Leishmanicidal Activity of Withanolides from Aureliana fasciculata var. fasciculata
Abstract
1. Introduction
2. Results and Discussion
3. Material and Methods
3.1. Botanical Material
3.2. Extraction and Isolation
3.3. ESI-MS Analysis
3.4. NMR Analysis
3.5. Parasites
3.5.1. Antipromastigote Activity
3.5.2. Antiamastigote Activity
3.6. Cytotoxic Study
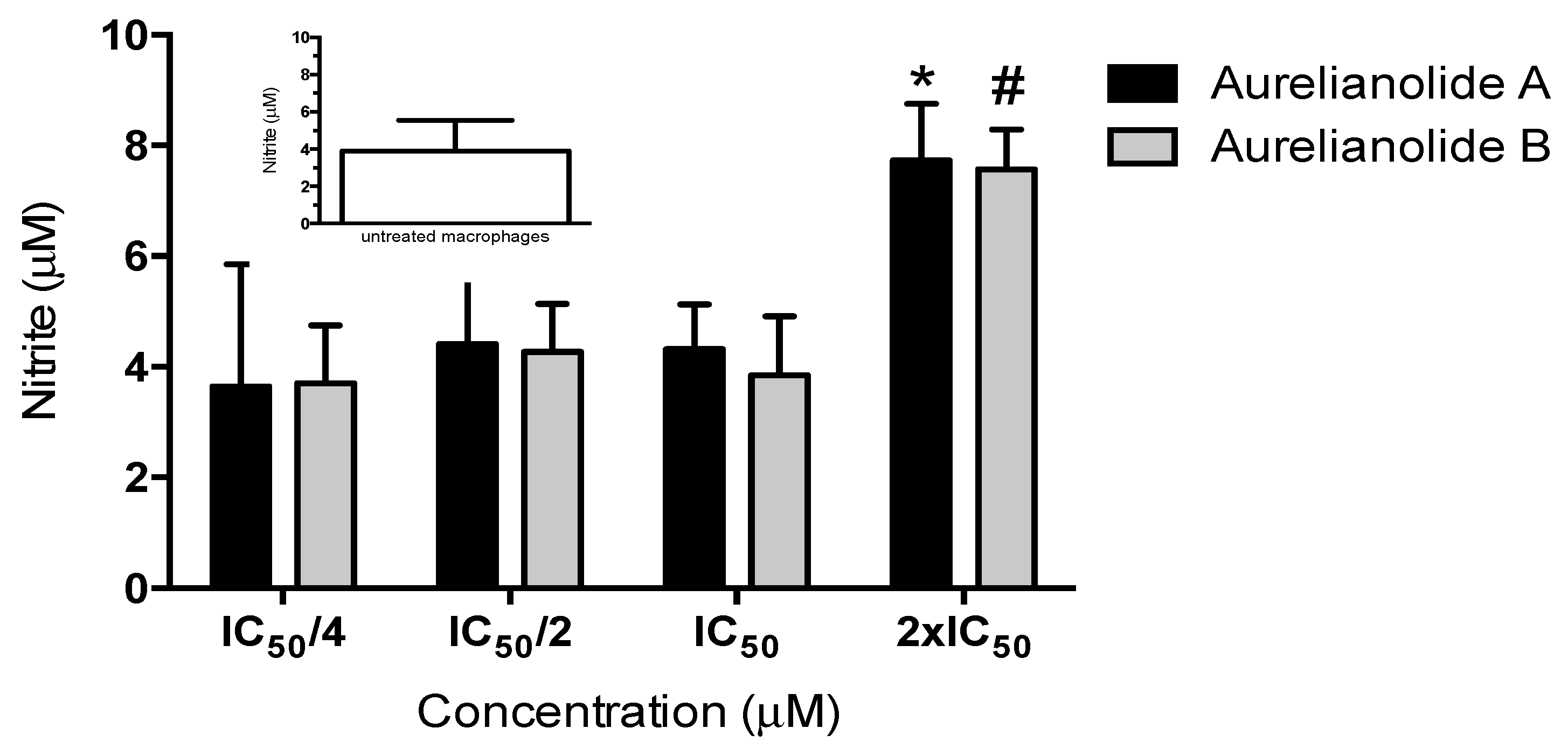
3.7. Nitric Oxide Production
3.8. In Silico ADMET properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; den Boer, M.; WHO Leishmaniasis Control Team. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef] [PubMed]
- Sundar, S.; Singh, A. Chemotherapeutics of visceral leishmaniasis: Present and future developments. Parasitology 2017, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- de Vries, H.J.C.; Reedjik, S.H.; Schallig, H.D.F.H. Cutaneous Leishmaniasis: Recent developments in diagnosis and management. Am. J. Clin. Dermatol. 2015, 16, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Berman, J.N. Chemotherapy of leishmaniasis: Recent advances in the treatment of visceral disease. Curr. Opin. Infect Dis. 1998, 11, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Gontijo, B.; Carvalho, M.L. American cutaneous leishmaniasis. Ver. Soc. Bras. Med. Trop. 2003, 36, 71–80. [Google Scholar] [CrossRef]
- Jhingran, A.; Chawla, B.; Saxena, S.; Barret, M.P.; Madhubala, R. Paronomycin: Uptake and resistance in Leishmania donovani. Mol. Biochem. Parasitol. 2009, 164, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Souza, V.C.; Lorenzi, H. Botânica Sistemática: Guia ilustrado para identificação das famílias de Angiospermas da flora brasileira, baseado em APG II.; Instituto Plantarum de Estudos da Flora: Nova Odessa, Brazil, 2005. [Google Scholar]
- Elabbar, F.A.; Nawill, M.A.B.; Ashraf, T.M.E. Extraction, separation and identification of compounds from leaves of Solanum elaeagnifolium Cav. (Solanaceae). Int. Curr. Pharm. J. 2014, 3, 234–239. [Google Scholar] [CrossRef]
- Hawkes, J.G. The economic importance of the family Solanaceae, In Solanaceae IV. Advances in Botany and Utilization; Royal Botanic Gardens: London, UK, 1999; pp. 1–8. [Google Scholar]
- Hunziker, A.T.; Barbosa, G. Estudios sobre Solanaceae XXX: Revisión de Aureliana. Darwiniana 1991, 30, 95–112. [Google Scholar]
- Almeida-Lafetá, R.; Ferreira, M.J.P.; Emerenciano, V.P.; Kaplan, M.A.C. Withanolides from Aureliana fasciculata var. fasciculata. Helv. Chim. Acta 2010, 93, 2478–2487. [Google Scholar]
- Dhar, N.; Razdan, S.; Rana, S.; Bhat, W.W.; Vishwakarma, R.; Lattoo, S.K.A. Decade of molecular understanding of withanolide biosynthesis and in vitro studies in Withania somnifera (L.) Dunal: Prospects and perspectives for pathway engineering. Front. Plant Sci. 2015, 6, 1031. [Google Scholar] [CrossRef] [PubMed]
- Vaishnavi, K.; Saxena, N.; Shah, N.; Singh, R.; Manjunath, K.; Uthayakumar, M.; Kanaujia, S.P.; Kaul, S.C.; Sekar, K.; Wadhwa, R. Differential activities of the two closely related withanolides, withaferin A and withanone: Bioinformatics and experimental evidences. PLoS ONE 2012, 7, e0044419. [Google Scholar] [CrossRef] [PubMed]
- Olmstead, R.G.; Bohs, L.; Migid, H.A.; Santiago-Valentin, E.; Garcia, V.F.; Collier, S.M. A molecular phylogeny of the Solanaceae. Taxon 2008, 54, 1159–1181. [Google Scholar]
- Zhang, H.; Samadi, A.K.; Cohen, M.S.; Timmermann, B.N. Antiproliferative withanolides from the Solanaceae: A structure–activity study. Pure Appl. Chem. 2012, 84, 1353–1367. [Google Scholar] [CrossRef] [PubMed]
- Glotter, E. Withanolides and related ergostane-type steroids. Nat. Prod. Rep. 1991, 8, 415–440. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Choi, S.U.; Choi, S.Z.; Son, M.W.; Lee, K.R. Withanolides from the rhizomes of Dioscorea japonica and their cytotoxicity. J. Agric. Food Chem. 2011, 59, 6980–6984. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.H.; Chou, K.J.; Wen, Z.H.; Wang, G.H.; Wu, Y.C.; Dai, C.F.; Sheu, J.H. Paraminabeolides A.−F, cytotoxic and anti-inflammatory marine withanolides from the soft coral Paraminabea acronocephala. J. Nat. Prod. 2011, 74, 1132–1141. [Google Scholar] [CrossRef] [PubMed]
- Kaileh, M.; Vanden, B.W.; Heyerick, A.; Horion, J.; Piette, J.; Libert, C.; De Keukeleire, D.; Essawi, T.; Haegeman, G. Withaferin A strongly elicits I kappa B kinase beta hyperphosphorylation concomitant with potent inhibition of its kinase activity. J. Biol. Chem. 2007, 282, 4253–4264. [Google Scholar] [CrossRef] [PubMed]
- Mulabagal, V.; Subbaraju, G.V.; Rao, C.V.; Sivaramakrishna, C.; DeWitt, D.L.; Holmes, D.; Sung, B.; Aggarwal, B.B.; Tsay, H.S.; Nair, M.G. Withanolide sulfoxide from aswagandha roots inhibits nuclear transcription factor-kappa-B, cyclooxygenase and tumor cell proliferation. Phytother. Res. 2009, 23, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Nagafuji, S.; Okabe, H.; Akahane, H.; Abe, F. Trypanocidal constituents in plants. Bio Pharm. Bull. 2004, 27, 193–197. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Dayakar, A.; Veronica, J.; Sundar, S.; Maurya, R. An in vitro study of apoptotic like death in Leishmania donovani promastigotes by withanolides. Parasitol. Int. 2013, 62, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Budhiraja, R.D.; Krishan, P.; Sudhir, S. Biological activity of withanolides. J. Sci. Ind. Res. 2000, 59, 904–911. [Google Scholar]
- Nicolás, F.G.; Reyes, G.; Audisio, M.C.; Uriburu, M.L.; González, S.; Barboza, G.E.; Nicotra, V.E. Withanolides with antibacterial activity from Nicandra john-tyleriana. J. Nat. Prod. 2015, 78, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Misico, R.I.; Viviana, E.; Nicotra, J.C.; Oberti, G.B.; Gil, R.R.; Burton, G. Withanolides and related steroids. Prog. Chem. Org. Nat. Prod. 2011, 94, 127–229. [Google Scholar] [PubMed]
- Kuroyanagi, M.I.; Murata, M.; Nakane, T.; Shirota, O.; Sekita, S.; Fuchino, H.; Shinwari, Z.K. Leishmanicidal activity withanolides from a Pakistani medicinal plant, Withania coagulans. Chem. Pharm. Bull. 2012, 60, 892–897. [Google Scholar] [CrossRef] [PubMed]
- Choudary, M.I.; Yousaf, S.; Ahmed, S.; Samreen, Y.K.; Rahman, A. Antileishmanial physalins from Physalis minima. Chem. Biodivers. 2005, 2, 1164–1173. [Google Scholar] [CrossRef] [PubMed]
- Bravo, B.J.A.; Sauvain, M.; Gimenez, T.A.; Balanza, E.; Serani, L.; Laprévote, O. Trypanocidal withanolides and withanolide Glycosides from Dunalia brachyacantha. J. Nat. Prod. 2001, 64, 720–725. [Google Scholar] [CrossRef]
- Murray, H.W.; Nathan, C.F. Macrophage microbicidal mechanisms in vivo: Reactive nitrogen versus oxygen intermediates in the killing of intracellular visceral Leishmania donovani. J. Exp. Med. 1999, 189, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, C.; Rollinghoff, M.; Diefenbach, A. Reactive oxygen and reactive nitrogen intermediates in innate and specific immunity. Curr. Opin. Immunol. 2000, 12, 64–76. [Google Scholar] [CrossRef]
- Carneiro, P.P.; Conceição, J.; Macedo, M.; Magalhães, V.; Carvalho, E.M.; Bacellar, O. The role of nitric oxide and reactive oxygen species in the killing of Leishmania braziliensis by monocytes from patients with Cutaneous Leishmaniasis. PLoS ONE 2016, 11, e0148084. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.Y.; Guo, R.; Li, T.; Liu, Y.; Wang, C.F.; Shu, Z.P.; Wang, Z.B.; Zhang, J.; Xia, Y.G. Five withanolides from the leaves of Datura metel L. and their inhibitory effects on Nitric Oxide production. Molecules 2014, 19, 4548–4559. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3e25. [Google Scholar] [CrossRef]
- Sundar, S.; Olliaro, P.L. Miltefosine in the treatment of leishmaniasis: Clinical evidence for informed clinical risk management. Ther. Clin. Risk Manag. 2007, 3, 733–740. [Google Scholar] [PubMed]
- McKerrow, J.H.; Lipinski, C.A. The rule of five should not impede anti-parasitic drug development. Int. J. Parasitol. Drugs Drug Resist. 2017, 7, 248–249. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.K.; Sinha, P.K.; Sundar, S.; Thakur, C.P.; Jha, T.K.; Pandey, K.; Das, V.R. Phase 4 trial of miltefosine for the treatment of Indian visceral leishmaniasis. J. Infect. Dis. 2007, 196, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Field, M.C.; Horn, D.; Fairlamb, A.H.; Ferguson, M.A.; Gray, D.W.; Read, K.D.; De Rycker, M.; Torrie, L.S.; Wyatt, P.G.; Wyllie, S.; et al. Anti-trypanossomtid drug discovery: An ongoing challenge and a continuing need. Nat. Rev. Microbiol. 2017, 15, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Soto, J.; Rea, J.; Balderrama, M.; Toledo, J.; Soto, P.; Valda, L.; Berman, J.D. Efficacy of miltefonsine for Bolivian cutaneous leishmaniasis. Am. J. Trop. Med. Hyg. 2008, 70, 210–211. [Google Scholar] [CrossRef]
- Uranw, S.; Ostyn, B.; Dorlo, T.P.; Hasker, E.; Dujardin, B.; Dujardin, J.C.; Rijal, S.; Boelaert, M. Adherence to miltefosine treatment for visceral leishmaniasis under routine conditions in Nepal. Trop. Med. Int. Health 2013, 18, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Van Bocxlaer, K.; Yardley, V.; Murdan, S.; Croft, S.L. Opical formulations of miltefosine for cutaneous leishmaniasis in a BALB/c mouse model. J. Pharm. Pharmacol. 2016, 68, 862–872. [Google Scholar] [CrossRef] [PubMed]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannebaum, S.R. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Cheng, F.; Li, W.; Zhou, Y.; Shen, J.; Wu, Z.; Liu, G.; Lee, PW. AdmetSAR: A comprehensive source and free tool for assessment of chemical ADMET properties. J. Chem. Inf. Model. 2012, 52, 3099–3105. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Valicherla, G.R.; Joshi, P.; Shahi, S.; Syed, A.A.; Gupta, A.P.; Hossain, Z.; Italiya, K.; Makadia, V.; Singh, S.K.; Wahajuddin, M.; Gayen, J.R. Determination of permeability, plasma protein binding, blood partitioning, pharmacokinetics and tissue distribution of Withanolide A in rats: A neuroprotective steroidal lactone. Drug Dev Res. 2018, 79, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.; Kallubai, M.; Sarkar, A.; Subramanyam, R. Elucidating the active interaction mechanism of phytochemicals withanolide and withanoside derivatives with human serum albumin. PLoS ONE 2018, 13, e0200053. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the isolated compounds are not available from the authors. |
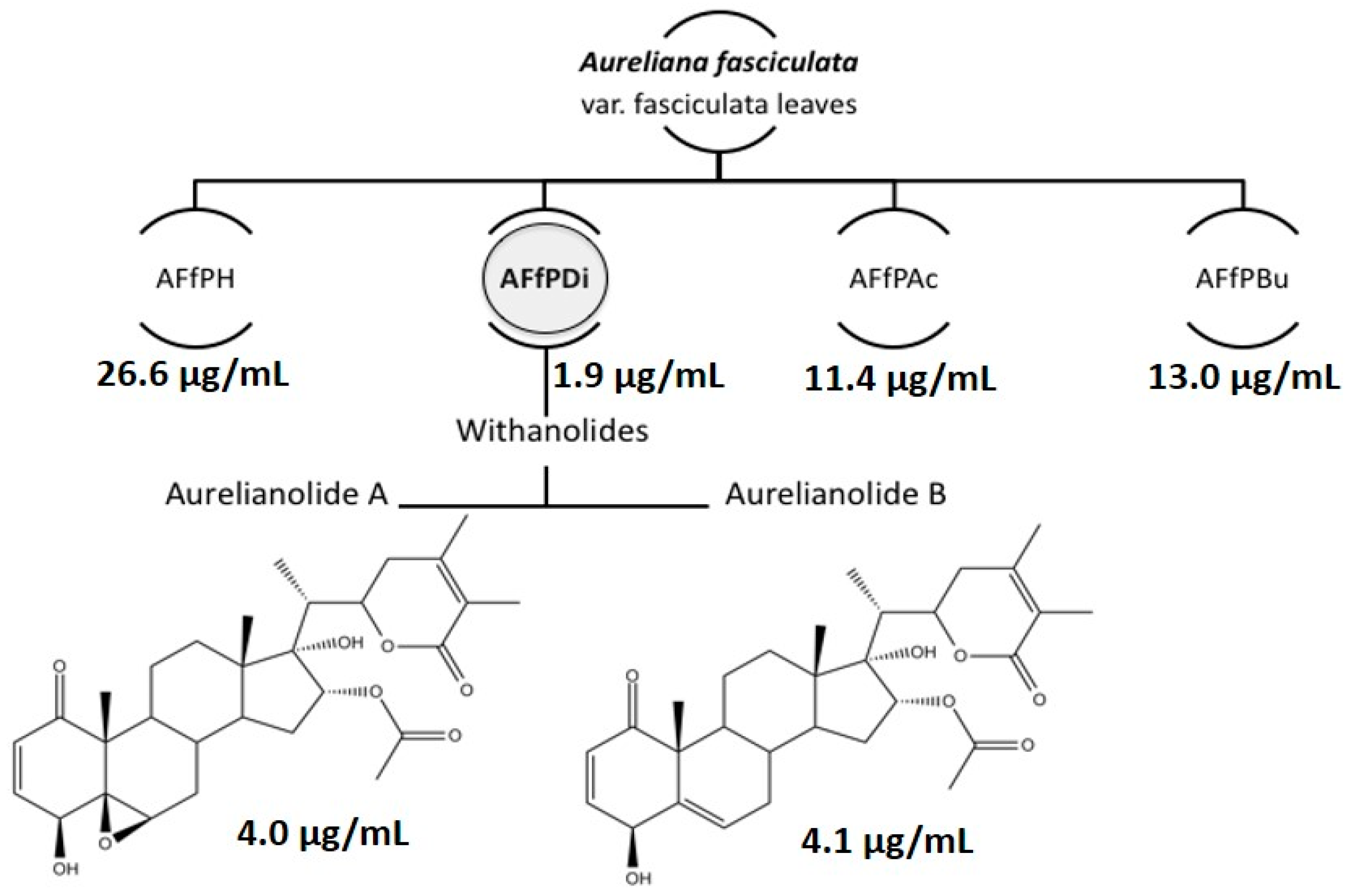
| L. amazonensis | J774 Macrophages (CC50) * | Selectivity Index (SI) | ||
|---|---|---|---|---|
| Promastigotes (IC50) * | Intracellular Amastigotes (IC50) * | |||
| AFfPH | 26.6 ± 0.1 | N.D. | N.D. | N.D. |
| AFfDi | 1.9 ± 0.7 | N.D. | N.D. | N.D. |
| AFfAc | 11.4 ± 0.1 | N.D. | N.D. | N.D. |
| AFfBu | 13.0 ± 0.1 | N.D. | N.D. | N.D. |
| Aurelianolide A | 4.0 ± 0.1 (7.6 ± 0.1) | 1.2 ± 0.1 (2.3 ± 0.1) | 6.7 ± 0.2 (12.7 ± 0.2) | 5.6 |
| Aurelianolide B | 4.1 ± 0.3 (7.9 ± 0.7) | 3.3 ± 0.1 (6.4 ± 0.1) | 6.7 ± 0.1 (13.1 ± 0.1) | 2.0 |
| Pentamidine | 2.8 ± 0.1 (4.8 ± 0.1) | 1.1 ± 0.1 (1.9 ± 0.1) | 5.0 ± 0.7 (8.5 ± 1.2) | 4.5 |
| Parameters | Aurelianolide A | Aurelianolide B | Miltefosine |
|---|---|---|---|
| MW | 528.642 | 512.643 | 407.576 |
| LogP | 3.037 | 3.825 | 5.6755 |
| #ACCEPTORS | 8 | 7 | 4 |
| #DONORS | 2 | 2 | 0 |
| Water solubility (log mg/l) | −4.924 | −5.329 | −5.673 |
| Parameters | Aurelianolide A | Aurelianolide B | Miltefosine |
|---|---|---|---|
| Absorption | |||
| Caco2 permeability (log Papp in 10−6 cm/s) | 1.31 | 1.474 | 1.153 |
| Intestinal absorption (human, %) | 91.459 | 90.543 | 94.987 |
| Skin Permeability (log Kp) | −3.101 | −3.638 | −2.702 |
| Distribution | |||
| VDss (human, l/kg) | 0.04 | 0.121 | 0.96 |
| Fraction unbound (human) | 0.223 | 0.171 | 0.238 |
| BBB permeability (log BB) | −0.801 | −0.602 | −0.345 |
| CNS permeability (log PS) | −3.278 | −3.033 | −3.172 |
| Metabolism | |||
| CYP2D6 substrate | No | No | No |
| CYP3A4 substrate | Yes | Yes | Yes |
| CYP1A2 inhibitor | No | No | No |
| CYP2C19 inhibitor | No | No | No |
| CYP2C9 inhibitior | No | No | No |
| CYP2D6 inhibitior | No | No | No |
| CYP3A4 inhibitior | No | No | No |
| Excretion | |||
| Total Clearance (log ml/min/kg) | 0.275 | 0.36 | 1.156 |
| Parameters | Aurelianolide A | Aurelianolide B | Miltefosine |
|---|---|---|---|
| AMES toxicity | No | No | No |
| Max. tolerated dose (human, log mg/kg/day) | −1.053 | −0.858 | 1.079 |
| hERG I inhibitor | No | No | No |
| hERG II inhibitor | Yes | Yes | Yes |
| Oral Rat Acute Toxicity (LD50) (mol/kg) | 2.518 | 2.284 | 2.211 |
| Oral Rat Chronic Toxicity (LOAEL) (log mg/kg_bw/day) | 1.786 | 1.692 | 1.34 |
| Hepatotoxicity | No | No | Yes |
| Skin Sensitisation | No | No | No |
| T. Pyriformis toxicity pIGC50 (log µg/l) | 0.299 | 0.340 | 1.054 |
| Minnow toxicity LC50 (log mM) | 0.503 | 0.109 | −2.403 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, S.C.d.M.; Pacheco, J.d.S.; Marques, A.M.; Veltri, E.R.P.; Almeida-Lafetá, R.d.C.; Figueiredo, M.R.; Kaplan, M.A.C.; Torres-Santos, E.C. Leishmanicidal Activity of Withanolides from Aureliana fasciculata var. fasciculata. Molecules 2018, 23, 3160. https://doi.org/10.3390/molecules23123160
Lima SCdM, Pacheco JdS, Marques AM, Veltri ERP, Almeida-Lafetá RdC, Figueiredo MR, Kaplan MAC, Torres-Santos EC. Leishmanicidal Activity of Withanolides from Aureliana fasciculata var. fasciculata. Molecules. 2018; 23(12):3160. https://doi.org/10.3390/molecules23123160
Chicago/Turabian StyleLima, Simone Cristina de M., Juliana da Silva Pacheco, André M. Marques, Eduardo Raul Pereira Veltri, Rita de Cássia Almeida-Lafetá, Maria Raquel Figueiredo, Maria Auxiliadora Coelho Kaplan, and Eduardo Caio Torres-Santos. 2018. "Leishmanicidal Activity of Withanolides from Aureliana fasciculata var. fasciculata" Molecules 23, no. 12: 3160. https://doi.org/10.3390/molecules23123160
APA StyleLima, S. C. d. M., Pacheco, J. d. S., Marques, A. M., Veltri, E. R. P., Almeida-Lafetá, R. d. C., Figueiredo, M. R., Kaplan, M. A. C., & Torres-Santos, E. C. (2018). Leishmanicidal Activity of Withanolides from Aureliana fasciculata var. fasciculata. Molecules, 23(12), 3160. https://doi.org/10.3390/molecules23123160






