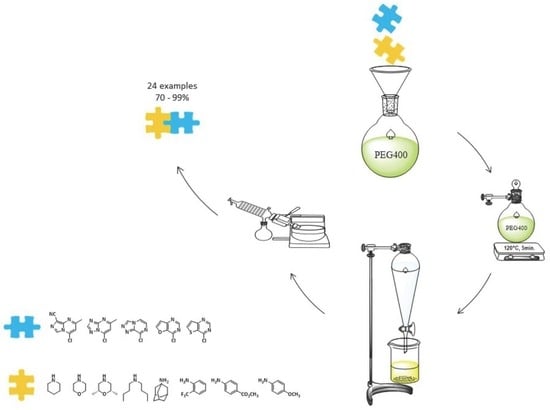
A Greener and Efficient Method for Nucleophilic Aromatic Substitution of Nitrogen-Containing Fused Heterocycles
Abstract
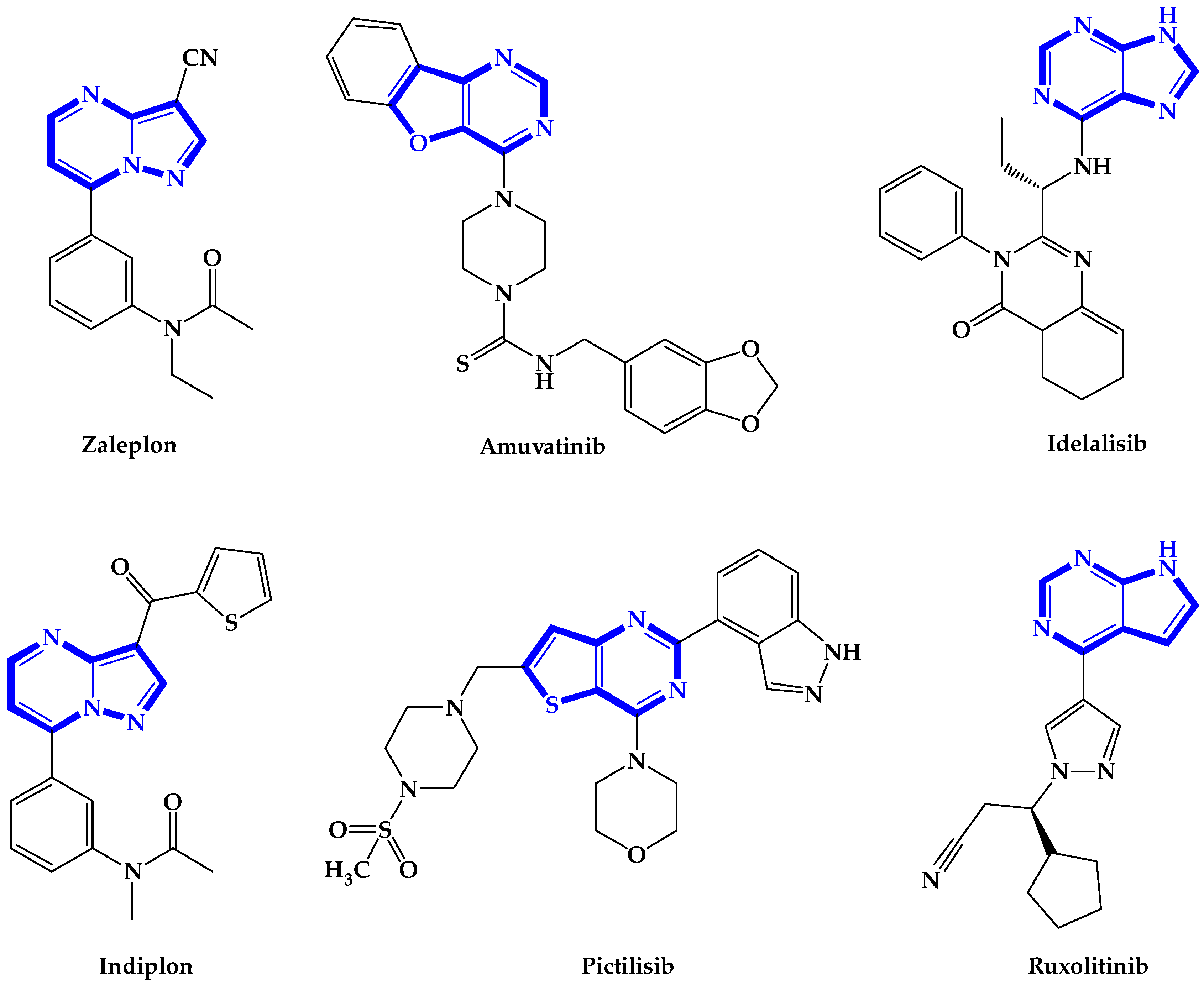
:1. Introduction
2. Results and Discussion
2.1. From 4-Chloro-2-methylimidazo[1,5-a]pyrimidine-8-carbonitrile
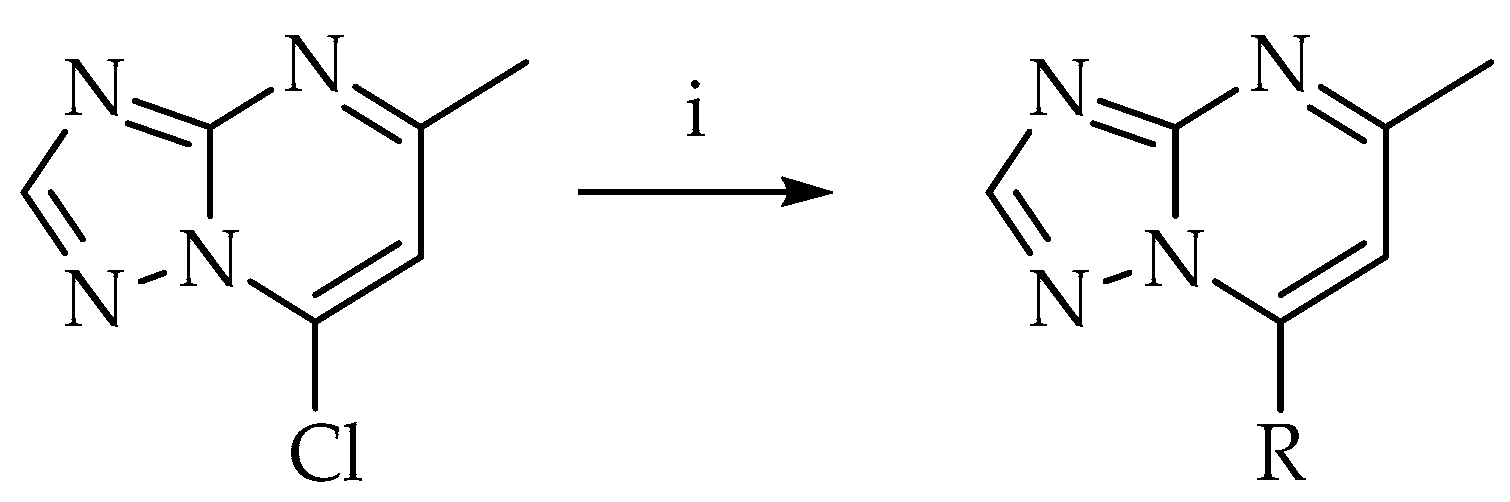
2.2. From 7-Chloro-5-methyl-[1,2,4]triazolo[1,5-a]pyrimidine
2.3. From 8-Chloro-[1,2,4]triazolo[4,3-a]pyrazine
2.4. From 4-Chlorofuro and thieno[3,2-d]pyrimidine
3. Materials and Methods
3.1. General Methods
3.2. General Procedure for the Synthesis of 1 to 24
4. Conclusions
Supplementary Materials
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bryan, M.C.; Dillon, B.; Hamann, L.G.; Hughes, G.J.; Kopach, M.E.; Peterson, E.A.; Pourashraf, M.; Raheem, I.; Richardson, P.; Richter, D.; et al. Sustainable Practices in Medicinal Chemistry: Current State and Future Directions. J. Med. Chem. 2013, 56, 6007–6021. [Google Scholar] [CrossRef] [PubMed]
- Watson, W.J.W. How do the fine chemical, pharmaceutical, and related industries approach green chemistry and sustainability? Green Chem. 2012, 14, 251–259. [Google Scholar] [CrossRef]
- Lombardino, J.G.; Lowe, J.A., III. A Guide to drug discovery: The Role of Medicinal Chemist in Drug Discovery-Then and Now. Nat. Rev. Drug Discov. 2004, 3, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: New York, NY, USA, 1998; p. 30. ISBN 9780198506980. [Google Scholar]
- Ashcroft, C.P.; Dunn, P.J.; Hayler, J.D.; Wells, A.S. Survey of Solvent Usage in Papers Published in Organic Process Research & Development 1997–2012. Org. Process Res. Dev. 2015, 19, 740–747. [Google Scholar] [CrossRef]
- Jimenez-Gonzalez, C.; Ponder, C.S.; Broxtermann, Q.B.; Manley, J.B. Using the Right Green Yardstick: Why Process Mass Intensity Is Used in the Pharmaceutical Industry To Drive More Sustainable Processes. Org. Process Res. Dev. 2011, 15, 912–917. [Google Scholar] [CrossRef]
- Jimenez-Gonzalez, C.; Curzons, A.D.; Constable, D.J.C.; Cunningham, V.L. Expanding GSK’s Solvent Selection Guide—application of life cycle assessment to enhance solvent selections. Clean Technol. Environ. Policy 2005, 7, 42–50. [Google Scholar] [CrossRef]
- Clarke, C.J.; Tu, W.-C.; Levers, O.; Bröhl, A.; Hallett, J.P. Green and Sustainable Solvents in Chemical Processes. Chem. Rev. 2018, 118, 747–800. [Google Scholar] [CrossRef] [PubMed]
- Laird, T. Green Chemistry is Good Process Chemistry. Org. Process Res. Dev. 2012, 16, 1–2. [Google Scholar] [CrossRef]
- Bisz, E.; Szostak, M. 2-Methyltetrahydrofuran: A Green Solvent for Iron-Catalyzed Cross-Coupling Reactions. ChemSusChem 2018. [Google Scholar] [CrossRef] [PubMed]
- Bisza, E.; Szostak, M. Cyclic ureas (DMI, DMPU) as efficient, sustainable ligands in iron-catalyzed C(sp2)–C(sp3) coupling of aryl chlorides and tosylates. Green Chem. 2017, 19, 5361–5366. [Google Scholar] [CrossRef]
- Harris, J.M.; Zalipsky, S. Polyethylene Glycol: Chemistry and Biological Application; ACS Books: Washington, DC, USA, 1997; ISBN 9780841235373. [Google Scholar]
- Harris, J.M. Poly(ethylene Glycol) Chemistry, Biotechnological and Biomedical Applications; Plenum Press: New York, NY, USA, 1992; ISBN 9781489907035. [Google Scholar]
- Vafaeezadeh, M.; Hashemi, M.M. Polyethylene glycol (PEG) as a green solvent for carbon–carbon bondformation reactions. J. Mol. Liq. 2015, 207, 73–79. [Google Scholar] [CrossRef]
- Colacino, E.; Martinez, J.; Lamaty, F.; Patrikeeva, L.S.; Khemchyan, L.L.; Ananikov, V.P.; Beletskaya, I.P. PEG as an alternative reaction medium in metal-mediated transformations. Coord. Chem. Rev. 2012, 256, 2893–2920. [Google Scholar] [CrossRef]
- Chen, J.; Spear, S.K.; Huddleston, J.G.; Rogers, R.D. Polyethylene glycol and solutions of polyethylene glycol as green reaction media. Green Chem. 2005, 7, 64–82. [Google Scholar] [CrossRef]
- Zhu, Y.; Romain, C.; Williams, C.K. Sustainable polymers from renewable resources. Nature 2016, 540, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Knop, K.; Hoogenboom, R.; Fischer, D.; Schubert, U.S. Poly(ethylene glycol) in Drug Delivery: Pros and Cons as Well as Potential Alternatives. Angew. Chem. Int. Ed. 2010, 49, 6288–6308. [Google Scholar] [CrossRef] [PubMed]
- Parrott, M.; Rule, S.; Kelleher, M.; Wilson, J. A Systematic Review of Treatments of Relapsed/Refractory Mantle Cell Lymphoma. Clin Lymphoma Myeloma Leuk. 2018, 18, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Samadder, P.; Aithal, R.; Belanc, O.; Krejci, L. Cancer TARGETases: DSB repair as a pharmacological target. Pharmacol. Ther. 2016, 161, 111–131. [Google Scholar] [CrossRef] [PubMed]
- Mortlock, A.; Foote, K.; Kettle, J.; Aquila, B. Kinase Inhibitors in Cancer Reference Module in Chemistry. Mol. Sci. Chem. Eng. Kinase Inhib. Cancer 2014. [Google Scholar] [CrossRef]
- Cherukupalli, S.; Hampannavar, G.A.; Chinnam, S.; Chandrasekaran, B.; Sayyad, N.; Kayamba, F.; Aleti, R.R.; Karpoormath, R. An appraisal on synthetic and pharmaceutical perspectives of pyrazolo[4,3-d]pyrimidine scaffold. Bioorg. Med. Chem. 2018, 26, 309–339. [Google Scholar] [CrossRef] [PubMed]
- Vitaku, E.; Smith, D.T.; Njardarson, J.T. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.D.; MacCoss, M.; Lawson, A.D.G. Rings in Drugs. J. Med. Chem. 2014, 57, 5845–5859. [Google Scholar] [CrossRef] [PubMed]
- Dumonteil, G.; Hiebel, M.-A.; Scherrmann, M.-C.; Berteina-Raboin, S. Iodine-catalyzed formation of substituted 2-aminobenzothiazole derivatives in PEG400. RSC Adv. 2016, 6, 73517–73521. [Google Scholar] [CrossRef]
- Hiebel, M.-A.; Berteina-Raboin, S. Iodine-catalyzed regioselective sulfenylation of imidazoheterocycles in PEG400. Green Chem. 2015, 17, 935–944. [Google Scholar] [CrossRef]
- Billault, I.; Pessel, F.; Petit, A.; Turgis, R.; Scherrmann, M.-C. Investigation of the copper(I) catalysed azide–alkyne cycloaddition reactions (CuAAC) in molten PEG2000. New J. Chem. 2015, 39, 1986–1995. [Google Scholar] [CrossRef]
- Tber, Z.; Hiebel, M.-A.; El Hakmaoui, A.; Akssira, M.; Guillaumet, G.; Berteina-Raboin, S. Metal Free Formation of Various 3-Iodo-1H-pyrrolo[3′,2′:4,5]imidazo-[1,2-a]pyridines and [1,2-b]Pyridazines and Their Further Functionalization. J. Org. Chem. 2015, 80, 6564–6573. [Google Scholar] [CrossRef] [PubMed]
- Hiebel, M.-A.; Fall, Y.; Scherrmann, M.-C.; Berteina-Raboin, S. Straightforward Synthesis of Various 2,3-Diarylimidazo[1,2-a]pyridines in PEG400 Medium through One-Pot Condensation and C–H Arylation. Eur. J. Org. Chem. 2014, 21, 4643–4650. [Google Scholar] [CrossRef]
- Turgis, R.; Billault, I.; Acherar, S.; Auge, J.; Scherrmann, M.-C. Total synthesis of high loading capacity PEG-based supports: Evaluation and improvement of the process by use of ultrafiltration and PEG as a solvent. Green Chem. 2013, 15, 1016–1029. [Google Scholar] [CrossRef]
- Arnould, M.; Hiebel, M.-A.; Massip, S.; Léger, J.M.; Jarry, C.; Berteina-Raboin, S.; Guillaumet, G. Efficient Metal-Free Synthesis of Various Pyrido[2′,1′:2,3]imidazo-[4,5-b]quinolones. Chem. Eur. J. 2013, 19, 12249–12253. [Google Scholar] [CrossRef] [PubMed]
- Prosa, N.; Turgis, R.; Piccardi, R.; Scherrmann, M.-C. Soluble Polymer-Supported Flow Synthesis: A Green Process for the Preparation of Heterocycles. Eur. J. Org. Chem. 2012, 11, 2188–2200. [Google Scholar] [CrossRef]
- Fresneau, N.; Hiebel, M.-A.; Agrofoglio, L.A.; Berteina-Raboin, S. Efficient Synthesis of Unprotected C-5-Aryl/Heteroaryl-2′-deoxyuridine via a Suzuki-Miyaura Reaction in Aqueous Media. Molecules 2012, 17, 14409–14417. [Google Scholar] [CrossRef] [PubMed]
- Fresneau, N.; Hiebel, M.-A.; Agrofoglio, L.A.; Berteina-Raboin, S. One-pot Sonogashira-cyclization protocol to obtain substituted furopyrimidine nucleosides in aqueous conditions. Tetrahedron Lett. 2012, 53, 1760–1763. [Google Scholar] [CrossRef]
- Alarcón-Espósito, J.; Tapia, R.A.; Contreras, R.; Campodónico, P.R. Changes in the SNAr reaction mechanism brought about by preferential solvation. RSC Adv. 2015, 5, 99322–99328. [Google Scholar] [CrossRef]
- Ormazabal-Toledo, R.; Santos, J.G.; Ríos, P.; Castro, E.A.; Campodónico, P.R.; Contreras, R. Hydrogen Bond Contribution to Preferential Solvation in SNAr Reactions. J. Phys. Chem. B 2013, 117, 5908–5915. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Salaski, E.J.; Berger, D.M.; Powell, D. Dramatic Effect of Solvent Hydrogen Bond Basicity on the Regiochemistry of SNAr Reactions of Electron-Deficient Polyfluoroarenes. Org. Lett. 2009, 11, 5662–5664. [Google Scholar] [CrossRef] [PubMed]
- Um, I.H.S.; Min, W.; Dust, J.M. Choice of Solvent (MeCN vs H2O) Decides Rate-Limiting Step in SNAr Aminolysis of 1-Fluoro-2,4-dinitrobenzene with Secondary Amines: Importance of Brønsted-Type Analysis in Acetonitrile. J. Org. Chem. 2007, 72, 8797–8803. [Google Scholar] [CrossRef] [PubMed]
- Nudelman, N.S.; Mancini, P.M.E.; Martinez, R.D.; Vottero, L.R. Solvents effects on aromatic nucleophilic substitutions. Part 5. Kinetics of the reactions of 1-fluoro-2,4-dinitrobenzene with piperidine in aprotic solvents. J. Chem. Soc. Perkin Trans. 1987, 2, 951–954. [Google Scholar] [CrossRef]
- Alarcón-Espósito, J.; Contreras, R.; Tapia, R.A.; Campodónico, P.R. Gutmann’s Donor Numbers Correctly Assess the Effect of the Solvent on the Kinetics of SNAr Reactions in Ionic Liquids. Chem. Eur. J. 2016, 22, 13347–13351. [Google Scholar] [CrossRef] [PubMed]
- Tanner, E.E.L.; Hawker, R.R.; Yau, H.M.; Croft, A.K.; Harper, J.B. Probing the importance of ionic liquid structure: A general ionic liquid effect on an SNAr process. Org. Biomol. Chem. 2013, 11, 7516–7521. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.C.; Masters, A.F.; Maschmeyer, T. Steric, hydrogen-bonding and structural heterogeneity effects on the nucleophilic substitution of N-(p-fluorophenyldiphenylmethyl)-4-picolinium chloride in ionic liquids. Org. Biomol. Chem. 2013, 11, 2534–2542. [Google Scholar] [CrossRef] [PubMed]
- D’Anna, F.; Marullo, S.; Noto, R. Aryl Azides Formation under Mild Conditions: A Kinetic Study in Some Ionic Liquid Solutions. J. Org. Chem. 2010, 75, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Newington, I.; Perez-Arlandis, J.M.; Welton, T. Ionic Liquids as Designer Solvents for Nucleophilic Aromatic Substitutions. Org. Lett. 2007, 9, 5247–5250. [Google Scholar] [CrossRef] [PubMed]
- Tormo, I.B.J.; Blettner, C.; Muller, B.; Gewehr, M.; Grammenos, W.; Grote, T.; Gypser, A.; Rheinheimer, J.; Schaefer, P.; Schieweck, F.; et al. Research Advances in Synthesis and Antifungal Activity of Pyrimidine Compounds. PCT/EP2004/004067, WO 2004092175 A1, 28 October 2004. [Google Scholar]
- Tang, W.; Shi, D.-Q. Synthesis and herbicidal activity of O,O-dialkyl N-[2-(5,7-dimethyl-[1,2,4]triazolo[1,5-a]pyrimidin-2-yloxy)benzoxyl]-1-amino-1-substitutedbenzyl phosphonates. J. Heterocyclic Chem. 2010, 47, 162–166. [Google Scholar] [CrossRef]
- Jiang, L.; Chen, C.; Zhou, Y.; Chen, Q.; Yang, G. Synthesis and Herbicidal Activities of Novel 1,2,4-Triazolo[1,5-a]-pyrimidine Containing Oxime Ether Moiety. Chin. J. Org. Chem. 2009, 29, 1392–1404. [Google Scholar]
- Qizhong, X.; Xuanfu, L.; Junhu, L.; Liang, B.; Xiaoping, B. Synthesis and Bioactivities of Novel 1,2,4-triazolo[1,5-a]Pyrimidine Derivatives Containing 1,2,4-triazole-5-thione Schiff Base Unit. Chin. J. Org. Chem. 2012, 32, 1255–1260. [Google Scholar] [CrossRef]
- Chen, Q.; Zhu, X.-L.; Jiang, L.-L.; Liu, Z.-M.; Yang, G.-F. Synthesis, antifungal activity and CoMFA analysis of novel 1,2,4-triazolo[1,5-a]pyrimidine derivatives. Eur. J. Med. Chem. 2008, 43, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, J.D.; Chudasama, C.J.; Patel, K.D. Pyrazole clubbed triazolo[1,5-a]pyrimidine hybrids as an anti-tubercular agents: Synthesis, in vitro screening and molecular docking study. Bioorg. Med. Chem. 2015, 23, 7711–7716. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, H.M.; El-Koussi, N.A.; Hassan, H.Y. Fluorinated 1,2,4-Triazolo[1,5-a]pyrimidine-6-carboxylic Acid Derivatives as Antimycobacterial Agents. Arch. Pharm. Chem. Life Sci. 2009, 342, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lee, M.; Peng, Z.; Blázquez, B.; Lastochkin, E.; Kumarasiri, M.; Bouley, R.; Chang, M.; Mobashery, S. Synthesis and Evaluation of 1,2,4-Triazolo[1,5-a]pyrimidines as Antibacterial Agents Against Enterococcus faecium. J. Med. Chem. 2015, 58, 4194–4203. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.-L.; Zhao, Y.-F.; Guo, S.-C.; Song, H.-S.; Wang, D.; Gong, P. Synthesis and Anti-tumor Activities of Novel [1,2,4]triazolo[1,5-a]pyrimidines. Molecules 2007, 12, 1136–1146. [Google Scholar] [CrossRef] [PubMed]
- Hassan, G.S.; El-Sherbeny, M.A.; El-Ashmawy, M.B.; Bayomi, S.M.; Maarouf, A.R.; Badria, F.A. Synthesis and antitumor testing of certain new fused triazolopyrimidine and triazoloquinazoline derivatives. Arab. J. Chem. 2017, 10, S1345–S1355. [Google Scholar] [CrossRef]
- Saito, T.; Obitsu, T.; Minamoto, C.; Sugiura, T.; Matsumura, N.; Ueno, S.; Kishi, A.; Katsumata, S.; Nakai, H.; Toda, M. Pyrazolo[1,5-a]pyrimidines, triazolo[1,5-a]pyrimidines and their tricyclic derivatives as corticotropin-releasing factor 1 (CRF1) receptor antagonists. Bioorg. Med. Chem. 2011, 19, 5955–5966. [Google Scholar] [CrossRef] [PubMed]
- Hougaard, C.; Hammami, S.; Eriksen, B.L.; Sørensen, U.S.; Jensen, M.L.; Strøbæk, D.; Christophersen, P. Evidence for a Common Pharmacological Interaction Site on KCa2 Channels Providing Both Selective Activation and Selective Inhibition of the Human KCa2.1 Subtype. Mol. Pharmacol. 2012, 81, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Uryu, S.; Tokuhiro, S.; Murasugi, T.; Oda, T. A novel compound, RS-1178, specifically inhibits neuronal cell death mediated by β-amyloid-induced macrophage activation in vitro. Brain Res. 2002, 946, 298–306. [Google Scholar] [CrossRef]
- Mustazza, C.; Del Giudice, M.R.; Borioni, A.; Gatta, F. Synthesis of pyrazolo[1,5-a]-, 1,2,4-triazolo[1,5-a]- and imidazo[1,2-a]pyrimidines related to zaleplon, a new drug for the treatment of insomnia. J. Heterocycl. Chem. 2001, 38, 1119–1129. [Google Scholar] [CrossRef]
- Sadana, A.K.; Mirza, Y.; Aneja, K.R.; Prakash, O. Hypervalent iodine mediated synthesis of 1-aryl/hetryl-1,2,4-triazolo[4,3-a] pyridines and 1-aryl/hetryl 5-methyl-1,2,4-triazolo[4,3-a]quinolines as antibacterial agents. Eur. J. Med. Chem. 2003, 38, 533–536. [Google Scholar] [CrossRef]
- Kalgutkar, A.S.; Hatch, H.L.; Kosea, F.; Nguyen, H.T.; Choo, E.F.; McClure, K.F.; Taylor, T.J.; Henne, K.R.; Kuperman, A.V.; Dombroski, M.A.; et al. Preclinical pharmacokinetics and metabolism of 6-(4-(2,5-difluorophenyl)oxazol-5-yl)-3-isopropyl-[1,2,4]-triazolo[4,3-a]pyridine, a novel and selective p38alpha inhibitor: Identification of an active metabolite in preclinical species and human liver microsomes. Biopharm. Drug Dispos. 2006, 27, 371–386. [Google Scholar] [CrossRef] [PubMed]
- McClure, K.F.; Abramov, Y.A.; Laird, E.R.; Barberia, J.T.; Cai, W.; Carty, T.J.; Cortina, S.R.; Danley, D.E.; Dipesa, A.J.; Donahue, K.M.; et al. Theoretical and Experimental Design of Atypical Kinase Inhibitors: Application to p38 MAP Kinase. J. Med. Chem. 2005, 48, 5728–5737. [Google Scholar] [CrossRef] [PubMed]
- Bektas, H.; Karaali, N.; Sahin, D.; Demirbas, A.; Karaoglu, S.A.; Demirbas, N. Synthesis and Antimicrobial Activities of Some New 1,2,4-Triazole Derivatives. Molecules 2010, 15, 2427–2438. [Google Scholar] [CrossRef] [PubMed]
- Lawson, E.C.; Hoekstra, W.J.; Addo, M.F.; Andrade-Gordon, P.; Damiano, B.P.; Kauffman, J.A.; Mitchell, J.A.; Maryanoff, B.E. 1,2,4-Triazolo[3,4-a]pyridine as a novel, constrained template for fibrinogen receptor (GPIIb/IIIa) antagonists. Bioorg. Med. Chem. Lett. 2001, 11, 2619–2622. [Google Scholar] [CrossRef]
- Moreau, S.; Coudert, P.; Rubat, C.; Vallee-Goyet, D.; Gardette, D.; Gramain, J.-C.; Couquelet, J. Synthesis and anticonvulsant properties of triazolo- and imidazopyridazinyl carboxamides and carboxylic acids. Bioorg. Med. Chem. 1998, 6, 983–991. [Google Scholar] [CrossRef]
- Nitlikar, L.H.; Darandale, S.N.; Shinde, D.B. Exploring the Unexplored Practical and Alternative Synthesis of 3-(Trifluoromethyl)-Triazolopiperazine the Key Intermediate for Sitagliptin. Lett. Org. Chem. 2013, 10, 348–352. [Google Scholar] [CrossRef]
- Elrazaz, E.Z.; Serya, R.A.T.; Ismail, N.S.M.; El Ella, D.A.A.; Abouzid, K.A.M. Thieno[2,3-d]pyrimidine based derivatives as kinase inhibitors and anticancer agents. Future J. Pharm. Sci. 2015, 1, 33–41. [Google Scholar] [CrossRef]
- Dinakaran, V.S.; Bomma, B.; Srinivasan, K.K. Fused pyrimidines: The heterocycle of diverse biological and pharmacological significance. Der Pharma Chem. 2012, 4, 255–265. [Google Scholar]
- Janeba, Z.; Holý, A.; Pohl, R.; Snoeck, R.; Andrei, G.; De Clercq, E.; Balzarini, J. Synthesis and biological evaluation of acyclic nucleotide analogues with a furo[2,3-d]pyrimidin-2(3H)-one base. Can. J. Chem. 2010, 88, 628–638. [Google Scholar] [CrossRef]
- Robins, M.J.; Nowak, I.; Rajwanshi, V.K.; Miranda, K.; Cannon, J.F.; Peterson, M.A.; Andrei, G.; Snoeck, R.; De Clercq, E.; Balzarini, J. Synthesis and Antiviral Evaluation of 6-(Alkyl-heteroaryl)furo[2,3-d]pyrimidin-2(3H)-one Nucleosides and Analogues with Ethynyl, Ethenyl, and Ethyl Spacers at C6 of the Furopyrimidine Core. J. Med. Chem. 2007, 50, 3897–3905. [Google Scholar] [CrossRef] [PubMed]
- McGuigan, C.; Barucki, H.; Blewett, S.; Carangio, A.; Erichsen, J.T.; Andrei, G.; Snoeck, R.; De Clercq, E.; Balzarini, J. Highly Potent and Selective Inhibition of Varicella-Zoster Virus by Bicyclic Furopyrimidine Nucleosides Bearing an Aryl Side Chain. J. Med. Chem. 2000, 43, 4993–4997. [Google Scholar] [CrossRef] [PubMed]
- Bhuiyan, M.M.H.; Rahman, K.M.M.; Hossain, M.K.; Rahim, M.A.; Hossain, M.I. Fused Pyrimidines. Part II: Synthesis and Antimicrobial activity of Some Furo[3,2-e]imidazo[1,2-c]pyrimidines and Furo[2,3-d]pyrimidines. Croat. Chem. Acta 2005, 78, 633–636. [Google Scholar]
- Gangjee, A.; Devraj, R.; McGuire, J.J.; Kisliuk, R.L.; Queener, S.F.; Barrows, L.R. Classical and Nonclassical Furo[2,3-d]pyrimidines as Novel Antifolates: Synthesis and Biological Activities. J. Med. Chem. 1994, 37, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Pyo, J.I.; Lee, S.H.; Cheong, C.S. A facile synthesis of some substituted furopyrimidine derivatives. J. Heterocycl. Chem. 2006, 43, 1129–1133. [Google Scholar] [CrossRef]
- Novinson, T.; O’Brien, D.E.; Robins, R.K. Synthesis of certain 8-cyano-2,4-disubstituted imidazo[1,5-a]pyrimidines. J. Heterocycl. Chem. 1974, 11, 873–878. [Google Scholar] [CrossRef]
- Levin, Y.A.; Sergeeva, E.M.; Kukhtin, V.A. Condensed heterocycles. V. Reaction of 4-chloro-6-methyl-1,2,4-triazolo[2,3-α] pyrimidine with some nitrogenous bases. Zhurnal Obshchei Khimii 1964, 34, 205–209. [Google Scholar]
- Reynolds, G.A.; VanAllan, J. A Structure of certain polyazaindenes. VII. 4-Amino-6-methyl-1,3,3a,7-tetraazaindene and its derivatives. J. Org. Chem. 1961, 26, 115–117. [Google Scholar] [CrossRef]
- Barelier, S.; Eidam, O.; Fish, I.; Hollander, J.; Figaroa, F.; Nachane, R.; Irwin, J.J.; Shoichet, B.K.; Siegal, G. Increasing Chemical Space Coverage by Combining Empirical and Computational Fragment Screens. ACS Chem. Biol. 2014, 9, 1528–1535. [Google Scholar] [CrossRef] [PubMed]
- Kemnitzer, W.; Sirisoma, N.; May, C.; Tseng, B.; Drewe, J.; Cai, S.X. Discovery of 4-anilino-N-methylthieno[3,2-d]pyrimidines and 4-anilino-N-methylthieno[2,3-d]pyrimidines as potent apoptosis inducers. Bioorg. Med. Chem. Lett. 2009, 19, 3536–3540. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of all compounds are available from the authors. |
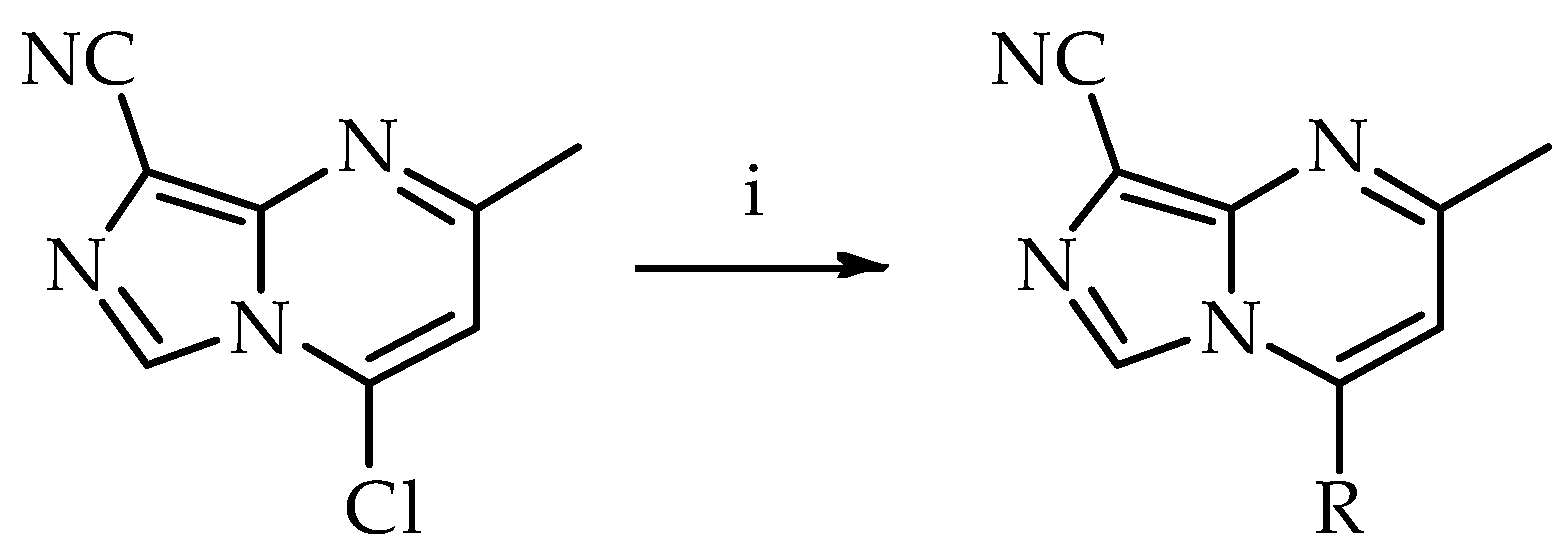


| Entry | Amine Reagent | Product | Yield |
|---|---|---|---|
| 1 |  |  | 1; 87% |
| 2 |  |  | 2; 92% |
| 3 |  |  | 3; 95% |
| 4 |  |  | 4; 81% |
| 5 |  |  | 5; 85% |
| 6 |  |  | 6; 70% |
| Entry | Amine Reagent | Product | Yield |
|---|---|---|---|
| 1 |  |  | 7; 79% |
| 2 |  |  | 8; 88% |
| 3 |  |  | 9; 89% |
| 4 |  |  | 10; 88% |
| Entry | Amine Reagent | Product | Yield |
|---|---|---|---|
| 1 |  |  | 11; 92% |
| 2 |  |  | 12; 99% |
| 3 |  |  | 13; 73% |
| 4 |  |  | 14; 75% |
| Entry | Amine Reagent | Product | Yield |
|---|---|---|---|
| 1 |  |  | 15; X = O, 99%
16; X = S, 86% |
| 2 |  |  | 17; X = O, 99%
18; X = S, 90% |
| 3 |  |  | 19; X = S, 83% |
| 4 |  |  | 20; X= S, 96% |
| 5 |  |  | 21; X = S, 77% |
| 6 |  |  | 22; X= S, 71% |
| 7 |  |  | 23; X = S, 79% |
| 8 |  |  | 24; X = S, 88% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campos, J.F.; Loubidi, M.; Scherrmann, M.-C.; Berteina-Raboin, S. A Greener and Efficient Method for Nucleophilic Aromatic Substitution of Nitrogen-Containing Fused Heterocycles. Molecules 2018, 23, 684. https://doi.org/10.3390/molecules23030684
Campos JF, Loubidi M, Scherrmann M-C, Berteina-Raboin S. A Greener and Efficient Method for Nucleophilic Aromatic Substitution of Nitrogen-Containing Fused Heterocycles. Molecules. 2018; 23(3):684. https://doi.org/10.3390/molecules23030684
Chicago/Turabian StyleCampos, Joana F., Mohammed Loubidi, Marie-Christine Scherrmann, and Sabine Berteina-Raboin. 2018. "A Greener and Efficient Method for Nucleophilic Aromatic Substitution of Nitrogen-Containing Fused Heterocycles" Molecules 23, no. 3: 684. https://doi.org/10.3390/molecules23030684
APA StyleCampos, J. F., Loubidi, M., Scherrmann, M.-C., & Berteina-Raboin, S. (2018). A Greener and Efficient Method for Nucleophilic Aromatic Substitution of Nitrogen-Containing Fused Heterocycles. Molecules, 23(3), 684. https://doi.org/10.3390/molecules23030684