Nutritional Composition and Antioxidant Properties of the Fruits of a Chinese Wild Passiflora foetida
Abstract
:1. Introduction
2. Material and Methods
2.1. Plant Material
2.2. Standards and Reagents
2.3. Physicochemical Characteristic
2.4. Nutritional Determinations
2.4.1. Proximate Analysis
2.4.2. Sugars Analysis
2.4.3. Organic Acids Analysis
2.4.4. Amino Acids Analysis
2.4.5. Minerals Analysis
2.4.6. Fatty Acids Analysis
2.5. Extraction of Phenolic Compounds
2.5.1. Extractable Phenolics (EP)
2.5.2. Non-Extractable Phenolics (NEP)
2.6. Measurements of Antioxidant Capacity and Functional Phytochemicals
2.6.1. Extraction and Quantification of Total Phenolic Contents (TPC) and Total Flavonoid Contents (TFC)
2.6.2. Antioxidant Activity by DPPH and ABTS
2.6.3. Chromatography and Mass Spectrometry
2.7. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Characteristics of the Edible Portion
3.2. Nutritional Composition
3.2.1. Sugars and Organic Acids
3.2.2. Amino Acids
3.2.3. Minerals
3.2.4. Fatty Acids
3.3. Functional Characterisation of the Powder
3.3.1. TPC, TFC and Antioxidant Activity
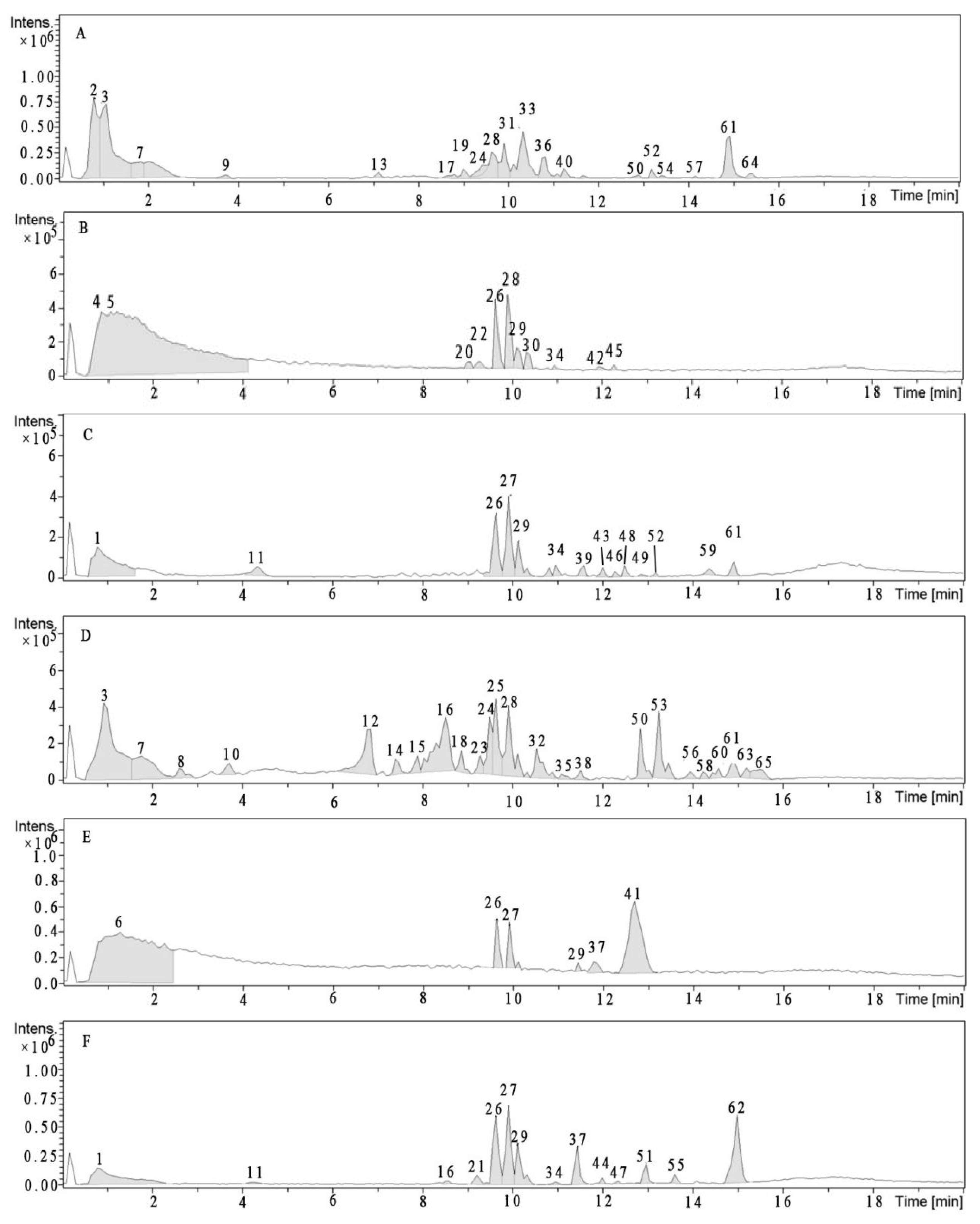
3.3.2. Identification of Polyphenolics
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Miroddi, M.; Calapai, G.; Navarra, M.; Minciullo, P.L.; Gangemi, S. Passiflora incarna L.: Ethnopharmacology, clinical application, safety and evaluation of clinical trials. J. Ethnopharmacol. 2013, 150, 791–804. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, S.; Arunachalam, K.; Parimelazhagan, T. Antioxidant, analgesic, anti-inflammatory and antipyretic effects of polyphenols from Passiflora subpeltata leaves-A promising species of Passiflora. Ind. Crop. Prod. 2014, 54, 272–280. [Google Scholar] [CrossRef]
- Dhawan, K.; Dhawan, S.; Sharma, A. Passiflora: A review update. J. Ethnopharmacol. 2004, 94, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Jawna-Zboinska, K.; Blecharz-Klin, K.; Joniec-Maciejak, I.; Wawer, A.; Pyrzanowska, J.; Piechal, A.; Mirowska-Guzel, D.; Widy-Tyszkiewicz, E. Passiflora incarnata L. improves spatial memory, reduces stress, and affects neurotransmission in rats. Phytother Res. 2016, 30, 781–789. [Google Scholar] [CrossRef] [PubMed]
- De Paula, R.C.M.; Soares, A.G.; Freitas, S.P. Volatile coumponds in passion fruit seed oil (Passiflora setacea BRS Perola do Cerrado and Passiflora alata BRS Doce Mel). Chem. Eng. Trans. 2015, 44, 103–108. [Google Scholar]
- Ramaiya, S.D.; Bujang, J.S.; Zakaria, M.H.; King, W.S.; Sahrir, M.A.S. Sugars, ascorbic acid, total phenolic content and total antioxidant activity in passion fruit (Passiflora) cultivars. J. Sci. Food Agric. 2013, 93, 1198–1205. [Google Scholar] [CrossRef] [PubMed]
- Vasco-Correa, J.; Zapata, A.D.Z. Enzymatic extraction of pectin from passion fruit peel (Passiflora edulis f. flavicarpa) at laboratory and bench scale. LWT Food Sci. Technol. 2017, 80, 280–285. [Google Scholar] [CrossRef]
- Padhye, M.D. Two types of embryo development in Passiflora foetida Linn. Curr. Sci. 1963, 32, 373–376. [Google Scholar]
- Nguyen, T.Y.; To, D.C.; Tran, M.H.; Lee, J.S.; Lee, J.H.; Kim, J.A.; Woo, M.H.; Min, B.S. Anti-inflammatory flavonoids isolated from Passiflora foetida. Nat. Prod. Commun. 2015, 10, 929–931. [Google Scholar] [PubMed]
- Mohanasundari, C.; Natarajan, D.; Srinivasan, K.; Umamaheswari, S.; Ramachandran, A. Antibacterial properties of Passiflora foetida L.—A common exotic medicinal plant. Afr. J. Biotechnol. 2007, 6, 2650–2653. [Google Scholar]
- Ahmad, N.; Chillara, R.; Kushwaha, P.; Khedgikar, V.; Karvande, A.; Choudhary, D.; Adhikary, S.; Maurya, R.; Trivedi, R. Evaluation of anti-osteoporotic activity of butanolic fraction from Passiflora foetida in ovariectomy-induced bone loss in mice. Biomed. Pharmacother. 2017, 88, 804–813. [Google Scholar] [CrossRef] [PubMed]
- Perez-Jimenez, J.; Saura-Calixto, F. Macromolecular antioxidants or non-extractable polyphenols in fruit and vegetables: Intake in four European countries. Food Res. Int. 2015, 74, 315–323. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis of AOAC International; Association of Official Analytical Chemists International: Arlington, VA, USA, 2005. [Google Scholar]
- Orqueda, M.E.; Rivas, M.; Zampini, I.C.; Alberto, M.R.; Torres, S.; Cuello, S.; Sayago, J.; Thomas-Valdes, S.; Jiménez-Aspee, F.; Schmeda-Hirschmann, G.; et al. Chemical and functional characterization of seed, pulp and skin powder from chilto (Solanum betaceum), an Argentine native fruit. Phenolic fractions affect key enzymes involved in metabolic syndrome and oxidative stress. Food Chem. 2017, 216, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, D.; You, Y.; Zeng, S.; Li, Y.; Tang, Q.; Han, G.; Liu, A.; Feng, C.; Li, C.; et al. Nutritional composition of boletus mushrooms from Southwest China and their antihyperglycemic and antioxidant activities. Food Chem. 2016, 211, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Darragh, A.J.; Moughan, P.J. The effect of hydrolysis time on amino acid analysis. J. AOAC Int. 2005, 88, 888–893. [Google Scholar] [PubMed]
- Drózdz, P.; Šežene, V.; Wójcik, J.; Pyrzynska, K. Evaluation of bioactive compounds, minerals and antioxidant activity of Lingonberry (Vaccinium vitis-idaea L.) fruits. Molecules 2018, 23, 53. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, A.; Lisciani, S.; Camilli, E.; Gabrielli, P.; Marconi, S.; Gambelli, L.; Marietta, L. Nutritional composition and antioxidant properties of traditional Italian dishes. Food Chem. 2017, 218, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, M.; de Camargo, A.C.; Shahidi, F. Antioxidants and bioactivities of free, esterified and insoluble-bound phenolics from berry seed meals. Food Chem. 2016, 197, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Antolak, H.; Czyzowska, A.; Sakac, M.; Mišan, A.; Duragic, O.; Kregiel, D. Phenolic compounds contained in little-known wild fruits as antiadhesive agents against the beverage-spoiling bacteria Asaia spp. Molecules 2017, 22, 1256. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.Y.; Tang, C.Y. Determination of total phenolic and flavonoid contents in selected fruits and vegetables, as well as their stimulatory effects on mouse splenocyte proliferation. Food Chem. 2007, 101, 140–147. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Byrne, D.H. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Gazola, A.C.; Costa, G.M.; Castellanos, L.; Ramos, F.A.; Reginatto, F.H.; de Lima, T.C.M.; Schenkel, E.P. Involvement of GABAergic pathway in the sedative activity of apigenin, the main flavonoid from Passiflora quadrangularis pericarp. Revista Brasileira De Farmacognosia-Brazilian. J. Pharmacogn. 2015, 25, 158–163. [Google Scholar] [CrossRef]
- De Oliveira, G.A.; de Castilhos, F.; Renard, C.M.G.C.; Bureau, S. Comparison of NIR and MIR spectroscopic methods for determination of individual sugars, organic acids and carotenoids in passion fruit. Food Res. Int. 2014, 60, 154–162. [Google Scholar] [CrossRef]
- World Health Organization, Food and Agriculture Organization of the United Nations, United Nations University. Protein and Amino Acid Requirements in Human Nutrition; World Health Organization: Geneva, Switzerland, 2007; pp. 1–265. [Google Scholar]
- Correa, R.C.G.; Peralta, R.M.; Haminiuk, C.W.I.; Maciel, G.M.; Bracht, A.; Ferreira, I.C.F.R. The past decade findings related with nutritional composition, bioactive molecules and biotechnological applications of Passiflora spp. (passion fruit). Trends. Food Sci. Technol. 2016, 58, 79–95. [Google Scholar] [CrossRef]
- Leterme, P.; Buldgen, A.; Estrada, F.; Londono, A.M. Mineral content of tropical fruits and unconventional foods of the Andes and the rain forest of Colombia. Food Chem. 2006, 95, 644–652. [Google Scholar] [CrossRef]
- Araujo, R.G.O.; Dias, F.D.S.; Macedo, S.M.; dos Santos, W.N.L.; Ferreira, S.L.C. Method development for the determination of manganese in wheat flour by slurry sampling flame atomic absorption spectrometry. Food Chem. 2007, 101, 397–400. [Google Scholar] [CrossRef]
- Kozlowska, M.; Gruczynska, E.; Scibisz, I.; Rudzinska, M. Fatty acids and sterols composition, and antioxidant activity of oils extracted from plant seeds. Food Chem. 2016, 213, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yang, F.; Zhang, C.; Ji, H.; Hong, P.; Deng, C. Optimization of process parameters for supercritical carbon dioxide extraction of Passiflora seed oil by response surface methodology. J. Supercrit. Fluids 2009, 48, 9–14. [Google Scholar] [CrossRef]
- Contreras-Calderon, J.; Calderon-Jaimes, L.; Guerra-Hernandez, E.; Garcia-Villanova, B. Antioxidant capacity, phenolic content and vitamin C in pulp, peel and seed from 24 exotic fruits from Colombia. Food Res. Int. 2011, 44, 2047–2053. [Google Scholar] [CrossRef]
- Zhang, H.; Jiang, L.; Ye, S.; Ye, Y.B.; Ren, F. Systematic evaluation of antioxidant capacities of the ethanolic extract of different tissues of jujube (Ziziphus jujuba Mill.) from China. Food Chem. Toxicol. 2010, 48, 1461–1465. [Google Scholar] [CrossRef] [PubMed]
- Spinola, V.; Pinto, J.; Castilho, P.C. Identification and quantification of phenolic compounds of selected fruits from Madeira Island by HPLC-DAD-ESI-MSn and screening for their antioxidant activity. Food Chem. 2015, 173, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Zucolotto, S.M.; Fagundes, C.; Reginatto, F.H.; Ramos, F.A.; Castellanos, L.; Duque, C.; Schenkel, E.P. Analysis of C-glycosyl flavonoids from South American Passiflora species by HPLC-DAD and HPLC-MS. Phytochem. Anal. 2012, 23, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Inbaraj, B.S.; Lu, H.; Kao, T.H.; Chen, B.H. Simultaneous determination of phenolic acids and flavonoids in Lycium barbarum Linnaeus by HPLC-DAD-ESI-MS. J. Pharm. Biomed. Anal. 2010, 51, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Gardana, C.; Scaglianti, M.; Pietta, P.; Simonetti, P. Analysis of the polyphenolic fraction of propolis from different sources by liquid chromatography-tandem mass spectrometry. J. Pharm. Biomed. Anal. 2007, 45, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Falcao, S.I.; Vale, N.; Gomes, P.; Domingues, M.R.M.; Freire, C.; Cardoso, S.M.; Vilas-Boas, M. Phenolic profiling of portuguese propolis by LC-MS spectrometry: Uncommon propolis rich in flavonoid glycosides. Phytochem. Anal. 2013, 24, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Carini, M.; Aldini, G.; Furlanetto, S.; Stefani, R.; Facino, R.M. LC coupled to ion-trap MS for the rapid screening and detection of polyphenol antioxidants from Helichrysum stoechas. J. Pharm. Biomed. Anal. 2001, 24, 517–526. [Google Scholar] [CrossRef]
- Vallverdu-Queralt, A.; Regueiro, J.; Martinez-Huelamo, M.; Alvarenga, J.F.R.; Leal, L.N.; Lamuela-Raventos, R.M. A comprehensive study on the phenolic profile of widely used culinary herbs and spices: Rosemary, thyme, oregano, cinnamon, cumin and bay. Food Chem. 2014, 154, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Shibano, M.; Lin, A.S.; Itokawa, H.; Lee, K.H. Separation and characterization of active flavonolignans of Silybum marianum by liquid chromatography connected with hybrid ion-trap and time-of-flight mass spectrometry (LC-MS/IT-TOF). J. Nat. Prod. 2007, 70, 1424–1428. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, S.C.; Castilho, P.C. Characterization of phenolic compounds in Helichrysum melaleucum by high-performance liquid chromatography with on-line ultraviolet and mass spectrometry detection. Rapid Commun. Mass Spectrom. 2010, 24, 1851–1868. [Google Scholar] [CrossRef] [PubMed]
- Kachlicki, P.; Einhorn, J.; Muth, D.; Kerhoas, L.; Stobiecki, M. Evaluation of glycosylation and malonylation patterns in flavonoid glycosides during LC/MS/MS metabolite profiling. J. Mass Spectrom. 2008, 43, 572–586. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
| Weight (g) | Fruit Shape Index | Color Parameters | ||||
|---|---|---|---|---|---|---|
| Length (cm) | Diameter (cm) | L/D a | L * | a * | b * | |
| 3.3 ± 0.2 (n = 25) | 1.62 ± 0.13 (n = 25) | 1.88 ± 0.13 (n = 25) | 0.86 ± 0.04 (n = 25) | 53 ± 1 (n = 6) | 17 ± 2 (n = 6) | 87 ± 2 (n = 6) |
| Compositions (Unit) | Data |
|---|---|
| Edible portion (g/100 g fresh fruit) | 62.73–89.85 |
| Inedible portion (g/100 g fresh fruit) | 10.15–37.27 |
| Water (g/100 g fresh fruit) | 76 ± 1 |
| Total fat (g/100 g fresh fruit) | 13 ± 2 |
| Total Protein (g/100 g fresh fruit) | 0.15 ± 0.01 |
| Ash (g/100 g fresh fruit) | 1.7 ± 0.3 |
| Total sugars (g/100 g fresh fruit) | 3.6 ± 0.1 |
| pH | 4.5 ± 0.1 |
| Total Energy (kJ) | 991 ± 88 |
| Sugars (g) | Per 100 g of FW | Percentage of Total Sugars (%) |
| Glucose | 1.6 ± 0.1 | 47.90 |
| Fructose | 1.6 ± 0.1 | 47.90 |
| Sucrose | 0.14 ± 0.01 | 4.20 |
| Total | 3.34 | |
| Organic Acids (mg) | Per 100 g of FW | Percentage of Total Organic Acids (%) |
| Oxalic acid | 0.07 ± 0.01 | 29.17 |
| Tartaric acid | 0.01 ± 0.00 | 4.17 |
| Malic acid | 0.01 ± 0.00 | 4.17 |
| Ascorbic acid | 0.03 ± 0.00 | 12.50 |
| Citric acid | 0.12 ± 0.01 | 50.00 |
| Total | 0.24 | |
| Amino Acids (mg) | Per 100 g of FW | Percentage of Total Amino Acids (%) |
| Aspartic acid | 110 ± 3 | 10.02 |
| Serine | 57 ± 2 | 5.20 |
| Glutamic | 203 ± 7 | 18.51 |
| Glycine | 63 ± 1 | 5.74 |
| Alanine | 58.2 ± 0.6 | 5.31 |
| Cystine | 18 ± 6 | 1.64 |
| Tyrosine | 17 ± 3 | 1.55 |
| Phenylalanine | 77 ± 4 | 7.02 |
| Arginine | 140 ± 4 | 12.76 |
| Proline | 46 ± 3 | 4.20 |
| Methionine | 10 ± 2 | 0.91 |
| Threonine | 40.7 ± 0.8 | 3.71 |
| Isoleucine | 40.4 ± 0.3 | 3.68 |
| Leucine | 74 ± 2 | 6.75 |
| Lysine | 58.6 ± 0.4 | 5.34 |
| Histidine | 28.6 ± 0.3 | 2.61 |
| Valine | 55.2 ± 0.7 | 5.03 |
| Cystine + Methionine | 28 ± 8 | 2.55 |
| Tyrosine + Phenylalanine | 118 ± 5 | 10.76 |
| EAA | 286 ± 1 | 26.07 |
| NEAA | 811 ± 133 | 73.93 |
| Total | 1097 | |
| Minerals (mg) | Per 100 g of FW | Percentage of Total Minerals (%) |
| Manganese | 0.32 ± 0.03 | 0.05 |
| Copper | 0.21 ± 0.02 | 0.04 |
| Iron | 0.89 ± 0.07 | 0.15 |
| Zinc | 1.03 ± 0.09 | 0.17 |
| Phosphorus | 86 ±7 | 14.44 |
| Sodium | 10± 2 | 1.68 |
| Magnesium | 40 ± 2 | 6.71 |
| Potassium | 451 ± 7 | 75.70 |
| Calcium | 6.3 ± 0.4 | 1.06 |
| Total | 595.75 | |
| Fatty Acids | Percentage of Total Fatty Acids (%) | |
| Myristic acid (C14:0) | 0.48 | |
| Palmitic acid (C16:0) | 18.18 | |
| Linoleic acid (C18:2, C9C12) | 44.69 | |
| Oleic acid (C18:1, C9) | 29.49 | |
| Stearic acid (C18:0) | 7.16 |
| P. foetida Powder | Polyphenolic Compounds mg/100g Powder | SC50 (mg/mL) g | ||||
|---|---|---|---|---|---|---|
| TPC | TFC | Total | DPPH | ABTS | ||
| Edible portion | EP | 45.4 ± 0.1 f | 239 ± 1 d | 284.4 | 5.88 ± 0.09 cd | 4.42 ± 0.04 d |
| Acid NEP | 608 ± 5 c | 814 ± 24 b | 1422 | 5.61 ± 0.07 bc | 7.6 ± 0.1 f | |
| Alkaline NEP | 218.5 ± 0.3 d | 1163 ± 2 a | 1381.5 | 2.75 ± 0.04 a | 0.447 ± 0.001 a | |
| Total | 871.9 | 2216 | 3087.6 | |||
| Inedible portion | EP | 203.6 ± 0.9 e | 753 ± 7 b | 956.6 | 6.3 ± 0.5 d | 3.17 ± 0.09 c |
| Acid NEP | 833 ± 5 a | 513 ± 61 c | 1346 | 5.26 ± 0.03 b | 9.8 ± 0.1 e | |
| Alkaline NEP | 622 ± 2 b | 84 ± 15 e | 706 | 6.38 ± 0.08 d | 2.062 ± 0.007 b | |
| Total | 1658.6 | 1350 | 3008.6 | |||
| Reference compound | Gallic acid | Rutin | Trolox | Trolox | ||
| Compound | TR (min) | (ESI)− (m/z Abundance) | Proposed Structure | Edible Portion | Inedible Portion | ||||
|---|---|---|---|---|---|---|---|---|---|
| EP | Acid NEP | Alkaline NEP | EP | Acid NEP | Alkaline NEP | ||||
| 1 | 0.79 | MS: 794.4155; MS/MS: 268.8039 | Unknown | + | + | ||||
| 2 | 0.81 | MS: 371.0882; MS/MS: 191.0144 | Caffeoylglucaric acid | + | |||||
| 3 | 0.96 | MS: 383.0517; MS/MS: 191.0162 | Quinine dimer | + | + | ||||
| 4 | 1.03 | MS: 481.0236; MS/MS: 191.0146 | Quinic acid derivative | ||||||
| 5 | 1.06 | MS: 208.9383; MS/MS: 96.9636 | Unknown | + | |||||
| 6 | 1.28 | MS: 358.8792; MS/MS: 264.9029 | Rosmarinic acid | + | |||||
| 7 | 1.75 | MS: 781.1470; MS/MS: 439.0800 | Unknown | + | + | ||||
| 8 | 2.60 | MS: 553.1413; MS/MS: 455.1866, 167.0313 | Caffeic acid derivative | + | |||||
| 9 | 3.65 | MS: 481.1134; MS/MS: 473.0759, 137.0221 | Silymarin | + | |||||
| 10 | 3.71 | MS:445.1406; MS/MS: 443.1954, 137.0217 | Lucuminic acid | + | |||||
| 11 | 4.34 | MS: 703.1230; MS/MS: 461.0935, 219.0506 | Hispidulin-7-O-hexoside derivative | + | + | ||||
| 12 | 6.80 | MS: 625.1631; MS/MS: 301.0937 | Caffeic acid derivative | + | |||||
| 13 | 7.12 | MS: 755.1698; MS/MS: 319.0830 | Quercetin 3-O-rhamnosyl-glucoside 7-O-rhamnoside | + | |||||
| 14 | 7.40 | MS: 593.1434; MS/MS: 473.1109, 353.0709 | Apigenin-6,8-di-C-glycoside (vicenin-2) | + | + | ||||
| 15 | 7.89 | MS: 417.1582; MS/MS: 206.0808 | Coumarylquinic acid derivative | + | |||||
| 16 | 8.51 | MS: 629.1147; MS/MS: 675.2466, 319.1335 | Unknown | + | |||||
| 17 | 8.79 | MS: 497.0879; MS/MS: 299.0581, 284.0329 | Chrysoeriol derivative | + | |||||
| 18 | 8.86 | MS: 671.2571; MS/MS: 249.1339 | Unknown | + | |||||
| 19 | 9.02 | MS: 600.3834; MS/MS: 285.0406 | Luteolin derivative | + | |||||
| 20 | 9.06 | MS: 285.0407; MS/MS: 149.0193 | Luteolin | + | |||||
| 21 | 9.21 | MS: 600.3899; MS/MS: 564.4132, 289.1176 | Unknown | + | |||||
| 22 | 9.27 | MS: 676.3797; MS/MS: 346.1848, 110.9766 | Unknown | + | |||||
| 23 | 9.28 | MS: 307.0856; MS/MS: 161.0402 | Unknown | + | |||||
| 24 | 9.42 | MS: 723.4727; MS/MS: 677.4750, 329.0685 | luteolin-3′-O-dirhamnoside-7-O-rhamnoside | + | + | ||||
| 25 | 9.59 | MS: 790.5349; MS/MS: 707.2673 | Unknown | + | |||||
| 26 | 9.62 | MS:713.4510; MS/MS: 677.4817 | Unknown | + | + | + | + | ||
| 27 | 9.66 | MS: 826.5030; MS/MS: 790.5349, 329.0708 | Unknown | + | + | + | |||
| 28 | 9.90 | MS: 836.5284; MS/MS: 790.5349 | Unknown | + | + | + | |||
| 29 | 10.11 | MS: 939.5540; MS/MS: 903.5825 | Unknown | + | + | + | + | ||
| 30 | 10.31 | MS: 313.0734; MS/MS: 283.0245 | Pinobanksin-3-O-acetate | + | |||||
| 31 | 10.32 | MS: 627.1411; MS/MS: 313.0737 | Unknown | + | |||||
| 32 | 10.56 | MS: 447.2273; MS/MS: 191.0530 | Vicenin-2 | + | |||||
| 33 | 10.78 | MS: 659.1250; MS/MS: 329.0690 | Unknown | + | |||||
| 34 | 10.94 | MS: 329.2362; MS/MS: 201.1069 | Kaempferol-methoxy-methyl ether | + | + | + | |||
| 35 | 11.09 | MS: 385.2271; MS/MS: 267.1972 | Feruloylglucaric acid | + | |||||
| 36 | 11.25 | MS: 343.0858; MS/MS: 313.0373, 285.0410 | Luteolin derivative | + | |||||
| 37 | 11.43 | MS: 323.2037; MS/MS: 287.2262 | Unknown | + | + | ||||
| 38 | 11.50 | MS: 311.1892; MS/MS: 267.1970 | Unknown | + | |||||
| 39 | 11.58 | MS: 723.4726; MS/MS: 379.2249 | Unknown | + | |||||
| 40 | 11.64 | MS: 379.0651; MS/MS: 328.0612, 313.0413 | Unknown | + | |||||
| 41 | 11.78 | MS: 501.1454; MS/MS: 389.1616, 367.1838 | Unknown | + | |||||
| 42 | 11.91 | MS: 593.1262; MS/MS: 417.1005, 209.0401 | Isoorientin | + | |||||
| 43 | 11.99 | MS: 653.2940; MS/MS: 653.2910 | Unknown | + | |||||
| 44 | 11.99 | MS: 639.4592; MS/MS: 337.2124 | Unknown | + | |||||
| 45 | 12.26 | MS: 540.2528; MS/MS: 425.2245, 110.9778 | Unknown | + | |||||
| 46 | 12.26 | MS: 653.2940; MS/MS: 653.2910 | Unknown | + | |||||
| 47 | 12.34 | MS: 433.2594; MS/MS: 287.2228, 163.0378 | p-coumaric acid derivative | + | |||||
| 48 | 12.48 | MS: 763.4315; MS/MS: 653.2859, 399.1997 | Unknown | + | |||||
| 49 | 12.83 | MS: 1187.2153; MS/MS: 739.4765, 324.7376 | Unknown | + | |||||
| 50 | 12.83 | MS: 721.3361; MS/MS: 397.1392, 277.2188 | Unknown | + | + | ||||
| 51 | 12.95 | MS: 447.2760; MS/MS: 415.2473 | Luteolin 8-C-hexoside (orientin) | + | |||||
| 52 | 13.17 | MS: 297.2446; MS/MS: 183.1340 | Unknown | + | + | ||||
| 53 | 13.24 | MS: 549.2817; MS/MS: 277.2167 | Unknown | + | |||||
| 54 | 13.38 | MS: 474.2656; MS/MS: 277.2171 | Unknown | + | |||||
| 55 | 13.60 | MS: 593.4174; MS/MS: 557.4400, 287.2226 | Kaempferol-3-O-rutinoside | + | |||||
| 56 | 13.94 | MS: 529.2990; MS/MS: 279.2320 | 1-O-Caffeoyl-5-O-feruloylquinic acid | + | |||||
| 57 | 14.15 | MS: 476.2824; MS/MS: 279.2334 | Unknown | + | |||||
| 58 | 14.22 | MS: 385.3012; MS/MS: 325.1847, 263.2395 | Unknown | + | |||||
| 59 | 14.36 | MS: 623.2774; MS/MS: 461.2278, 446.2038 | Isorhamnetin 3-O-glucoside 7-O-rhamnoside | + | |||||
| 60 | 14.57 | MS: 341.2741; MS/MS: 339.2020 | Pinobanksin-3-O-butyrate | + | |||||
| 61 | 14.88 | MS: 339.2374; MS/MS: 163.1074 | p-coumaric acid derivative | + | + | + | |||
| 62 | 14.97 | MS: 607.4342; MS/MS: 607.4342 | Spinosin | + | |||||
| 63 | 15.19 | MS: 487.3090; MS/MS: 341.3043 | Caffeic acid-O-hexoside-O-rhamnoside | + | |||||
| 64 | 15.40 | MS: 743.4882; MS/MS: 739.4757, 389.1516, 268.7993 | Unknown | + | |||||
| 65 | 15.47 | MS: 671.2448; MS/MS: 277.2184 | Unknown | + | |||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.; Wei, X.-Q.; Li, M.-Y.; Duan, X.-W.; Sun, Y.-M.; Yang, R.-L.; Su, X.-D.; Huang, R.-M.; Wang, H. Nutritional Composition and Antioxidant Properties of the Fruits of a Chinese Wild Passiflora foetida. Molecules 2018, 23, 459. https://doi.org/10.3390/molecules23020459
Song Y, Wei X-Q, Li M-Y, Duan X-W, Sun Y-M, Yang R-L, Su X-D, Huang R-M, Wang H. Nutritional Composition and Antioxidant Properties of the Fruits of a Chinese Wild Passiflora foetida. Molecules. 2018; 23(2):459. https://doi.org/10.3390/molecules23020459
Chicago/Turabian StyleSong, Ya, Xiao-Qun Wei, Mei-Ying Li, Xue-Wu Duan, Yuan-Ming Sun, Rui-Li Yang, Xiang-Dong Su, Ri-Ming Huang, and Hong Wang. 2018. "Nutritional Composition and Antioxidant Properties of the Fruits of a Chinese Wild Passiflora foetida" Molecules 23, no. 2: 459. https://doi.org/10.3390/molecules23020459
APA StyleSong, Y., Wei, X.-Q., Li, M.-Y., Duan, X.-W., Sun, Y.-M., Yang, R.-L., Su, X.-D., Huang, R.-M., & Wang, H. (2018). Nutritional Composition and Antioxidant Properties of the Fruits of a Chinese Wild Passiflora foetida. Molecules, 23(2), 459. https://doi.org/10.3390/molecules23020459








