Simultaneous Quantitation of Isoprenoid Pyrophosphates in Plasma and Cancer Cells Using LC-MS/MS
Abstract
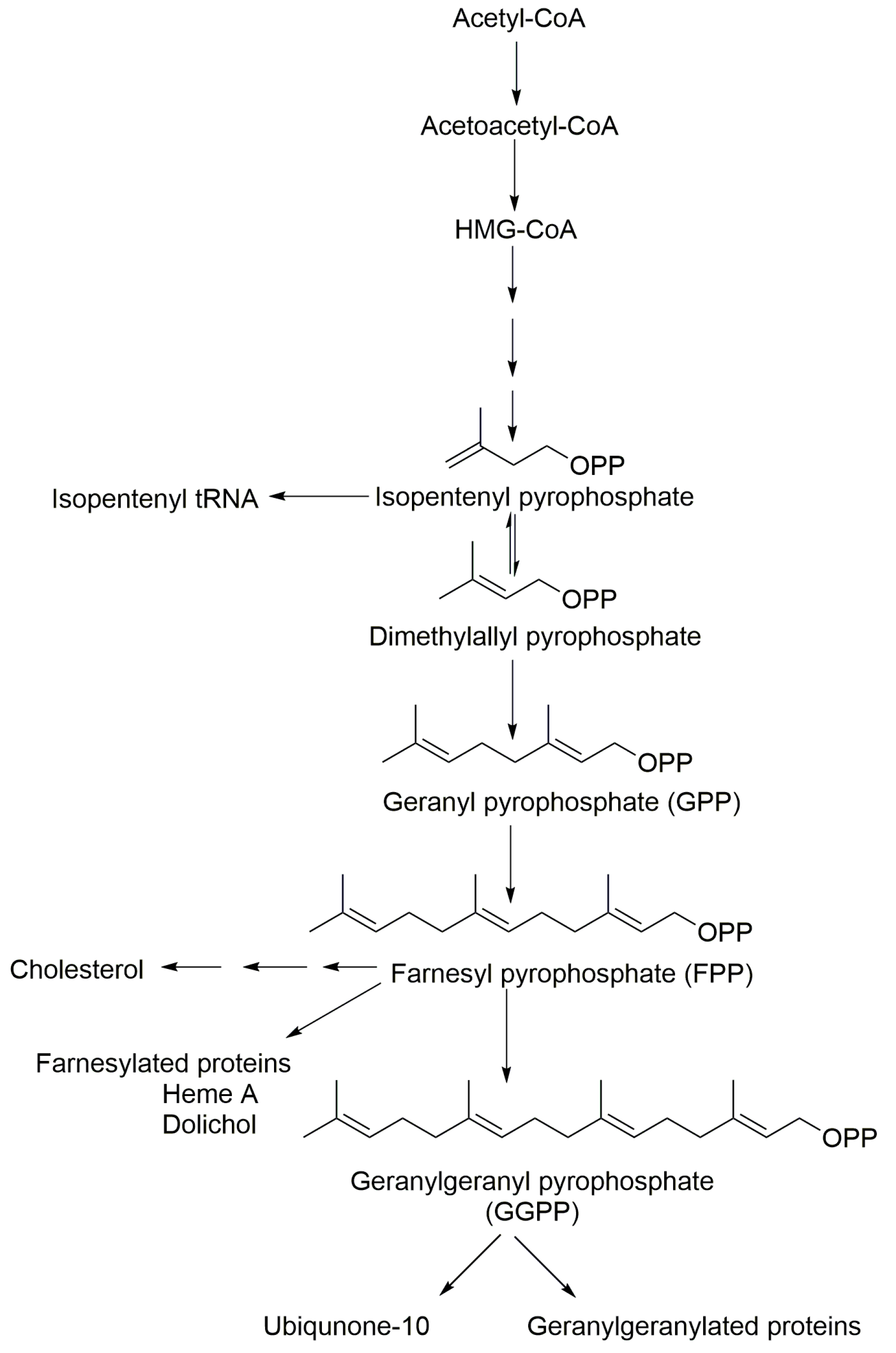
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.1.1. Cell Culture
2.1.2. Chemicals
2.2. Liquid Chromatographic and MS/MS Conditions
2.3. Charcoal-Stripped Plasma Preparation
2.4. Preparation of Stock, Calibration Standard and Quality Control Sample Preparation
2.5. Plasma Sample Preparation
2.6. Cell Sample Preparation
2.7. Method Validation
2.8. Selectivity and Specificity
2.9. Sensitivity
2.10. Accuracy and Precision
2.11. Recovery and Matrix Effect
2.12. Calibration Curve
2.13. Plasma Stability
2.14. Application of the Method
3. Results and Discussion
3.1. Chromatographic and Mass Spectrometric Conditions Optimization
3.2. Assay Validation
3.2.1. Specificity and Selectivity
3.2.2. Accuracy and Precision
3.3. Calibration Curve and Carry-Over
3.4. Recovery and Matrix Effect
3.5. Plasma Stability
3.6. Application of the Method for Samples Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| DMAPP | dimethyl allyl pyrophosphate |
| DMEM | Dulbecco’s modified Eagle’s medium |
| FPP | farnesyl pyrophosphate |
| FPPS | farnesyl pyrophosphate synthase |
| GGPP | geranylgeranyl pyrophosphate |
| GPP | geranyl pyrophosphate |
| GGDPS | geranylgeranyl diphosphate synthase |
| HMG-CoA | 3-hydroxy-3-methylglutaryl-CoA |
| IBP | isoprenoid biosynthetic pathway |
| IPP | isopentenyl pyrophosphate |
| IS | internal standard |
| LOD | limit of detection |
| LC-MS/MS | high-performance liquid chromatography–tandem mass spectrometry |
| MVA | mevalonate |
| MVAP | 5-phosphomevalonate |
| MPD | mevalonate pyrophosphate decarboxylase |
| MRM | multiple reaction monitoring |
References
- Buhaescu, I.; Izzedine, H. Mevalonate pathway: A review of clinical and therapeutical implications. Clin. Biochem. 2007, 40, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Haney, S.; Wills, V.; Wiemer, D.; Holstein, S. Recent advances in the development of mammalian geranylgeranyl diphosphate synthase inhibitors. Molecules 2017, 22, 886. [Google Scholar] [CrossRef] [PubMed]
- Eastell, R.; Walsh, J.S.; Watts, N.B.; Siris, E. Bisphosphonates for postmenopausal osteoporosis. Bone 2011, 49, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.E.; McCloskey, E.V. Bisphosphonates in oncology. Bone 2011, 49, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Morgan, G.J.; Davies, F.E.; Gregory, W.M.; Cocks, K.; Bell, S.E.; Szubert, A.J.; Navarro-Coy, N.; Drayson, M.T.; Owen, R.G.; Feyler, S.; et al. First-line treatment with zoledronic acid as compared with clodronic acid in multiple myeloma (MRC Myeloma IX): A randomised controlled trial. Lancet 2010, 376, 1989–1999. [Google Scholar] [CrossRef]
- Chan, K.K.W.; Oza, A.M.; Siu, L.L. The statins as anticancer agents. Clin. Cancer Res. 2003, 9, 10–19. [Google Scholar] [PubMed]
- Mijimolle, N.; Velasco, J.; Dubus, P.; Guerra, C.; Weinbaum, C.A.; Casey, P.J.; Campuzano, V.; Barbacid, M. Protein farnesyltransferase in embryogenesis, adult homeostasis, and tumor development. Cancer Cell 2005, 7, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Haney, S.L.; Chhonker, Y.S.; Varney, M.L.; Talmon, G.; Murry, D.J.; Holstein, S.A. Preclinical investigation of a potent geranylgeranyl diphosphate synthase inhibitor. Investig. New Drugs 2018, 36, 810–818. [Google Scholar] [CrossRef]
- Allen, C.; Kortagere, S.; Tong, H.; Matthiesen, R.A.; Metzger, J.I.; Wiemer, D.F.; Holstein, S.A. Olefin isomers of a triazole bisphosphonate synergistically inhibit geranylgeranyl diphosphate synthase. Mol. Pharmacol. 2017, 91, 229–236. [Google Scholar] [CrossRef]
- Cole, S.L.; Vassar, R. Isoprenoids and Alzheimer’s disease: A complex relationship. Neurobiol. Dis. 2006, 22, 209–222. [Google Scholar] [CrossRef]
- Eckert, G.P.; Hooff, G.P.; Strandjord, D.M.; Igbavboa, U.; Volmer, D.A.; Müller, W.E.; Wood, W.G. Regulation of the brain isoprenoids farnesyl-and geranylgeranylpyrophosphate is altered in male Alzheimer patients. Neurobiol. Dis. 2009, 35, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, A.C.R.G.; Resende, R.; Oliveira, C.R.; Pereira, C.M.F. Cholesterol and statins in Alzheimer’s disease: Current controversies. Exp. Neurol. 2010, 223, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.K. Isoprenoids as mediators of the biological effects of statins. J. Clin. Investig. 2002, 110, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Hori, Y.; Kikuchi, A.; Isomura, M.; Katayama, M.; Miura, Y.; Fujioka, H.; Kaibuchi, K.; Takai, Y. Post-translational modifications of the C-terminal region of the rho protein are important for its interaction with membranes and the stimulatory and inhibitory GDP/GTP exchange proteins. Oncogene 1991, 6, 515–522. [Google Scholar] [PubMed]
- Casey, P.J.; Seabra, M.C. Protein prenyltransferases. J. Biol. Chem. 1996, 271, 5289–5292. [Google Scholar] [CrossRef] [PubMed]
- McTaggart, S.J. Isoprenylated proteins. Cell. Mol. Life Sci. 2006, 63, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Kuder, C.H.; Wasko, B.M.; Hohl, R.J. Quantitative determination of isopentenyl diphosphate in cultured mammalian cells. Anal. Biochem. 2013, 433, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Hooff, G.P.; Wood, W.G.; Kim, J.-H.; Igbavboa, U.; Ong, W.-Y.; Muller, W.E.; Eckert, G.P. Brain isoprenoids farnesyl pyrophosphate and geranylgeranyl pyrophosphate are increased in aged mice. Mol. Neurobiol. 2012, 46, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Hooff, G.P.; Volmer, D.A.; Wood, W.G.; Müller, W.E.; Eckert, G.P. Isoprenoid quantitation in human brain tissue: A validated HPLC–fluorescence detection method for endogenous farnesyl-(FPP) and geranylgeranylpyrophosphate (GGPP). Anal. Bioanal. Chem. 2008, 392, 673–680. [Google Scholar] [CrossRef]
- Tong, H.; Holstein, S.A.; Hohl, R.J. Simultaneous determination of farnesyl and geranylgeranyl pyrophosphate levels in cultured cells. Anal. Biochem. 2005, 336, 51–59. [Google Scholar] [CrossRef]
- Tong, H.; Wiemer, A.J.; Neighbors, J.D.; Hohl, R.J. Quantitative determination of farnesyl and geranylgeranyl diphosphate levels in mammalian tissue. Anal. Biochem. 2008, 378, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Saisho, Y.; Morimoto, A.; Umeda, T. Determination of farnesyl pyrophosphate in dog and human plasma by high-performance liquid chromatography with fluorescence detection. Anal. Biochem. 1997, 252, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, H.; Iguchi, M.; Jinno, F. Bioanalysis of farnesyl pyrophosphate in human plasma by high-performance liquid chromatography coupled to triple quadrupole tandem mass spectrometry and hybrid quadrupole Orbitrap high-resolution mass spectrometry. Anal. Bioanal. Chem. 2017, 409, 3551–3560. [Google Scholar] [CrossRef] [PubMed]
- Hooff, G.P.; Patel, N.; Wood, W.G.; Müller, W.E.; Eckert, G.P.; Volmer, D.A. A rapid and sensitive assay for determining human brain levels of farnesyl-(FPP) and geranylgeranylpyrophosphate (GGPP) and transferase activities using UHPLC–MS/MS. Anal. Bioanal. Chem. 2010, 398, 1801–1808. [Google Scholar] [CrossRef] [PubMed]
- Henneman, L.; van Cruchten, A.G.; Denis, S.W.; Amolins, M.W.; Placzek, A.T.; Gibbs, R.A.; Kulik, W.; Waterham, H.R. Detection of nonsterol isoprenoids by HPLC–MS/MS. Anal. Biochem. 2008, 383, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Henneman, L.; van Cruchten, A.G.; Kulik, W.; Waterham, H.R. Inhibition of the isoprenoid biosynthesis pathway; detection of intermediates by UPLC–MS/MS. Biochim. Biophys. Acta 2011, 1811, 227–233. [Google Scholar] [CrossRef]
- Iwamura, T.; Taniguchi, S.; Kitamura, N.; Yamanari, H.; Kojima, A.; Hidaka, K.; Setoguchi, T.; Katsuki, T. Correlation between CA19-9 production in vitro and histological grades of differentiation in vivo in clones isolated from a human pancreatic cancer cell line (SUIT-2). J. Gastroenterol. Hepatol. 1992, 7, 512–519. [Google Scholar] [CrossRef]
- Chhonker, Y.S.; Haney, S.L.; Matthiesen, R.A.; Wiemer, D.F.; Holstein, S.A.; Murry, D.J. Quantitative determination of a potent geranylgeranyl diphosphate synthase inhibitor using LC–MS/MS: Derivatization and application. J. Pharm. Biomed. Anal. 2018, 153, 22–28. [Google Scholar] [CrossRef]
- Department of Health and Human Services Food and Drug Administration, Center for Drug Evaluation and Research (CDER) Center for Veterinary Medicine (CVM). Guidance for Industry, Bioanalytical Method Validation; FDA: Rockville, MD, USA, 2013.
- Van Eeckhaut, A.; Lanckmans, K.; Sarre, S.; Smolders, I.; Michotte, Y. Validation of bioanalytical LC–MS/MS assays: Evaluation of matrix effects. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2009, 877, 2198–2207. [Google Scholar] [CrossRef]
- Matuszewski, B.K.; Constanzer, M.L.; Chavez-Eng, C.M. Matrix effect in quantitative LC/MS/MS analyses of biological fluids: A method for determination of finasteride in human plasma at picogram per milliliter concentrations. Anal. Chem. 1998, 70, 882–889. [Google Scholar] [CrossRef]
- Gachet, M.S.; Rhyn, P.; Bosch, O.G.; Quednow, B.B.; Gertsch, J. A quantitiative LC-MS/MS method for the measurement of arachidonic acid, prostanoids, endocannabinoids, N-acylethanolamines and steroids in human plasma. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2015, 976, 6–18. [Google Scholar] [CrossRef] [PubMed]
- Strassburg, K.; Huijbrechts, A.M.; Kortekaas, K.A.; Lindeman, J.H.; Pedersen, T.L.; Dane, A.; Berger, R.; Brenkman, A.; Hankemeier, T.; van Duynhoven, J.; et al. Quantitative profiling of oxylipins through comprehensive LC-MS/MS analysis: Application in cardiac surgery. Anal Bioanal. Chem 2012, 404, 1413–1426. [Google Scholar] [CrossRef] [PubMed]
- Prasain, J.K.; Arabshahi, A.; Taub, P.R.; Sweeney, S.; Moore, R.; Sharer, J.D.; Barnes, S. Simultaneous quantification of F2-isoprostanes and prostaglandins in human urine by liquid chromatography tandem-mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2013, 913, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.; Tomaru, K.; Matsumoto, N.; Watanabe, S.; Higashi, T. LC/ESI-MS/MS method for determination of salivary eicosapentaenoic acid concentration to arachidonic acid concentration ratio. Biomed. Chromatogr. 2015, 30, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Thakare, R.; Chhonker, Y.S.; Gautam, N.; Nelson, A.; Casaburi, R.; Criner, G.; Dransfield, M.T.; Make, B.; Schmid, K.K.; Rennard, S.I.; et al. Simultaneous LC–MS/MS analysis of eicosanoids and related metabolites in human serum, sputum and BALF. Biomed. Chromatogr. 2018, 32, e4102. [Google Scholar] [CrossRef] [PubMed]
- Deems, R.; Buczynski, M.W.; Bowers-Gentry, R.; Harkewicz, R.; Dennis, E.A. Detection and quantitation of eicosanoids via high performance liquid chromatography-electrospray ionization-mass spectrometry. In Methods in Enzymology; Brown, H.A., Ed.; Academic Press: Cambridge, MA, USA, 2007; Volume 432, pp. 59–82. [Google Scholar]
- Levison, B.S.; Zhang, R.; Wang, Z.; Fu, X.; Didonato, J.A.; Hazen, S.L. Quantification of fatty acid oxidation products using online high-performance liquid chromatography tandem mass spectrometry. Free Radic. Biol. Med. 2013, 59, 2–13. [Google Scholar] [CrossRef]
- Gouveia-Figueira, S.; Nording, M.L. Validation of a tandem mass spectrometry method using combined extraction of 42 oxylipins and 15 endocannabinoid-related compounds including prostamides from biological matrices. Prostaglandins Other Lipid Mediat. 2015, 121, 110–121. [Google Scholar] [CrossRef]
- Idborg, H.; Pawelzik, S.C.; Perez-Manso, M.; Björk, L.; Hamrin, J.; Herlenius, E.; Jakobsson, P.J. Evaluation of urinary prostaglandin E2 metabolite as a biomarker in infants with fever due to viral infection. Prostaglandins Leukot. Essent. Fatty Acids 2014, 91, 269–275. [Google Scholar] [CrossRef]
- Jian, W.; Edom, R.W.; Xue, X.; Huang, M.Q.; Fourie, A.; Weng, N. Quantitation of leukotriene B4 in human sputum as a biomarker using UPLC-MS/MS. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2013, 932, 59–65. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, G.; Clarke, P.A.; Huang, J.T.J.; Takahashi, E.; Muirhead, D.; Steenwyk, R.C.; Lin, Z. Simultaneous and high-throughput quantitation of urinary tetranor PGDM and tetranor PGEM by online SPE-LC–MS/MS as inflammatory biomarkers. J. Mass Spectrom. 2011, 46, 705–711. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
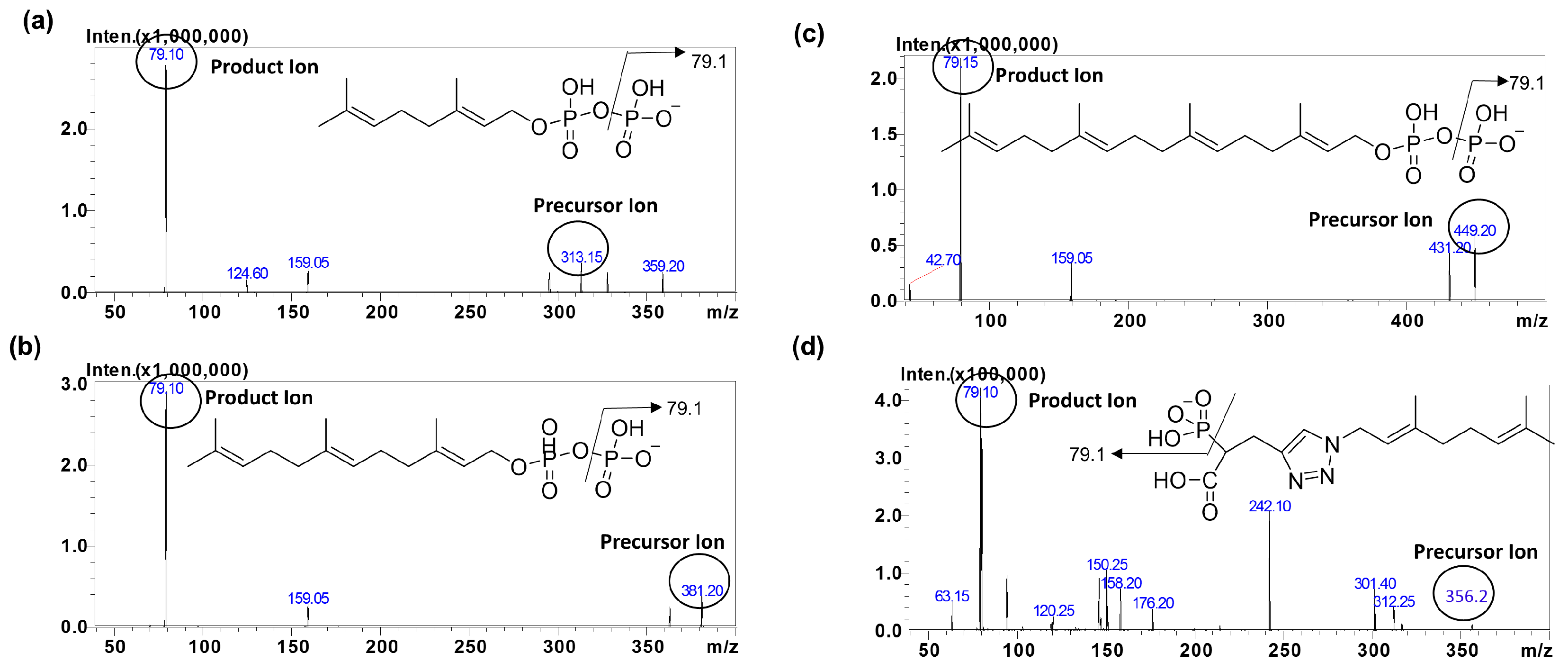
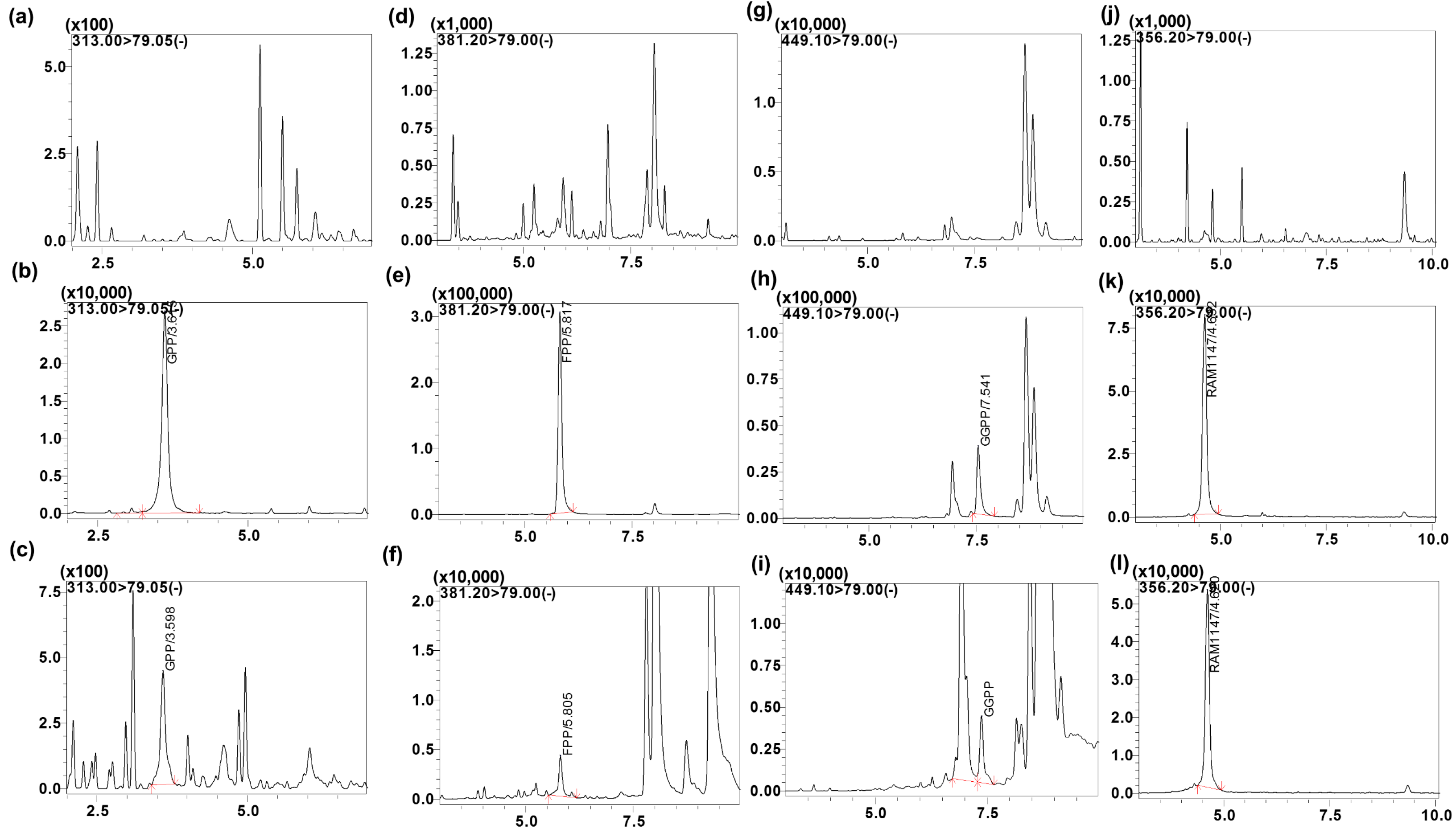
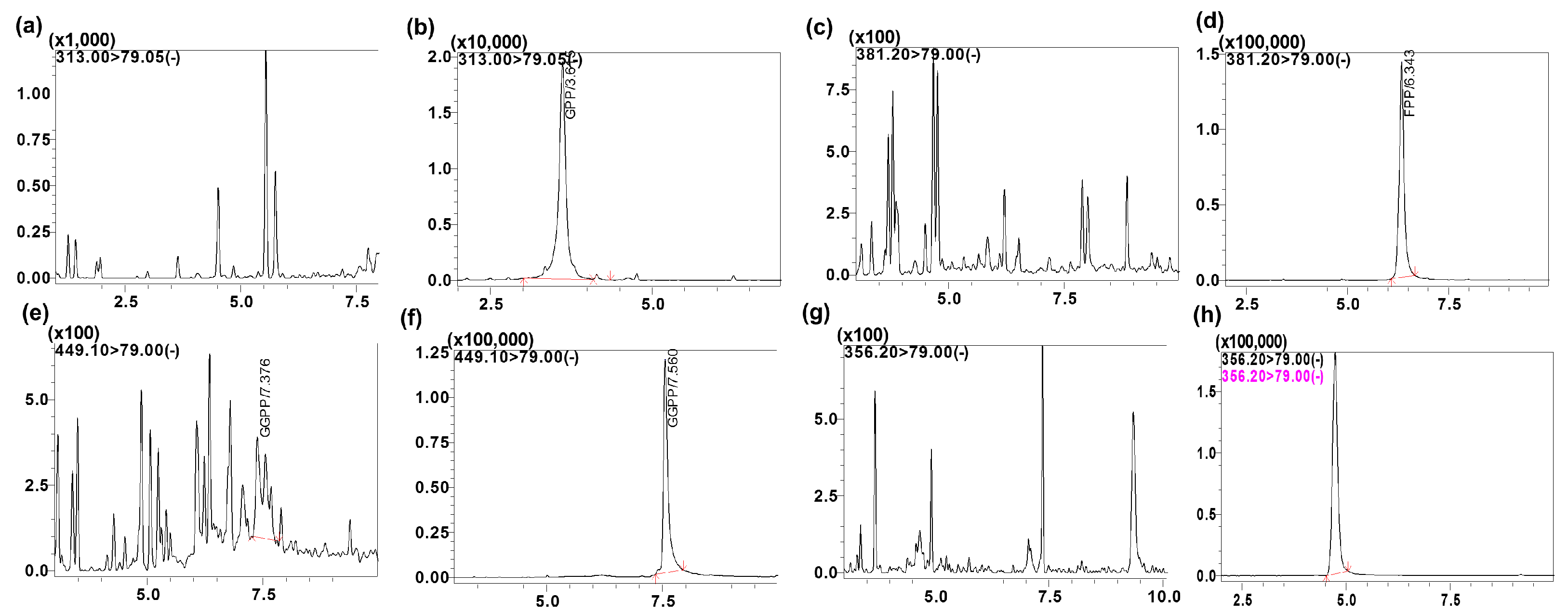
| Analytes | MRM Transition m/z (Q1→Q3) | Q1 (V) | CE (V) | Q3 (V) | Retention Time |
|---|---|---|---|---|---|
| GPP | 313.1→79.1 | 11 | 25 | 10 | 3.6 |
| FPP | 381.2→79.1 | 14 | 35 | 26 | 5.8 |
| GGPP | 449.2→79.1 | 15 | 25 | 22 | 7.5 |
| RAM1147 | 356.2→79.1 | 27 | 55 | 24 | 4.6 |
| Bio-Matrix | Analytes | LLOQ (0.04 ng/mL) | LQC (1 ng/mL) | MQC (5 ng/mL) | HQC (20 ng/mL) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Accuracy | % RSD | Accuracy | % RSD | Accuracy | % RSD | Accuracy | % RSD | ||
| Charcoalated Plasma | GPP | 97.8 | 8.2 | 102.3 | 3.2 | 98.3 | 7.6 | 102.5 | 7.8 |
| FPP | 91.9 | 7.4 | 92.5 | 4.7 | 104.6 | 8.5 | 108.4 | 2.9 | |
| GGPP | 100.7 | 6.2 | 112.7 | 8.3 | 97.2 | 5.4 | 91.1 | 9.1 | |
| Buffer | GPP | 101.2 | 6.6 | 105.2 | 5.8 | 97.8 | 10 | 97.3 | 1.8 |
| FPP | 110.2 | 2.5 | 112.5 | 7.7 | 101.3 | 2.5 | 95.2 | 4.9 | |
| GGPP | 94.4 | 7.8 | 98.4 | 2.9 | 105.2 | 5.4 | 103.1 | 7.8 | |
| Analyte | Charcoalated Human Plasma | Buffer | ||
|---|---|---|---|---|
| LQC | HQC | LQC | HQC | |
| GPP | 55.1 ± 4.2 | 59.1 ± 5.9 | 85.1 ± 9.1 | 81.5 ± 9.9 |
| FPP | 63.8 ± 5.9 | 64.2 ± 4.4 | 87.8 ± 10.5 | 84.8 ± 8.9 |
| GGPP | 40.2 ± 4.4 | 47.3 ± 5.5 | 74.5 ± 8.7 | 88.5 ± 5.7 |
| Analyte | % Stability (Mean ± SD) | ||
|---|---|---|---|
| Auto-Sampler (4 °C, 36 h) | Long-Term (−80 ± 5 °C, 30 days) | Bench-Top (20 °C, 8 h) | |
| GPP | 96.4 ± 4.6 | 91.3 ± 7.8 | 103.4 ± 8.6 |
| FPP | 93.5 ± 9.6 | 102.5 ± 9.5 | 98.5 ± 9.1 |
| GGPP | 104.4 ± 6.7 | 93.6 ± 7.3 | 109.2 ± 5.2 |
| Cell Lines | GPP | FPP | GGPP |
|---|---|---|---|
| Concentration (Mean ± SD) (nM/106 cells, n = 3) | |||
| AsPC1 | 0.28 ± 0.08 | 0.84 ± 0.28 | 1.96 ± 0.60 |
| MiaPaca | BLQ | 1.11 ± 0.12 | 2.31 ± 0.76 |
| BxPC3 | 0.61 ± 0.10 | 1.49 ± 0.33 | 2.67 ± 0.75 |
| Panc1 | 0.68 ± 0.11 | 1.86 ± 0.11 | 9.96 ± 1.08 |
| S2013 | 0.29 ± 0.08 | 0.77 ± 0.07 | 2.00 ± 0.95 |
| Capan-1 | BLQ | 0.50 ± 0.22 | 0.29 ± 0.06 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chhonker, Y.S.; Haney, S.L.; Bala, V.; Holstein, S.A.; Murry, D.J. Simultaneous Quantitation of Isoprenoid Pyrophosphates in Plasma and Cancer Cells Using LC-MS/MS. Molecules 2018, 23, 3275. https://doi.org/10.3390/molecules23123275
Chhonker YS, Haney SL, Bala V, Holstein SA, Murry DJ. Simultaneous Quantitation of Isoprenoid Pyrophosphates in Plasma and Cancer Cells Using LC-MS/MS. Molecules. 2018; 23(12):3275. https://doi.org/10.3390/molecules23123275
Chicago/Turabian StyleChhonker, Yashpal S., Staci L. Haney, Veenu Bala, Sarah A. Holstein, and Daryl J. Murry. 2018. "Simultaneous Quantitation of Isoprenoid Pyrophosphates in Plasma and Cancer Cells Using LC-MS/MS" Molecules 23, no. 12: 3275. https://doi.org/10.3390/molecules23123275
APA StyleChhonker, Y. S., Haney, S. L., Bala, V., Holstein, S. A., & Murry, D. J. (2018). Simultaneous Quantitation of Isoprenoid Pyrophosphates in Plasma and Cancer Cells Using LC-MS/MS. Molecules, 23(12), 3275. https://doi.org/10.3390/molecules23123275






