Abstract
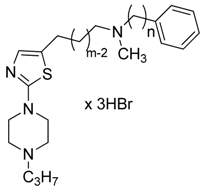
H3 receptors present on histaminergic and non-histaminergic neurons, act as autoreceptors or heteroreceptors controlling neurotransmitter release and synthesis. Previous, studies have found that the compound N-methyl-N-3-phenylalkyl-2-[2-(4-n-propylpiperazin-1-yl)-1,3-thiazol-5-yl]ethan-1-amine (ADS-531, 2c) exhibits high in vitro potency toward H3 guinea pig jejunal receptors, with pA2 = 8.27. To optimize the structure of the lead compound ADS-531, a series of 5-substituted-2-thiazol-4-n-propylpiperazines 3 were synthesized and subjected to in vitro pharmacological characterization; the alkyl chain between position 2 of the thiazole ring and the terminal secondary N-methylamino function was elongated from three to four methylene groups and the N-methylamino functionality was substituted by benzyl-, 2-phenylethyl-, and 3-phenyl-propyl- moieties. SAR studies on novel non-imidazole, 5-substituted-2-thiazol-4-n-propyl-piperazines 3 showed that the most active compound 3a (pA2 = 8.38), additionally possessed a weak competitive H1-antagonistic activity. Therefore, compound ADS-531, which did not exhibit any H1-antagonistic activity, was chosen for further evaluation for its affinity to the recombinant rat and human histamine H3 receptors (rH3R and hH3R, respectively). ADS-531 exhibited nanomolar affinity for both rH3R and hH3R receptors. It was also shown that, ADS-531 given subchronically to rats (s.c. 3 mg/kg, 5 days) penetrated the brain, where it affected dopamine, noradrenaline and serotonin concentration; however, it did not affect histamine concentration nor feeding behavior.
1. Introduction
The H3 receptors mediate the diverse biological effects of the neurotransmitter histamine [1] and they are widely expressed in the mammalian brain, particularly in areas involved in cognitive processes and arousal, i.e., the cerebral cortex, hippocampus, basal ganglia, and hypothalamus [2,3]. H3 receptors are located on histaminergic or non-histaminergic neurons, respectively acting as autoreceptors or heteroreceptors, controlling the release and synthesis of histamine [4] and of multiple neurotransmitters such as acetylcholine [5], norepinephrine [6] and dopamine [7]. These data suggested that H3 antagonists could affect a number of behaviors and be useful in the treatment of cognitive deficits associated with a variety of disease states including Alzheimer’s disease (AD) [8], attention deficit hyperactivity disorder (ADHD) [9], schizophrenia [10], and obesity [11].
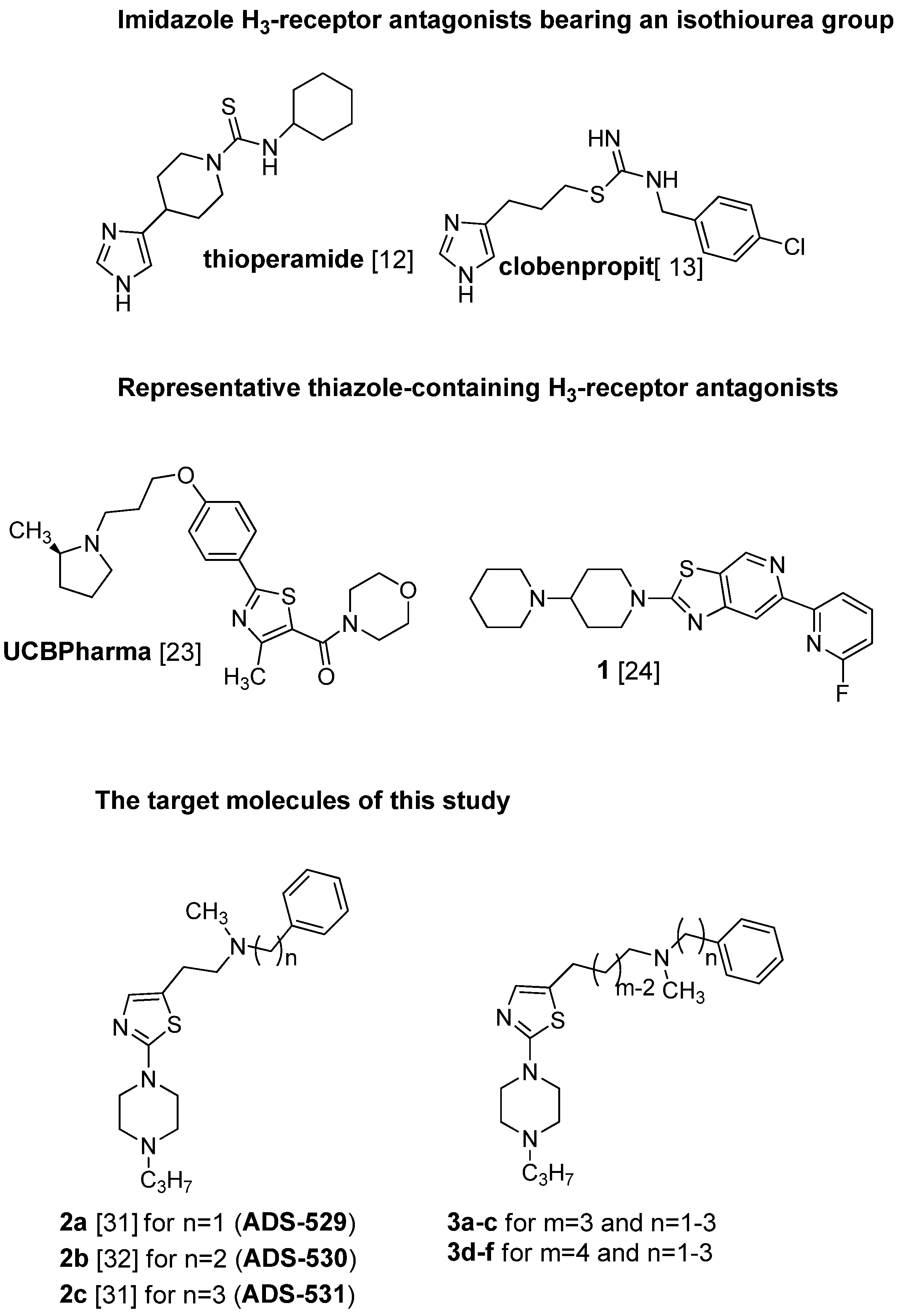
The first generation of active histamine H3 receptor antagonists were designed on the basis of a structural modification of the endogenous ligand, histamine, wherein the imidazole ring plays an important role [12]. Widely known representatives are thioperamide [13], and clobenpropit [14], both containing an isothiourea group (Figure 1).
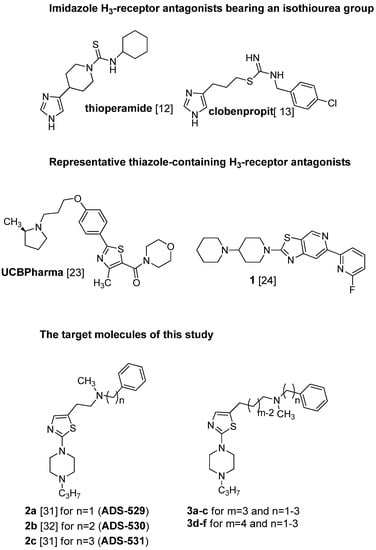
Figure 1.
Structures of some known histamine H3-receptor antagonists and the target molecules of this study.
Many ligands of this type have found utility in experimental studies as pharmacological tools [15]. However, the presence of an imidazole ring with strong hydrogen bond acceptor and donor properties causes low bioavailability and greatly limits penetration of the blood-brain barrier [16,17]. Among others, these compounds bind to the heme iron in CYP enzymes [18], and when co-administrated with other interacting drugs, can lead to adverse side-effects through drug-drug interactions [19]. Following these discoveries, and the successful cloning of the H3 receptor by Lovenberg et al. [20] in 1999, the intensive search for non-imidazole-based compounds was resumed, as these compounds may offer improvements in binding affinity and CNS penetration [21,22]. Despite significant differences in molecular weight and polarity, a general pharmacophore model has been developed for non-imidazole H3-antagonists. They are usually characterized by a basic group, often a tertiary amine, connected through an aliphatic spacer to a second pharmacophoric fragment which includes, a lipophilic substituent (often with a phenyl-like structure), depending on the series; some pronounced examples where the phenyl moiety was successfully replaced by different heterocyclic rings, one being a structurally modified thiazole ring are given in the literature [23,24]; (UCBPharma and compound 1, Figure 1). The lipophilic part is accompanied by another displaying high chemical diversity, such as an H-donor/acceptor, a 2nd basic part or acid group or lipophilic residue [25]. These efforts have resulted in a number of non-imidazole antagonists with high selectivity and specificity. The successful replacement of the imidazole moiety with pyrrolidine, piperidine, piperazine and other basic tertiary amines has been reported in patent applications and chemistry papers [26,27,28,29]. Recently, compound BF2.649 (Wakix®), an H3 inverse agonist, carrying the characteristic 3-aminopropan-1-ol functionality in its structure, has undergone clinical studies, and been approved and registered as a drug against narcolepsy [30]. Our previous study described two series of N-methyl-2-[2-(4-propylpiperazin-1-yl)-1,3-thiazol-5-yl]- [31] and N-methyl-2-[2-(4-propylpiperazin-1-yl)-1,3-thiazol-4-yl]ethan-1-amine derivatives [32] in which the terminal secondary N-methylamino function has been substituted with ω-aliphatic and ω-phenylaliphatic moieties (carbon chains of varying lengths from one to five methylene groups) with moderate to pronounced affinity for the histamine H3 receptor. It was shown by comparison of the homologous pairs of both series, that the presence of the aforementioned substituents at position 5 in the thiazole ring is favorable for histamine H3 receptor antagonist activity, whereas substitution at position 4 typically leads to a strong decrease of activity. The highest affinity for these series was seen for the derivative bearing N-methyl-N-phenylpropyl moiety [31], (compound ADS-531; pA2 = 8.27; Figure 1) and slightly lower affinity for the one carrying an N-methyl-N-benzyl or N-methyl-N-phenylethyl substituent [31] (2a, pA2 = 7.76; 2b, pA2 = 7.61). The most active compound ADS-531, which did not exhibit any H1-antagonistic activity, was chosen as the lead compound for further structural modification.
The aim of the present study was, hence, to optimize the structure of the lead compound 2c. To this end, a series of 5-substituted-2-thiazol-4-n-propylpiperazines (3; Figure 1) were synthesized and their pharmacological properties functionally evaluated with an in vitro test system using guinea pig jejunum preparations [33]. In this series, the alkyl chain between position 2 of the thiazole ring and the terminal secondary N-methylamino function was elongated from three to four methylene groups. The N-methylamino group was substituted by benzyl-, 2-phenylethyl-, and 3-phenylpropyl- substituents, these substituents being found to have the highest potency in previously described series of thiazoles [31]. Furthermore, compounds with the highest potency at the H3 receptor were also tested for H1 antagonistic effects in vitro, according to standard methods, using guinea pig ileum [34].
The results of SARs, together with previously described data, indicated that compound ADS-531, devoid of the antagonistic activity at the H1 receptor, was the most active of the group of compounds 2a–c. This compound was evaluated for its affinity for the recombinant rat and human histamine H3 receptors (rH3R and hH3R, respectively), transiently expressed in HEK-293T cells. Additionally, derivative ADS-531 was subjected to in vivo evaluation of its impact on feeding behavior and brain neurotransmitter systems after repeated peripheral administration to rats. Postmortem analyses of the rat brain tissues were also carried out to determine the activities of MAO-A, MAO-B, and HNMT.
2. Results and Discussion
2.1. Chemistry
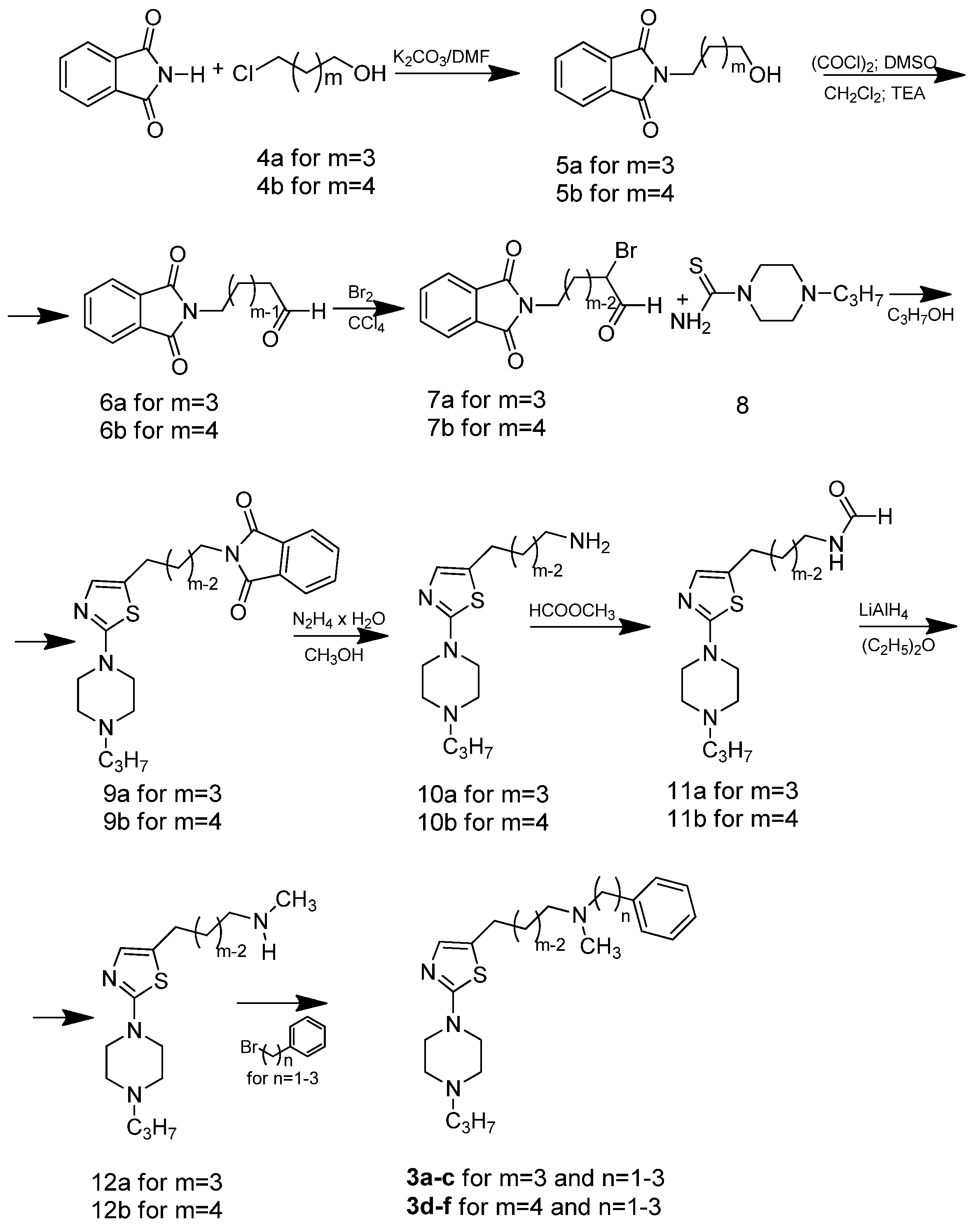
The general synthetic procedure used in this study is illustrated in Scheme 1. The central building blocks of the title compounds (compounds 3a–c and 3d–f) were 3-[2-(4-propylpiperazin-1-yl)-1,3-thiazol-5-yl]propan-1-amine (10a) and 4-[2-(4-propylpiperazin-1-yl)-1,3-thiazol-5-yl]butan-1-amine (10b), respectively (Scheme 1). The phthalimidobutanols 5a and 5b were prepared from ω-chloroalkanols 4a and 4b and phthalimide to yield 70% and 62% of the desired compounds, respectively.
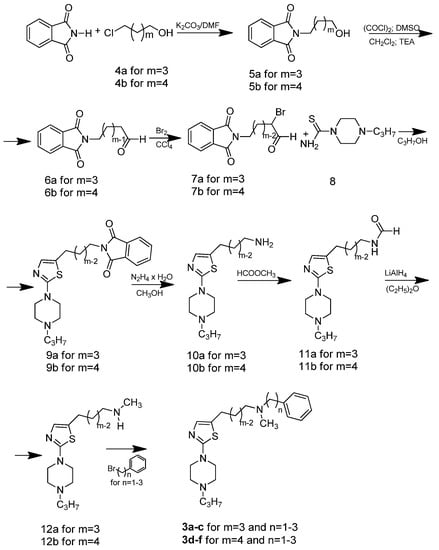
Scheme 1.
Synthetic routes to compounds 3a–f.
The ω-phthalimidoalkanals 6a and 6b were obtained from ω-phthalimidoalkanols 5a and 5b by reaction with oxalyl chloride/dimethyl sulfoxide, followed by proton abstraction with triethylamine and hydrolysis to the corresponding aldehydes. Bromination of the aldehydes was performed in carbon tetrachloride and after identification with NMR, the crude 2-bromo-ω-phtalimidoalkanals 7a and 7b were used in the cyclization reaction (Scheme 1). Ring closure of the crude 2-bromo-ω-phtalimidoalkanals 7a and 7b with 1-(4-n-propyl)piperazine thioamide (8) was performed in anhydrous DMF under an argon atmosphere giving high yields of the desired 5-(ω-phthalimidoalkyl)thiazoles 9a, 9b. Subsequent hydrazinolysis, basification with sodium hydroxide and extraction with chloroform led to the production of pure amines 10a, 10b. Derivatives 12a and 12b were prepared from compound 10a, 10b by two-step synthesis including formylation with methyl formate to compounds 11a, 11b and finally reduction with LiAlH4 in dry ethyl ether. Propan-1-amines 3a–c and butan-1-amines 3d–f were synthesized from compounds 12a, 12b by alkylation with the corresponding primary phenyloalkyl halides in the presence of K2CO3 in DMF followed by purification by column chromatography. All free bases were treated with methanolic HBr and the hydrobromides were precipitated with dry diethyl ether. The 1-(4-n-propyl)piperazine thioamide (8) was directly obtained by the reaction of the 1-n-propylpiperazine dihydrobromidewith potassium thiocyanate in aqueous solution [31]. 1H- and 13C-NMR spectra for all newly synthesized compounds are shown in the Supplementary Material.
6-Chlorohexanol, 7-chloroheptanol, phthalimide, oxalyl chloride, dimethyl sulfoxide (DMSO), 1-n-propylpiperazinedihydrobromide, triethylamine, methyl formate, lithium aluminum hydride(LiAlH4), benzyl bromide, 1-bromo-2-phenylethane and 1-bromo-3-phenylpropane and all solvents were purchased from Sigma-Aldrich (Saint Louis, MO, USA) or Alfa Aesar (Haverhill, MA, USA) and used without any purification.
2.2. Pharmacology
2.2.1. In Vitro Pharmacological Studies
H3 Antagonistic Activity for Compounds 2a–c and 3a–f
The compounds were tested in vitro as H3 receptor antagonists against H3 agonist-induced inhibition of the electrically evoked contraction of the guinea-pig jejunum [33]. The potency of the newly synthesized compounds 3a–f are reported in Table 1, as well as the previously described data for compounds 2a,c [31] and 2b [32]. Derivatives 3a–f show moderate to pronounced antagonist activity at H3-receptor. Propan-1-amines 3a–c (Table 1) and butan-1-amines 3d–f (Table 1) were synthesized to optimize the structure of the lead compound ADS-531 and the complementary 2a–c derivatives series [31,32]. In this series, the alkyl chain between position 2 of the thiazole ring and the terminal secondary N-methylamino function was elongated from three to four methylene groups. The N-methylamino functionality was substituted by benzyl-, 2-phenylethyl-, and 3-phenylpropyl- substituents, with these showing the highest potency in previously described series 2a–c. A comparison of homologous triplets, carrying benzyl substituents (compounds 2a, 3a, 3d), found derivative 3a (pA2 = 8.38) to have a higher potency than its analogs 2a, 3d (pA2 = 7.76 and 7.46, respectively). In the case of derivatives bearing a 2-phenylethyl substituent (compounds 2b, 3b, and 3e) the potency increases slightly with increasing alkyl chain length (pA2 = 7.61, 7.81 and 7.95, respectively). The highest potency, for the series of derivatives carrying of 3-phenylpropyl- substituent (compounds 2c, 3c, and 3f), is seen for 2c (pA2 = 8.27), but an increase in the alkyl chain length to two methylene groups resulted in a decrease of antagonist activity for compound 3c (pA2 = 7.46), while the activity increased again when the chain was further lengthened to three methylene groups (3f; pA2 = 7.91).

Table 1.
H3- and H1-antagonistic potency of compounds 2a–c and 3a–f as tested in the in vitro test system in the guinea pig jejunum.
Differences are observed within the 2a–c and 3a–c series. In the series of derivatives containing an ethyl linker between position 2 of the thiazole ring and the terminal secondary N-methylamino function (compounds 2a–c), the compound bearing a 3-phenylpropyl-residue (2c; pA2 = 8.27) shows the highest potency at the H3-receptor. Shortening the alkyl chain to two methylenes (compound 2b) or one methylene group (compound 2a) leads to a compound with a lower potency (pA2 = 7.61 and pA2 = 7.76, respectively). These results are in contrast to the results obtained for a series containing a propyl linker (compounds 3a–c). The compound carrying a benzyl substituent (3a; pA2 = 8.38) shows the highest potency at the H3 receptor, while the derivative with a 3-phenylpropyl moiety (3c; pA2 = 7.46) shows the lowest antagonist activity. Compounds 3d–f, containing a butyl linker, show moderate potency at H3-receptor, independent of the alkyl chain length in the ω-phenylalkyl substituent (pA2 ranging from 7.91 to 7.97).
To summarize, the obtained results indicated that elongation of the alkyl chain from two to three methylene groups between position 2 of the thiazole ring and the terminal secondary N-methylamino function resulted in compound 3a (bearing a benzyl substituent, and propyl linker). This compound demonstrated slightly higher potency than the parent compound 2c (bearing a 3-phenylpropyl moiety, and an ethyl linker) but in contrast to 3a, 2c did not possess any activity at H1.
H1 Antagonistic Activity for Compounds 3a, and 3d
The final compounds showing the highest potency for the H3 receptors were also tested for H1 antagonistic effects in vitro, following standard methods, using the guinea pig ileum [34]. Compounds 3a, and 3d show weak, but competitive H1-antagonistic activity with pA2 = 5.5, and pA2 = 6.25 (Table 1), respectively (for pyrilamine pA2 = 8.66).
2.2.2. Histamine H3 Receptor Affinity
Additionally, the affinity of the most active compound ADS-531 was evaluated by measuring the displacement curve of [3H]-Nα-methylhistamine at the rat (rH3R) and human histamine H3 receptor (hH3R) in HEK-293Tcell membranes as described by Bongers [35].
Saturation of Rat and Human H3 Receptors
To determine the total and non-specific binding, membranes expressing rH3R or hH3R were incubated with different concentrations of [3H]-Nα-MH (0–20 nM) in the absence or presence of unlabeled thioperamide (10 μM) for two hours at 25 °C. The reaction was terminated by rapid filtration on GF/C 96 well plates and the levels of the bound radioligand were measured by scintillometry. Specific binding was defined as the difference between the total and non-specific binding conditions. A representative graph of the saturation of rat and human H3R can be found in the Supplementary Material.
Analysis of the [3H]-Nα-MH saturation binding yielded at rH3R a KD value of 2.72 ± 0.34 nM and a Bmax value of 2715 ± 445 fmol/mg protein and at hH3R a KD value of 0.9 ± 0.08 nM and a Bmax value of 632 ± 52 fmol/mg protein.
Competitive Binding of H3 Receptor Ligands
The affinity of ADS-531, histamine and thioperamide—the reference compound—were determined by measuring the displacement curves of [3H]-Nα-methylhistamine binding to the rat and human histamine H3 receptor expressed in HEK-293T membranes. Derivative ADS-531 possesses a slightly lower nanomolar affinity for the rat H3R (pKi 7.5 ± 0.1) than thioperamide (pKi 7.9 ± 0.1), and slightly higher than histamine (pKi = 7.3 ± 0.1). A significantly higher affinity is observed for ADS-531 at the human H3R (pKi 8.5 ± 0.1) than of thioperamide (pKi 7.2 ± 0.1) and pKi of histamine (7.7 ± 0.1). Representative graphs of competition binding of H3R ligands on the rat and human H3 receptors are shown in the Supplementary Material (Section 2.2.2; Figures S2 and S3, respectively).
2.2.3. Verification of In Vivo Activity of Compound ADS-531
The brain histaminergic system participates in the regulation of feeding behavior, and of the four histamine receptors, H1 and H3 play an important role. Their activation is a critical part of the regulatory mechanism behind the diurnal rhythm of food consumption, as well as energy intake and expenditure [36,37,38,39]. Studies have shown that the central administration of histamine and likewise H1 receptor agonists, lowered food intake. Also, the strategies leading to enhanced synaptic histamine availability—i.e., the blockade of the H3 receptor or inhibition of histamine catabolism—caused hypophagia while the administration of H1 antagonists resulted in hyperphagia [37]. An in vivo evaluation was therefore performed on the impact of compound ADS-531 on brain neurotransmitter systems. Given that the compound enters the CNS and blocks H3R, its peripheral administration should result in neuronal histamine release. The released histamine, in turn, acting via H1R, would induce loss of appetite, resulting in a decrease of food intake. To ensure conclusive results, a five-day course of treatment was chosen with daily monitoring of consumption at 9 a.m. Any influence of subchronic administration of ADS-531 on cerebral amine neurotransmitters concentrations and/or the activities of catabolic enzymes, monoamine oxidases A and B and histamine N-methyltransferase would be disclosed by post-mortem analyses of the brain tissues of the treated rats. In our in vivo studies, Lewis rats were used as subjects, and ciproxifan was used as a reference instead of thioperamide, because the latter demonstrated lower bioavailability due to restricted brain penetration [40].
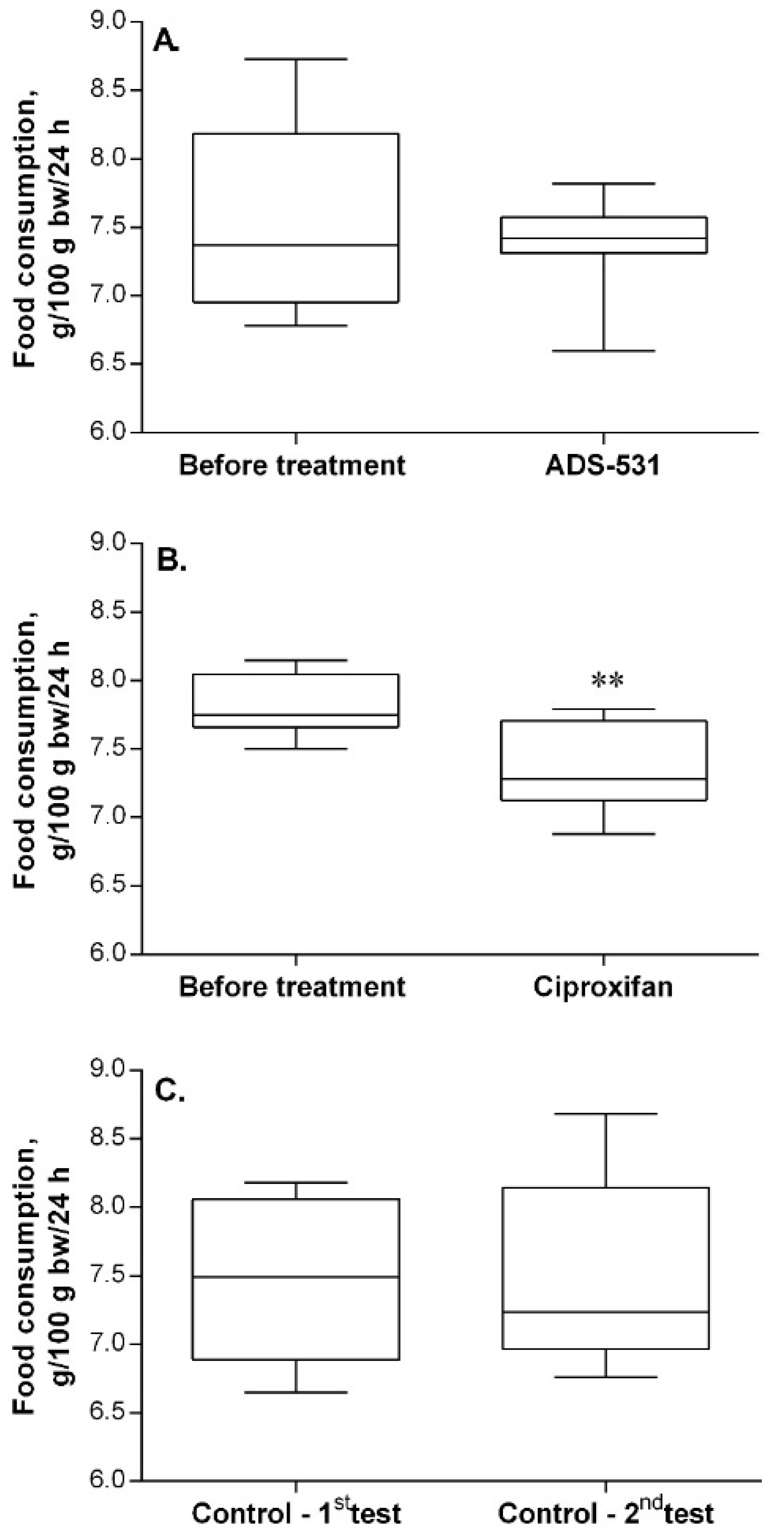
As can be seen in Figure 2, five-day treatment with ADS-531 did not influence food intake by the rats, whereas treatment with ciproxifan caused a significant decrease in consumption. It is important to note that the treatment was preceded by an adaptive period to experimental conditions.
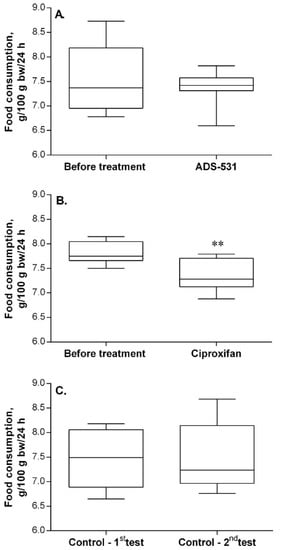
Figure 2.
The effect of ADS-531 (s.c. 3 mg/kg/daily for five days), the newly synthesized histamine H3 receptor antagonist (chart A), and of the reference ciproxifan (s.c. 3 mg/kg/daily for five days; chart B) on food consumption. Consumption by untreated rats (chart C). Median (the line in the middle of the box) and the range of values (whiskers) are given for eight rats. Paired t-test, ** p < 0.01 vs. before treatment.
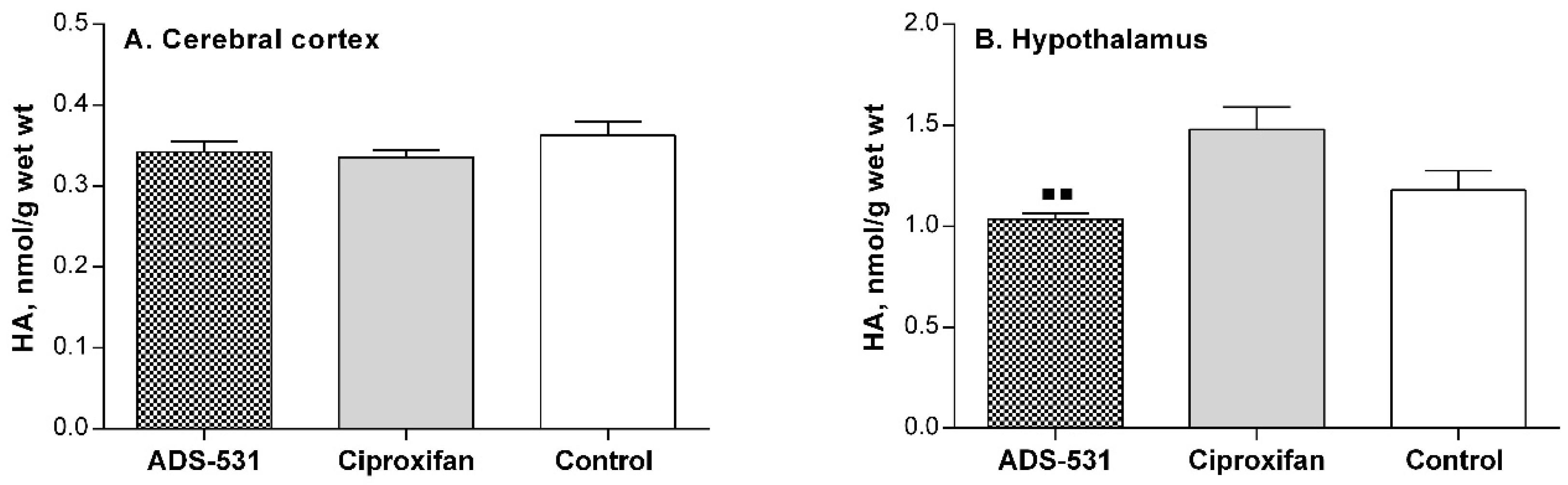
No significant changes were observed in CNS histamine concentration (Figure 3), nor in the enzyme activities related to histamine catabolism in the brain tissue of the sacrificed animals (Table 2). In the hypothalamus (Figure 3B), where the histamine cell bodies are located, the amine concentration in the ciproxifan-treated rats tended to be higher than in the untreated controls and was significantly higher than in ADS-531 injected rats. This could suggest some enhancement of histamine synthesis by ciproxifan, following the presumed enhanced release of histamine to the synapses with its anorectic effects; this observation agrees closely with the observed intravital decrement of food intake (Figure 2B). Histamine is metabolized in the mammalian brain exclusively by the N-methylation pathway, involving histamine N-methyltransferase at the first step, followed by monoamine oxidase B, which catalyzes the oxidative deamination of N-telemethylhistamine. Neither of the two drugs used affected this pathway (Table 2).
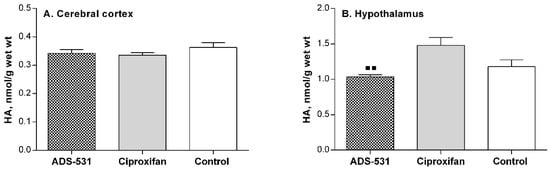
Figure 3.
Cerebral histamine concentration in rats subchronically treated with newly synthesized ADS-531 (s.c. 3 mg/kg/daily for 5 days) histamine H3 receptor antagonist or the reference ciproxifan (s.c. 3 mg/kg/daily for five days). One-way ANOVA and Tukey’s multiple comparisons tests: ■■ vs. ciproxifan, p < 0.01.

Table 2.
The effect of subchronic administration of ADS-531 (s.c. 3 mg/kg/daily for five days) or ciproxifan (s.c. 3 mg/kg/daily for 5 days) on cerebral MAOs and HNMT activities.
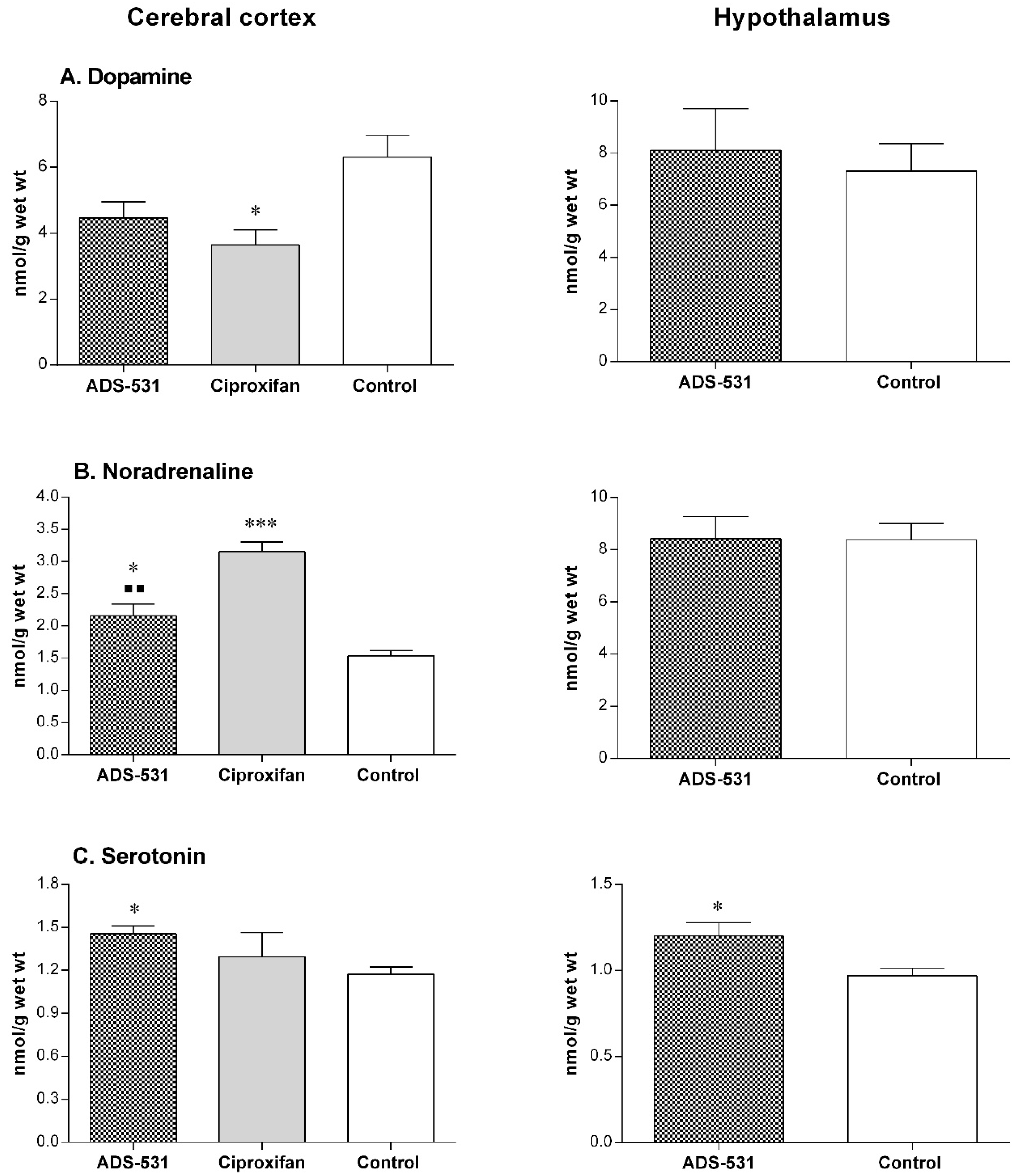
One way ANOVA and Tukey’s multiple comparisons test showed no statistically significant differences. The apparent lack of the effects of the tested compound on the histaminergic system was by no means caused by the lack of its ability to cross the blood-brain barrier. The data presented in Figure 4 clearly indicates that ADS-531 caused alterations in the concentrations of dopamine, noradrenaline, and serotonin.
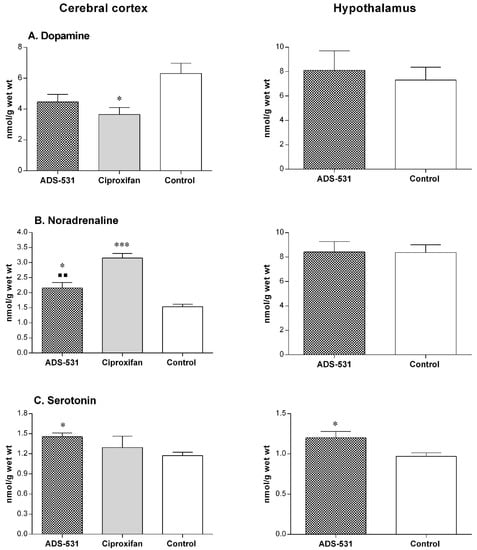
Figure 4.
The cerebral concentration of biogenic amines’ in rats subchronically treated with reference Ciproxifan (s.c. 3 mg/kg/daily for five days) and ADS-531 (s.c. 3 mg/kg/daily for five days), the newly synthesized histamine H3 receptor antagonist. DA—dopamine, NA—noradrenaline, 5-HT—serotonin. The values are means ± SEM for five to eight rats. One-way ANOVA and Tukey’s multiple comparisons test (cerebral cortex) or Unpaired t test (hypothalamus): * vs. Control, ■ vs. Ciproxifan; a single symbols means p < 0.05, two symbols: p < 0.01, three symbols: p < 0.001.
An increase of 5HT and NA level throughout the brain with the exception of the hypothalamus indicates decreased serotonergic and noradrenergic activity and a concomitant increase in DA system activity. These findings may suggest that ADS-531 is likely to show some agonistic activity to serotonin autoreceptors, thereby modifying dopamine and noradrenaline release [41].
3. Conclusions
ADS-531 was found to exhibit the highest in vitro affinity toward the H3 guinea pig jejunal receptors with pA2 = 8.27. In competition radioligand binding studies at the rat histamine H3 receptor, compound ADS-531 (pKi 7.5 ± 0.1) showed slightly lower nanomolar affinity than the reference compound—thioperamide (pKi = 7.9), and slightly higher than histamine (pKi = 7.3 ± 0.1). Significantly higher affinity was observed for ADS-531 at the human H3R (pKi = 8.5 ± 0.1) than thioperamide (pKi = 7.2 ± 0.1) and pKi of histamine (7.7 ± 0.1). ADS-531 given parenterally for five days did not influence the food intake in rats. No significant changes were observed in histamine concentration, nor in the enzyme activities related to histamine metabolism examined in the brain. The apparent lack of the effects of the tested compound on the histaminergic system was by no means caused by the lack of its ability to cross the blood-brain barrier. The presented data leaves no doubt that ADS-531 caused alterations in the concentrations of dopamine, noradrenaline, and serotonin. The high potency and affinity for H3 receptors and in vivo activity suggest that further study on ADS-531 is merited.
4. Experimental Section
4.1. General Information
All melting points (m.p.) were measured in open capillaries on an electrothermal apparatus and are uncorrected. Infrared spectra (IR) were measured on a FT-IR vegus spectrophotometer (Thermo Nicolet, city, state abbrev if USA, country). For all compounds, 1H-NMR spectra were recorded on a Mercury VX 300 MHz spectrometer (Varian, city, state abbrev if USA, country). Chemical shifts are expressed in ppm downfield from internal TMS as a reference. 1H-NMR data are reported in order: multiplicity (br, broad; s, singlet; d, doublet; t, triplet; m, multiplet; * exchangeable by D2O) number of protons, and approximate coupling constant in Hertz. 13C-NMR spectra were recorded on an Avance III 600 MHz spectrometer (Bruker, city, state abbrev if USA, country). Elemental analysis (C, H, N) for all compounds was measured on Series II CHNS/O Analyzer 2400 (Perkin Elmer, city, state abbrev if USA, country) and were within ±0.4% of theoretical values. TLC was performed on silica gel 60 F254 plates (Merck, city, state abbrev if USA, country). Flash column chromatography was carried out using silica gel 60Å 50 μm (J. T. Baker B. V., Phillipsburg, NJ, USA), employing the same eluent as was indicated by TLC. All obtained final free bases were treated with methanolic HBr, the hydrobromide was precipitated with dry diethyl ether and crystallized twice from ethanol.
4.2. Chemistry
4.2.1. General Procedure for the Preparation of Compounds 5a,b
A solution of phthalimide (14.7 g, 0.1 mol) and 5-chloropentanol (11.25 g, 0.1 mol) or 6-chloro-hexanol (12.45 g, 0.1 mol) in anhydrous DMF (100 mL) was heated at 150 °C with vigorous stirring in the presence of finely-powdered anhydrous K2CO3 (13.8 g, 0.1 mol) for 12 h. After cooling, the inorganic materials were filtered off and the solvent was evaporated in vacuo (2 mmHg, 100 °C). The residue was dissolved in 150 mL of ethyl acetate, and after standing overnight at 5 °C, the solution was filtered and concentrated in vacuo. The residue was taken up in 80 mL of CHCl3. After extraction with 60 mL of 5% aqueous solution of NaHCO3 and 3 × 60 mL of H2O, the solvent was dried over Na2SO4 and removed in vacuo, giving:
2-(5-Hydroxypentyl)-1H-isoindole-1,3(2H)-dione (5a). A sticky oil (on standing for a prolonged period the viscous oil crystallized giving a solid), yield 70% (16.32 g); m.p. 41–43 °C; Rf = 0.39 (CHCl3/CH3OH 20:1); 1H-NMR (300 MHz, CDCl3): δ = 1.40–1.43 (m, 4H, CH2C), 1.53–1.58 (m, CH2CH2OH), 1.66–1.71 (m, 3H, CH2CH2N, OH), 3.61–3.63 (t, J = 6.6 Hz, 2H, CH2N), 3.66–3.68 (t, J = 7.2 Hz, 2H, CH2OH), 7.68–7.71 (m, 2H, Harom); 7.81–7.84 (m, 2H, H) ppm.
2-(6-Hydroxyhexyl)-1H-isoindole-1,3(2H)-dione (5b). A sticky oil, yield 62% (15.33 g); Rf = 0.40 (CHCl3/CH3OH 20:1); 1H-NMR (300 MHz, CDCl3): δ = 1.40–1.43 (m, 4H, CH2C), 1.53–1.58 (m, CH2CH2OH), 1.66–1.71 (m, 3H, CH2CH2N, OH), 3.61–3.63 (t, J = 6.6 Hz, 2H, CH2N), 3.66–3.68 (t, J = 7.2 Hz, 2H, CH2OH), 7.68–7.71 (m, 2H, H), 7.81–7.84 (m, 2H, H) ppm.
4.2.2. General Procedure for the Preparation of Compounds 6a,b
To a well-stirred solution of oxalyl chloride (6.08 mL, 0.07 mol) in dry CH2Cl2 (120 mL), a solution of anhydrous DMSO (5.69 mL, 0.08 mol) in CH2C12 (200 mL) was added under an argon atmosphere at −70 °C at such a rate that the temperature was maintained at −70 °C. After the addition was completed, stirring was continued for 15 min, and then, a solution of 5a (7.7 g, 0.033 mol) or 5b (8.16 g, 0.033 mol) in dry CH2C12 (70 mL) was added while keeping the temperature at −70 °C. The reaction mixture was stirred for another 30 min at −70 °C, and Et3N (17.1 mL, 0.122 mol) was added. The mixture was allowed to warm to ambient temperature, and 100 mL of 5% aqueous solution of HCl was added and stirring was continued for 20 min. The organic layer was separated and extracted with H2O to the almost neutral reaction, dried over anhydrous Na2SO4, filtered, and concentrated in vacuo to yield the crude product 6a and 6b. The obtained viscous oils were stored under argon and used without further purification for the preparation of 7a and 7b:
5-(1,3-Dioxo-1,3-dihydro-2H-isoindol-2-yl)pentanal (6b). A sticky oil, 82.0% (7.63 g) based on 1H-NMR); Rf = 0.25 (CHCl3/CH3OH 100:1); 1H-NMR (300 MHz, CDCl3): δ = 1.62–1.77 (m, 4H, CCH2CH2C), 2.50–2.52 (t, J = 7.2, J = 1.8 Hz, 2H, CH2CHO), 3.64–3.72 (t, J = 7.2 Hz, CH2N), 7.69–7.74 (m, 2H, H ar.), 7.82–7.86 (m, 2H, H), 9.69–9.71 (t, J = 1.8 Hz, CHO) ppm.
6-(1,3-Dioxo-1,3-dihydro-2H-isoindol-2-yl)hexanal (6b). A sticky oil, 79.0% (8.09 g) based on 1H-NMR); Rf = 0.46 (CHCl3/CH3OH 100:1); 1H-NMR (300 MHz, CDCl3): δ ppm: 1.28–1.35 (m, 2H, CH2C), 1.58–1.66 (m, 4H, CH2CH2CHO, CH2CH2N), 2.34–2.39 (m, 2H, CH2CHO), 3.58–3.62 (t, J = 7.2 Hz, CH2N), 7.62–7.68 (m, 2H, H), 7.24–7.78 (m, 2H, H), 9.67–9.68 (t, J = 1.8 Hz, CHO) ppm.
4.2.3. General Procedure for the Preparation of Compounds 7a,b
Small portions of a solution of Br2 (4.8 g, 0.03 mol) in acetonitrile (10 mL) were added to a stirred solution of 6a (6.93 g, 0.03 mol) or 6b (7.36 g, 0.03 mol) in CCl4 (130 mL) under an argon atmosphere. The reaction mixture was stirred for two hours at ambient temperature, and then 5% aqueous solution of NaHCO3 (200 mL) and CHCl3 (50 mL) were added followed by stirring for 15 min. The organic phase was separated and extracted under an argon atmosphere with H2O till neutral reaction. After drying over anhydrous Na2SO4 and filtration, the solvent was removed in vacuo. The resulting viscous oils were used without further purification for the preparation of 9a and 9b.
2-Bromo-5-(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)pentanal (7a). A sticky oil (containing 9.3 g of 7a), yield 76.0% (based on 1H-NMR); Rf = 0.44 (CHCl3/CH3OH 100:1); 1H-NMR (300 MHz, CDCl3): δ = 2.08–2.13 (m, 2H, CH2CH2CH2), 2.49–2.54 (m, 2H, CH2CHBr), 3.74–3.76 (t, J = 7.2 Hz, 2H, CH2N), 4.33–4.36 (m, 1H, CHCHO), 7.72–7.74 (m, 2H), 7.84–7.87 (m, 2H), 9.44 (d, J = 2.4 Hz, 1H, CHO) ppm.
2-Bromo-6-(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)hexanal (7b). A sticky oil (containing 9.7 g of 7b) yield 74.7% (based on 1H-NMR); Rf = 0.46 (CHCl3/CH3OH 100:1); 1H-NMR (300 MHz, CDCl3): δ = 1.72–1.80 (m, 4H, CH2C), 1.96–2.11 (m, 2H, CH2CHBrCHO), 3.68–3.73 (m, 2H, CH2N), 4.20–4.26 (m, 1H, CHBr), 7.82–7.85 (m, 2H, Harom.), 7.85–7.87 (m, 2H, Harom.), 9.43–9.44 (d, J = 2.7 Hz, 1H, CHO) ppm.
4.2.4. General Procedure for the Preparation of Compounds 9a,b
A solution of 4-propylpiperazine-1-carbothioamide (8, 4.12 g, 0.022 mol) in anhydrous DMF (250 mL) was added to a stirred solution of 7a (7.06 g, 0.022 mol) or 7b (7.2 g, 0.022 mol) in anhydrous DMF (250.0 mL) under an argon atmosphere. The reaction mixture was heated at 95 °C for nine hours. After cooling, the solvent was removed in vacuo. The residue was dissolved in n-propanol and cooled to 5 °C. The precipitate was filtered off and washed with ether. The hydrobromide product was obtained as a brown solid. The free base was obtained as follows: the hydrobromide was mixed with saturated aqueous potassium carbonate solution over night at room temperature. The solid was filtered, washed with water, ether and air dried to leave a light brown solid. The crude product was purified by silica gel flash-column chromatography, giving:
2-[3-(4-n-Propylpiperazin-1-yl)propyl]-1H-isoindole-1,3(2H)-dione (9a). A white solid, yield 34% (2.98 g); Rf = 0.51 (CHCl3/CH3OH 15:1); m.p.: 80–81 °C; (m.p.dihydrobromide: 258–260 °C), 1H-NMR (300 MHz, CDCl3): δ = 0.89–0.94 (t, J = 7.2 Hz, 3H, CH3); 1.46–1.56 (sx, J = 7.2 Hz, 2H, CH3CH2), 1.97–2.02 (p, J = 7.2 Hz, 2H, CH2CH2N), 2.32–2.35 (m, 2H, CH2N), 2.49–2.58 (m, 4H, CH2piperazine), 2.72–2.74 (t, J = 7.2 Hz, 2H, CH2thiazole), 3.41–3.43 (m, 4H, CH2piperazine), 3.75–3.76 (t, J = 7.2 Hz, CHN(CO)2), 6.87 (s, 1H, Hthiazole), 7.70–7.72 (m, 2H, Harom), 7.82–7.84 (m, 2H, Harom.) ppm.
2-[4-(4-n-Propylpiperazin-1-yl)butyl]-1H-isoindole-1,3(2H)-dione (9b). A white solid, yield 38% (3.44 g); Rf = 0.23 (CHCl3/CH3OH 20:1); m.p.: 102–104 °C; (m.p.dihydrobromide: 257–259 °C); 1H-NMR (300 MHz, CDCl3): δ = 0.89–0.94 (t, J = 7.2 Hz, 3H, CH3), 1.49–1.74 (m, 6H, CH2C), 2.30–2.35 (m, 2H, CH2N), 2.52–2.54 (m, 4H, Hpiperazine), 2.66–2.69 (m, 2H, CH2thiazole), 3.42–3.44 (m, 4H, Hpiperazine), 3.67–3.69 (t, J = 7.2 Hz, CH2NCO), 6.80–6.81 (t, J = 1.2 Hz, CHthiazole), 7.68–7.70 (m, 2H, Harom.); 7.79–7.82 (m, 2H, Harom) ppm.
4.2.5. General Procedure for the Preparation of Compounds 10a,b
A solution of 9a (3.19 g, 0.008 mol) or 9b (3.30 g, 0.008 mol) and of N2H4·H2O (0.8 g, 0.016 mol) in MeOH (50 mL) was refluxed for nine hours. The solvent was evaporated and the remaining material was dissolved in 30 mL of methylene chloride. After cooling, the crystallized phthalhydrazide was filtered off. Concentration in vacuo provided a white sticky semi-solid, which was purified by column chromatography on silica gel, giving:
3-[2-(4-Propylpiperazin-1-yl)-1,3-thiazol-5-yl]propan-1-amine (10a). A sticky oil, yield 60% (1.29 g); Rf = 0.33 (CHCl3/MeOH/NH3aq 60:10:1); m.p.treehydrobromide: 240–242 °C; 1H-NMR (300 MHz, CDCl3) δ = 0.91–0.93 (t, J = 7.2 Hz, 3H, CH3), 1.39 (sbr, 2H, NH2), 1.49–1.56 (sx, J = 7.2 Hz, 2H, CHCH2), 1.71–1.76 (p, J = 7.2 Hz, 2H, CCH2C), 2.33–2.36 (m, 2H, CH2N), 2.52–2.54 (m, 4H, CH2piperazine), 2.70–2.72 (t, J = 7.2 Hz, 2H, CH2thiazole), 2.74–2.76 (t, J = 7.2 Hz, 2H, CH2NH2), 3.43–3.45 (m, 4H, CH2piperazine), 6.84 (s, 1Hthiazole) ppm.
4-[2-(4-Propylpiperazin-1-yl)-1,3-thiazol-5-yl]butan-1-amine (10b). A sticky oil, yield 62% (1.40 g): Rf = 0.18 (CHCl3/MeOH/NH3aq 60:10:1); m.p.treehydrobromide: 222–224 °C; 1H-NMR (300 MHz, CDCl3) δ = 0.87–0.92 (t, J = 7.5 Hz, 3H, CH3), 1.45–1.63 (m, 6H, CH2C), 2.25 (sbr, 2H, NH2), 2.29–2.35 (m, 2H, CH2N-propyl), 2.50–2.53 (m, 4H, Hpiperazine), 2.62–2.72 (m, 4H, CH2thiazole, CH2N), 3.40–3.43 (m, 4H, CH2piperazine), 6.81 (s, 1H, CHthiazole) ppm.
4.2.6. General Procedure for the Preparation of Compounds 11a,b
A solution of 10a (1.85 g, 0.0069 mol) or 10b (1.95 g, 0.0069 mol) and methyl formate (16.33 g, 16.0 mL, 0.27 mol) in MeOH (6 mL), was heated under an argon atmosphere at 32 °C for 48 h. The solvent was evaporated to yield the crude product 11a and 11b. The obtained viscous oils were stored under argon and used without further purification for the preparation of 7a and 7b.
N-{3-[2-(4-Propylpiperazin-1-yl)-1,3-thiazol-5-yl]propyl}formamide (11a). A sticky oil (on standing for a prolonged period the viscous oil crystallized giving the solid, m.p. 57–59 °C), yield 91.0% (1.87 g; based on 1H-NMR); Rf = 0.5 (CHCl3/CH3OH 9:1); 1H-NMR (300 MHz, CDCl3) δ = 0.91–0.93 (t, J = 7.2 Hz, 3H, CH3), 1.50–1.56 (sx, J = 7.2 Hz, 2H, CH3CH2), 1.80–1.85 (p, J = 7.2 Hz, 2H, CCH2C), 2.32–2.36 (m, 2H, CH2N), 2.53–2.55 (m, 4H, CH2piperazine), 2.69–2.72 (t, J = 7.2 Hz, 2H, CH2thiazole), 3.32–3.35 (q, J = 6.6 Hz, 2H, CH2NHCHO), 3.43–3.46 (m, 4H, CH2piperazine), 6.06 (br, 1H, NH), 6.84 (s, 1H, Hthiazole), 8.16 (s, 1H, CHO) ppm.
N-{4-[2-(4-Propylpiperazin-1-yl)-1,3-thiazol-5-yl]butyl}formamide(11b). A sticky oil (on standing for a prolonged period the viscous oil crystallized giving the solid, m.p. 58–60 °C), yield 83.0% (1.77 g, based on 1H NMR); Rf = 0.58 (CHCl3/CH3OH/NH3aq 60:10:1); 1H-NMR (300 MHz, CDCl3): δ = 0.91–0.93 (t, J = 7.4 Hz, 3H, CH3), 1.50–1.64 (m, 6H, CH2-C), 2.34–2.36 (m, 2H, CH2Npiperazine), 2.53–2.57 (m, 4H, CH2piperazine), 2.67–2.69 (t, J = 6.7 Hz, CH2thiazole), 3.30–3.33 (t, J = 7.6 Hz, 2H, CH2NH); 3.44–3.45 (m, 4H, CH2piperazine); 5.61 (sbr, 1H, NH); 6.83 (s, 1H, Hthiazole); 8.16 (s, 1H, CHO) ppm.
N-{4-[2-(4-Propylpiperazin-1-yl)-1,3-thiazol-5-yl]butyl}formamide (11b). A sticky oil (on standing for a prolonged period the viscous oil crystallized giving the solid, m.p. 58–60 °C), yield 83.0% (1.77 g, based on 1H NMR); Rf = 0.58 (CHCl3/CH3OH/NH3aq 60:10:1); 1H-NMR (300 MHz, CDCl3): δ = 0.91–0.93 (t, J = 7.4 Hz, 3H, CH3), 1.50–1.64 (m, 6H, CH2-C), 2.34–2.36 (m, 2H, CH2Npiperazine), 2.53–2.57 (m, 4H, CH2piperazine), 2.67–2.69 (t, J = 6.7 Hz, CH2thiazole), 3.30–3.33 (t, J = 7.6 Hz, 2H, CH2NH); 3.44–3.45 (m, 4H, CH2piperazine); 5.61 (sbr, 1H, NH); 6.83 (s, 1H, Hthiazole); 8.16 (s, 1H, CHO) ppm.
4.2.7. General Procedure for the Preparation of Compounds 12a,b
To a vigorous stirred suspension of 11a (0.98 g, 0.0033 mol) or 11b (1.02 g, 0.0033 mol) in anhydrous ethyl ether (200 mL), LiAlH4 (0.35 g, 0.009 mol) was added. The mixture was stirred at room temperature for two hours, and quenched by dropwise addition of water (0.7 mL), 5% of NaOH solution (0.6 mL), and water (0.2 mL). The suspension was stirred for 30 min, and filtered. The filter cake was washed with ether (2 × 50 mL). The combined organic extracts were washed with water (3 × 50 mL), dried (Na2SO4), and filtered. The solvent was evaporated and remaining material was purified by column chromatography on silica gel, giving:
N-Methyl-3-[2-(4-propylpiperazin-1-yl)-1,3-thiazol-5-yl]propan-1-amine (12a). A white solid, yield 85% (1.15 g); (m.p. 38–40 °C; Rf = 0.24 (CHCl3/CH3OH/NH3aq 60:10:1); 1H-NMR (300 MHz, CDCl3): δ = 0.91–0.93 (t, J = 7.2 Hz, 3H, CH3). 1.23 (sbr, 1H, NH). 1.50–1.55 (sx, J = 7.8 Hz, 2H, CH2CH3), 1.75–1.85 (p, J = 7.2 Hz, 2H, CH2CH2CH2), 2.33–2.35 (m, 2H, CH2N), 2.42 (s, 3H, CH3N), 2.52–2.55 (m, 4H, CH2piperazine), 2.61–2.63 (t, J = 7.2 Hz, 2H, CH2NHCH3), 2.69–2.72 (t, J = 7.2 Hz, 2H, CH2thiazole), 3.43–3.46 (m, 4H, CH2piperazine), 6.84 (s, 1H, Hthiazole) ppm; IR (nujol): 3301, 2934, 2843, 2789, 1670, 1519, 1451, 1376, 1278, 1232, 1145, 1037, 988, 912, 842, 802, 751 cm−1.
N-Methyl-4-[2-(4-n-propylpiperazin-1-yl)-1,3-thiazol-5-yl]butan-1-amine (12b). A sticky oil, yield 58.3% (0.57 g); Rf = 0.16 (CHCl3/CH3OH/NH3aq 60:10:1); 1H-NMR (300 MHz, CDCl3): δ = 0.91–0.93 (t, J = 7.4 Hz, 3H, CH3), 1.50–1.65 (m, 6H, CH2C), 2.33–2.35 (m, 4H, CH2Npiperazine), 2.42 (s, 3H, NCH3), 2.52–2.54 (m, 4H, CH2piperazine), 2.57–2.59 (t, J = 7.0 Hz, 2H, CH2NCH3); 2.65–2.68 (t, J = 7.4 Hz, 2H, CH2thiazole); 3.43–3.47 (m, 4H, CH2piperazine); 6.83 (s, 1H, Hthiazole) ppm; IR (nujol): 3298, 3045, 2933, 2874, 2845, 2812, 1520, 1453, 1376, 1341, 1277, 1232, 1168, 1145, 1100, 1037, 988, 890, 843, 802, 752 cm−1.
4.2.8. General Procedure for the Preparation of Compounds 3a–f
The corresponding phenylalkyl bromide (0.001 mol) was added to a solution of 12a (0.283 g, 0.001 mol) or 12b (0.296 g, 0.001 mol) in the presence of potassium carbonate (0.002 mol) in acetonitrile (20.0 mL). The reaction mixture was stirred for ninety-six hours at room temperature. The solvent was evaporated and water (25 mL) was added to the residue the mixture was extracted with dichloromethane (3 × 25 mL). The water layer was discarded and the solvent was dried over Na2SO4 and filtered. The solvent was evaporated and the crude product was purified by column chromatography on silica gel, giving:
N-Benzyl-N-methyl-3-[2-(4-n-piperazin-1-yl)-1,3-thiazol-5-yl]propan-1-amine (3a). A sticky oil, yield 74.0% (0.28 g); Rf = 0.16 (CHCl3/CH3OH 6:1); m.p.trihydrobromide = 254–255 °C; 1H-NMR (300 MHz, CDCl3): δ = 0.91–0.93 (t, J = 7.2 Hz, 3H, CH3), 1.50–1.56 (sx, J = 7.8 Hz, 2H, CH2CH3), 1.77–1.81 (p, J = 7.6 Hz, 2H, CH2CH2CH2), 2.17 (s, 3H, CH3), 2.32–2.35 (m, 2H, CH2N), 2.41–2.43 (t, J = 7.2 Hz, 2H, CH2NCH3), 2.52–2.54 (m, 4H, CH2piperazine.), 2.68–2.71 (t, J = 7.2 Hz, 2H, CH2thiazole), 3.43–3.47 (m, 4H, CH2piperazine), 3.51 (s, 2H, CH2Ph), 6.82 (s, 1H, Hthiazole), 7.21–7.24 (m, 1H), 7.28–7.31 (m, 4H) ppm; 13C-NMR (75 MHz, CDCl3): δ = 11.83, 19.94, 24.68, 29.05, 42.04, 48.38, 52.37, 56.35, 60.53, 62.34, 126.82, 127.49, 128.13, 128.91, 135.41, 139.20, 170.59 ppm. IR (nujol): 3027, 2938, 2874, 2786, 1519, 1453, 1376, 1280, 1231, 1168, 1145, 1004, 911, 845, 737, 699 cm−1. Anal. calcd for C21H35 Br3N4S (M = 615.34): C 40.99, H 5.73, N9.11, found: C 40.81, H 5.76, N 8.90.
N-Methyl-N-(2-phenylethyl)-3-[2-(4-n-propylpiperazin-1-yl)-1,3-thiazol-5-yl]propan-1-amine (3b). A sticky oil; yield 82.0% (0.32 g); Rf = 0.45 (CHCl3/CH3OH 6:1); m.p.trihydrobromide = 255–256 °C; 1H-NMR (300 MHz, CDCl3): δ = 0.90–0.93 (t, J = 7.2 Hz, 3H, CH3), 1.50–1.56 (sx, J = 7.2 Hz, 2H, CH2CH3), 1.73–1.78 (p, J = 7.2 Hz, 2H, CH2CH2CH2), 2.29 (s, 3H, NCH3), 2.32–2.35 (m, 2H, CH2N), 2.42–2.45 (t, J = 7.2 Hz, 2H, CH2NCH3), 2.52–2.54 (m, 4H, CH2piperazine), 2.59–2.61 (m, 2H, NCH2CH2Ph), 2.64–2.66 (t, J = 7.2 Hz, 2H, CH2thiazole), 2.74–2.77 (m, 2H, CH2Ph), 3.43–3.45 (m, 4H, CH2piperazine), 6.82 (s, 1H, Hthiazole), 7.18–7.19 (m, 3H, H), 7.26–7.28 (m, 2H, H) ppm; 13C-NMR (75 MHz, CDCl3): δ = 11.84, 19.95, 24.77, 28.93, 33.81, 42.11, 48.40, 52.37, 56.50, 59.52, 60.55, 125.88, 127.41, 128.28, 128.65, 135.47, 140.57, 170.63 ppm; IR (nujol): 3027, 2938, 2874, 2786, 1519, 1453, 1376, 1280, 1231, 1168, 1145, 1004, 911, 845, 737, 699 cm−1. Anal. calcd for C22H37 Br3N4S × H2O (M = 647.38): C 40.81, H 6.07, N 8.65, found: C 41.06, H 6.13, N 8.48.
N-Methyl-N-(3-phenylpropyl)-3-[2-(4-n-propylpropylpiperazin-1-yl)-1,3-thiazol-5-yl]-propan-1-amine (3c). A sticky oil; yield 52.0% (0.22 g); Rf = 0.24 (CHCl3/CH3OH 6:1); m.p.trihydrobromide monohydrate = 252–254 °C; 1H-NMR (300 MHz, CDCl3): δ = 1.51–1.55 (sx, J = 7.6 Hz, 2H, CCH2C); 1.73–1.80 (m, 2H, CCH2C); 2.21 (s, 3H, CH3); 2.33–2.38 (m, 6H CH2N, CH2Ph); 2.51–2.54 (m, 4H CH2piperazine); 2.61–2.64 (t, J = 7.9 Hz, 2H, CH2thiazole); 2.66–2.69 (t, J = 7.3 Hz, 2H, CH2N), 3.43–3.45 (m, 4H, CH2piperaz), 6.84 (s, 1H, Hthiazole); 7.17–7.18 (m, 3H, Harom), 7.25–7.28 (m, 2H, Harom); 13C-NMR (CDCl3): δ = 12.07, 20.17, 25.08, 29.11, 29.11, 29.16, 33.85, 42.29, 52.59, 56.89, 57.45, 60.77, 125.88, 127.66, 128.46, 128.56, 135.63, 142.48, 170.84. IR (nujol): 3025, 2939, 2874, 2788, 1519, 1453, 1376, 1279, 1231, 1168, 1135, 1032, 1004, 911, 843, 802, 751, 699 cm−1. Anal. calcd for C23H39Br3N4S (M = 643.385): C 42.93, H 6.11, N 8.71, found: C 42.75, H 5.85, N 8.76.
N-Benzyl-N-methyl-4-[2-(4-n-propylpiperazin-1-yl)-1,3-thiazol-5-yl]butan-1-amine (3d). A sticky oil; yield 81.0% (0.313 g); Rf = 0.38 (CHCl3/CH3OH/NH3aq 100:10:1); m.p.trihydrobromide dihydrate = 196–197 °C; 1H-NMR (300 MHz, CDCl3): δ = 0.90–0.93 (t, J = 7.4 Hz, 3H, CH3), 1.48–1.63 (m, 6H, CH2C), 2.17 (s, 3H, NCH3), 2.29–2.38 (m, 4H, CH2N), 2.49–2.53 (m, 4H, CH2piperazine); 2.62–2.64 (t, J = 7.0 Hz, 2H, CH2thiazole), 3.43–3.44 (m, 4H, CH2piperazine), 3.46 (s, 2H, CH2Ph 6.82), 6.81 (s, 1H, Hthiazole); 7.23–7.31 (m, 5H) ppm; 13C-NMR (75 MHz, CDCl3): δ = 12.11, 20.13, 26.78, 27.08, 29.19, 42.37, 48.58, 52.56, 57.64, 60.84, 62.54, 127.00, 127.89, 128.31, 128.43, 135.51, 139.41, 170.74 ppm; IR (nujol): 3027, 2935, 2874, 2785, 1519, 1453, 1376, 1278, 1231, 1168, 1145, 1037, 1004, 911, 843, 802, 738, 699 cm−1. Anal. calcd for C22H37 Br3N4S × 2H2O (M = 665.392): C 39.71, H 5.91, N 8.43, found: C 40.03, H 5.94, N 8.46.
N-Methyl-N-(2-phenylethyl)-4-[2-(4-n-propylpiperazin-1-yl)-1,3-thiazol-5-yl]butan-1-amine (3e). A sticky oil yield 42.0% (0.168 g); Rf = 0.16(CHCl3/CH3OH/NH3aq 200:10:1); mptrihydrobromide monohydrate = 196–197 °C; 1H-NMR (300 MHz, CDCl3): δ = 0.81–0.86 (t, J = 7.2 Hz, 3H, CH3), 1.40–1.50 (m, 6H, CH2C), 2.21–2.24 (m, 5H, NCH3, CH2Npropyl), 2.42–2.45 (m, 4H, CH2piperazine), 2.51–2.57 (m, 4H, CH2N), 3.34–3.37 (m, 4H, CH2piperazine) 6.75 (s, 1H, Hthiazole), 7.09–7.22 (m, 5H, H) ppm; 13C-NMR (75 MHz, CDCl3): δ = 12.08, 20.10, 26.64, 27.11, 29.31, 33.84, 42.25, 48.43, 52.44, 57.25, 59.61, 60.61, 125.89, 127.51, 128.27, 128.60, 135.28, 140.33, 170.44 ppm; IR (nujol): 3027, 2935, 2875, 2785, 1519, 1453, 1376, 1278, 1231, 1168, 1145, 1037, 1004, 911, 843, 802, 738, 699 cm−1. Anal. calcd forC23H39Br3N4S × H2O (M = 661.402): C 41.76, H 6.24, N 8.42, found: C 41.82, H 6.27, N 8.51.
N-Methyl-N-(3-phenylpropyl)-4-[2-(4-n-propylpiperazin-1-yl)-1,3-thiazol-5-yl]butan-1-amine (3f). A sticky oil yield 24.0% (0.098 g); Rf = 0.18 (CHCl3/CH3OH/NH3aq 100:10:1); mptrihydrobromide = 208–210 °C; 1H-NMR (300 MHz, CDCl3): δ = 0.89–0.94 (t, J = 7.2 Hz, 3H, CH3), 1.49–1.59 (m, 6H, CH2C), 1.79–1.82 (m, 2H, NCH2CH2CH2Ph), 2.20 (s, 3H, NCH3), 2.31–2.38 (m, 6H, CH2Npropyl, CH2NCH3), 2.51–2.54 (m, 4H, CH2piperazine), 2.59–2.68 (m, 4H, CH2thiazole, CH2-Ph), 3.42–3.45 (m, 4H, CH2piperazine), 6.83 (s, 1H, Hthiazole), 7.14–7.29 (m, 5H, H) ppm; 13C-NMR (75 MHz, CDCl3): δ = 12.17, 20.22, 26.80, 29.21, 29.48, 33.89, 42.36, 48.55, 52.57, 57.39, 57.52, 60.74, 125.74, 127.70, 128.31, 128.40, 135.36, 142.27, 170.57 ppm; IR (nujol): 3027, 2935, 2875, 2785, 1519, 1453, 1376, 1278, 1231, 1168, 1145, 1037, 1004, 911, 843, 802, 738, 699 cm−1. Anal. calcd for C24H41Br3N4S (M = 657.416): C 43.84, H 6.29, N 8.52, found: C 43.51, H 6.32, N 8.53.
4.3. In Vitro Pharmacology
4.3.1. H3 Antagonistic Activity for Compounds 3a–f
In the first step, all obtained compounds were tested for H3 antagonistic effects in vitro, according to standard methods, based on electrically contracting guinea pig jejunum [33]. Male guinea pigs weighing 300–400 g were sacrificed and a portion of the small intestine, 20–50 cm proximal to the ileocaecal valve (jejunum), was removed and placed in Krebs buffer (composition (mM) NaCl 118; KCl 5.6; MgSO4 1.18; CaCl2 2.5; NaH2PO4 1.28; NaHCO3 25; glucose 5.5 and indomethacin (1 × 10−6 mol/L)). Whole jejunum segments (2 cm) were prepared and mounted between two platinum electrodes (4 mm apart) in 20 mL Krebs buffer, continuously gassed with 95% O2:5% CO2 and maintained at 37 °C. Contractions were recorded isotonically under 1.0 g tension with Hugo Sachs Hebel-Messvorsatz (Tl-2)/HF-modem (Hugo Sachs Elektronik, Hugstetten, Germany) connected to a pen recorder. The equilibration took place for one hour with washings every 10 min. The muscle segments were then stimulated at a maximum between 15 and 20 V, continuously at a frequency of 0.1 Hz for a duration of 0.5 ms, with rectangular-wave electrical pulses, delivered by a Grass Stimulator S-88 (Grass Instruments Co., Quincy, MA, USA). After 30 min of stimulation, pyrilamine (1 × 10−5 mol/L concentration in organ bath) was added, followed by (R)-α-methylhistamine five minutes later, and then cumulative concentration-response curves (half-log increments) of (R)-α-methylhistamine, H3-agonist, were recorded until no further change in response was found. Five minutes before adding the tested compounds, pyrilamine (1 × 10−5 mol/L concentration in an organ bath) was added, after another 20 min cumulative concentration-response curves (half-log increments) of (R)-α-methylhistamine, an H3-agonist, were recorded until no further change in response was found. Statistical analysis was carried out with the Students’ t-test. In all tests, p < 0.05 was considered statistically significant. The potency of an antagonist is expressed by its pA2 value, calculated from the Schild [33] regression analysis where at least three concentrations were used. The pA2 values were compared with the potency of thioperamide.
4.3.2. H1 Antagonistic Activity for Compounds 3a,d
Selected final compounds were tested for H1 antagonistic effects in vitro, following standard methods, using the guinea pig ileum [34]. The donors were male guinea pigs (300–400 g) as mentioned above. The excised ileum was placed in phosphate buffer at room temperature (pH 7.4) containing (mM) NaCl (136.9); KCl (2.68); NaHPO4 (7.19). The intraluminal content was flushed, and segments about 2 cm in lenght were cut and mounted for isotonic contractions in water mixed with 20 mL organ baths filled with oxygenated (O2:CO2 = 95:5, v/v) Krebs buffer containing (mM) NaCl (117.5); KCl (5.6); MgSO4 (1.18); CaCl2 (2.5); NaH2PO4 (1.28); NaHCO3 (25); glucose (5.5) and indomethacin (1 × 10−6 mol/L) at 37 °C under a constant load of 0.5 g. After a 30 min equilibration period with washings every 10 min, a submaximal priming dose of histamine (1 mM) was given and washed out (standard washing procedure: 3 changes of buffer during 30 min). After washing out, the antagonistic activity of given compounds was measured by recording a Concentration Response Curve (CRC) for histamine in the presence of the tested compounds 3a and 3d, which were added 5 min before histamine. This procedure was repeated with higher concentrations of the compounds. The antagonism was of a competitive nature causing a parallel shift of the CRC. The pA2-values were calculated according to Arunlakshana and Schild [34]. The pA2 values were compared with the affinity of pyrilamine.
4.3.3. Antagonist Binding to the Rat rH3R and Human hH3R
Cell Culture and Transfection
Human Embryonic Kidney cells (HEK293T) were cultured in DMEM supplemented with 10% Fetal Bovine Serum and 100 IU·mL−1 penicillin and 100 μg·mL−1 streptomycins at 37 °C and 5% CO2. The day prior transfection two million cells were seeded in 10 cm dishes. Approximately four million cells were transfected by the polyethyleneimine (PEI) method with 5 μg of cDNA in a ratio of 1:4 (DNA:PEI). Briefly, 0.5 μg of pcDNA3-rH3R or pcDNA3.1-hH3R and 4.5 μg of empty plasmid (pcDNA3.1) were mixed with 20 μg of 25 kDa linear PEI in 500 μL of 150 mM NaCl and incubated for 30 min at 22 °C. Meanwhile, medium from 10 cm dishes was replaced with fresh culture medium and the transfection mix was subsequently added drop-wise to the cells and incubated for 48 h at 37 °C and 5% CO2.
Crude Membrane Extracts
Forty-eight hours after transfection cells were washed with ice-cold phosphate buffered saline (PBS) scrapped and the homogenate centrifuged for 10 min at ~2000 g, 4 °C. The supernatant was aspirated and cell pellets were resuspended in 1 mL ice-cold PBS and centrifuged again under same conditions, the supernatant aspirated and the membranes stored at −20 °C until further use.
[3H]-Nα-methylhistamine Binding
Frozen cell pellets were dissolved in 50 mM Tris-HCl buffer (pH 7.4), homogenized by sonication (40 Watt Labsonic 1510) for 3 to 5 s and kept on ice until use. Cell homogenates were incubated in presence of increasing concentrations of [3H]-NαMH (0–20 nM) in 50 mM Tris-HCl binding buffer for saturation binding. Total and non-specific binding was determined in the absence or presence of excess non-labeled thioperamide (10 μM), respectively. For the competition binding assay, homogenates were incubated with increasing concentrations of receptor ligands (10−11 to 10−4 M) and ~2.5 nM of [3H]-NαMH. All assays were incubated at 25 °C for two hours on a shaking table (600 rpm). The reaction was terminated by rapid filtration into 0.5% polyethyleneimine pre-soaked glass fiber C plates (GF/C Perkin Elmer) followed by three washes with ice-cold Tris-HCl buffer (pH 7.4 at 4 °C). The plates were dried for one hour at 50 °C and scintillation liquid were added to each well (25 μL). Retained radioactivity was determined by liquid scintillation counting in a Wallac Microbeta (Perkin Elmer). Protein determination for Bmax estimation was performed with a Pierce BCA protein assay kit and measured by spectrophotometry in Power Wave X340 (Bio-Tek Instruments Inc., BioTek, Winooski, VT, USA).
Chemicals
Dubelcco’s Modified Eagles Medium (DMEM), Phosphate Buffered Saline (PBS) Trizma Base, polyethyleneimine solution (50%, PEI) were purchased from Sigma Aldrich. Fetal Bovine Serum (FBS, Bodinco BV, Alkmaar, The Netherlands), Penicillin/Streptomycin (streptomycin 10,000 IU·mL−1; penicillin and 10,000 μg·mL−1 (Thermo Fisher Scientific, p/a Perbio Science BVBA, Etten-Leur, Netherlands), linear 25 kDa polyethyleneimine (PEI, Polysciences, Warrington, PA, USA), [3H]-N-α-methylhistamine (specific activity 79.7 Ci/mmol, Perkin Elmer), thioperamide, (Abcam, Cambridge, MA, USA), histamine (TCI).
Verification of In Vivo Activity of Compound ADS-531
The H3 antagonistic activity of ADS-531 toward the brain histamine receptors was assessed in vivo by the intravital study of feeding behavior and then, by post-mortem analyses of neurotransmitter systems in the brain tissues from the treated rats (the ethic approval number: 86/LB696/2013).
For the first stage, food intakes were measured daily during subchronic drugs administration. H3R antagonists show the ability to inhibit appetite. Given that the compound enters the CNS and blocks H3R, more histamine should be released. The released amine acting via H1R would exert an anorectic effect. All animal experimental procedures were performed in accordance with EU directives and local ethical regulations. Male Lewis rats 9–10 weeks of age were used. Metabolic cages (Tecniplast, Buguggiate, Italy) were used to measure feed consumption. The cages have a standardized size that allows the animal to move inside freely. Throughout the adaptive period and the experimental one, access to feed and fluid was unlimited. The illumination cycle was stable at 12 h light on, 12 h light off. Rats were individually placed in the cages four days before the onset of treatment to adapt to a new environment and conditions of housing. The consumption and excretion were recorded every morning and the monitoring continued as long as the rats stayed in metabolic cages. The treatment ran for five days from the fifth day. Subcutaneous injections of either ADS 531 or ciproxifan used as a reference (3 mg/kg/day to n = 8 rats) in the study group and physiological saline (0.2 mL/day to n = 8 rats) in case of the control group. Records of consumption were taken as g of feed per 100 g body mass.
4.4. Post-Mortem Biochemical Analyses
Post-mortem brain analyses included estimation of the amine neurotransmitters concentrations as well as the activities of the degradative enzymes HNMT and MAO-A and MAO-B. The rats were sacrificed 24 h after the last injection. The brains were collected, separated into hypothalamus and the cerebral cortex according to the Glowinski and Iversen method [42], immediately frozen in liquid nitrogen and kept at −80 °C until assayed. The amine concentrations: dopamine, noradrenaline, and serotonin were measured by RIA kits and histamine by a Research ELISA kit (DIAsource ImmunoAssays S.A. Louvain-la-Neuve, Place du Levant, Belgium). The enzyme activities of histamine N-methyltransferase and monoamine oxidase A and B were estimated by radioisotopic assays according to Taylor and Snyder [43] and Fowler and Tipton [44] with modifications by Gomez et al. [45], respectively. For MAO-A serotonin (5-[2-14C]-hydroxytryptamine binoxalate) and for MAO-B β-[ethyl-1-14C]-phenylethylamine hydrochloride were used as substrates and respectively, clorgyline (MAO-A) and deprenyl (MAO-B) (final conc. 10−9 M) as the enzymes inhibitors. Protein was measured by Lowry method [46].
Supplementary Materials
The following are available online.
Acknowledgments
This study was supported by departmental sources of the Medical University of Lodz grant numbers 503/3-016-01/503-31-001; 503/5-087-02/503-01 and COST Action CA 15135. The authors would like to thank Mieczyslaw Wośko, president of the pharmaceutical company Polfarmex SA, for providing financial support for the purchase of the necessary reagents for in vivo studies and also to thank H. Stark, from Heinrich-Heine-Universität Düsseldorf, Germany for kindly donating ciproxifan for use as the reference compound for the in vivo study.
Author Contributions
K. Walczyński was responsible for the supervision and development of the whole project. R. Guryn performed the chemical syntheses of the newly synthesized compounds. M. Staszewski performed preliminary pharmacological studies in vitro, for both the H3 and H1 receptor. A. Stasiak performed the extended pharmacological studies in vivo, elaborated and described the results. D. McNaught Flores performed the hH3 and rH3 binding affinity test, elaborated and described the results. A. W. Fogel coordinated the advanced pharmacological studies in vivo and interpreted the obtained results. R. Leurs coordinated the hH3 and rH3 binding affinity test and interpreted the obtained results.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Brown, R.E.; Stevens, D.R.; Haas, H.L. The physiology of brain histamine. Prog. Neurobiol. 2001, 63, 637–672. [Google Scholar] [CrossRef]
- Martinez-Mir, M.I.; Pollard, H.; Moreau, J.; Arrang, J.-M.; Ruat, M.; Traiffort, E.; Schwartz, J.-C.; Palacios, J.M. Three histamine receptors (H1, H2, and H3) visualized in the brain of human and non-human primates. Brain Res. 1990, 526, 322–327. [Google Scholar] [CrossRef]
- Pollard, H.; Moreau, J.; Arrang, J.-M.; Schwartz, J.-C. A detailed autoradiographic mapping of histamine H3 receptors in rat brain areas. Neuroscience 1993, 52, 169–189. [Google Scholar] [CrossRef]
- Arrang, J.-M.; Garbarg, M.; Schwartz, J.-C. Auto-inhibition of brain histamine release mediated by a novel class (H3) of histamine receptor. Nature 1983, 302, 832–837. [Google Scholar] [CrossRef] [PubMed]
- Blandina, P.; Giorgetti, M.; Bartolini, L.; Cecchi, M.; Timmerman, H.; Leurs, R.; Pepeu, G.; Giovannini, M.G. Inhibition of cortical acetylcholine release and cognitive performance by histamine H3 receptor activation in rats. Br. J. Pharmacol. 1996, 119, 1656–1664. [Google Scholar] [CrossRef] [PubMed]
- Schlicker, E.; Behling, A.; Lümmen, G.; Göthert, M. Histamine H3A-receptor-mediated inhibition of noradrenaline release in the mouse brain cortex. Naunyn Schmiedebergs Arch. Pharmacol. 1992, 345, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Schlicker, E.; Malinowska, B.; Kathmann, M.; Gothert, M. Intermediate affinity, and potency of clozapine and low affinity of other neuroleptics and of antidepressants at H3 receptors. Fund. Clin. Pharmacol. 1994, 8, 128–137. [Google Scholar] [CrossRef]
- Passani, M.B.; Cangioli, I.; Bocciottini, L.; Mannaioni, P.F. Thioperamide, and cimetidine modulate acetylcholine release from the amygdala of freely moving rats. Inflamm. Res. 2000, 49, S43–S44. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, S.; Imaizumi, M.; Onodera, K. Effects of thioperamide, a histamine H3-receptor antagonist, on a scopolamine-induced learning deficit using an elevated plus-maze test in mice. Life Sci. 1995, 57, 2137–2144. [Google Scholar] [CrossRef]
- Pillot, C.; Ortiz, J.; Héron, A.; Ridray, S.; Schwartz, J.-C.; Arrang, J.-M. Ciproxifan, a Histamine H3-Receptor Antagonist/Inverse Agonist, Potentiates Neurochemical and Behavioral Effects of Haloperidol in the Rat. J. Neurosci. 2002, 22, 7272–7280. [Google Scholar] [PubMed]
- Takahashi, K.; Suwa, H.; Ishikawa, T.; Kotani, H. Targeted disruption of H3 receptors results in changes in brain histamine tone leading to an obese phenotype. J. Clin. Investig. 2002, 110, 1791–1799. [Google Scholar] [CrossRef] [PubMed]
- Stark, H.; Kathmann, M.; Schlicker, E.; Schunack, W.; Schlegel, B.; Sippl, W. Medicinal chemical and pharmacological aspects of imidazole-containing histamine H3 receptor antagonists. Mini-Rev. Med. Chem. 2004, 4, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Arrang, J.-M.; Garbarg, M.; Lancelot, J.-C.; Lecomte, J.-M.; Pollard, H.; Robba, M.; Schunack, W.; Schwartz, J.-C. Highly potent and selective ligands for histamine H3-receptors. Nature 1987, 327, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Van der Goot, H.; Schepers, M. J-P.; Sterk, G.-J.; Timmerman, H. Isothiourea analogues of histamine as potent agonists or antagonists of the histamine H3-receptor. Eur. J. Med. Chem. 1992, 27, 511–517. [Google Scholar] [CrossRef]
- Van der Goot, H.; Timmerman, H. Selective ligands as tools to study histamine receptors. Eur. J. Med. Chem. 2000, 35, 5–20. [Google Scholar] [CrossRef]
- Liedtke, S.; Flau, K.; Kathmann, M.; Schlicker, E.; Stark, H.; Meier, G.; Schunack, W. Replacement of imidazole by a piperidine moiety differentially affects the potency of histamine H3-receptor antagonists. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2003, 367, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Chadha, H.S.; Abraham, M.A.; Mitchel, R.C. Physicochemical analysis of the factors governing distribution of solutes between blood and brain. Bioorg. Med. Chem. Lett. 1994, 4, 2511–2516. [Google Scholar] [CrossRef]
- Stephanos, J.J. Drug-Protein Interactions. Two-Site Binding of Heterocyclic Ligands to a Monomeric Hemoglobin. J. Inorg. Biochem. 1996, 62, 155–169. [Google Scholar] [CrossRef]
- Zhang, M.; Ballard, M.E.; Pan, L.; Roberts, S.; Faghih, R.; Cowart, T.A.; Esbenshade, G.B.; Fox, M.; Decker, M.W.; Hancock, A.A.; et al. Lack of cataleptogenic potentiation with non-imidazole H3 receptor antagonists reveals potential drug-drug interactions between imidazole-based H3 receptor antagonists and antipsychotic drugs. Brain Res. 2005, 1045, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Lovenberg, T.W.; Roland, B.L.; Wilson, S.J.; Jiang, X.; Pyati, J.; Huvar, A.; Jackson, M.R.; Erlander, M.G. Cloning and functional expression of the human histamine H3 receptor. Mol. Pharmacol. 1999, 55, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Celanire, S.; Wijtmans, M.; Talaga, P.; Leurs, R.; de Esch, I.J.P. Keynote review: Histamine H3 receptor antagonists reach out for the clinic. Drug Discov. Today 2005, 10, 1613–1627. [Google Scholar] [CrossRef]
- Leurs, R.; Bakker, R.A.; Timmerman, H.; de Esch, I.J.P. The histamine H3 receptor: From gene cloning to H3 receptor drugs. Nat. Rev. Drug Discov. 2005, 4, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Denonne, F.; Atienzar, F.; Célanire, S.; Christophe, B.; Delannois, F.; Delaunoy, C.; Delporte, M.-L.; Durieu, V.; Gillard, M.; Lallemand, B.; et al. Phenyl-oxazoles, a new family of inverse agonists at the H(3) histamine receptor. ChemMedChem 2010, 5, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.U.; Palani, A.; Chen, X.; Huang, Y.; Aslanian, R.G.; West, R.E.; Williams, M.S., Jr.; Wu, R.-L.; Hwa, J.; Sondey, C.; et al. Synthesis and structure-activity relationships of 2-(1,4′-bipiperidin-1′-yl)thiazolopyridine as H3 receptor antagonists. Bioorg. Med. Chem. Lett. 2009, 19, 6176–6180. [Google Scholar] [CrossRef] [PubMed]
- Sander, K.; von Coburg, Y.; Camelin, J.-C.; Ligneau, X.; Rau, O.; Schubert-Zsilavecz, M.; Schwartz, J.-C.; Stark, H. Acidic elements in histamine H(3) receptor antagonists. Bioorg. Med. Chem. Lett. 2010, 20, 1581–1584. [Google Scholar] [CrossRef] [PubMed]
- Esbenshade, T.A.; Fox, C.B.; Cowart, M.D. Histamine H3 Receptor Antagonists: Preclinical Promise for Treating Obesity and Cognitive Disorders. Mol. Interv. 2006, 6, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Gemkov, M.J.; Davenport, A.J.; Harich, S.; Ellenbroek, B.A.; Cesura, A.; Hallett, A. The histamine H3 receptor as a therapeutic drug target for CNS disorders. Drug Discov. Today 2009, 14, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Kuhne, S.; Wijtmans, M.; Lim, H.D.; Leurs, R.; de Esch, I.J.P. Several down, a few to go: Histamine H3 receptor ligands making the final push towards the market? Expert Opin. Investig. Drugs 2011, 20, 1629–1648. [Google Scholar] [CrossRef] [PubMed]
- Berlin, M.; Boyce, C.W.; De Lera Ruiz, M. Histamine H3 Receptor as a Drug Discovery Target. J. Med. Chem. 2011, 54, 26–53. [Google Scholar] [CrossRef] [PubMed]
- Ligneau, X.; Perrin, D.; Landais, L.; Camelin, J.C.; Calmels, T.P.; Berrebi-Bertrand, I.; Lecomte, J.M.; Parmentrier, R.; Anaclet, C.; Lin, J.S.; et al. BF2.649 [1-{3-[3-(4-Chlorophenyl)propoxy]propyl}piperidine, Hydrochloride], a Nonimidazole Inverse Agonist/Antagonist at the Human Histamine H3 Receptor: Preclinical Pharmacology. J. Pharmacol. Exp. Ther. 2007, 320, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Frymarkiewicz, A.; Walczyński, K. Non-imidazole histamine H3 ligands, Part IV: SAR of 1-[2-thiazol-5-yl-(2-aminoethyl)]-4-n-propylpiperazine derivatives. Eur. J. Med. Chem. 2009, 44, 1674–1681. [Google Scholar] [CrossRef] [PubMed]
- Guryn, R.; Staszewski, M.; Walczyński, K. Non-imidazole histamine H3 ligands: Part V. synthesis and preliminary pharmacological investigation of 1-[2-thiazol-4-yl- and 1-[2-thiazol-5-yl-(2-aminoethyl)]-4-n-propylpiperazine derivatives. Med. Chem. Res. 2013, 22, 3640–3652. [Google Scholar] [CrossRef] [PubMed]
- Vollinga, R.C.; Zuiderveld, O.P.; Scheerens, H.; Bast, A.; Timmerman, H. A simple and rapid in vitro test system for the screening of histamine H3 ligands. Methods Find. Exp. Clin. Pharmacol. 1992, 105, 747–751. [Google Scholar]
- Arunlakshana, O.; Schild, H.O. Some quantitative uses of drug antagonists. Br. J. Pharmacol. 1959, 14, 48–55. [Google Scholar] [CrossRef]
- Bongers, G.; Sallmen, T.; Passani, M.B.; Mariottini, C.; Wendelin, D.; Lozada, A.; van Marle, A.; Navis, M.; Blandina, P.; Bakker, R.A.; et al. The Akt/GSK-3b axis as a new signaling pathway of the histamine H3 receptor. J. Neurochem. 2007, 103, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Clineschmidt, B.V.; Lotti, V.J. Histamine: Intraventricular injection suppresses ingestive behavior of the cat. Arch. Int. Pharmacodyn. Ther. 1973, 206, 288–298. [Google Scholar] [PubMed]
- Lecklin, A.; Etu-Seppälä, P.; Stark, H.; Tuomisto, L. Effects of intracerebroventricularly infused histamine and selective H1, H2 and H3 agonists on food and water intake and urine flow in Wistar rats. Brain Res. 1998, 793, 279–288. [Google Scholar] [CrossRef]
- Masaki, T.; Chiba, S.; Yasuda, T.; Noguchi, H.; Kakuma, T.; Watanabe, T.; Yoshimatsu, H. Involvement of hypothalamic histamine H1 receptor in the regulation of feeding rhythm and obesity. Diabetes 2004, 53, 2250–2260. [Google Scholar] [CrossRef] [PubMed]
- Provensi, G.; Blandina, P.; Passani, M.B. The histaminergic system as a target for the prevention of obesity and metabolic syndrome. Neuropharmacology 2016, 106, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Ligneau, X.; Lin, J.S.; Vanni-Mercier, G.; Jouvet, M.; Muir, J.L.; Ganellin, C.R.; Stark, H.; Elz, S.; Schunack, W.; Schwartz, J.-C. Neurochemical and Behavioral Effects of Ciproxifan, a Potent Histamine H3-Receptor Antagonist. JPET 1998, 297, 658–666. [Google Scholar]
- Fink, K.B.; Göthert, M. 5-HT receptor regulation of neurotransmitter release. Pharmacol. Rev. 2007, 59, 360–417. [Google Scholar] [CrossRef] [PubMed]
- Glowinski, J.; Iversen, L.L. Regional studies of catecholamines in the rat Brain-I. The disposition of [3H]norepinephrine, [3H]dopamine and [3H]dopa in various region of the brain. J. Neurochem. 1966, 13, 655–669. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.M.; Snyder, S.H. Isotopic microassay of histamine, histidine, histidine decarboxylase and histamine methyltransferase in brain tissue. J. Neurochem. 1972, 19, 1343–1358. [Google Scholar] [CrossRef] [PubMed]
- Fowler, C.J.; Tipton, K.F. The Concentration dependence of the oxidation of tyramine by the two forms of rat liver mitochondrial monoamine oxidase. Biochem. Pharmacol. 1981, 30, 3329–3332. [Google Scholar] [CrossRef]
- Gómez, N.; Balsa, D.; Unzeta, M. A comparative study of some kinetic and molecular properties of microsomal and mitochondrial monoamine oxidase. Biochem. Pharmacol. 1988, 37, 3407–3413. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).