Chromosome Doubling-Enhanced Biomass and Dihydrotanshinone I Production in Salvia miltiorrhiza, A Traditional Chinese Medicinal Plant
Abstract
:1. Introduction
2. Results
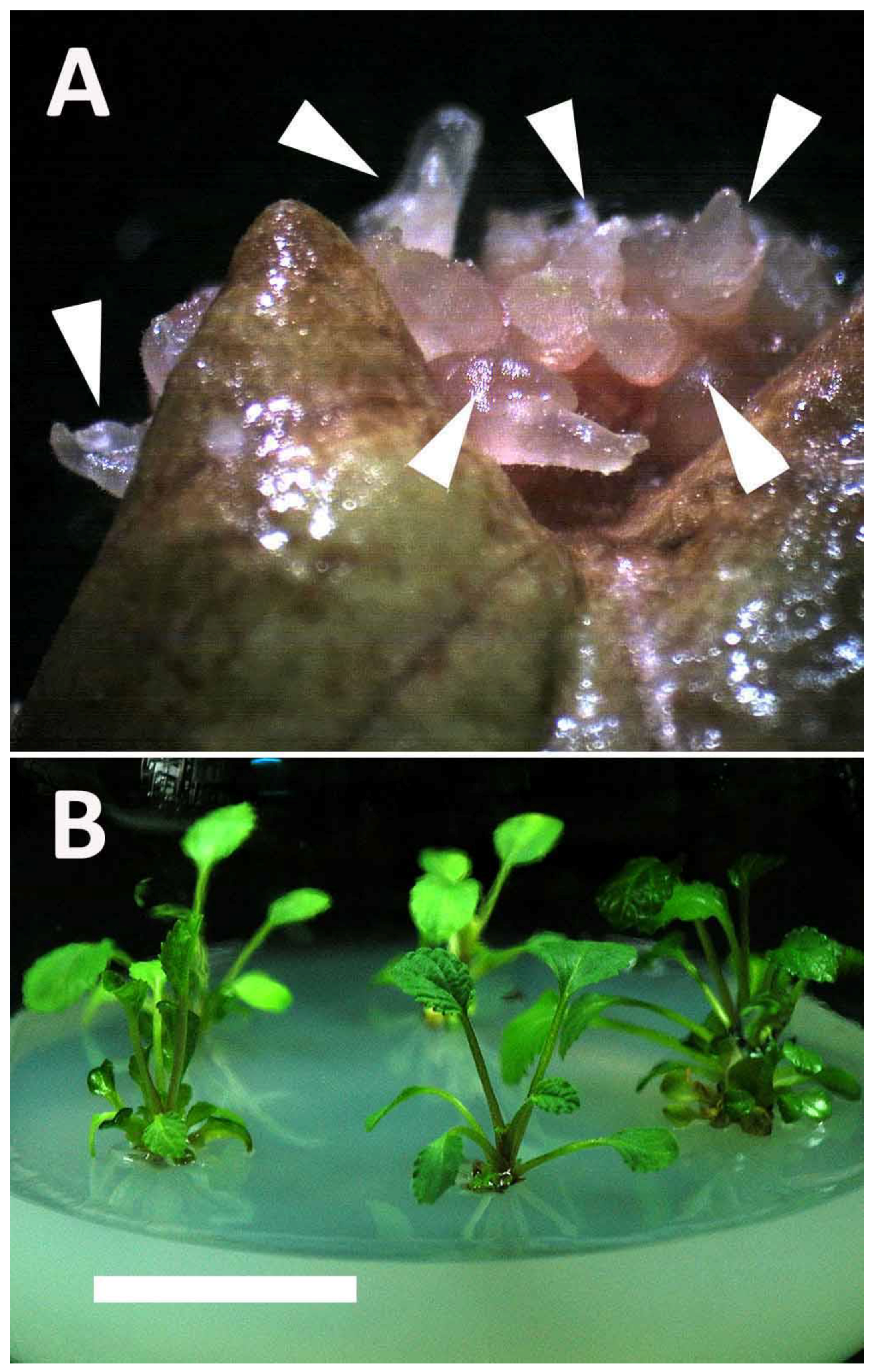
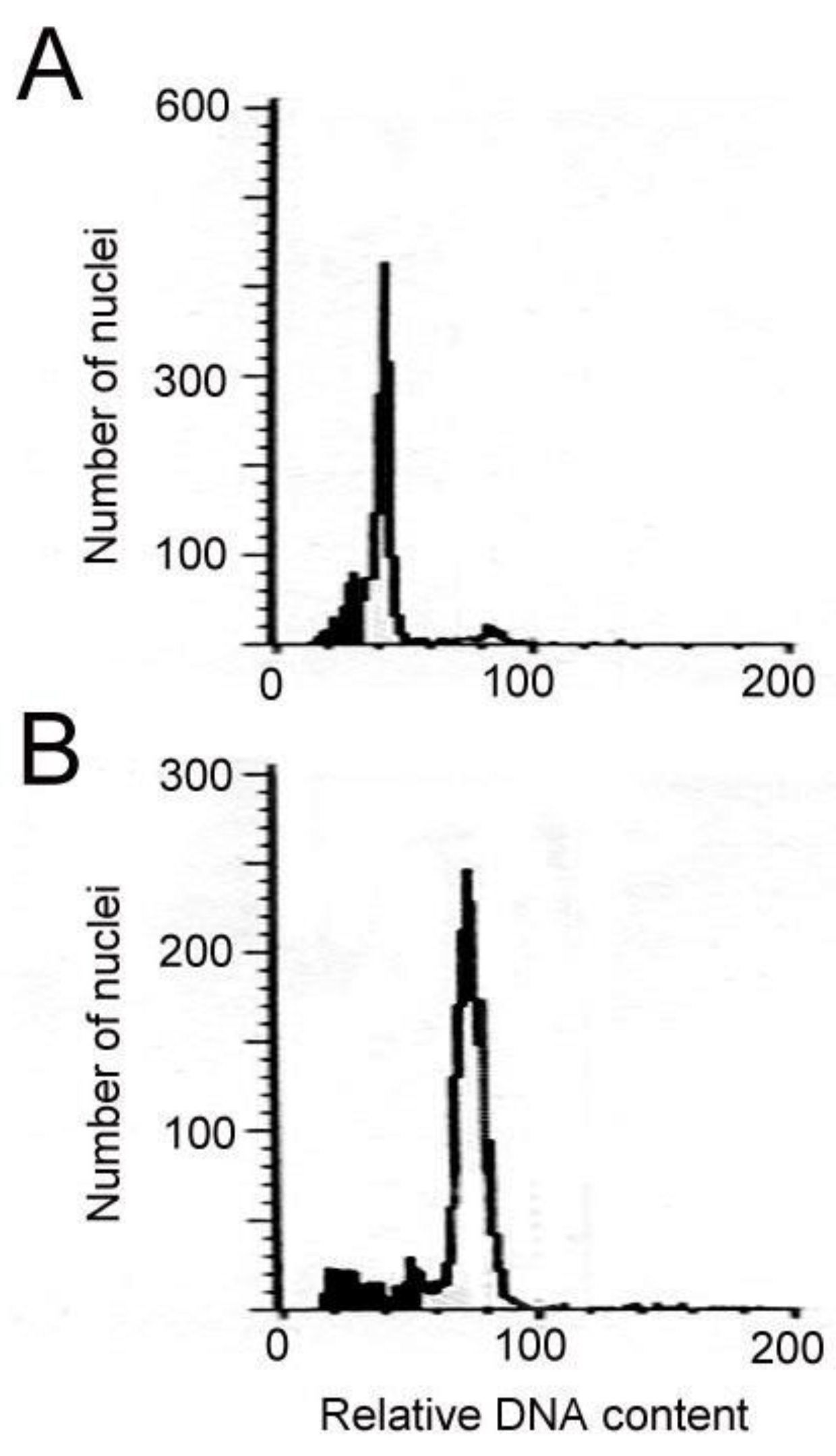
2.1. Induction and Identification of Polyploidy
2.2. In Vitro Growth of Tetraploids
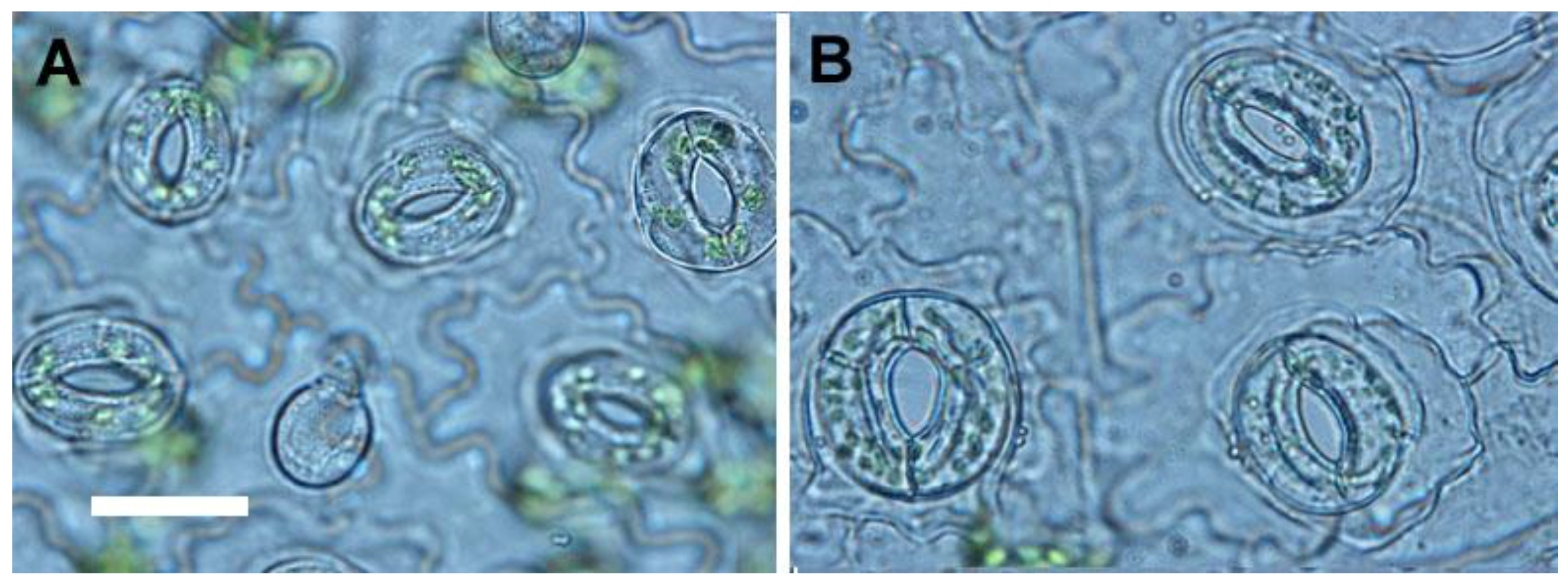
2.3. Growth Characteristics of Ex Vitro Plants
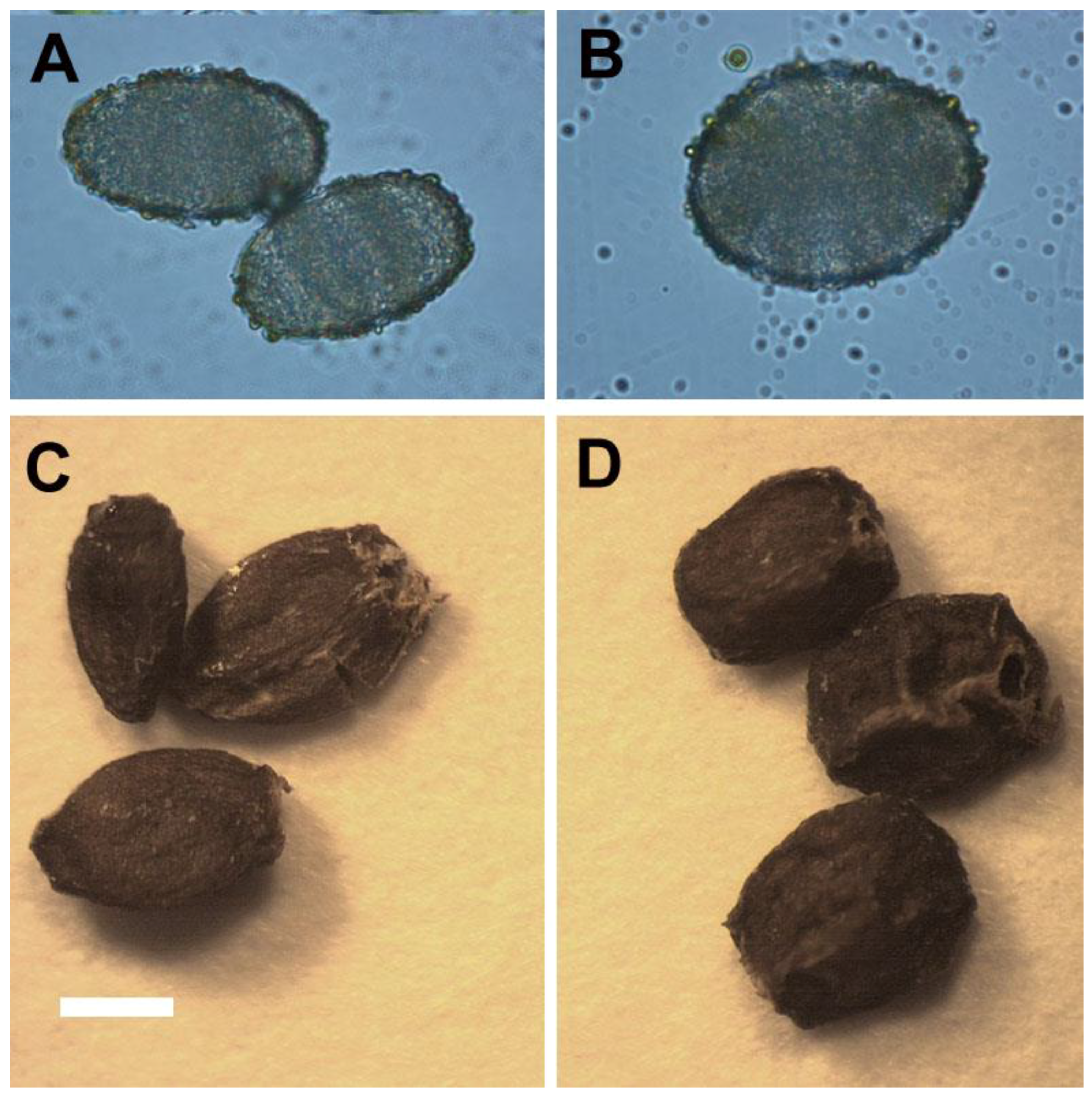
2.4. Reproductive Behaviors of Ex Vitro Plants
2.5. The Stability of Polyploidy
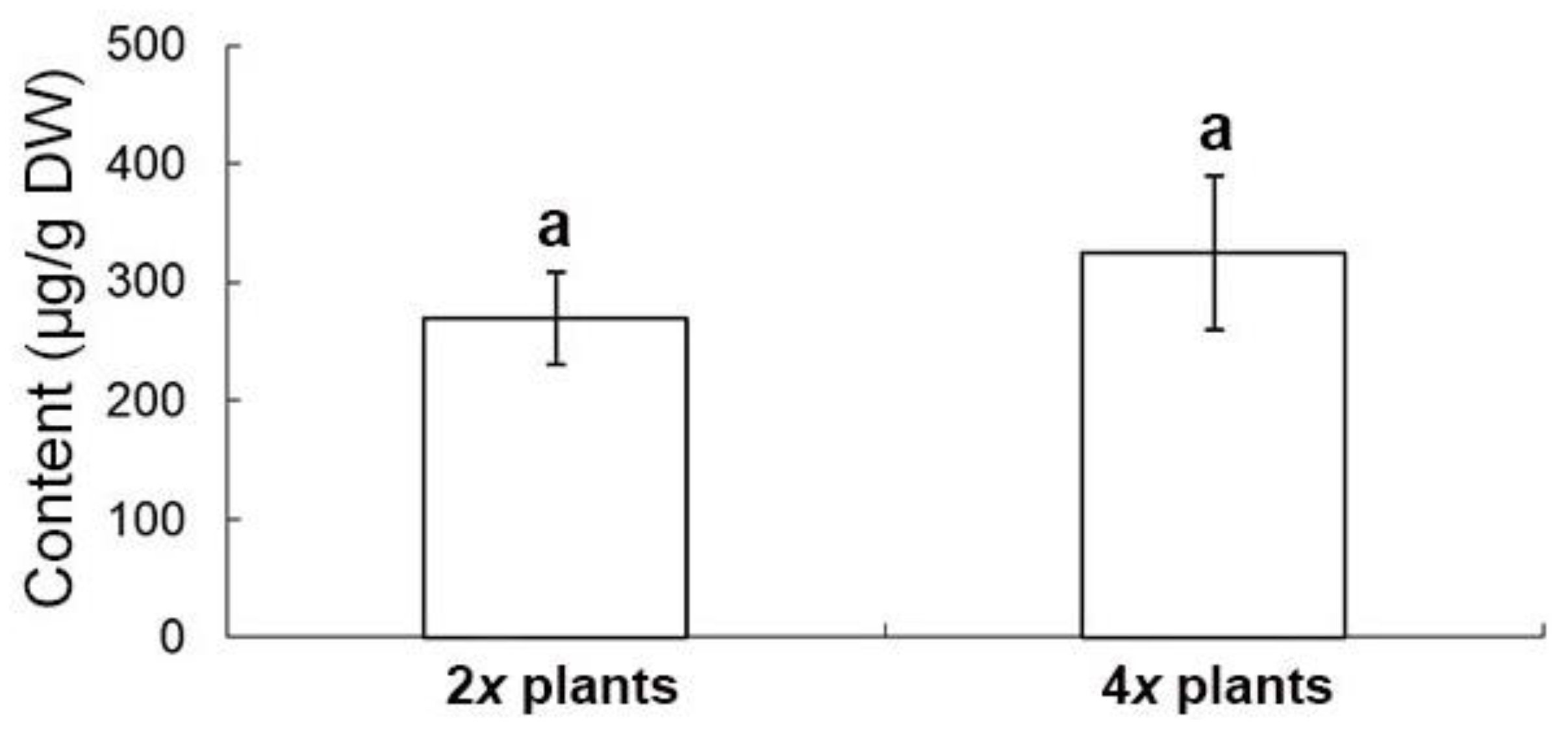
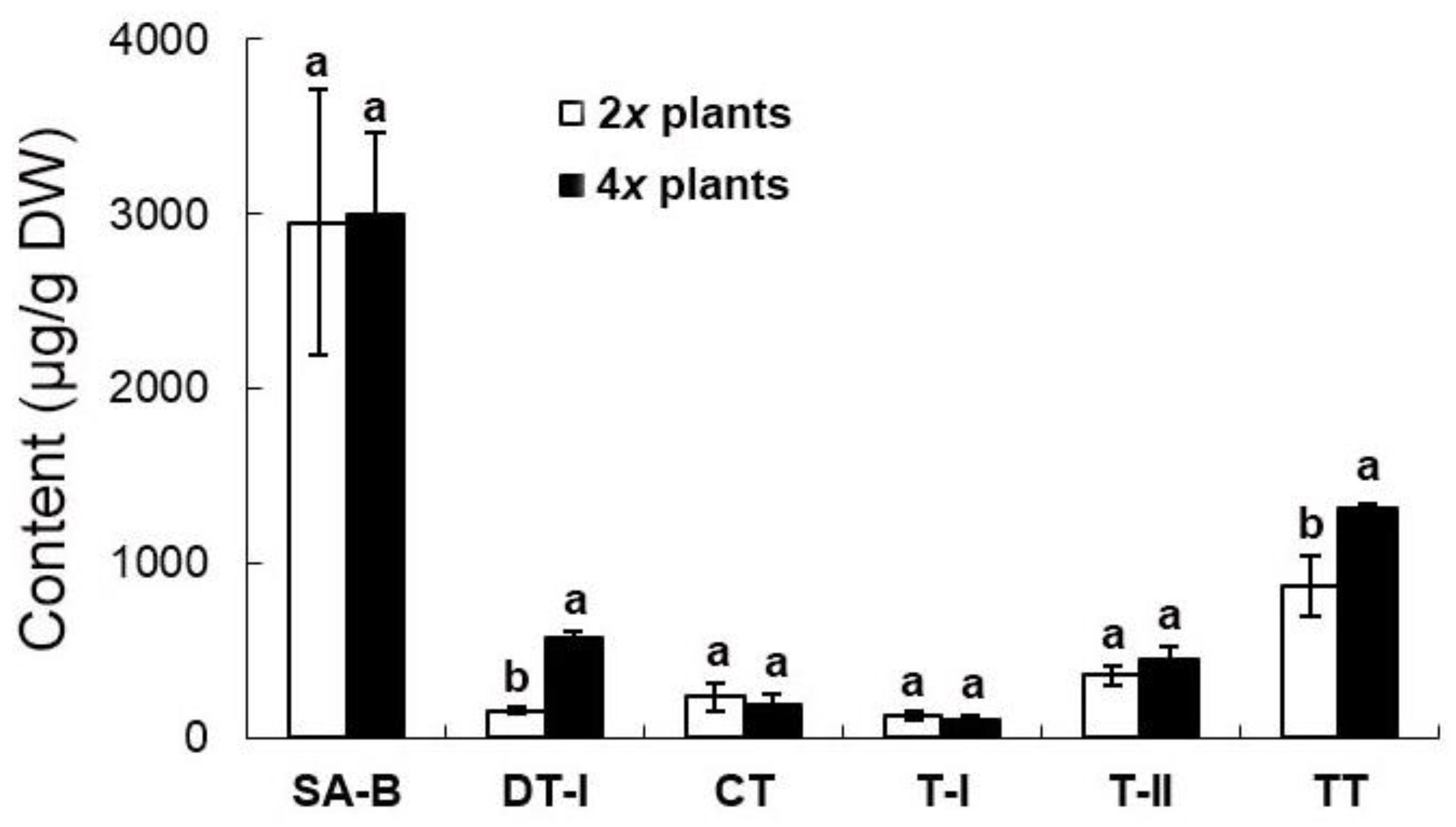
2.6. Evaluation of Medicinal Constituents in Ex Vitro Plants
3. Discussion
4. Materials and Methods
4.1. Plant Source
4.2. Obtaining the Donor Plants
4.3. Polyploidy Induction via Direct Shoot Formation
4.4. Analysis of Flow Cytometry
4.5. Analysis of Stomata
4.6. Acclimatization
4.7. Evaluation of Ploidy Stability
4.8. Evaluation of Bioactive Compounds
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Gao, S.L.; Zhu, D.N.; Cai, Z.H.; Xu, D.R. Autotetrapolid plants from colchicine-treated bud culture of Salvia miltiorrhiza. Plant Cell Tissue Organ Cult. 1996, 47, 73–77. [Google Scholar] [CrossRef]
- Wang, J.W.; Wu, J.Y. Tanshinone biosynthesis in Salvia miltiorrhiza and production in plant tissue cultures. Appl. Microbiol. Biotechnol. 2010, 88, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.G.; Song, L.; Liu, M.; Hu, Z.B.; Wang, Z.T. Advancement in analysis of Salvia miltiorrhiza Radix et Rhizoma (Danshen). J. Chromatogr. A 2009, 1216, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xiong, X.; Feng, B. Cardiovascular effects of salvianolic acid B. J. Evid. Based Complement. Altern. Med. 2013, 2013, 247948. [Google Scholar] [CrossRef]
- Zhang, H.S.; Wang, S.Q. Salvianolic acid B from Salvia miltiorrhiza inhibits tumor necrosis factor-α (TNF-α)-induced MMP-2 upregulation in human aortic smooth muscle cells via suppression of NAD(P)H oxidase-derived reactive oxygen species. J. Mol. Cell. Cardiol. 2006, 41, 138–148. [Google Scholar] [CrossRef]
- Cai, Z.H.; Gao, S.L.; Xu, D.R. Research on rapid-propagation technology of Salvia miltiorrhiza in vitro culture. J. China Pharm. Univ. 1991, 22, 65–68. [Google Scholar]
- Nilanthi, D.; Chen, X.L.; Zhao, F.C.; Yang, Y.S.; Wu, H. Induction of tetraploids from petiole explants through colchicine treatments in Echinacea purpurea L. J. Biomed. Biotechnol. 2009, 2009, 343485. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Hu, C.G.; Yao, J.L. Tetraploidization of diploid Dioscorea results in activation of the antioxidant defense system and increased heat tolerance. J. Plant Physiol. 2010, 167, 88–94. [Google Scholar] [CrossRef]
- Cai, X.; Kang, X.Y. In vitro tetraploid induction from leaf explants of Populus pseudo-simonii Kitag. Plant Cell Rep. 2011, 30, 1771–1778. [Google Scholar] [CrossRef]
- Dhooghe, E.; Van Laere, K.; Eeckhaut, T.; Leus, L.; Van Huylenbroeck, J. Mitotic chromosome doubling of plant tissues in vitro. Plant Cell Tissue Organ Cult. 2011, 104, 359–373. [Google Scholar] [CrossRef]
- Chung, H.H.; Shi, S.K.; Huang, B.; Chen, J.T. Enhanced agronomic traits and medicinal constituents of autotetraploids in Anoectochilus formosanus Hayata, a top-grade medicinal orchid. Molecules 2017, 22, 1907. [Google Scholar] [CrossRef] [PubMed]
- Tsai, K.L.; Chen, E.G.; Chen, J.T. Thidiazuron-induced efficient propagation of Salvia miltiorrhiza through in vitro organogenesis and medicinal constituents of regenerated plants. Acta Physiol. Plant 2016, 38, 29. [Google Scholar] [CrossRef]
- Adaniya:, S.; Shirai, D. In vitro induction of tetraploid ginger (Zingiber officinale Roscoe) and its pollen fertility and germinability. Sci. Hortic. 2001, 88, 277–287. [Google Scholar] [CrossRef]
- Roy, A.; Leggett, G.; Koutoulis, A. In vitro tetraploid induction and generation of tetraploids from mixoploids in hop (Humulus lupulus L.). Plant Cell Rep. 2001, 20, 489–495. [Google Scholar]
- Gao, S.L.; Chen, B.J.; Zhu, D.N. In vitro production and identification of autotetraploids of Scutellaria baicalensis. Plant Cell Tissue Organ Cult. 2002, 70, 289–293. [Google Scholar] [CrossRef]
- Rey, H.; Sansberro, B.; Collavino, M.; Davina, J.; Gonzalez, A.; Mroginski, L. Colchicine, trifluralin and oryzalin promoted development of somatic embryos in Ilex paraguariensis (Aquifoliaceae). Euphytica 2002, 123, 49–56. [Google Scholar] [CrossRef]
- Petersen, K.K.; Hagberg, P.; Kristiansen, K. Colchicine and oryzalin mediated chromosome doubling in different genotypes of Miscanthus sinensis. Plant Cell Tissue Organ Cult. 2003, 73, 137–146. [Google Scholar] [CrossRef]
- De Carvalho, J.F.R.P.; de Carvalho, C.R.; Otoni, W.C. In vitro induction of polyploidy in annatto (Bixa orellana). Plant Cell Tissue Organ Cult. 2005, 80, 69–75. [Google Scholar] [CrossRef]
- Chen, L.L.; Gao, S.L. In vitro tetraploid induction and generation of tetraploids from mixoploids in Astragalus membranaceus. Sci. Hortic. 2007, 112, 339–344. [Google Scholar] [CrossRef]
- Rubuluza, T.; Nikolova, R.V.; Smith, M.T.; Hannweg, K. In vitro induction of tetraploids in Colophospermum mopane by colchicine. S. Afr. J. Bot. 2007, 73, 259–261. [Google Scholar] [CrossRef]
- Thorpe, T.A. Morphogenesis and regeneration. In Plant Cell and Tissue Culture; Vasil, K.I., Thorpe, T.A., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1994; pp. 17–36. [Google Scholar]
- Chuang, M.T.; Ho, F.M.; Wu, C.C.; Zhuang, S.Y.; Lin, S.Y.; Suk, F.M.; Liang, Y.C. 15,16-Dihydrotanshinone I., a compound of Salvia miltiorrhiza Bunge, induces apoptosis through inducing endoplasmic reticular stress in human prostate carcinoma cells. J. Evid. Based Complement. Altern. Med. 2011, 2011, 865435. [Google Scholar]
- Suk, F.M.; Jou, W.J.; Lin, R.J.; Lin, S.Y.; Tzeng, F.Y.; Liang, Y.C. 15,16-Dihydrotanshinone I-induced apoptosis in human colorectal cancer cells: Involvement of ATF3. Anticancer Res. 2013, 33, 3222–3231. [Google Scholar]
- Xu, Y.P.; Geng, L.J.; Zhao, S.J. Biosynthesis of bioactive ingredients of Salvia miltiorrhiza and advanced biotechnologies for their production. Biotechnol. Equip. 2018. [Google Scholar] [CrossRef]
- Yang, D.; Fang, Y.; Xia, P.; Zhang, X.; Liang, Z. Diverse responses of tanshinone biosynthesis to biotic and abiotic elicitors in hairy root cultures of Salvia miltiorrhiza and Salvia castanea Diels f. tomentosa. Gene 2018, 643, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Gantait, S.; Mandal, N.; Bhattacharyya, S.; Das, P.K. Induction and identification of tetrapolids using in vitro colchicine treatment of Gerbera jamesonii Bolus cv. Sciella. Plant Cell Tissue Organ Cult. 2011, 106, 485–493. [Google Scholar] [CrossRef]
- Sugiyama, S.I. Polyploidy and cellular mechanisms changing leaf size: Comparison of diploid and autotetraploid populations in two species of Lolium. Ann. Bot. 2005, 96, 931–938. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, V.M.; Forni-Martins, E.R.; Magalhães, P.M.; Alves, M.N. Chromosomal and morphological studies of diploid and polyploid cytotypes of Stevia rebaudiana (Bertoni) (Eupatorieae, Asteraceae). Genet. Mol. Biol. 2004, 27, 215–222. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Duncan, D.B. Multiple range and multiple F test. Food Nutr. Sci. Biom. 1955, 11, 1–42. [Google Scholar] [CrossRef]


| Colchicine (mg/L) | 0.5 mg/L BA | 0.5 mg/L TDZ | ||
|---|---|---|---|---|
| Number of Shoot/Explants | Polyploidy (%) | Number of Shoot/Explants | Polyploidy (%) | |
| 0 | 9.3 ± 2.5 a | 0 | 33.3 ± 1.5 a | 0 |
| 0.5 | 3.7 ± 0.6 b | 9.1 | 13.7 ± 1.5 b | 39.0 |
| 1 | 1.3 ± 0.6 c | 0 | 11.0 ± 1.0 c | 18.2 |
| 2 | 0c | 0 | 5.0 ± 1.0 d | 13.33 |
| 3 | 0c | 0 | 1.3 ± 0.6 e | 0 |
| 4 | 0c | 0 | 0.7 ± 0.6 e | 0 |
| Treatment | Fresh Weight (g) | Shoot Length (mm) | No. of Leaves | Root Length (cm) | Root Diameter (mm) |
|---|---|---|---|---|---|
| Diploid | 5.29 ± 1.24 b | 2.53 ± 0.14 b | 15.3 ± 0.8 b | 8.70 ± 1.20 a | 0.80 ± 0.03 b |
| Tetraploid | 9.96 ± 1.62 a | 3.15 ± 0.17 a | 18.5 ± 0.9 a | 4.46 ± 0.87 b | 1.09 ± 0.04 a |
| Treatment | Stomata Frequency (No./Microscopic Field) | Stomata Size | |
|---|---|---|---|
| Length (μm) | Width (μm) | ||
| Diploid | 29.0 ± 1.2 a | 11.5 ± 0.4 b | 14.7 ± 0.4 b |
| Tetraploid | 13.6 ± 0.4 b | 17.8 ± 0.5 a | 19.1 ± 0.2 a |
| Treatment | Leaflet Length (cm) | Leaflet Width (cm) | Leaflet Area (cm2) | Leaflet Shape |
|---|---|---|---|---|
| Diploid | 3.75 ± 0.11 a | 2.43 ± 0.07 b | 5.20 ± 0.27 b | Ovate |
| Tetraploid | 3.64 ± 0.12 b | 3.01 ± 0.11 a | 5.91 ± 0.31 a | Orbiculate or elliptical |
| Treatment | Pollen | Seed | ||
|---|---|---|---|---|
| Length (μm) | Width (μm) | Length (mm) | Width (mm) | |
| Diploid | 30.8 ± 0.7 b | 21.7 ± 0.7 b | 0.47 ± 0.01 a | 0.29 ± 0.01 b |
| Tetraploid | 38.9 ± 1.2 a | 30.0 ± 0.8 a | 0.49 ± 0.02 a | 0.38 ± 0.01 a |
| Characteristics | 10th Generation Vegetative Clonal 4x Plants Obtained from Nodal Stem Segments | Seed-Derived 4x Plants Obtained via Self-Pollination |
|---|---|---|
| Level of ploidy (proved by flow cytometry) | 4x | 4x |
| Stoma frequency (no./microscopic field) | 13.6 ± 1.1 a | 13.8 ± 1.1 a |
| Stoma length (μm) | 17.8 ± 0.8 a | 18.0 ± 1.0 a |
| Stoma width (μm) | 19.0 ± 1.9 a | 19.2 ± 1.5 a |
| Leaflet shpae | Elliptical to orbiculate | Elliptical to orbiculate |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, E.G.; Tsai, K.-L.; Chung, H.-H.; Chen, J.-T. Chromosome Doubling-Enhanced Biomass and Dihydrotanshinone I Production in Salvia miltiorrhiza, A Traditional Chinese Medicinal Plant. Molecules 2018, 23, 3106. https://doi.org/10.3390/molecules23123106
Chen EG, Tsai K-L, Chung H-H, Chen J-T. Chromosome Doubling-Enhanced Biomass and Dihydrotanshinone I Production in Salvia miltiorrhiza, A Traditional Chinese Medicinal Plant. Molecules. 2018; 23(12):3106. https://doi.org/10.3390/molecules23123106
Chicago/Turabian StyleChen, Elena Gamboa, Kang-Lun Tsai, Hsiao-Hang Chung, and Jen-Tsung Chen. 2018. "Chromosome Doubling-Enhanced Biomass and Dihydrotanshinone I Production in Salvia miltiorrhiza, A Traditional Chinese Medicinal Plant" Molecules 23, no. 12: 3106. https://doi.org/10.3390/molecules23123106
APA StyleChen, E. G., Tsai, K.-L., Chung, H.-H., & Chen, J.-T. (2018). Chromosome Doubling-Enhanced Biomass and Dihydrotanshinone I Production in Salvia miltiorrhiza, A Traditional Chinese Medicinal Plant. Molecules, 23(12), 3106. https://doi.org/10.3390/molecules23123106






