Isothiocyanates: An Overview of Their Antimicrobial Activity against Human Infections
Abstract
1. Introduction
2. Isothiocyanates in Nature
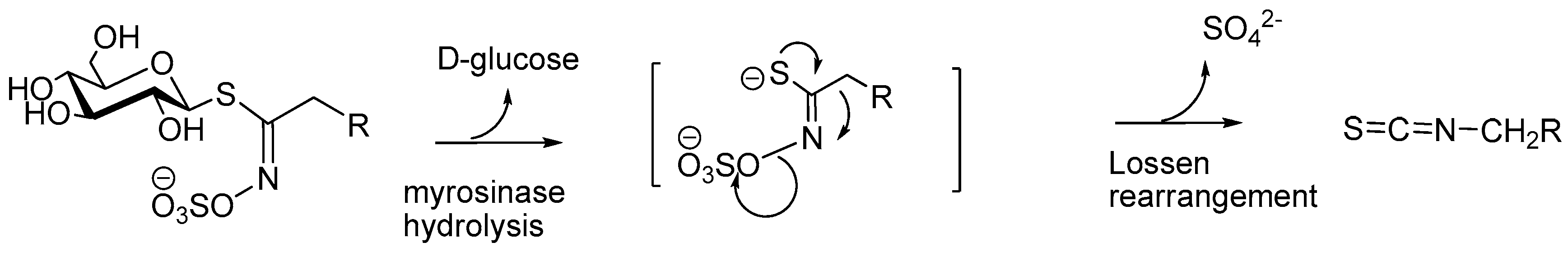
3. ITCs Metabolism
4. Isothiocyanates Antimicrobial Properties against Human Pathogens
4.1. Helicobacter Pylori
4.2. Clostridium Difficile and Clostridium Perfringens
4.3. Campylobacter Jejuni
4.4. Salmonella Enterica
4.5. Escherichia coli
4.6. Pseudomonas Aeruginosa
4.7. Staphylococcus Aureus and Methicillin-Resistant S. aureus (MRSA)
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Petrovska, B.B. Historical review of medicinal plants’ usage. Pharmacogn. Rev. 2012, 6, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Pezzuto, J.M. Natural products as a vital source for the discovery of cancer chemotherapeutic and chemopreventive agents. Med. Princ. Pract. 2016, 25, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Sarkar, B.; Cholia, R.P.; Gautam, N.; Dhiman, M.; Mantha, A.K. Ape1/ref-1 as an emerging therapeutic target for various human diseases: Phytochemical modulation of its functions. Exp. Mol. Med. 2014, 46, e106. [Google Scholar] [CrossRef] [PubMed]
- Dillard, C.J.; German, J.B. Phytochemicals: Nutraceuticals and human health. J. Sci. Food Agric. 2000, 80, 1744–1756. [Google Scholar] [CrossRef]
- Hammer, K.A.; Carson, C.F.; Riley, T.V. Antimicrobial activity of essential oils and other plant extracts. J. Appl. Microbiol. 1999, 86, 985–990. [Google Scholar] [CrossRef] [PubMed]
- Arora, D.S.; Kaur, J. Antimicrobial activity of spices. Int. J. Antimicrob. Agents 1999, 12, 257–262. [Google Scholar] [CrossRef]
- Saladino, F.; Bordin, K.; Luciano, F.B.; Franzón, M.F.; Mañes, J.; Meca, G. Antimicrobial activity of the glucosinolates. In Glucosinolates, 1st ed.; Ramawat, K.G., Mérillon, J.-M., Eds.; Reference Series in Phytochemistry; Springer: Cham, Germany, 2017; pp. 249–274. [Google Scholar]
- Tiwari, B.K.; Valdramidis, V.P.; O’Donnell, C.P.; Muthukumarappan, K.; Bourke, P.; Cullen, P.J. Application of natural antimicrobials for food preservation. J. Agric. Food Chem. 2009, 57, 5987–6000. [Google Scholar] [CrossRef] [PubMed]
- Romero, C.D.; Chopin, S.F.; Buck, G.; Martinez, E.; Garcia, M.; Bixby, L. Antibacterial properties of common herbal remedies of the southwest. J. Ethnopharmacol. 2005, 99, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Guil-Guerrero, J.L.; Ramos, L.; Moreno, C.; Zuniga-Paredes, J.C.; Carlosama-Yepez, M.; Ruales, P. Antimicrobial activity of plant-food by-products: A review focusing on the tropics. Livest. Sci. 2016, 189, 32–49. [Google Scholar] [CrossRef]
- Dufour, V.; Stahl, M.; Baysse, C. The antibacterial properties of isothiocyanates. Microbiol. Soc. 2015, 161, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Brabban, A.D.; Edwards, C. The effects of glucosinolates and their hydrolysis products on microbial growth. J. Appl. Bacteriol. 1995, 79, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Blažević, I.; Montaut, S.; Burčul, F.; Rollin, P. Glucosinolates: Novel sources and biological potential. In Glucosinolates, 1st ed.; Ramawat, K.G., Mérillon, J.-M., Eds.; Reference Series in Phytochemistry; Springer: Cham, Germany, 2017; pp. 3–60. [Google Scholar]
- Ishida, M.; Hara, M.; Fukino, N.; Kakizaki, T.; Morimitsu, Y. Glucosinolate metabolism, functionality and breeding for the improvement of brassicaceae vegetables. Breed. Sci. 2014, 64, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Haristoy, X.; Angioi-Duprez, K.; Duprez, A.; Lozniewski, A. Efficacy of sulforaphane in eradicating Helicobacter pylori in human gastric xenografts implanted in nude mice. Antimicrob. Agents Chemother. 2003, 47, 3982–3984. [Google Scholar] [CrossRef] [PubMed]
- Yanaka, A.; Zhang, S.H.; Tauchi, M.; Suzuki, H.; Shibahara, T.; Matsui, H.; Nakahara, A.; Tanaka, N. Daily intake of sulforaphane-rich broccoli sprouts prevents progression of high salt diet-induced gastric atrophy in H. pylori-infected C57/BL6 mice in vivo. Gastroenterology 2003, 124, A5. [Google Scholar] [CrossRef]
- Jin, Y.; Wang, M.; Rosen, R.T.; Ho, C.T. Thermal degradation of sulforaphane in aqueous solution. J. Agric. Chem. 1999, 47, 3121–3123. [Google Scholar] [CrossRef]
- Fahey, J.W.; Zalcmann, A.T.; Talalay, P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 2001, 56, 5–51. [Google Scholar] [CrossRef]
- Agerbirk, N.; Olsen, C.E. Glucosinolate structures in evolution. Phytochemistry 2012, 77, 16–45. [Google Scholar] [CrossRef] [PubMed]
- Iori, R.; Rollin, P.; Streicher, H.; Thiem, J.; Palmieri, S. The myrosinase-glucosinolate interaction mechanism studied using some synthetic competitive inhibitors. FEBS Lett. 1996, 385, 87–90. [Google Scholar] [CrossRef]
- Bones, A.M.; Rossiter, J.T. The myrosinase-glucosinolate system, its organisation and biochemistry. Physiol. Plant. 1996, 97, 194–208. [Google Scholar] [CrossRef]
- Bones, A.M.; Rossiter, J.T. The enzymic and chemically induced decomposition of glucosinolates. Phytochemistry 2006, 67, 1053–1067. [Google Scholar] [CrossRef] [PubMed]
- Traka, M.; Mithen, R. Glucosinolates, isothiocyanates and human health. Phytochem. Rev. 2009, 8, 269–282. [Google Scholar] [CrossRef]
- Sivapalan, T.; Melchini, A.; Saha, S.; Needs, P.W.; Traka, M.H.; Tapp, H.; Dainty, J.R.; Mithen, R.F. Bioavailability of glucoraphanin and sulforaphane from high-glucoraphanin broccoli. Mol. Nutr. Food Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Abd Rani, N.Z.; Husain, K.; Kumolosasi, E. Moringa genus: A review of phytochemistry and pharmacology. Front. Pharmacol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Jung, I.L. Soluble extract from Moringa oleifera leaves with a new anticancer activity. PLoS ONE 2014, 9, e95492. [Google Scholar] [CrossRef] [PubMed]
- Giacoppo, S.; Rajan, T.S.; Iori, R.; Rollin, P.; Bramanti, P.; Mazzon, E. The alpha-cyclodextrin complex of the Moringa isothiocyanate suppresses lipopolysaccharide-induced inflammation in RAW 264.7 macrophage cells through Akt and p38 inhibition. Inflamm. Res. 2017, 66, 487–503. [Google Scholar] [CrossRef] [PubMed]
- Rajan, T.S.; Giacoppo, S.; Iori, R.; De Nicola, G.R.; Grassi, G.; Pollastro, F.; Bramanti, P.; Mazzon, E. Anti-inflammatory and antioxidant effects of a combination of cannabidiol and moringin in LPS-stimulated macrophages. Fitoterapia 2016, 112, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Padla, E.P.; Solis, L.T.; Levida, R.M.; Shen, C.C.; Ragasa, C.Y. Antimicrobial Isothiocyanates from the Seeds of Moringa oleifera Lam. Z. Naturforsch. C 2012, 67, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Dzotam, J.K.; Touani, F.K.; Kuete, V. Antibacterial and antibiotic-modifying activities of three food plants (Xanthosoma mafaffa Lam., Moringa oleifera (L.) Schott and Passiflora edulis Sims) against multidrug-resistant (MDR) Gram-negative bacteria. BMC Complement. Altern. Med. 2016, 16, 9. [Google Scholar] [CrossRef] [PubMed]
- Anwar, F.; Latif, S.; Ashraf, M.; Gilani, A.H. Moringa oleifera: A food plant with multiple medicinal uses. Phytother. Res. 2007, 21, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Hashem, F.A.; Wahba, H.E. Isothiocyanates in myrosinase treated herb extract of Cleome chrysantha Decne. and their antimicrobial activities. Phytother. Res. 2000, 14, 284–287. [Google Scholar] [CrossRef]
- Muhaidat, R.; Al-Qudah, M.A.; Al-Shayeb, A.; Jacob, H.J.; Al-Jaber, H.I.; Hussein, E.; Al-Tarawneh, I.N.; Abu Orabi, S.T. Chemical profile and antibacterial activity of crude fractions and essential oils of Capparis ovata Desf. and Capparis spinosa L. (Capparaceae). Int. J. Integr. Biol. 2013, 14, 39–47. [Google Scholar]
- Barba, F.J.; Nikmaram, N.; Roohinejad, S.; Khelfa, A.; Zhu, Z.; Koubaa, M. Bioavailability of glucosinolates and their breakdown products: Impact of processing. Front. Nutr. 2016, 3, 24. [Google Scholar] [CrossRef] [PubMed]
- Bollard, M.; Stribbling, S.; Mitchell, S.; Caldwell, J. The disposition of allyl isothiocyanate in the rat and mouse. Food Chem Toxicol. 1997, 35, 933–943. [Google Scholar] [CrossRef]
- Conaway, C.C.; Jiao, D.; Kohri, T.; Liebes, L.; Chung, F.L. Disposition and pharmacokinetics of phenethyl isothiocyanate and 6-phenylhexyl isothiocyanate in f344 rats. Drug Metab. Dispos. 1999, 27, 13–20. [Google Scholar] [PubMed]
- Rouzaud, G.; Young, S.A.; Duncan, A.J. Hydrolysis of glucosinolates to isothiocyanates after ingestion of raw or microwaved cabbage by human volunteers. Cancer Epidemiol. Biomarker. Prev. 2004, 13, 125–131. [Google Scholar] [CrossRef]
- Getahun, S.M.; Chung, F.L. Conversion of glucosinolates to isothiocyanates in humans after ingestion of cooked watercress. Cancer Epidemiol. Biomark. Prev. 1999, 8, 447–451. [Google Scholar]
- Walker, J.C. Studies on disease resistance in the onion. Proc. Natl. Acad. Sci. USA 1925, 11, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Delaquis, P.J.; Mazza, G. Antimicrobial properties of isothiocyanates in food preservation. Food Technol. 1995, 49, 73–84. [Google Scholar]
- Larkin, R.P.; Griffin, T.S. Control of soilborne potato diseases using Brassica green manures. Crop Prot. 2007, 26, 1067–1077. [Google Scholar] [CrossRef]
- Fahey, J.W.; Haristoy, X.; Dolan, P.M.; Kensler, T.W.; Scholtus, I.; Stephenson, K.K.; Talalay, P.; Lozniewski, A. Sulforaphane inhibits extracellular, intracellular, and antibiotic-resistant strains of Helicobacter pylori and prevents benzo[a]pyrene-induced stomach tumors. Proc. Natl. Acad. Sci. USA 2002, 99, 7610–7615. [Google Scholar] [CrossRef] [PubMed]
- Ganin, H.; Rayo, J.; Amara, N.; Levy, N.; Krief, P.M.; Meijler, M. Sulforaphane and erucin, naturalisothiocyanates from broccoli, inhibit bacterial quorum sensing. Med. Chem. Comm. 2013, 4, 175–179. [Google Scholar] [CrossRef]
- Nowicki, D.; Rodzik, O.; Herman-Antosiewicz, A.; Szalewska-Palasz, A. Isothiocyanates as effective agents against enterohemorrhagic Escherichia coli: Insight to the mode of action. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Dias, C.; Aires, A.; Bennett, R.N.; Rosa, E.A.S.; Saavedra, M.J. First study on antimicriobial activity and synergy between isothiocyanates and antibiotics against selected gram-negative and gram-positive pathogenic bacteria from clinical and animal source. Med. Chem. 2012, 8, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.G.; Lee, H.S. Growth-inhibiting activities of phenethyl isothiocyanate and its derivatives against intestinal bacteria. J. Food Sci. 2009, 74, M467–M471. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, S.J.; Mutters, N.T.; Blessing, B.; Günther, F. Natural isothiocyanates express antimicrobial activity against developing and mature biofilms of Pseudomonas aeruginosa. Fitoterapia 2017, 119, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.; Abreu, A.C.; Ferreira, C.; Saavedra, M.J.; Simoes, L.C.; Simoes, M. Antibacterial activity and mode of action of selected glucosinolate hydrolysis products against bacterial pathogens. J. Food Sci. Technol. 2015, 52, 4737–4748. [Google Scholar] [CrossRef] [PubMed]
- Aires, A.; Mota, V.R.; Saavedra, M.J.; Rosa, E.A.S.; Bennett, R.N. The antimicrobial effects of glucosinolates and their respective enzymatic hydrolysis products on bacteria isolated from the human intestinal tract. J. Appl. Microbiol. 2009, 106, 2086–2095. [Google Scholar] [CrossRef] [PubMed]
- Dias, C.; Aires, A.; Saavedra, M.J. Antimicrobial activity of isothiocyanates from cruciferous plants against methicillin-resistant Staphylococcus aureus (MRSA). Int. J. Mol. Sci. 2014, 15, 19552–19561. [Google Scholar] [CrossRef] [PubMed]
- Sofrata, A.; Santangelo, E.M.; Azeem, M.; Borg-Karlson, A.K.; Gustafsson, A.; Pütsep, K. Benzyl isothiocyanate, a major component from the roots of Salvadora persica is highly active against Gram-negative bacteria. PLoS ONE 2011, 6, e23045. [Google Scholar] [CrossRef] [PubMed]
- Dufour, V.; Alazzam, B.; Ermel, G.; Thepaut, M.; Rossero, A.; Tresse, O.; Baysse, C. Antimicrobial activities of isothiocyanates against Campylobacter jejuni isolates. Front. Cell. Infect. Microbiol. 2012, 2, 53. [Google Scholar] [CrossRef] [PubMed]
- Dufour, V.; Stahl, M.; Rosenfeld, E.; Stintzi, A.; Baysse, C. Insights into the mode of action of benzyl isothiocyanate on Campylobacter jejuni. Appl. Environ. Microbiol. 2013, 79, 6958–6968. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.J.; Dockery, C.R.; Crosby, M.; Chavarria, K.; Patterson, B.; Giedd, M. Antibacterial activities of wasabi against Escherichia coli O157:H7 and Staphylococcus aureus. Front. Microbiol. 2016, 7, 1043. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.M.; Preston, J.F., III; Wei, C.I. Antibacterial mechanism of allyl isothiocyanate. J. Food Prot. 2000, 63, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Luciano, F.B.; Holley, R.A. Enzymatic inhibition by allyl isothiocyanate and factors affecting its antimicrobial action against Escherichia coliO157:H7. Int. J. Food Microbiol. 2009, 131, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, T.H.; Bragason, S.K.; Phipps, R.K.; Christensen, L.D.; van Gennip, M.; Alhede, M.; Skindersoe, M.; Larsen, T.O.; Hoiby, N.; Bjarnsholt, T.; et al. . Food as a source for quorum sensing inhibitors: Iberin from horseradish revealed as a quorum sensing inhibitor of Pseudomonas aeruginosa. Appl. Environ. Microbiol. 2012, 78, 2410–2421. [Google Scholar] [CrossRef] [PubMed]
- Saleh, N.M.; Mabrouk, M.I.; Salem-Bekhit, M.M.; Hafez, E.H. Challenge of Moringa peregrine Forssk as an antimicrobial agent against multi-drug-resistant Salmonella sp. Biotechnol. Biotechnol. Eq. 2017, 31, 380–386. [Google Scholar] [CrossRef]
- Pal, S.K.; Mukherjee, P.K.; Saha, K.; Pal, M.; Saha, B.P. Antimicrobial action of the leaf extract of Moringa oleifera Lam. Anc. Sci. Life 1995, 14, 197–199. [Google Scholar] [PubMed]
- Rahman, M.M.; Rahman, M.M.; Akhter, S.; Jamal, M.A.; Pandeya, D.R.; Haque, M.A.; Alam, M.F.; Rahman, A. Control of coliform bacteria detected from diarrhea associated patients by extracts of Moringa oleifera. Nepal Med. Coll. J. 2010, 12, 12–19. [Google Scholar] [PubMed]
- Rahman, M.M.; Islam Sheik, M.M.; Sharmin, S.A.; Islam, M.S.; Rahman, M.A.; Rahman, M.M.; Alam, M.F. Antibacterial activity of leaves juice and extracts of Moringa oleifera Lam. (2n = 28) against some human pathogenic bacteria. Chiang-Mai Univ. J. Nat. Sci. 2009, 8, 219–227. [Google Scholar]
- Peixoto, J.R.O.; Silva, G.C.; Costa, R.A.; Fontenelle, J.L.D.; Vieira, G.H.F.; Filho, A.A.F.; Vieira, R.H.S.D.F. In vitro antibacterial effect of aqueous and ethanolic Moringa leaf extracts. Asian Pac. J. Trop. Med. 2011, 4, 201–204. [Google Scholar] [CrossRef]
- Zaffer, M.; Ahmad, S.; Sharma, R.; Mahajan, S.; Gupta, A.; Agnihotri, R.K. Antibacterial activity of bark extracts of Moringa oleiferaLam. against some selected bacteria. Pak. J. Pharm. Sci. 2014, 27, 1857–1862. [Google Scholar] [PubMed]
- Dalukdeniya, D.A.C.K.; De Silva, K.L.S.R.; Rathnayaka, R.M.U.S.K. Antimicrobial activity of different extracts of leaves bark and roots of Moringa oleifera (Lam). Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 687–691. [Google Scholar] [CrossRef]
- Vieira, G.H.F.; Mourao, J.A.; Angelo, A.M.; Costa, R.A.; Vieira, R.H.S.D. Antibacterial effect (in vitro) of Moringa oleifera and Annona muricata against Gram positive and Gram negative bacteria. Rev. Inst. Med. Trop. Sao Paulo 2010, 52, 129–132. [Google Scholar] [CrossRef]
- Walter, A.; Samuel, W.; Peter, A.; Joseph, O. Antibacterial activity of moringa oleifera and moringa stenopetala methanol and n-hexane seed extracts on bacteria implicated in water borne diseases. Afr J Microbiol. Res. 2011, 5, 153–157. [Google Scholar]
- Galuppo, M.; Nicola, G.R.; Iori, R.; Dell’Utri, P.; Bramanti, P.; Mazzon, E. Antibacterial activity of glucomoringin bioactivated with myrosinase against two important pathogens affecting the health of long-term patients in hospitals. Molecules 2013, 18, 14340–14348. [Google Scholar] [CrossRef] [PubMed]
- Björkholm, N.; Zhukhovitsky, V.; Löfman, C.; Hultén, K.; Enroth, H.; Block, M.; Rigo, R.; Falk, P.; Engstrand, L. Helicobacter pylori entry into human gastric epithelial cells: A potential determinant of virulence, persistence, and treatment failures. Helicobacter 2000, 5, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Brenciaglia, M.I.; Fornara, A.M.; Scaltrito, M.M.; Dubini, F. Helicobacter pylori: Cultivability and antibiotic susceptibility of coccoid forms. Int. J. Antimicrob. Agents 2000, 13, 237–241. [Google Scholar] [CrossRef]
- Engstrand, L.; Graham, D.; Scheynius, A.; Genta, R.M.; El-Zaatari, F. Is the sanctuary where Helicobacter pylori avoids antibacterial treatment intracellular? Am. J. Clin. Pathol. 1997, 108, 504–509. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ohba, R.; Iijima, K. Pathogenesis and risk factors for gastric cancer after Helicobacter pylori eradication. World J.Gastrointest. Oncol. 2016, 8, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.W.; Stephenson, K.K.; Wade, K.L.; Talalay, P. Urease from Helicobacter pylori is inactivated by sulforaphane and other isothiocyanates. Biochem. Biophys. Res. Commun. 2013, 435, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ha, N.C.; Oh, S.T.; Sung, J.Y.; Cha, K.A.; Lee, M.H.; Oh, B.H. Supramolecular assembly and acid resistance of Helicobacter pylori urease. Nat. Struct. Biol. 2001, 8, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.T.; Mobley, H.L. Purification and N-terminal analysis of urease from Helicobacter pylori. Infect. Immun. 1990, 58, 992–998. [Google Scholar] [PubMed]
- Rood, J.I.; McClane, B.A.; Songer, J.G.; Titball, R.W. The Clostridia: Molecular Biology and Pathogenesis; Academic Press: London, UK, 1997. [Google Scholar]
- Rupnik, M.; Wilcox, M.H.; Gerding, D.N. Clostridium difficile infection: New developments in epidemiology and pathogenesis. Nat. Rev. Microbiol. 2009, 7, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Borriello, S.P.; Welch, A.R.; Larson, H.E.; Barclay, F.; Stringer, M.F.; Bartholomew, B.A. Enterotoxigenic Clostridium perfringens: A possible cause of antibiotic-associated diarrhoea. Lancet 1984, 1, 305–307. [Google Scholar] [CrossRef]
- Savidge, T.C.; Pan, W.H.; Newman, P.; O’Brien, M.; Anton, P.M.; Pothoulakis, C. Clostridium difficile toxin B is an inflammatory enterotoxin in human intestine. Gastroenterology 2003, 125, 413–420. [Google Scholar] [CrossRef]
- Niilo, L. Clostridium perfringens in animal disease: a review of current knowledge. Can. Vet. J. 1980, 21, 141–148. [Google Scholar] [PubMed]
- Park, H.W.; Choi, K.D.; Shin, I.S. Antimicrobial activity of isothiocyanates (ITCs) extracted from horseradish (Armoracia rusticana) root against oral microorganisms. Biocontrol. Sci. 2013, 18, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Rzewuska, K.; Korsak, D.; Maćkiw, E. Antibiotic resistance of bacteria Campylobacter sp. Przeglad Epidemiol. 2010, 64, 63–68. [Google Scholar]
- Engberg, J.; Aarestrup, F.M.; Taylor, D.E.; Gerner-Smidt, P.; Nachamkin, I. Quinolone and macrolide resistance in Campylobacter jejuni and C. coli: Resistance mechanisms and trends in human isolates. Emerg. Infect. Dis. 2001, 7, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Dingle, K.E.; Colles, F.M.; Ure, R.; Wagenaar, J.A.; Duim, B.; Bolton, F.J.; Fox, A.J.; Wareing, D.R.A.; Maiden, M.C.J. Molecular characterization of Campylobacter jejuni clones: A basis for epidemiologic investigation. Emerg. Infect. Dis. 2002, 8, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Feasey, N.A.; Dougan, G.; Kingsley, R.A.; Heyderman, R.S.; Gordon, M.A. Invasive non-typhoidal Salmonella disease: An emerging and neglected tropical disease in Africa. Lancet 2012, 379, 2489–2499. [Google Scholar] [CrossRef]
- Escobar-Paramo, P.; Clermont, O.; Blanc-Potard, A.B.; Bui, H.; Le Bouguenec, C.; Denamur, E. A specific genetic background is required for acquisition and expression of virulence factors in Escherichia coli. Mol. Biol. Evol. 2004, 21, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Nataro, J.P.; Kaper, J.B. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 1998, 11, 142–201. [Google Scholar] [PubMed]
- Pacheco, A.R.; Sperandio, V. Shiga toxin in enterohemorrhagic E.coli: Regulation and novel anti-virulence strategies. Front. Cell. Infect. Microbiol. 2012, 2, 81. [Google Scholar] [CrossRef] [PubMed]
- Lebeaux, D.; Ghigo, J.M.; Beloin, C. Biofilm-related infections: Bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiol. Mol. Biol. Rev. 2014, 78, 510–543. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M. Biofilm formation: a clinically relevant microbiological process. Clin. Infect. Dis. 2001, 33, 1387–1392. [Google Scholar] [CrossRef] [PubMed]
- Bridier, A.; Sanchez-Vizuete, P.; Guilbaud, M.; Piard, J.-C.; Naitali, M.; Briandet, R. Biofilm-associated persistence of food-borne pathogens. Food Microbiol. 2015, 45, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Velez Perez, A.L.; Schmidt-Malan, S.M.; Kohner, P.C.; Karau, M.J.; Greenwood-Quaintance, K.E.; Patel, R. In vitro activity of ceftolozane/tazobactam against clinical isolates of Pseudomonas aeruginosa in the planktonic and biofilm states. Diagn. Microbiol. Infect. Dis. 2016, 85, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Defez, C.; Fabbro-Peray, P.; Bouziges, N.; Gouby, A.; Mahamat, A.; Daurès, J.P.; Sotto, A. Risk factors for multidrug-resistant Pseudomonas aeruginosa nosocomial infection. J. Hosp. Infect. 2004, 57, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Van Delden, C.; Iglewski, B.H. Cell-to-cell signaling and Pseudomonas aeruginosa infections. Emerg. Infect. Dis. 1998, 4, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Tian, X.L. Quorum sensing and bacterial social interactions in biofilms. Sensors 2012, 12, 2519–2538. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.; McDermott, C.; Anoopkumar-Dukie, S.; McFarland, A.J.; Forbes, A.; Perkins, A.V.; Davey, A.K.; Chess-Williams, R.; Kiefel, M.J.; Arora, D.; et al. Cellular effects of pyocyanin, a secreted virulence factor of Pseudomonas aeruginosa. Toxins 2016, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Zulianello, L.; Canard, C.; Kohler, T.; Caille, D.; Lacroix, J.S.; Meda, P. Rhamnolipids are virulence factors that promote early infiltration of primary human airway epithelia by Pseudomonas aeruginosa. Infect. Immun. 2006, 74, 3134–3147. [Google Scholar] [CrossRef] [PubMed]
- Mittal, R.; Aggarwal, S.; Sharma, S.; Chhibber, S.; Harjai, K. Urinary tract infections caused by Pseudomonas aeruginosa: A minireview. J. Infect. Public Health 2009, 2, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.J.; Yoon, S.S.; Bae, I.K.; Jeong, S.H.; Kim, J.M.; Lee, K. Risk factors for mortality in patients with bloodstream infections caused by carbapenem-resistant Pseudomonas aeruginosa: Clinical impact of bacterial virulence and strains on outcome. Diagn. Microbiol. Infect. Dis. 2014, 80, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Thammavongsa, V.; Schneewind, O.; Missiakas, D. Recurrent infections and immune evasion strategies of Staphylococcus aureus. Curr. Opin. Microbiol. 2012, 15, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, C.P.; Daniels, M.; Zhao, F.; Alegre, M.-L.; Chong, A.S.; Daum, R.S. Protective immunity against recurrent Staphylococcus aureus skin infection requires antibody and interleukin-17A. Infect. Immun. 2014, 82, 2125–2134. [Google Scholar] [CrossRef] [PubMed]
- Libert, M.; Elkholti, M.; Massaut, J.; Karmali, R.; Mascart, G.; Cherifi, S. Risk factors for meticillin resistance and outcome of Staphylococcus aureus bloodstream infection in a Belgian university hospital. J. Hosp. Infect. 2008, 68, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.; Smith, D.L.; Laxminarayan, R. Hospitalizations and deaths caused by methicillin-resistant Staphylococcus aureus, United States, 1999–2005. Emerg. Infect. Dis. 2007, 13, 1840–1846. [Google Scholar] [CrossRef] [PubMed]
- Elixhauser, A.; Steiner, C. Infections with methicillin-resistant Staphylococcus aureus (MRSA) in U.S. Hospitals, 1993–2005: Statistical brief #35. In Healthcare Cost and Utilization Project (HCUP) Statistical Briefs; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2006. [Google Scholar]
Sample Availability: Samples of the compounds not are available from the authors. |
| Glucosinolate | Acronym | Side Chain | Derived ITC | Acronym |
|---|---|---|---|---|
| Glucocapparin | GCA | Methyl | Methyl ITC | MITC |
| Sinigrin | SIN | 2-Propenyl | Allyl ITC | AITC |
| Glucoerucin | GER | 4-Methylsulfanylbutyl | Erucin | ERN |
| Glucoraphanin | GRA | 4-Methylsulfinylbutyl | Sulforaphane | SFN |
| Glucotropaeolin | GTL | Benzyl | Benzyl ITC | BITC |
| Gluconasturtiin | GST | 2-Phenylethyl | Phenethyl ITC | PEITC |
| Glucomoringin | GMG | 4-(α-l-Rhamnopyranosyloxy)benzyl ITC | Moringin | MGN |
| Glucobrassicin | GBS | Indol-3-ylmethyl | Indole-3-carbinol (*) | I3C |
| ITCs | ITCs Source | Sensible Bacterial | Mechanism of Action | Reference |
|---|---|---|---|---|
| Sulforaphane SFN | broccoli seed extract | H. pylori | NA | [43] |
| Sulforaphane SFN | Synthetic | P. aeruginosa, EHEC E. coli strains, S. aureus | SFN inhibits bacterial quorum sensing, affects the pyocyanin production and exerts anti-biofilm activity against P. aeruginosa. SFN inhibits EHEC E. coli Shiga toxin production. | [44,45,46] |
| Phenethyl isothiocyanate PEITC | Sinapis alba seed | C. difficile ATCC 9689 and C. perfringens ATCC 13124 | PEITC mechanism of action against Clostridia species appears to be correlated with aromatic structure. | [47] |
| Synthetic | P. aeruginosa; S. aureus, E. coli CECT 434 | PEITC affects E. coli CECT 434, P. aeruginosa ATCC 10145, S. aureus CECT 976 cellular membrane integrity. PEITC reduce P. aeruginosa biofilm development. | [46,48,49,50,51] | |
| Benzyl isothiocyanate BITC | Salvadora persica root | S. enterica serotype Typhimurium | Essential oil rich in BITC, induces the loss of bacterial membrane integrity. | [52] |
| Benzyl isothiocyanate BITC | Synthetic | C. difficile; C. perfringens; C. jejuni; EHEC E. coli; P. aeruginosa; S. aureus | BITC inhibits EHEC E. coli Shiga toxin production. BITC induces the loss of MRSA membrane integrity and potential. BITC influences C.jejuni redox balance and metabolism up to death. BITC mechanism of action against Clostridia species appears to be correlated with aromatic structure. BITC reduces the metabolic activity of P. aeruginosa into the mature biofilm | [45,46,47,48,50,51,53,54] |
| Allyl isothiocyanate AITC | Wasabia japonica | E. coli O157:H7; S.aureus | NA | [55] |
| Allyl isothiocyanate AITC | Synthetic | C. jejuni E. coli O157:H7; E. coli CECT 434 P. aeruginosa; S. aureus | AITC affectes E. coli O157: H7 cell membrane integrity. AITC displays inhibitory action against thioredoxin reductase and acetate kinase of E. coli O157:H7 interacting with the sulfhydryl groups of the enzymes. AITC inhibites EHEC E. coli Shiga toxin production. AITC affects E. coli CECT 434, P. aeruginosa ATCC 10145, S. aureus CECT 976 cellular membrane integrity.AITC aliphatic structure impares its ability to counteract MRSA growth compared to the other ITCs. AITC reduces the metabolic activity of P. aeruginosa into the mature biofilm. | [45,48,49,51,53,55,56,57] |
| Erucin ERN | Synthetic | P. aeruginosa; S. aureus | ERN inhibits P. aeruginosa quorum sensing nd affects the production of pyocyanin. | [44,46] |
| Iberin IBN | Armoracia rusticana | P. aeruginosa | IBN inhibits P.aeruginosa quorum sensing and affects the production of pyocyanin and rhamnolipid | [58] |
| Iberin IBN | Synthetic | S. aureus | NA | [46] |
| Moringa peregrina seed extract | S. enterica isolates including MDR strains | Moringa peregrina aqueous seed extract induces the loss of cell wall integrity membrane potential. | [59] | |
| Moringa oleifera leaf extract | E. coli, P. aeruginosa S. aureus | NA | [60,61,62,63] | |
| Moringa oleifera bark extract | S. aureus, S. enterica | NA | [64,65] | |
| Moringa oleifera seed extract | S. aureus, S. enetrica | NA | [66,67] | |
| Moringin MGN | Moringa oleifera seed extract | S. aureus | NA | [30,68] |
| Moringa stenopetala seed extract | S. enterica | NA | [67] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romeo, L.; Iori, R.; Rollin, P.; Bramanti, P.; Mazzon, E. Isothiocyanates: An Overview of Their Antimicrobial Activity against Human Infections. Molecules 2018, 23, 624. https://doi.org/10.3390/molecules23030624
Romeo L, Iori R, Rollin P, Bramanti P, Mazzon E. Isothiocyanates: An Overview of Their Antimicrobial Activity against Human Infections. Molecules. 2018; 23(3):624. https://doi.org/10.3390/molecules23030624
Chicago/Turabian StyleRomeo, Letizia, Renato Iori, Patrick Rollin, Placido Bramanti, and Emanuela Mazzon. 2018. "Isothiocyanates: An Overview of Their Antimicrobial Activity against Human Infections" Molecules 23, no. 3: 624. https://doi.org/10.3390/molecules23030624
APA StyleRomeo, L., Iori, R., Rollin, P., Bramanti, P., & Mazzon, E. (2018). Isothiocyanates: An Overview of Their Antimicrobial Activity against Human Infections. Molecules, 23(3), 624. https://doi.org/10.3390/molecules23030624






