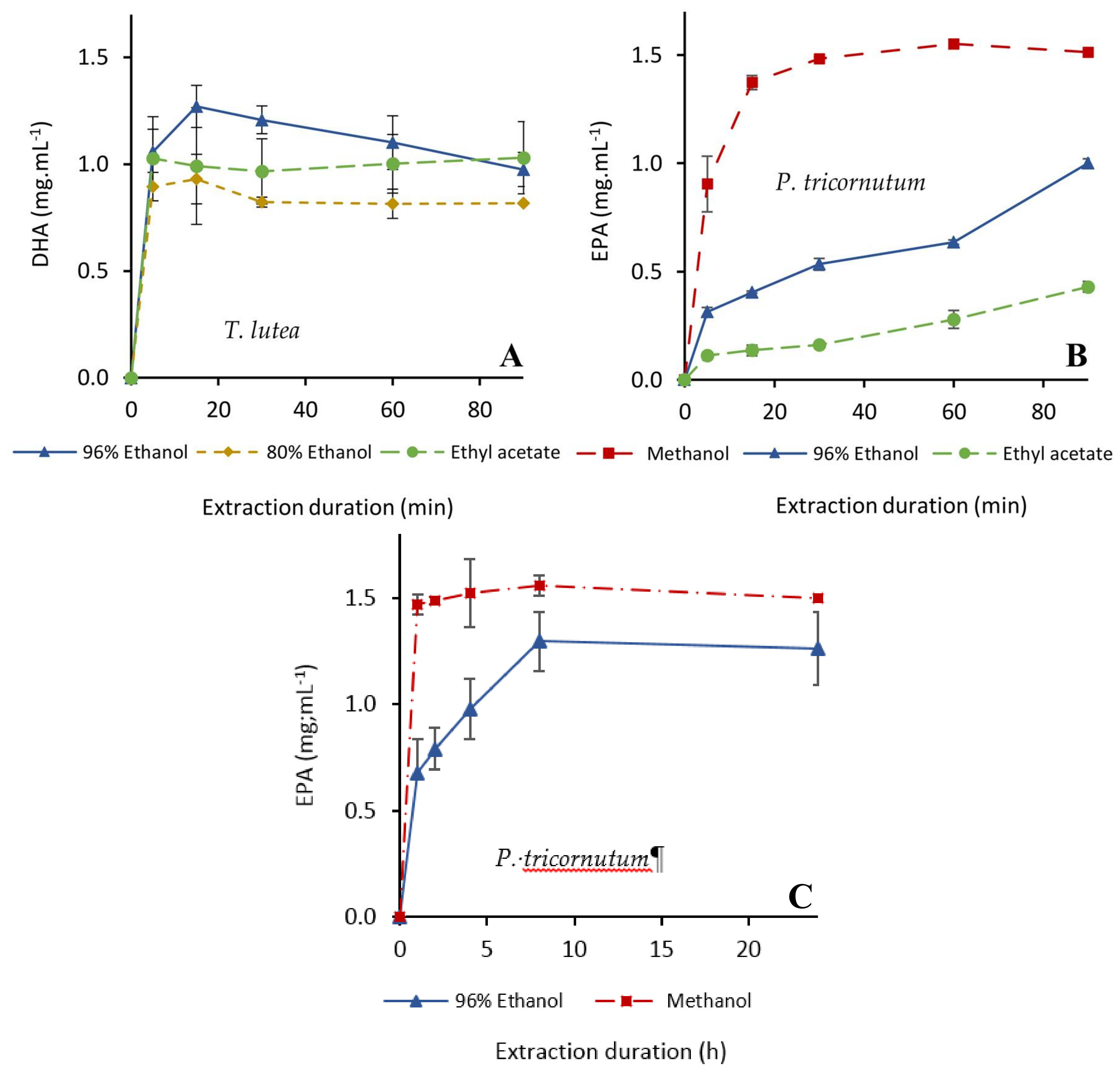
2.2.2. Extraction of PUFAs
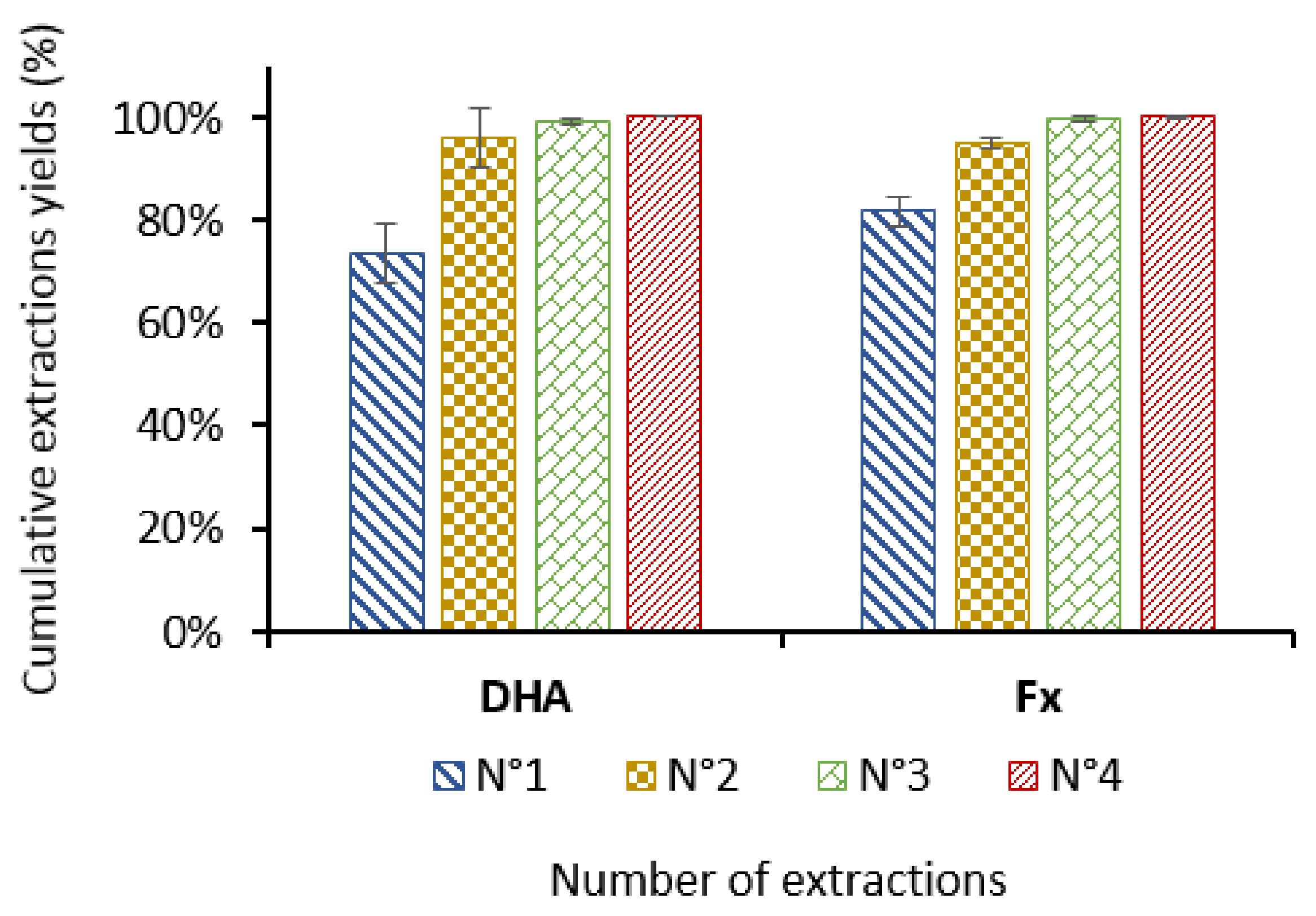
2.3. Ethanol/Biomass Ratio
3. Materials and Methods
3.1. Biological Material and Culture Conditions
3.2. Standards and Reagents
3.3. Extractions
3.4. Analyses
3.4.1. Fucoxanthin
3.4.2. PUFAs
3.4.3. Particle Size Distribution
3.5. Scanning Electron Microscopy (SEM)
3.6. Statistical Analysis
3.7. COSMO-SAC Calculations
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ALA | Linolenic acid |
| BHT | Butylated hydroxytoluene |
| DHA | Docosahexaenoic acid |
| DW | Dry weight |
| EPA | Eicosapentaenoic acid |
| FAMEs | Fatty acid methyl esters |
| Fx | Fucoxanthine |
| GC-FID | Gas chromatography with flame ionization detector |
| HPLC | High performance liquid chromatography |
| logP | octanol/water partition coefficient |
| PUFAs | Long chain polyunsaturated fatty acids |
| SDA | Stearidonic acid |
| SEM | Scanning electron microscopy. |
References
- WHO|10 Facts on Obesity. Available online: http://www.who.int/features/factfiles/obesity/en/ (accessed on 24 January 2018).
- Data and Statistics. Available online: http://www.euro.who.int/en/health-topics/noncommunicable-diseases/diabetes/data-and-statistics (accessed on 13 February 2018).
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Semaw, F.A.M.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet Lond. Engl. 2014, 384, 766–781. [Google Scholar] [CrossRef]
- Wan-Loy, C.; Siew-Moi, P. Marine Algae as a Potential Source for Anti-Obesity Agents. Mar. Drugs 2016, 14, 222. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Tao, N.; Wang, X.; Xiao, J.; Wang, M. Marine-derived bioactive compounds with anti-obesity effect: A review. J. Funct. Foods 2016, 21, 372–387. [Google Scholar] [CrossRef]
- Ríos-Hoyo, A.; Gutiérrez-Salmeán, G. New Dietary Supplements for Obesity: What We Currently Know. Curr. Obes. Rep. 2016, 5, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Abidov, M.; Ramazanov, Z.; Seifulla, R.; Grachev, S. The effects of Xanthigen in the weight management of obese premenopausal women with non-alcoholic fatty liver disease and normal liver fat. Diabetes Obes. Metab. 2010, 12, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Polus, A.; Zapala, B.; Razny, U.; Gielicz, A.; Kiec-Wilk, B.; Malczewska-Malec, M.; Sanak, M.; Childs, C.E.; Calder, P.C.; Dembinska-Kiec, A. Omega-3 fatty acid supplementation influences the whole blood transcriptome in women with obesity, associated with pro-resolving lipid mediator production. Biochim. Biophys. Acta 2016, 1861, 1746–1755. [Google Scholar] [CrossRef] [PubMed]
- Ruzickova, J.; Rossmeisl, M.; Prazak, T.; Flachs, P.; Sponarova, J.; Veck, M.; Tvrzicka, E.; Bryhn, M.; Kopecky, J. Omega-3 PUFA of marine origin limit diet-induced obesity in mice by reducing cellularity of adipose tissue. Lipids 2004, 39, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Wang, L. Omega-3 and omega-6 fatty acids: Role in body fat gain and development of obesity. N. Am. J. Med. Sci. 2015, 8, 163–171. [Google Scholar]
- Simopoulos, A.P.; DiNicolantonio, J.J. The importance of a balanced ω-6 to ω-3 ratio in the prevention and management of obesity. Open Heart 2016, 3, e000385. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Rong, Y.; Bao, L.; Nie, B.; Ren, G.; Zheng, C.; Amin, R.; Arnold, R.D.; Jeganathan, R.B.; Huggins, K.W. Suppression of adipocyte differentiation and lipid accumulation by stearidonic acid (SDA) in 3T3-L1 cells. Lipids Health Dis. 2017, 16, 181. [Google Scholar] [CrossRef] [PubMed]
- Jin, E.; Juergen, E.W.P.; Lee, H.K.; Hyun, S.M.; Chang, M. Xanthophylls in microalgae: From biosynthesis to biotechnological mass production and application. J. Microbiol. Biotechnol. 2003, 13, 165–174. [Google Scholar]
- Guedes, A.C.; Amaro, H.M.; Malcata, F.X. Microalgae as sources of carotenoids. Mar. Drugs 2011, 9, 625–644. [Google Scholar] [CrossRef] [PubMed]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Sivagnanam, S.P.; Yin, S.; Choi, J.H.; Park, Y.B.; Woo, H.C.; Chun, B.S. Biological properties of fucoxanthin in oil recovered from two brown seaweeds using supercritical CO2 extraction. Mar. Drugs 2015, 13, 3422–3442. [Google Scholar] [CrossRef] [PubMed]
- Fung, A.; Hamid, N.; Lu, J. Fucoxanthin content and antioxidant properties of Undaria pinnatifida. Food Chem. 2013, 136, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Huang, L.; Yu, J.; Xiang, S.; Wang, J.; Zhang, J.; Yan, X.; Cui, W.; He, S.; Wang, Q. Fucoxanthin, a marine carotenoid, reverses scopolamine-Induced cognitive impairments in mice and inhibits acetylcholinesterase in vitro. Mar. Drugs 2016, 14, 67. [Google Scholar] [CrossRef] [PubMed]
- Grasa-López, A.; Miliar-García, Á.; Quevedo-Corona, L.; Paniagua-Castro, N.; Escalona-Cardoso, G.; Reyes-Maldonado, E.; Jaramillo-Flores, M.-E. Undaria pinnatifida and fucoxanthin ameliorate lipogenesis and markers of both inflammation and cardiovascular dysfunction in an animal model of diet-induced obesity. Mar. Drugs 2016, 14, 148. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-T.; Chou, H.-N.; Huang, C. Dietary fucoxanthin increases metabolic rate and upregulated mRNA expressions of the PGC-1alpha network, mitochondrial biogenesis and fusion genes in white adipose tissues of mice. Mar. Drugs 2014, 12, 964–982. [Google Scholar] [CrossRef] [PubMed]
- Asai, A.; Yonekura, L.; Nagao, A. Low bioavailability of dietary epoxyxanthophylls in humans. Br. J. Nutr. 2008, 100, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Gammone, M.A.; D’Orazio, N. Anti-obesity activity of the marine carotenoid fucoxanthin. Mar. Drugs 2015, 13, 2196–2214. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Takahashi, N.; Kawada, T.; Miyashita, K. Fucoxanthin and its metabolite, fucoxanthinol, suppress adipocyte differentiation in 3T3-L1 cells. Int. J. Mol. Med. 2006, 18, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Wan, X.; Jiang, M.; Hu, C.; Hu, H.; Huang, F. Metabolic engineering of microorganisms to produce omega-3 very long-chain polyunsaturated fatty acids. Prog. Lipid Res. 2014, 56, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Jaswir, I.; Noviendri, D.; Salleh, H.M.; Miyashita, K. Fucoxanthin extractions of brown seaweeds and analysis of their lipid fraction in methanol. Food Sci. Technol. Res. 2012, 18, 251–257. [Google Scholar] [CrossRef]
- Noviendri, D.; Jaswir, I.; Salleh, H.M.; Taher, M.; Miyashita, K.; Ramli, N. Fucoxanthin extraction and fatty acid analysis of Sargassum binderi and S. duplicatum. J. Med. Plants Res. 2011, 5, 2405–2412. [Google Scholar]
- Billakanti, J.M.; Catchpole, O.J.; Fenton, T.A.; Mitchell, K.A.; MacKenzie, A.D. Enzyme-assisted extraction of fucoxanthin and lipids containing polyunsaturated fatty acids from Undaria pinnatifida using dimethyl ether and ethanol. Process Biochem. 2013, 48, 1999–2008. [Google Scholar] [CrossRef]
- Yang, Y.-H.; Du, L.; Hosokawa, M.; Miyashita, K.; Kokubun, Y.; Arai, H.; Taroda, H. Fatty acid and lipid class composition of the microalga Phaeodactylum tricornutum. J. Oleo Sci. 2017, 66, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Ryckebosch, E.; Bermúdez, S.P.C.; Termote-Verhalle, R.; Bruneel, C.; Muylaert, K.; Parra-Saldivar, R.; Foubert, I. Influence of extraction solvent system on the extractability of lipid components from the biomass of Nannochloropsis gaditana. J. Appl. Phycol. 2014, 26, 1501–1510. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Huang, R.; Li, T.; Zhou, J.; Cen, K. Biodiesel from wet microalgae: Extraction with hexane after the microwave-assisted transesterification of lipids. Bioresour. Technol. 2014, 170, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Aresta, M.; Dibenedetto, A.; Carone, M.; Colonna, T.; Fragale, C. Production of biodiesel from macroalgae by supercritical CO2 extraction and thermochemical liquefaction. Environ. Chem. Lett. 2005, 3, 136–139. [Google Scholar] [CrossRef]
- Kim, S.M.; Kang, S.-W.; Kwon, O.-N.; Chung, D.; Pan, C.-H. Fucoxanthin as a major carotenoid in Isochrysis aff. galbana: Characterization of extraction for commercial application. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 477–483. [Google Scholar] [CrossRef]
- Kim, S.M.; Jung, Y.-J.; Kwon, O.-N.; Cha, K.H.; Um, B.-H.; Chung, D.; Pan, C.-H. A potential commercial source of fucoxanthin extracted from the microalga Phaeodactylum tricornutum. Appl. Biochem. Biotechnol. 2012, 166, 1843–1855. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Wang, K.; Wan, L.; Li, A.; Hu, Q.; Zhang, C. Production, characterization, and antioxidant activity of fucoxanthin from the marine diatom Odontella aurita. Mar. Drugs 2013, 11, 2667–2681. [Google Scholar] [CrossRef] [PubMed]
- Capello, C.; Fischer, U.; Hungerbühler, K. What is a green solvent? A comprehensive framework for the environmental assessment of solvents. Green Chem. 2007, 9, 927–934. [Google Scholar] [CrossRef]
- Byrne, F.P.; Jin, S.; Paggiola, G.; Petchey, T.H.M.; Clark, J.H.; Farmer, T.J.; Hunt, A.J.; Robert McElroy, C.; Sherwood, J. Tools and techniques for solvent selection: Green solvent selection guides. Sustain. Chem. Process. 2016, 4, 7. [Google Scholar] [CrossRef]
- Pye, C.C.; Ziegler, T. An implementation of the conductor-like screening model of solvation within the Amsterdam density functional package. Theor. Chem. Acc. 1999, 101, 396–408. [Google Scholar] [CrossRef]
- Chen, W.-L.; Hsieh, C.-M.; Yang, L.; Hsu, C.-C.; Lin, S.-T. A Critical Evaluation on the Performance of COSMO-SAC Models for Vapor–Liquid and Liquid–Liquid Equilibrium Predictions Based on Different Quantum Chemical Calculations. Ind. Eng. Chem. Res. 2016, 55, 9312–9322. [Google Scholar] [CrossRef]
- Muller-Feuga, A.; Lemar, M.; Vermel, E.; Pradelles, R.; Rimbaud, L.; Valiorgue, P. Appraisal of a horizontal two-phase flow photobioreactor for industrial production of delicate microalgae species. J. Appl. Phycol. 2012, 24, 349–355. [Google Scholar] [CrossRef]
- Zhu, C.J.; Lee, Y.K. Determination of biomass dry weight of marine microalgae. J. Appl. Phycol. 1997, 9, 189–194. [Google Scholar] [CrossRef]
- Cordoba, M.; Arredondo-Vega, B.; Carreon-Palau, L. Evaluation of growth, cell size and biomass of Isochrysis aff. galbana (T-ISO) with two LED regimes. Results Biol. 2013, 4, 7–15. [Google Scholar]
- Willis, A.; Chiovitti, A.; Dugdale, T.M.; Wetherbee, R. Characterization of the extracellular matrix of Phaeodactylum tricornutum (Bacillariophyceae): Structure, composition, and adhesive characteristics. J. Phycol. 2013, 49, 937–949. [Google Scholar] [PubMed]
- Tibaldi, E.; Chini Zittelli, G.; Parisi, G.; Bruno, M.; Giorgi, G.; Tulli, F.; Venturini, S.; Tredici, M.R.; Poli, B.M. Growth performance and quality traits of European sea bass (D. labrax) fed diets including increasing levels of freeze-dried Isochrysis sp. (T-ISO) biomass as a source of protein and n-3 long chain PUFA in partial substitution of fish derivatives. Aquaculture 2015, 440, 60–68. [Google Scholar] [CrossRef]
- Huerlimann, R.; Steinig, E.J.; Loxton, H.; Zenger, K.R.; Jerry, D.R.; Heimann, K. Effects of growth phase and nitrogen starvation on expression of fatty acid desaturases and fatty acid composition of Isochrysis aff. galbana (TISO). Gene 2014, 545, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Ryckebosch, E.; Bruneel, C.; Termote-Verhalle, R.; Goiris, K.; Muylaert, K.; Foubert, I. Nutritional evaluation of microalgae oils rich in omega-3 long chain polyunsaturated fatty acids as an alternative for fish oil. Food Chem. 2014, 160, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Patil, V.; Källqvist, T.; Olsen, E.; Vogt, G.; Gislerød, H.R. Fatty acid composition of 12 microalgae for possible use in aquaculture feed. Aquac. Int. 2007, 15, 1–9. [Google Scholar] [CrossRef]
- Qiao, H.; Cong, C.; Sun, C.; Li, B.; Wang, J.; Zhang, L. Effect of culture conditions on growth, fatty acid composition and DHA/EPA ratio of Phaeodactylum tricornutum. Aquaculture 2016, 452, 311–317. [Google Scholar] [CrossRef]
- Mulders, K.J.M.; Lamers, P.P.; Martens, D.E.; Wijffels, R.H. Phototrophic pigment production with microalgae: Biological constraints and opportunities. J. Phycol. 2014, 50, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-C. Solvent extraction of fucoxanthin from Phaeodactylum tricornutum. Sep. Sci. Technol. 2014, 49, 410–415. [Google Scholar] [CrossRef]
- Shang, Y.F.; Kim, S.M.; Lee, W.J.; Um, B.-H. Pressurized liquid method for fucoxanthin extraction from Eisenia bicyclis (Kjellman) Setchell. J. Biosci. Bioeng. 2011, 111, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Susanto, E.; Fahmi, A.S.; Agustini, T.W.; Rosyadi, S.; Wardani, A.D. Effects of different heat processing on fucoxanthin, antioxidant activity and colour of Indonesian brown seaweeds. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Surakarta, Indonesia, 10–12 August 2017; Volume 55, p. 012063. [Google Scholar]
- Muller-Feuga, A. Photosynthetic Reactor for Cultivating Microorganisms, and Method for Cultivating Microorganisms. World Patent WO2010109108 A1, 2010. [Google Scholar]


| Compounds | T. lutea | P. tricornutum |
|---|---|---|
| Linoleic acid | 17.8 ± 0.1 | 0.4 ± 0.1 |
| Stearidonic acid | 46.2 ± 1.4 | 0.4 ± 0.1 |
| Eicosapentaenoic acid | 1.8 ± 0.1 | 29.1 ± 1.3 |
| Docosahexaenoic acid | 23.0 ± 0.4 | 1.2 ± 0.1 |
| Total PUFAs ω3 | 88.8 ± 1.8 | 31.1 ± 1.5 |
| Fx | 18.8 ± 0.5 | 13.3 ± 0.3 |
| Species | Molecules | Extractions Parameters | Durations to Achieve Different Target Extraction Yields (min) 1 | |||
|---|---|---|---|---|---|---|
| Solvent | Ratio Solvent/Biomass (v/w) | 70% | 90% | Maximum 2 | ||
| T. lutea | Fx | 96% ethanol | 20:1 | <5 | 15–30 | 60 (100%) |
| 10:1 | <5 | 60 | 60 (90%) | |||
| 5:1 | 5–15 | 60–90 | 90 (91%) | |||
| 80% ethanol | 20:1 | <5 | <5 | 5 (90%) | ||
| 100% ethyl acetate | 20:1 | 5–15 | - | 90 (87%) | ||
| Docosahexaenoic acid | 96% ethanol | 20:1 | <5 | 5–15 | 15 (100%) | |
| 10:1 | 15–30 | - | 60 (83%) | |||
| 5:1 | 30–60 | - | 60 (72%) | |||
| 80% ethanol | 20:1 | 5 | - | 15 (73%) | ||
| 100% ethyl acetate | 20:1 | <5 | - | 5 (81%) | ||
| P. tricornutum | Fx | 100% methanol | 20:1 | 5–15 | 15–30 | 60 (100%) |
| 96% ethanol | 20:1 | 120–240 | 240–480 | 1440 (95%) | ||
| 10:1 | 240–480 | 480–1440 | 1440 (97%) | |||
| 5:1 | 240–480 | 480–1440 | 1440 (91%) | |||
| Eicosapentaenoic acid | 100% methanol | 20:1 | 5–15 | 15–30 | 60 (100%) | |
| 96% ethanol | 20:1 | 240–480 | - | 480 (89%) | ||
| 10:1 | 480–1440 | - | 1440 (77%) | |||
| 5:1 | - | - | 1440 (68%) | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delbrut, A.; Albina, P.; Lapierre, T.; Pradelles, R.; Dubreucq, E. Fucoxanthin and Polyunsaturated Fatty Acids Co-Extraction by a Green Process. Molecules 2018, 23, 874. https://doi.org/10.3390/molecules23040874
Delbrut A, Albina P, Lapierre T, Pradelles R, Dubreucq E. Fucoxanthin and Polyunsaturated Fatty Acids Co-Extraction by a Green Process. Molecules. 2018; 23(4):874. https://doi.org/10.3390/molecules23040874
Chicago/Turabian StyleDelbrut, Antoine, Pierre Albina, Théo Lapierre, Rémi Pradelles, and Eric Dubreucq. 2018. "Fucoxanthin and Polyunsaturated Fatty Acids Co-Extraction by a Green Process" Molecules 23, no. 4: 874. https://doi.org/10.3390/molecules23040874
APA StyleDelbrut, A., Albina, P., Lapierre, T., Pradelles, R., & Dubreucq, E. (2018). Fucoxanthin and Polyunsaturated Fatty Acids Co-Extraction by a Green Process. Molecules, 23(4), 874. https://doi.org/10.3390/molecules23040874





