Synthesis, In Vitro Biological Evaluation, and Oxidative Transformation of New Flavonol Derivatives: The Possible Role of the Phenyl-N,N-Dimethylamino Group
Abstract
1. Introduction
2. Results and Discussion
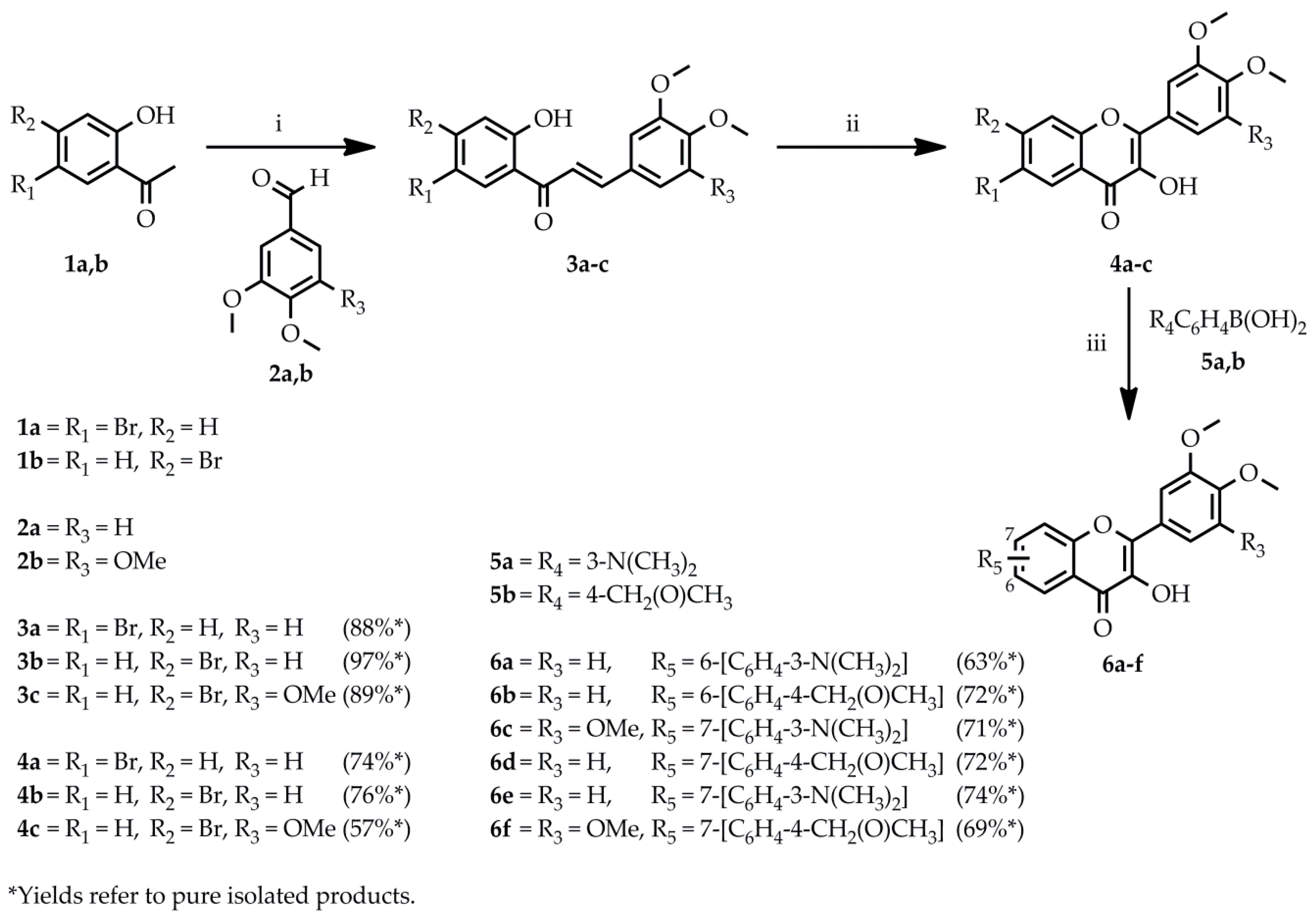
2.1. Chemistry
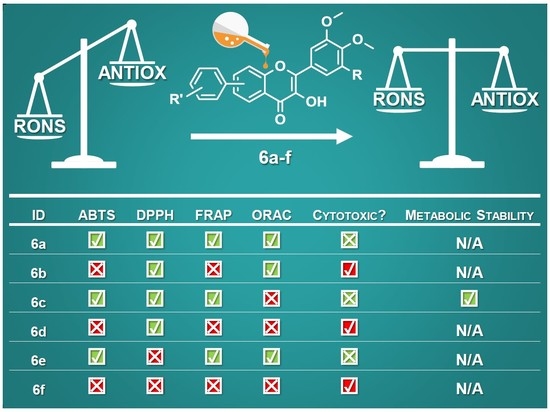
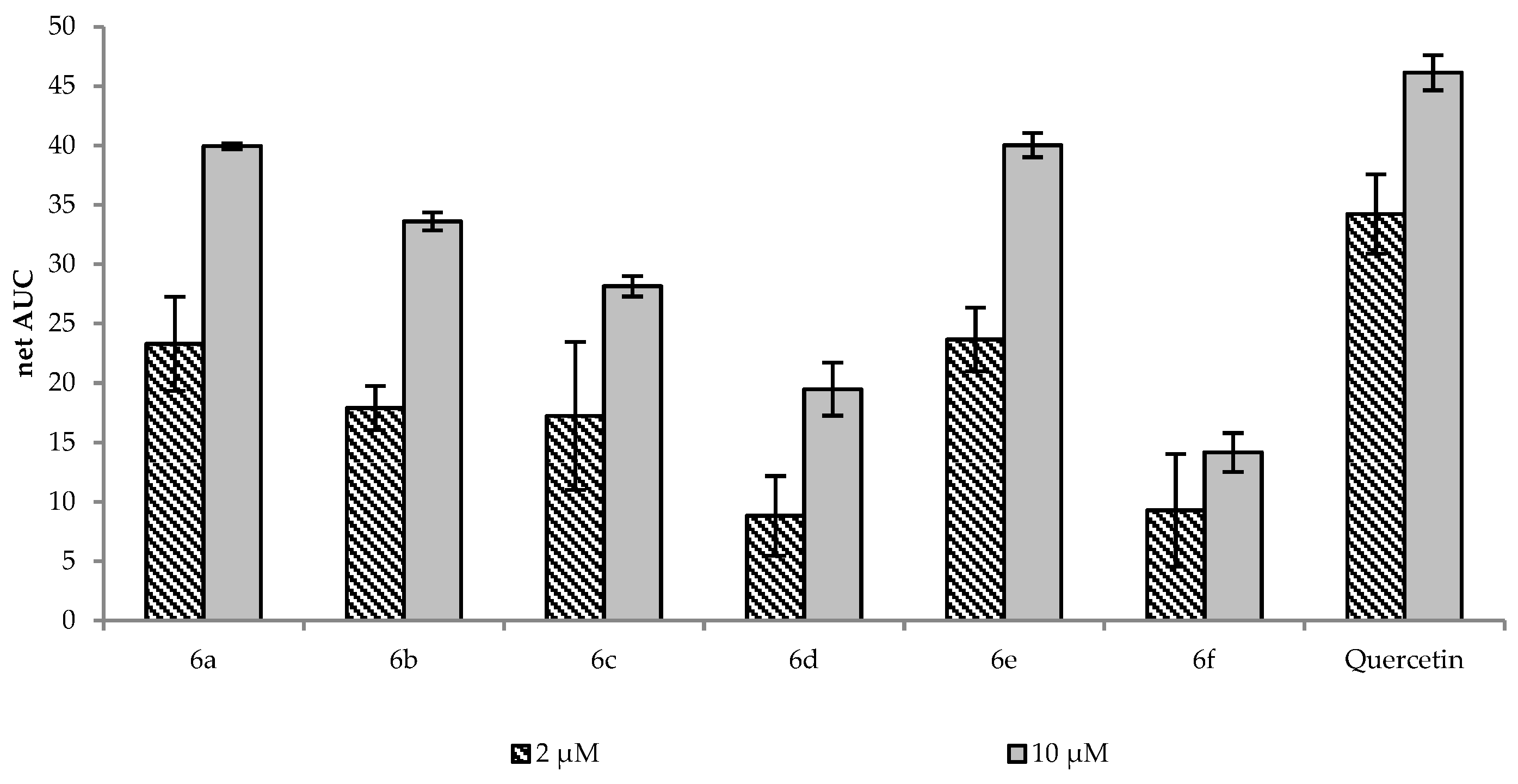
2.2. Antioxidant Activity
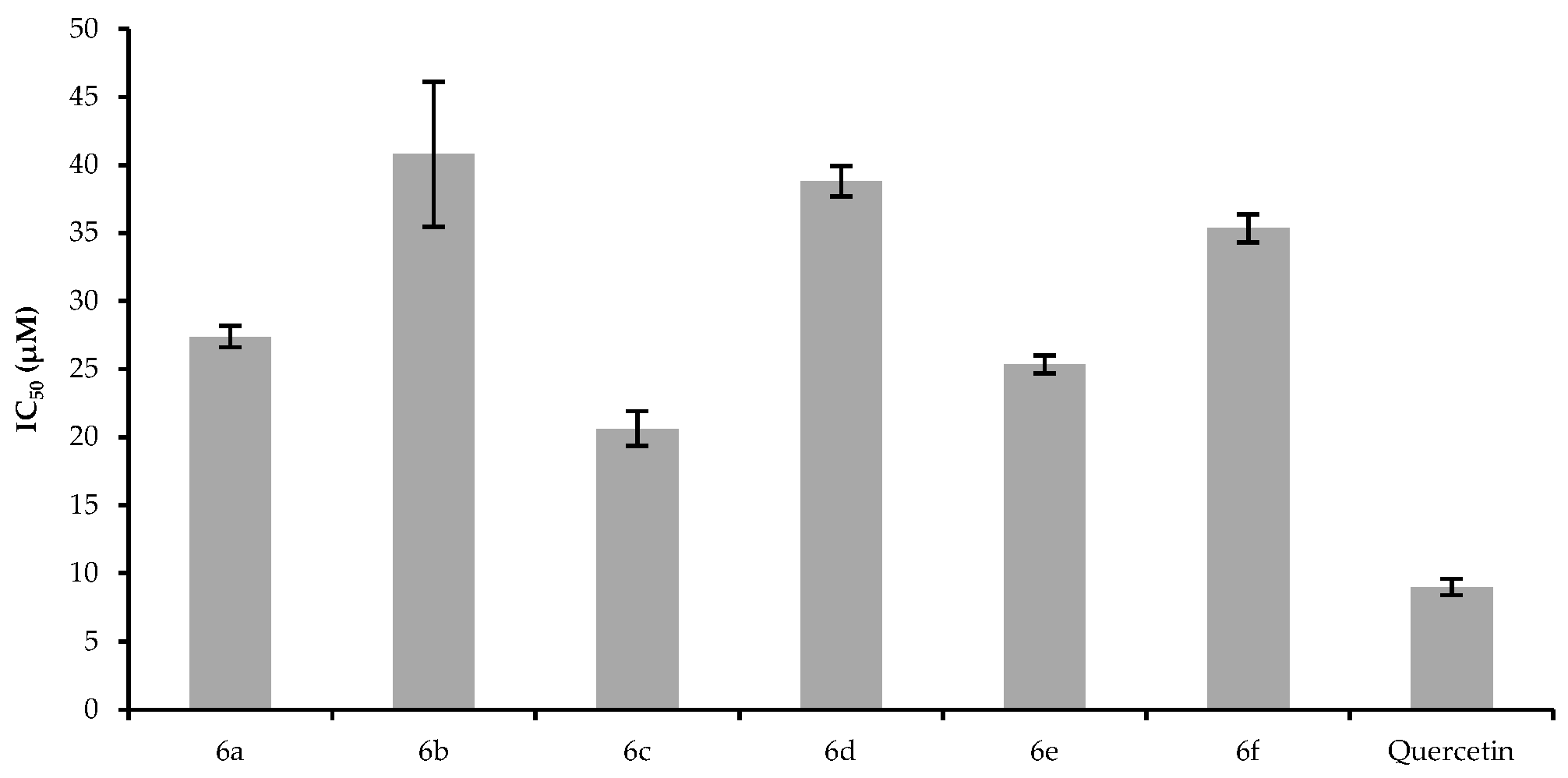
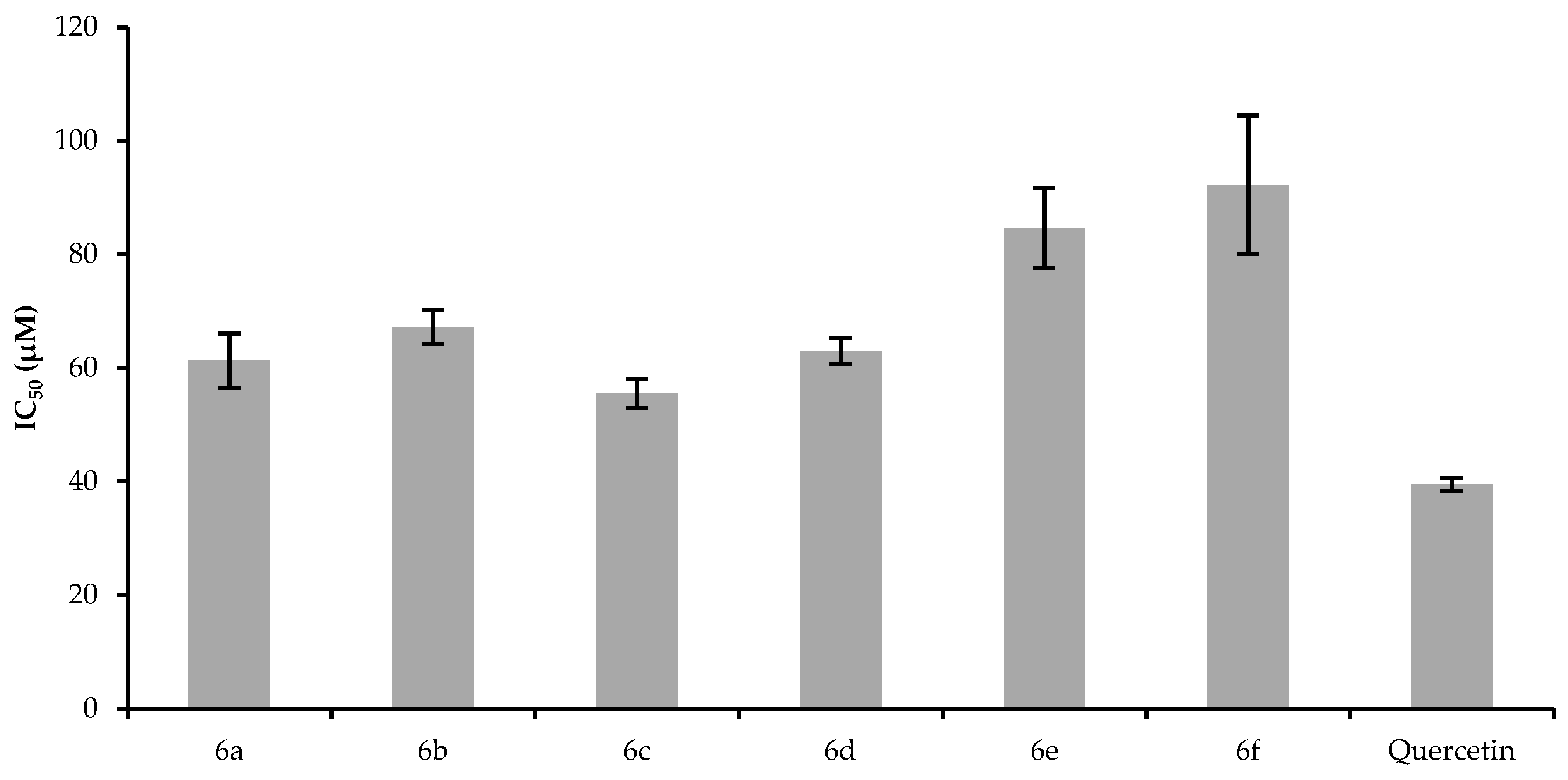
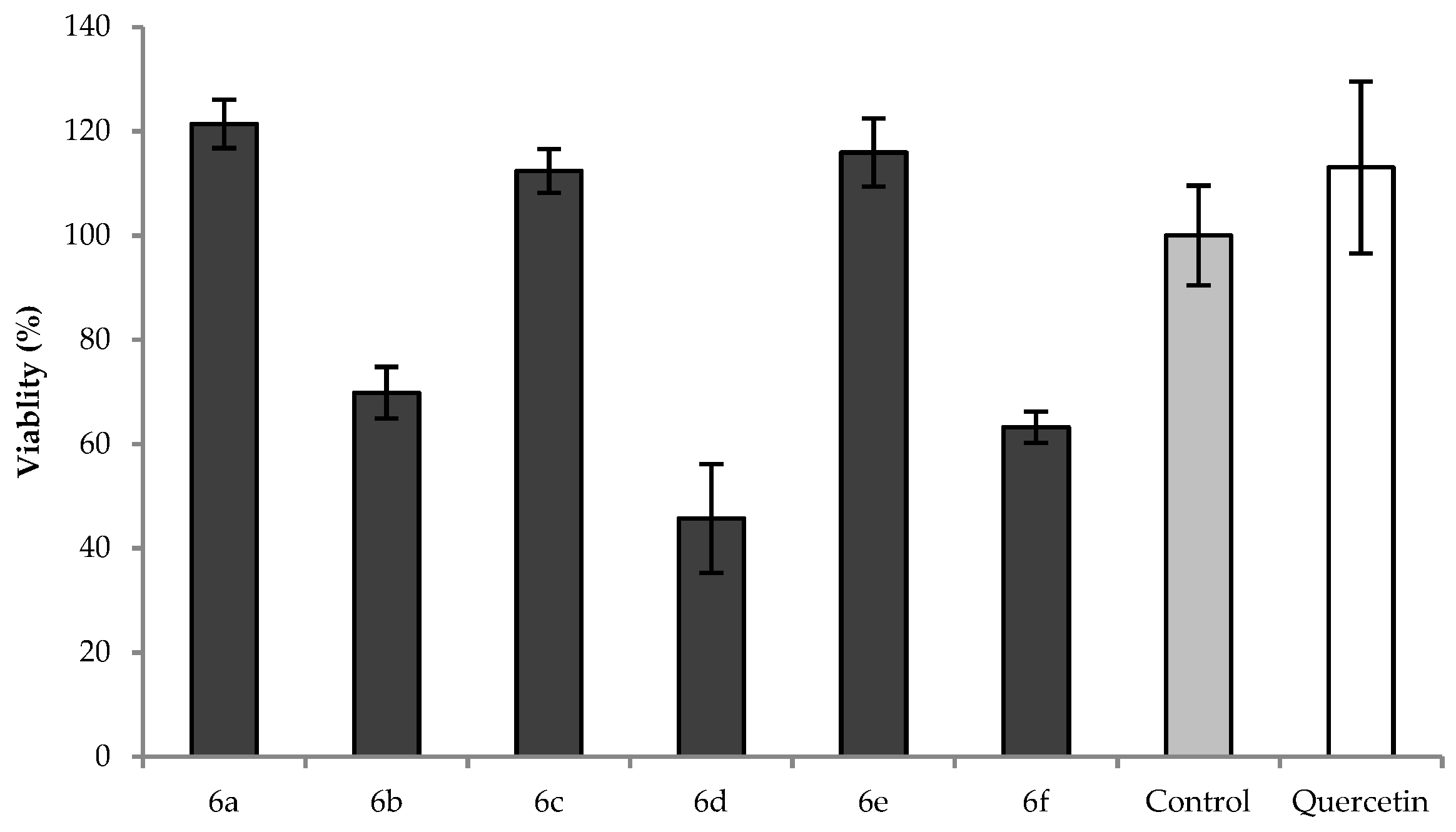
2.3. Cytotoxic Effect
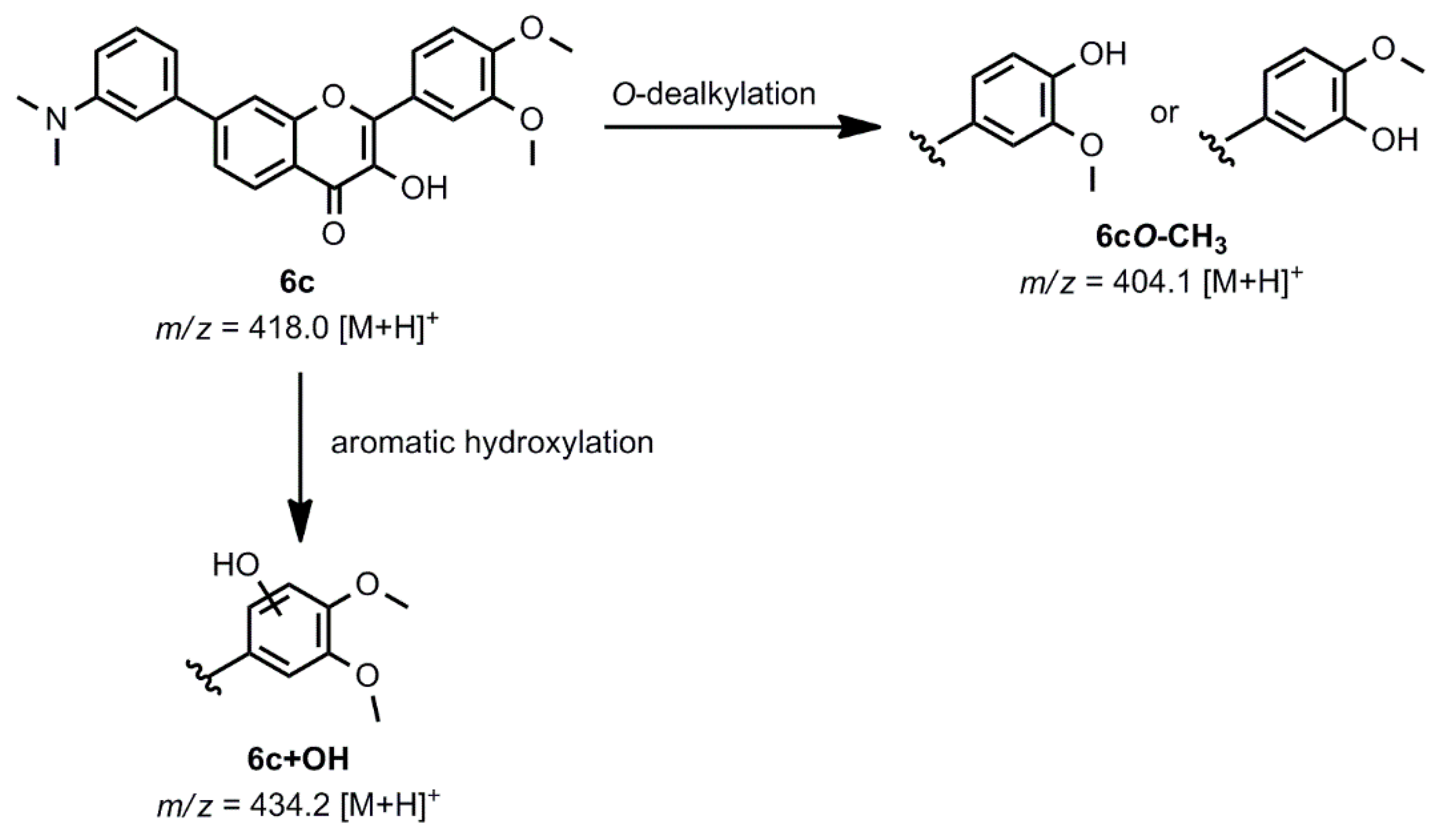
2.4. Oxidative Transformation
3. Materials and Methods
3.1. Chemistry
3.1.1. General Information
3.1.2. General Procedure for the Synthesis of 3a–c
3.1.3. General Procedure for the Synthesis of 4a–c
3.1.4. General Procedure for the Synthesis of 6a–f
3.2. Antioxidant Activity and Cytotoxicity
3.2.1. Chemicals
3.2.2. ABTS Assay
3.2.3. DPPH Assay
3.2.4. FRAP Assay
3.2.5. ORAC Assay
3.2.6. MTT Assay
3.3. Oxidative Transformation
Chemical Fenton Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Joseph, N.; Zhang-James, Y.; Perl, A.; Faraone, S.V. Oxidative Stress and ADHD: A Meta-Analysis. J. Atten. Disord. 2015, 19, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Oxidative stress and cancer: Have we moved forward? Biochem. J. 2007, 401, 1–11. [Google Scholar] [CrossRef]
- Hwang, O. Role of oxidative stress in Parkinson’s disease. Exp. Neurobiol. 2013, 22, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Bonomini, F.; Tengattini, S.; Fabiano, A.; Bianchi, R.; Rezzani, R. Atherosclerosis and oxidative stress. Histol. Histopathol. 2008, 23, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Dhalla, A.K.; Seneviratne, C.; Singal, P.K. Oxidative stress and heart failure. Mol. Cell Biochem. 1995, 147, 77–81. [Google Scholar] [CrossRef]
- Ramond, A.; Godin-Ribuot, D.; Ribuot, C.; Totoson, P.; Koritchneva, I.; Cachot, S.; Levy, P.; Joyeux-Faure, M. Oxidative stress mediates cardiac infarction aggravation induced by intermittent hypoxia. Fundam. Clin. Pharmacol. 2013, 27, 252–261. [Google Scholar] [CrossRef]
- Andre, C.M.; Larondelle, Y.; Evers, D. Dietary Antioxidants and Oxidative Stress from a Human and Plant Perspective: A Review. Curr. Nutr. Food Sci. 2010, 6, 2–12. [Google Scholar] [CrossRef]
- Hercberg, S.; Galan, P.; Preziosi, P.; Alfarez, M.-J.; Vazquez, C. The potential role of antioxidant vitamins in preventing cardiovascular diseases and cancers. Nutrition 1998, 14, 513–520. [Google Scholar] [CrossRef]
- Cadenas, E.; Packer, L. Handbook of Antioxidants; Marcel Dekker: New York, NY, USA, 2002. [Google Scholar]
- Cushnie, T.P.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Di Carlo, G.; Mascolo, N.; Izzo, A.A.; Capasso, F. Flavonoids: Old and new aspects of a class of natural therapeutic drugs. Life Sci. 1999, 65, 337–353. [Google Scholar] [CrossRef]
- Luisa Helena, C.; Leila, Z.; Elga Heloisa, A.; Maria Santos Reis Bonorino, F.; Poliane, F.; Rosangela Guollo, D.; Moacir Geraldo, P.; Fatima Regina Mena Barreto, S. Flavonoids: Prospective Drug Candidates. Mini-Rev. Med. Chem. 2008, 8, 1429–1440. [Google Scholar] [CrossRef]
- Shahidi, F.; Wanasundara, P.K. Phenolic antioxidants. Crit. Rev. Food Sci. Nutr. 1992, 32, 67–103. [Google Scholar] [CrossRef] [PubMed]
- Federico, D.; Abin-Carriquiry Juan, A.; Arredondo, F.; Echeverry, C.; Rivera-Megret, F. Neuroprotective Actions of Flavones and Flavonols: Mechanisms and Relationship to Flavonoid Structural Features. Cent. Nerv. Syst. Agents Med. Chem. 2013, 13, 30–35. [Google Scholar] [CrossRef]
- Gomez-Pinilla, F. Brain foods: The effects of nutrients on brain function. Nat. Rev. Neurosci. 2008, 9, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.-H.; Chen, P.-N.; Hsieh, Y.-H.; Lin, C.-Y.; Cheng, F.-Y.; Chiu, P.-C.; Chu, S.-C.; Hsieh, Y.-S. 3-Hydroxyflavone inhibits human osteosarcoma U2OS and 143B cells metastasis by affecting EMT and repressing u-PA/MMP-2 via FAK-Src to MEK/ERK and RhoA/MLC2 pathways and reduces 143B tumor growth in vivo. Food Chem. Toxicol. 2016, 97, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Csepanyi, E.; Szabados-Furjesi, P.; Kiss-Szikszai, A.; Frensemeier, L.M.; Karst, U.; Lekli, I.; Haines, D.D.; Tosaki, A.; Bak, I. Antioxidant Properties and Oxidative Transformation of Different Chromone Derivatives. Molecules 2017, 22, 588. [Google Scholar] [CrossRef]
- Bendary, E.; Francis, R.R.; Ali, H.M.G.; Sarwat, M.I.; El Hady, S. Antioxidant and structure–activity relationships (SARs) of some phenolic and anilines compounds. Ann. Agric. Sci. 2013, 58, 173–181. [Google Scholar] [CrossRef]
- Culhaoglu, B.; Capan, A.; Boga, M.; Ozturk, M.; Ozturk, T.; Topcu, G. Antioxidant and Anticholinesterase Activities of Some Dialkylamino Substituted 3-Hydroxyflavone Derivatives. Med. Chem. 2017, 13, 254–259. [Google Scholar] [CrossRef]
- Dauzonne, D.; Folléas, B.; Martinez, L.; Chabot, G.G. Synthesis and in vitro cytotoxicity of a series of 3-aminoflavones. Eur. J. Med. Chem. 1997, 32, 71–82. [Google Scholar] [CrossRef]
- Liu, G.; Ge, Z.; Zhao, M.; Zhou, Y. Design, Synthesis and cytotoxic activities of novel aliphatic amino-substituted flavonoids. Molecules 2013, 18, 14070–14084. [Google Scholar] [CrossRef]
- Chen, L.; Teng, H.; Xie, Z.; Cao, H.; Cheang, W.S.; Skalicka-Woniak, K.; Georgiev, M.I.; Xiao, J. Modifications of dietary flavonoids towards improved bioactivity: An update on structure–activity relationship. Crit. Rev. Food Sci. Nutr. 2018, 58, 513–527. [Google Scholar] [CrossRef]
- Walle, T.; Ta, N.; Kawamori, T.; Wen, X.; Tsuji, P.A.; Walle, U.K. Cancer chemopreventive properties of orally bioavailable flavonoids--methylated versus unmethylated flavones. Biochem. Pharmacol. 2007, 73, 1288–1296. [Google Scholar] [CrossRef]
- Wen, X.; Walle, T. Methylated flavonoids have greatly improved intestinal absorption and metabolic stability. Drug Metab. Dispos. 2006, 34, 1786–1792. [Google Scholar] [CrossRef]
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant and prooxidant behavior of flavonoids: Structure-activity relationships. Free Radic. Biol. Med. 1997, 22, 749–760. [Google Scholar] [CrossRef]
- Kim, B.T.; Chun, J.C.; Hwang, K.J. Synthesis of Dihydroxylated Chalcone Derivatives with Diverse Substitution Patterns and Their Radical Scavenging Ability toward DPPH Free Radicals. Bull. Korean Chem. Soc. 2008, 29, 1125–1130. [Google Scholar] [CrossRef]
- Fukuhara, K.; Nakanishi, I.; Kansui, H.; Sugiyama, E.; Kimura, M.; Shimada, T.; Urano, S.; Yamaguchi, K.; Miyata, N. Enhanced Radical-Scavenging Activity of a Planar Catechin Analogue. J. Am. Chem. Soc. 2002, 124, 5952–5953. [Google Scholar] [CrossRef]
- Deng, D.; Zhang, J.; Cooney, J.M.; Skinner, M.A.; Adaim, A.; Jensen, D.J.; Stevenson, D.E. Methylated polyphenols are poor “chemical” antioxidants but can still effectively protect cells from hydrogen peroxide-induced cytotoxicity. FEBS Lett. 2006, 580, 5247–5250. [Google Scholar] [CrossRef]
- Luo, W.; Wang, T.; Hong, C.; Yang, Y.C.; Chen, Y.; Cen, J.; Xie, S.Q.; Wang, C.J. Design, synthesis and evaluation of 4-dimethylamine flavonoid derivatives as potential multifunctional anti-Alzheimer agents. Eur. J. Med. Chem. 2016, 122, 17–26. [Google Scholar] [CrossRef]
- Jeong, J.M.; Choi, C.H.; Kang, S.K.; Lee, I.H.; Lee, J.Y.; Jung, H. Antioxidant and chemosensitizing effects of flavonoids with hydroxy and/or methoxy groups and structure-activity relationship. J. Pharm. Pharm. Sci. 2007, 10, 537–546. [Google Scholar] [CrossRef]
- Johansson, T.; Weidolf, L.; Jurva, U. Mimicry of phase I drug metabolism—Novel methods for metabolite characterization and synthesis. Rapid Commun. Mass Spectrom. 2007, 21, 2323–2331. [Google Scholar] [CrossRef]
- Lohmann, W.; Karst, U. Biomimetic modeling of oxidative drug metabolism: Strategies, advantages and limitations. Anal. Bioanal. Chem. 2008, 391, 79–96. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Sugahara, S.; Ueda, Y.; Fukuhara, K.; Kamamuta, Y.; Matsuda, Y.; Murata, T.; Kuroda, Y.; Kabata, K.; Ono, M.; Igoshi, K.; et al. Antioxidant Effects of Herbal Tea Leaves from Yacon (Smallanthus sonchifolius) on Multiple Free Radical and Reducing Power Assays, Especially on Different Superoxide Anion Radical Generation Systems. J. Food Sci. 2015, 80, C2420–2429. [Google Scholar] [CrossRef]
- Clarke, G.; Ting, K.N.; Wiart, C.; Fry, J. High Correlation of 2,2-diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging, Ferric Reducing Activity Potential and Total Phenolics Content Indicates Redundancy in Use of All Three Assays to Screen for Antioxidant Activity of Extracts of Plants from the Malaysian Rainforest. Antioxidants (Basel) 2013, 2, 1–10. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |


| ID | R | R’ | Position of R‘-Ph |
|---|---|---|---|
| 6a | H | 3-N(CH3)2 | 6 |
| 6b | H | 4-CH2(O)CH3 | 6 |
| 6c | H | 3-N(CH3)2 | 7 |
| 6d | H | 4-CH2(O)CH3 | 7 |
| 6e | OMe | 3-N(CH3)2 | 7 |
| 6f | OMe | 4-CH2(O)CH3 | 7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szabados-Furjesi, P.; Pajtas, D.; Barta, A.; Csepanyi, E.; Kiss-Szikszai, A.; Tosaki, A.; Bak, I. Synthesis, In Vitro Biological Evaluation, and Oxidative Transformation of New Flavonol Derivatives: The Possible Role of the Phenyl-N,N-Dimethylamino Group. Molecules 2018, 23, 3161. https://doi.org/10.3390/molecules23123161
Szabados-Furjesi P, Pajtas D, Barta A, Csepanyi E, Kiss-Szikszai A, Tosaki A, Bak I. Synthesis, In Vitro Biological Evaluation, and Oxidative Transformation of New Flavonol Derivatives: The Possible Role of the Phenyl-N,N-Dimethylamino Group. Molecules. 2018; 23(12):3161. https://doi.org/10.3390/molecules23123161
Chicago/Turabian StyleSzabados-Furjesi, Peter, David Pajtas, Aliz Barta, Evelin Csepanyi, Attila Kiss-Szikszai, Arpad Tosaki, and Istvan Bak. 2018. "Synthesis, In Vitro Biological Evaluation, and Oxidative Transformation of New Flavonol Derivatives: The Possible Role of the Phenyl-N,N-Dimethylamino Group" Molecules 23, no. 12: 3161. https://doi.org/10.3390/molecules23123161
APA StyleSzabados-Furjesi, P., Pajtas, D., Barta, A., Csepanyi, E., Kiss-Szikszai, A., Tosaki, A., & Bak, I. (2018). Synthesis, In Vitro Biological Evaluation, and Oxidative Transformation of New Flavonol Derivatives: The Possible Role of the Phenyl-N,N-Dimethylamino Group. Molecules, 23(12), 3161. https://doi.org/10.3390/molecules23123161