MgONPs Can Boost Plant Growth: Evidence from Increased Seedling Growth, Morpho-Physiological Activities, and Mg Uptake in Tobacco (Nicotiana tabacum L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Characterization of Nanoparticles
2.2. Seed Germination Test
2.3. Tobacco Plants Growth and Greenhouse Conditions
2.4. Chlorophyll Determination
2.5. Determination of Antioxidant Enzymes, MDA, Protein and Relative Water Contents
2.6. Elemental Analysis
2.7. Structural Observation Using Paraffin Sections
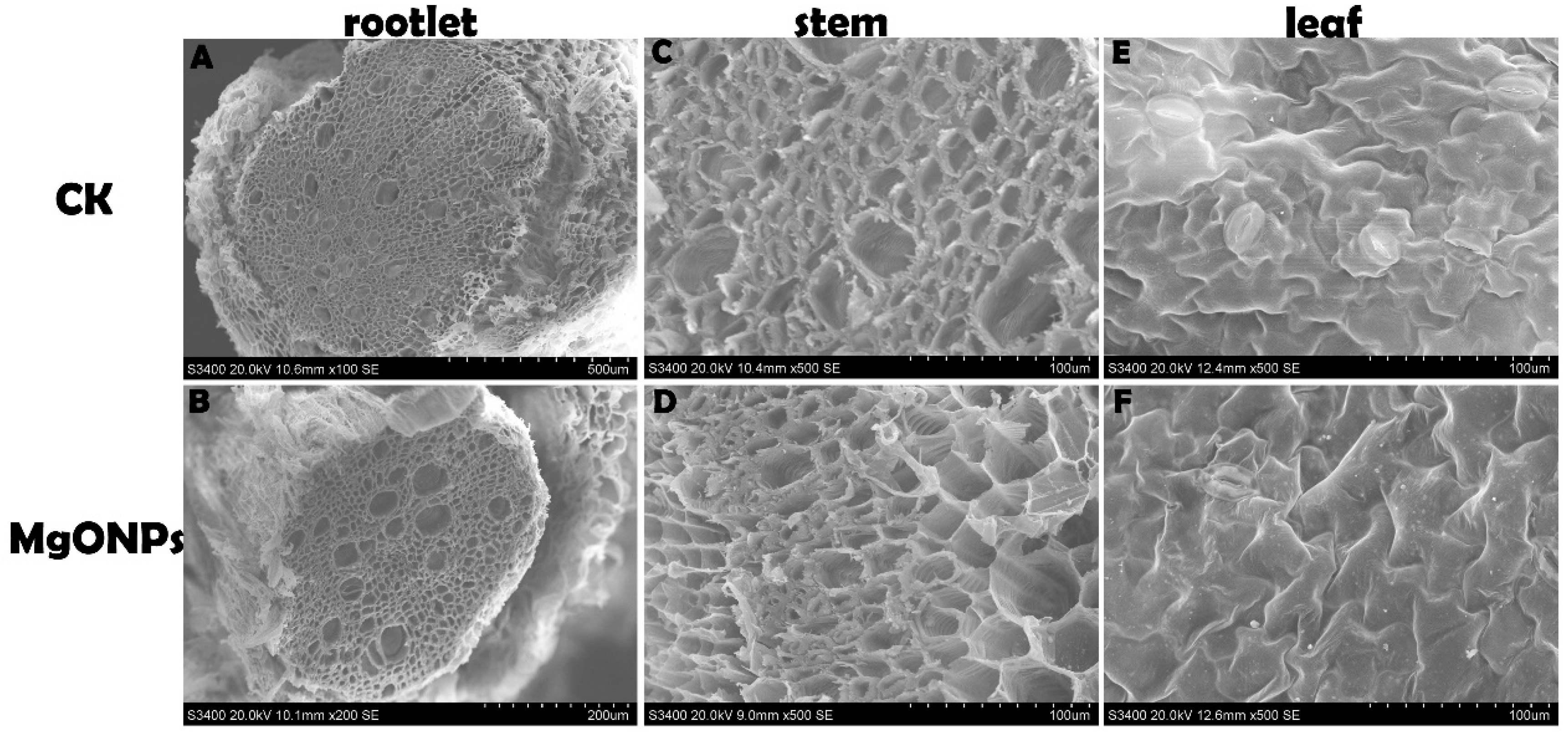
2.8. SEM Observation
2.9. Statistical Analysis
3. Results and Discussion
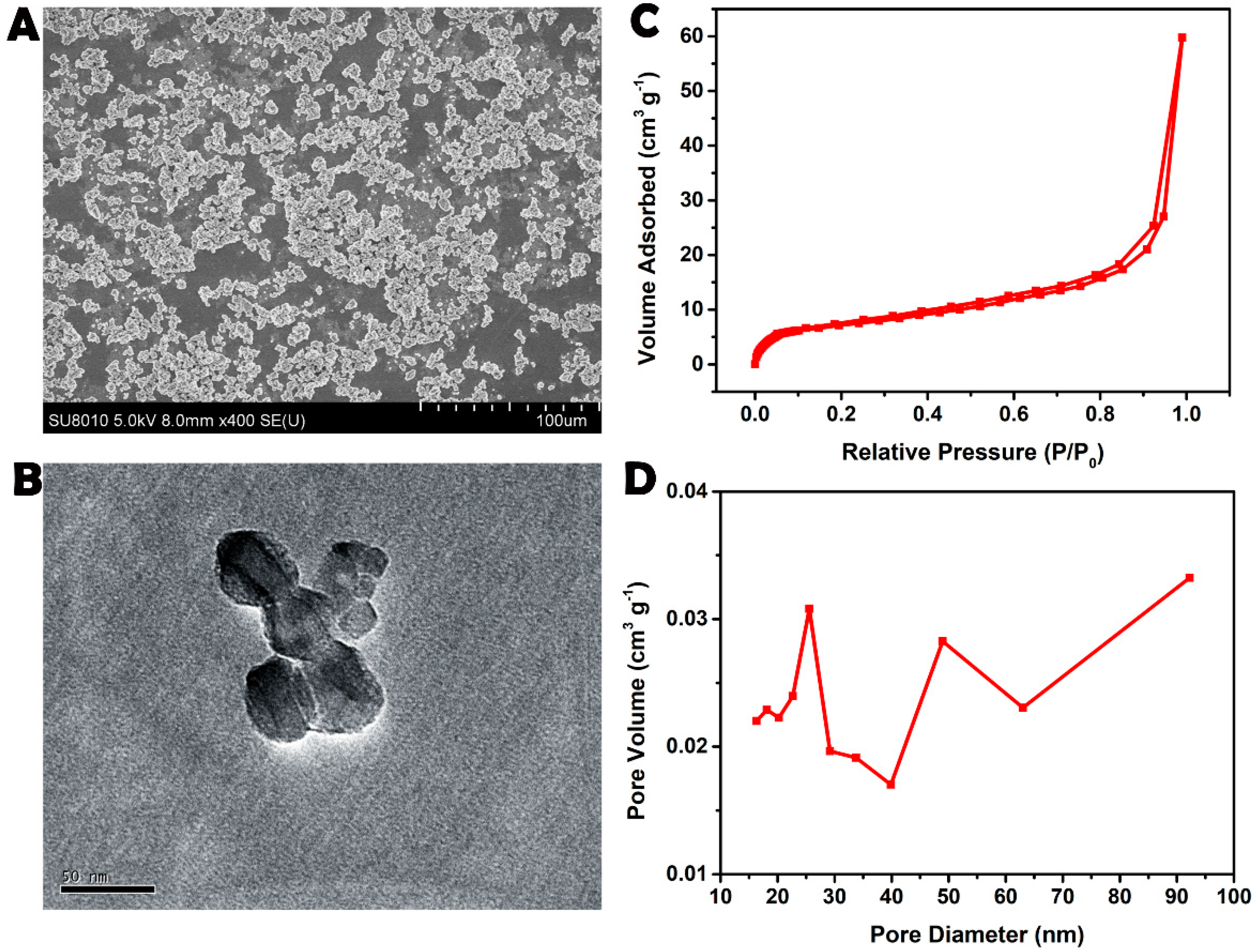
3.1. Materials and Characterization of the NPs
3.2. Seed Germination
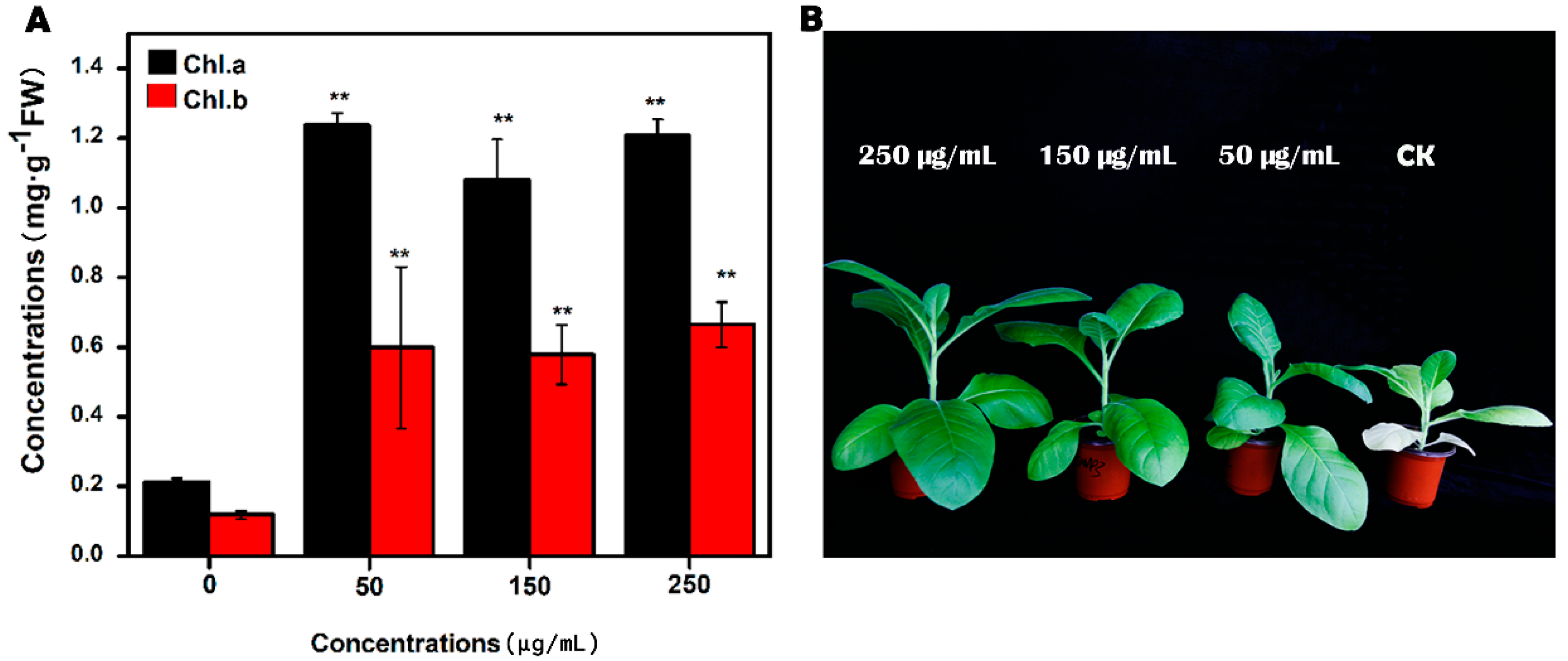
3.3. Effect of MgNPs on Tobacco Growth and Chlorophyll Content
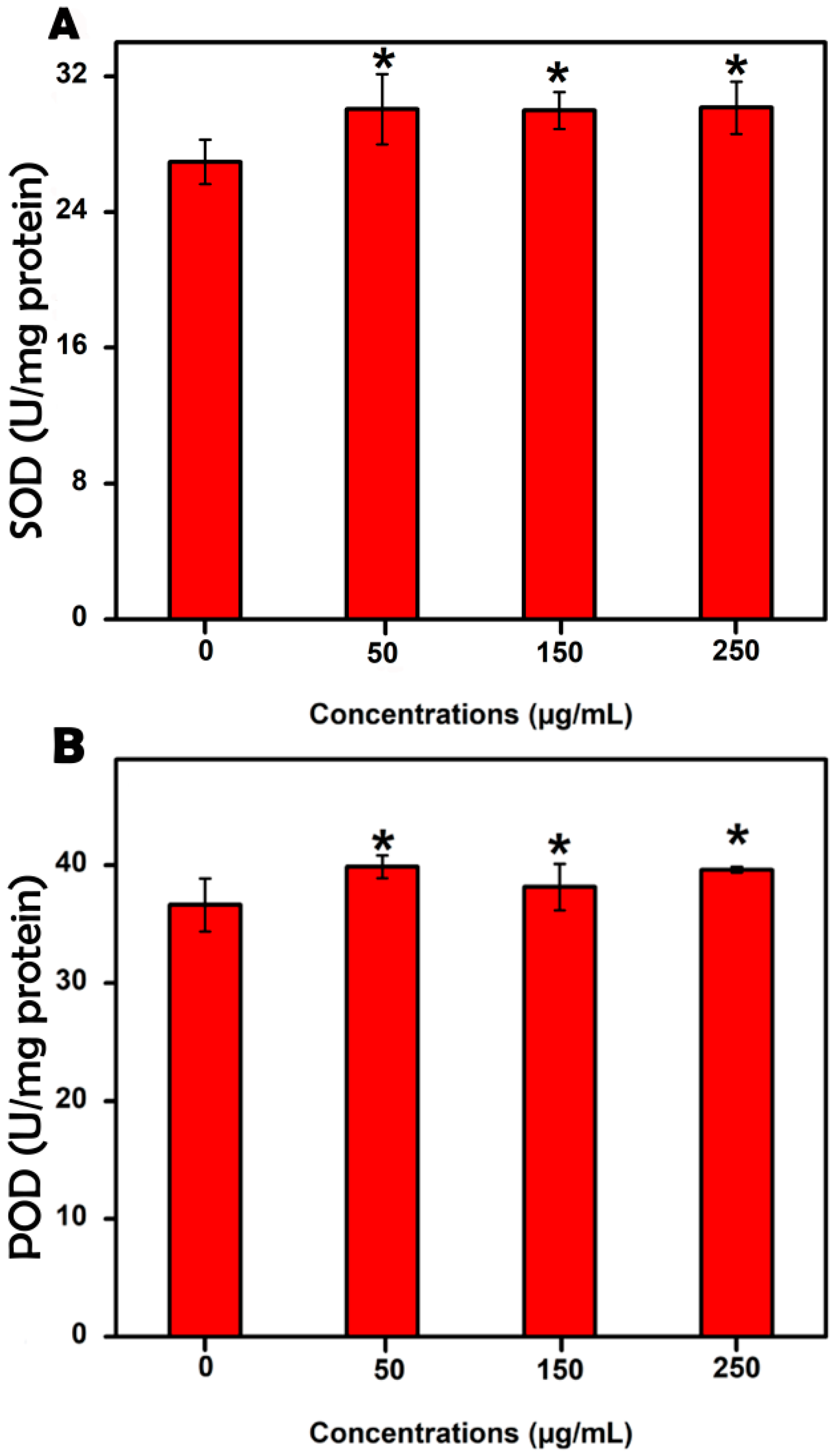
3.4. Evaluation of the Antioxidant Enzyme Activities
3.5. Malondialdehyde, Relative Water and Protein Content Detection
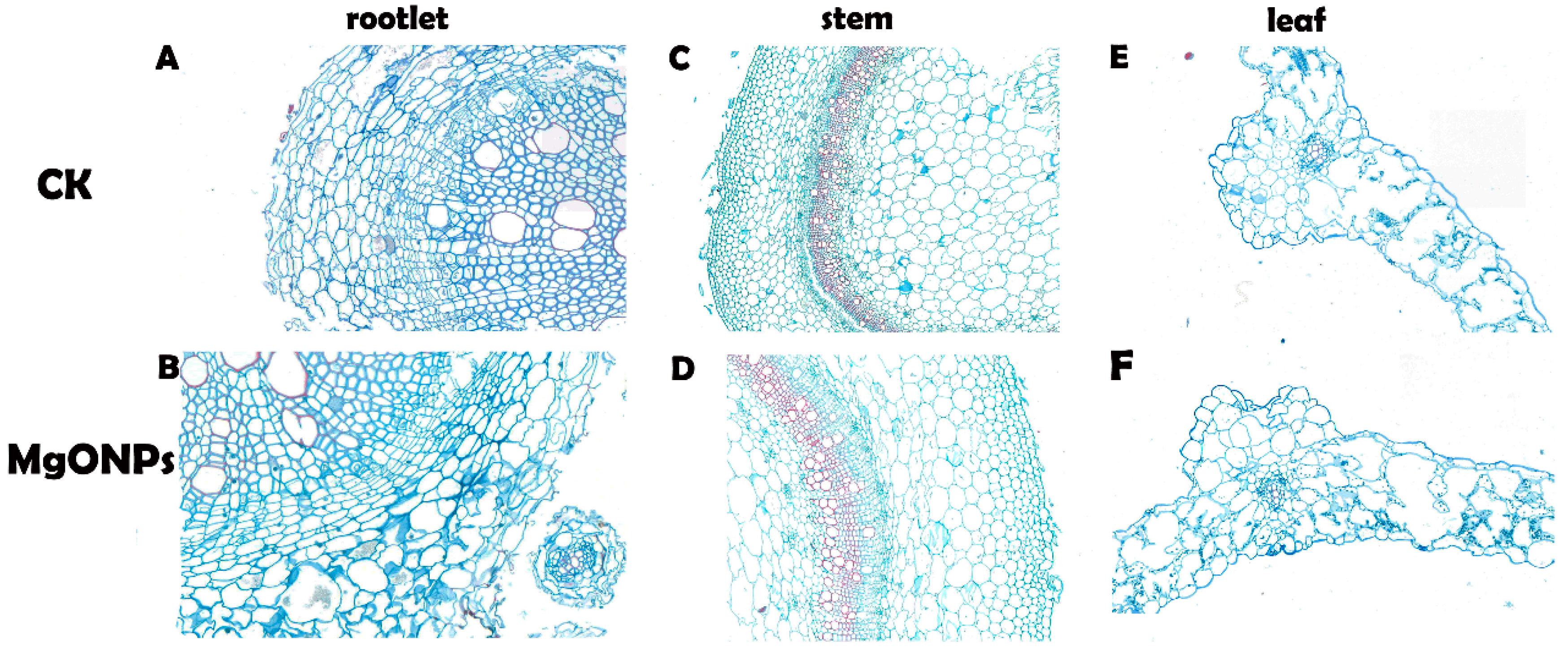
3.6. Morphological and Anatomical Analyses
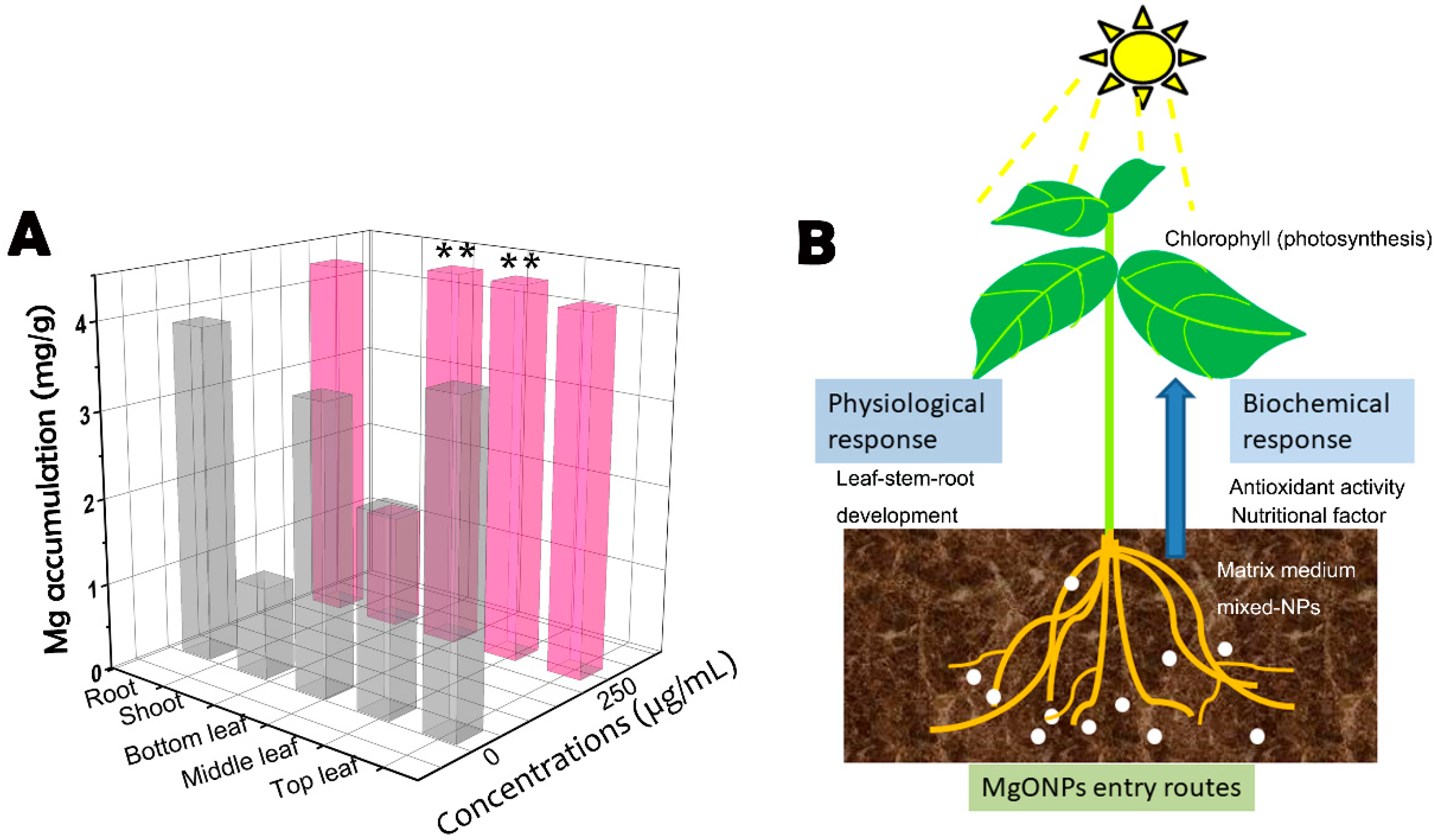
3.7. Uptake and Translocation of MgONPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rodrigues, S.M.; Demokritou, P.; Dokoozlian, N.; Hendren, C.O.; Karn, B.; Mauter, M.S.; Sadik, O.A.; Safarpour, M.; Unrine, J.M.; Viers, J.; et al. Nanotechnology for sustainable food production: Promising opportunities and scientific challenges. Environ. Sci. Nano 2017, 4, 767–781. [Google Scholar] [CrossRef]
- Gogos, A.; Knauer, K.; Bucheli, T.D. Nanomaterials in Plant Protection and Fertilization: Current State, Foreseen Applications, and Research Priorities. J. Agric. Food Chem. 2012, 60, 9781–9792. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Qin, X.; Zeng, G. Biodiversity change behind wide applications of nanomaterials? Nano Today 2017, 17, 11–13. [Google Scholar] [CrossRef]
- Capaldi Arruda, S.C.; Diniz Silva, A.L.; Galazzi, R.M.; Azevedo, R.A.; Zezzi Arruda, M.A. Nanoparticles applied to plant science: A review. Talanta 2015, 131, 693–705. [Google Scholar] [CrossRef]
- Zhao, P.; Cao, L.; Ma, D.; Zhou, Z.; Huang, Q.; Pan, C. Translocation, distribution and degradation of prochloraz-loaded mesoporous silica nanoparticles in cucumber plants. Nanoscale 2018, 10, 1798–1806. [Google Scholar] [CrossRef]
- Rai, P.K.; Kumar, V.; Lee, S.; Raza, N.; Kim, K.-H.; Ok, Y.S.; Tsang, D.C.W. Nanoparticle-plant interaction: Implications in energy, environment, and agriculture. Environ. Inter. 2018, 119, 1–19. [Google Scholar] [CrossRef]
- Raliya, R.; Nair, R.; Chavalmane, S.; Wang, W.-N.; Biswas, P. Mechanistic evaluation of translocation and physiological impact of titanium dioxide and zinc oxide nanoparticles on the tomato (Solanum lycopersicum L.) plant. Metallomics 2015, 7, 1584–1594. [Google Scholar] [CrossRef]
- Tan, W.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Interaction of titanium dioxide nanoparticles with soil components and plants: Current knowledge and future research needs–a critical review. Environ. Sci. Nano 2018, 5, 257–278. [Google Scholar] [CrossRef]
- Raliya, R.; Biswas, P.; Tarafdar, J.C. TiO2 nanoparticle biosynthesis and its physiological effect on mung bean (Vigna radiata L.). Biotechnol. Rep. 2015, 5, 22–26. [Google Scholar] [CrossRef]
- Das, P.; Barua, S.; Sarkar, S.; Chatterjee, S.K.; Mukherjee, S.; Goswami, L.; Das, S.; Bhattacharya, S.; Karak, N.; Bhattacharya, S.S. Mechanism of toxicity and transformation of silver nanoparticles: Inclusive assessment in earthworm-microbe-soil-plant system. Geoderma 2018, 314, 73–84. [Google Scholar] [CrossRef]
- De la Rosa, G.; Lopez-Moreno, M.L.; Hernandez-Viezcas, J.; Montes, M.O.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Toxicity and biotransformation of ZnO nanoparticles in the desert plants Prosopis juliflora-velutina, Salsola tragus and Parkinsonia florida. Int. J. Nanotechnol. 2011, 8, 492–506. [Google Scholar] [CrossRef]
- Zuverza-Mena, N.; Martinez-Fernandez, D.; Du, W.; Hernandez-Viezcas, J.A.; Bonilla-Bird, N.; Lopez-Moreno, M.L.; Komarek, M.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Exposure of engineered nanomaterials to plants: Insights into the physiological and biochemical responses-A review. Plant Physiol. Biochem. 2017, 110, 236–264. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Chen, J.; Liu, Z.; Wang, H.; Yang, H.; Ding, W. Magnesium Oxide Nanoparticles: Effective Agricultural Antibacterial Agent Against Ralstonia solanacearum. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Avellan, A.; Laughton, S.; Vaidya, R.; Rodrigues, S.M.; Casman, E.A.; Lowry, G.V. CuO Nanoparticle Dissolution and Toxicity to Wheat (Triticum aestivum) in Rhizosphere Soil. Environ. Sci. Technol. 2018, 52, 2888–2897. [Google Scholar] [CrossRef] [PubMed]
- Burman, U.; Saini, M.; Praveen, K. Effect of zinc oxide nanoparticles on growth and antioxidant system of chickpea seedlings. Toxicol. Environ. Chem. 2013, 95, 605–612. [Google Scholar] [CrossRef]
- Wang, W.-N.; Tarafdar, J.C.; Biswas, P. Nanoparticle synthesis and delivery by an aerosol route for watermelon plant foliar uptake. J. Nanopart. Res. 2013, 15, 1417. [Google Scholar] [CrossRef]
- Guo, S.; Li, D.; Zhang, L.; Li, J.; Wang, E. Monodisperse mesoporous superparamagnetic single-crystal magnetite nanoparticles for drug delivery. Biomaterials 2009, 30, 1881–1889. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Hong, F.S.; Lu, S.P.; Liu, C. Effect of nano-TiO2 on strength of naturally and growth aged seeds of spinach. Biol. Trace Elem. Res. 2005, 104, 83–91. [Google Scholar] [CrossRef]
- Clement, L.; Hurel, C.; Marmier, N. Toxicity of TiO2 nanoparticles to cladocerans, algae, rotifers and plants–Effects of size and crystalline structure. Chemosphere 2013, 90, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Ruttkay-Nedecky, B.; Krystofova, O.; Nejdl, L.; Adam, V. Nanoparticles based on essential metals and their phytotoxicity. J. Nanobiotechnol. 2017, 15, 33. [Google Scholar] [CrossRef] [PubMed]
- Jhansi, K.; Jayarambabu, N.; Reddy, K.P.; Reddy, N.M.; Suvarna, R.P.; Rao, K.V.; Kumar, V.R.; Rajendar, V. Biosynthesis of MgO nanoparticles using mushroom extract: Effect on peanut (Arachis hypogaea L.) seed germination. Biotech 2017, 7, 263. [Google Scholar] [CrossRef] [PubMed]
- Moynier, F.; Fujii, T. Theoretical isotopic fractionation of magnesium between chlorophylls. Sci. Rep. 2017, 7, 6973. [Google Scholar] [CrossRef] [PubMed]
- Morteza, E.; Moaveni, P.; Farahani, H.A.; Kiyani, M. Study of photosynthetic pigments changes of maize (Zea mays L.) under nano Tio(2) spraying at various growth stages. Springerplus 2013, 2, 247. [Google Scholar] [CrossRef] [PubMed]
- Juhel, G.; Batisse, E.; Hugues, Q.; Daly, D.; van Pelt, F.N.A.M.; O’Halloran, J.; Jansen, M.A.K. Alumina nanoparticles enhance growth of Lemna minor. Aquat. Toxicol. 2011, 105, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Krishnaraj, C.; Jagan, E.G.; Ramachandran, R.; Abirami, S.M.; Mohan, N.; Kalaichelvan, P.T. Effect of biologically synthesized silver nanoparticles on Bacopa monnieri (Linn.) Wettst. plant growth metabolism. Process Biochem. 2012, 47, 651–658. [Google Scholar] [CrossRef]
- Hernandez-Viezcas, J.A.; Castillo-Michel, H.; Andrews, J.C.; Cotte, M.; Rico, C.; Peralta-Videa, J.R.; Ge, Y.; Priester, J.H.; Holden, P.A.; Gardea-Torresdey, J.L. In Situ Synchrotron X-ray Fluorescence Mapping and Speciation of CeO2 and ZnO Nanoparticles in Soil Cultivated Soybean (Glycine max). Acs Nano 2013, 7, 1415–1423. [Google Scholar] [CrossRef]
- Yuan, L.; Richardson, C.J.; Ho, M.; Willis, C.W.; Colman, B.P.; Wiesner, M.R. Stress Responses of Aquatic Plants to Silver Nanoparticles. Environ. Sci. Technol. 2018, 52, 2558–2565. [Google Scholar] [CrossRef]
- Li, X.; Ke, M.; Zhang, M.; Peijnenburg, W.J.G.M.; Fan, X.; Xu, J.; Zhang, Z.; Lu, T.; Fu, Z.; Qian, H. The interactive effects of diclofop-methyl and silver nanoparticles on Arabidopsis thaliana: Growth, photosynthesis and antioxidant system. Environ. Pollut. 2018, 232, 212–219. [Google Scholar] [CrossRef]
- Lee, S.; Kim, S.; Kim, S.; Lee, I. Assessment of phytotoxicity of ZnO NPs on a medicinal plant, Fagopyrum esculentum. Environ. Sci. Pollut. Res. 2013, 20, 848–854. [Google Scholar] [CrossRef]
- Cruz de Carvalho, M.H. Drought stress and reactive oxygen species: Production, scavenging and signaling. Plant Signal. Behav. 2008, 3, 156–165. [Google Scholar] [CrossRef]
- Ding, L.; Zhao, H.-m.; Zeng, W.-J.; Li, Q.; Wang, Y.; Wang, S.-q. Physiological responses of five plants in northwest China arid area under drought stress. Yingyong Shengtai Xuebao 2017, 28, 1455–1463. [Google Scholar] [PubMed]
- Qian, H.; Zhu, K.; Lu, H.; Lavoie, M.; Chen, S.; Zhou, Z.; Deng, Z.; Chen, J.; Fu, Z. Contrasting silver nanoparticle toxicity and detoxification strategies in Microcystis aeruginosa and Chlorella vulgaris: New insights from proteomic and physiological analyses. Sci. Total Environ. 2016, 572, 1213–1221. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Cao, T.; Ni, L.; Xie, P.; Li, Z. Carbon, nitrogen and antioxidant enzyme responses of Potamogeton crispus to both low light and high nutrient stresses. Environ. Exp. Bot. 2010, 68, 44–50. [Google Scholar] [CrossRef]
- Wen, Y.; Chen, H.; Shen, C.; Zhao, M.; Liu, W. Enantioselectivity Tuning of Chiral Herbicide Dichlorprop by Copper: Roles of Reactive Oxygen Species. Environ. Sci. Technol. 2011, 45, 4778–4784. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Tan, W.; Yin, Y.; Ji, R.; Peralta-Videa, J.R.; Guo, H.; Gardea-Torresdey, J.L. Differential effects of copper nanoparticles/microparticles in agronomic and physiological parameters of oregano (Origanum vulgare). Sci. Total Environ. 2018, 618, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Foltete, A.-S.; Masfaraud, J.-F.; Bigorgne, E.; Nahmani, J.; Chaurand, P.; Botta, C.; Labille, J.; Rose, J.; Ferard, J.-F.; Cotelle, S. Environmental impact of sunscreen nanomaterials: Ecotoxicity and genotoxicity of altered TiO2 nanocomposites on Vicia faba. Environ. Pollut. 2011, 159, 2515–2522. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-L.; Zhang, Y.-X.; Liu, H.-Z.; Xin, H. Phytotoxicity of Copper Oxide Nanoparticles to Metabolic Activity in the Roots of Rice. Huanjing Kexue 2014, 35, 1968–1973. [Google Scholar]
- Wang, Z.-Y.; Yu, X.-L.; Gao, D.-M.; Feng, W.-Q.; Xing, B.-S.; Li, F.-M. Effect of Nano-rutile TiO2 and Multiwalled Carbon Nanotubes on the Growth of Maize (Zea mays L.) Seedlings and the Relevant Antioxidant Response. Huanjing Kexue 2010, 31, 480–487. [Google Scholar]
- Rico, C.M.; Majumdar, S.; Duarte-Gardea, M.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Interaction of Nanoparticles with Edible Plants and Their Possible Implications in the Food Chain. J. Agri. Food Chem. 2011, 59, 3485–3498. [Google Scholar] [CrossRef]
- Kole, C.; Kole, P.; Randunu, K.M.; Choudhary, P.; Podila, R.; Ke, P.C.; Rao, A.M.; Marcus, R.K. Nanobiotechnology can boost crop production and quality: First evidence from increased plant biomass, fruit yield and phytomedicine content in bitter melon (Momordica charantia). Bmc Biotechnol. 2013, 13, 37. [Google Scholar] [CrossRef]
- Cakmak, I.; Marschner, H. Magnesium-deficiency and high light-intensity enhance activities of superoxide-dismutase, ascorbate peroxidase, and glutathione-reductase in bean-leaves. Plant Physiol. 1992, 98, 1222–1227. [Google Scholar] [CrossRef] [PubMed]
- Smirnoff, N. Tansley Review No. 52. The role of active oxygen in the response of plants to water deficit and desiccation. New Phytol. 1993, 125, 27–58. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, X.; Chen, Y.; Sommerfeld, M.; Hu, Q. Toxicity assessment of manufactured nanomaterials using the unicellular green alga Chlamydomonas reinhardtii. Chemosphere 2008, 73, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Krizkova, S.; Ryant, P.; Krystofova, O.; Adam, V.; Galiova, M.; Beklova, M.; Babula, P.; Kaiser, J.; Novotny, K.; Novotny, J.; et al. Multi-instrumental analysis of tissues of sunflower plants treated with silver(I) ions–Plants as bioindicators of environmental pollution. Sensors 2008, 8, 445–463. [Google Scholar] [CrossRef] [PubMed]
- García-Gómez, C.; Babin, M.; Obrador, A.; Álvarez, J.M.; Fernández, M.D. Integrating ecotoxicity and chemical approaches to compare the effects of ZnO nanoparticles, ZnO bulk, and ZnCl 2 on plants and microorganisms in a natural soil. Environmental Sci. Pollut. Res. 2015, 22, 16803–16813. [Google Scholar] [CrossRef] [PubMed]
- Zafar, H.; Ali, A.; Zia, M. CuO nanoparticles inhibited root growth from Brassica nigra seedlings but induced root from stem and leaf explants. Appl. Biochem. Biotechnol. 2017, 181, 365–378. [Google Scholar] [CrossRef]
- Mosa, K.A.; El-Naggar, M.; Ramamoorthy, K.; Alawadhi, H.; Elnaggar, A.; Wartanian, S.; Ibrahim, E.; Hani, H. Copper Nanoparticles Induced Genotoxicty, Oxidative Stress, and Changes in Superoxide Dismutase (SOD) Gene Expression in Cucumber (Cucumis sativus) Plants. Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef]
- Raliya, R.; Franke, C.; Chavalmane, S.; Nair, R.; Reed, N.; Biswas, P. Quantitative Understanding of Nanoparticle Uptake in Watermelon Plants. Front. Plant Sci. 2016, 7, 1288. [Google Scholar] [CrossRef]
- Corredor, E.; Testillano, P.S.; Coronado, M.-J.; Gonzalez-Melendi, P.; Fernandez-Pacheco, R.; Marquina, C.; Ricardo Ibarra, M.; de la Fuente, J.M.; Rubiales, D.; Perez-de-Luque, A.; et al. Nanoparticle penetration and transport in living pumpkin plants: In situ subcellular identification. BMC Plant Biol. 2009, 9, 45. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, L.; Liu, M.; Liu, Z.; Yang, H.; Sun, X.; Chen, J.; Xiang, S.; Ding, W. MgONPs Can Boost Plant Growth: Evidence from Increased Seedling Growth, Morpho-Physiological Activities, and Mg Uptake in Tobacco (Nicotiana tabacum L.). Molecules 2018, 23, 3375. https://doi.org/10.3390/molecules23123375
Cai L, Liu M, Liu Z, Yang H, Sun X, Chen J, Xiang S, Ding W. MgONPs Can Boost Plant Growth: Evidence from Increased Seedling Growth, Morpho-Physiological Activities, and Mg Uptake in Tobacco (Nicotiana tabacum L.). Molecules. 2018; 23(12):3375. https://doi.org/10.3390/molecules23123375
Chicago/Turabian StyleCai, Lin, Minghong Liu, Zhongwei Liu, Huikuan Yang, Xianchao Sun, Juanni Chen, Shunyu Xiang, and Wei Ding. 2018. "MgONPs Can Boost Plant Growth: Evidence from Increased Seedling Growth, Morpho-Physiological Activities, and Mg Uptake in Tobacco (Nicotiana tabacum L.)" Molecules 23, no. 12: 3375. https://doi.org/10.3390/molecules23123375
APA StyleCai, L., Liu, M., Liu, Z., Yang, H., Sun, X., Chen, J., Xiang, S., & Ding, W. (2018). MgONPs Can Boost Plant Growth: Evidence from Increased Seedling Growth, Morpho-Physiological Activities, and Mg Uptake in Tobacco (Nicotiana tabacum L.). Molecules, 23(12), 3375. https://doi.org/10.3390/molecules23123375




