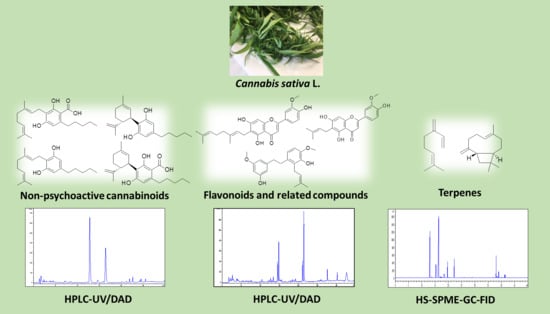
New Methods for the Comprehensive Analysis of Bioactive Compounds in Cannabis sativa L. (hemp)
Abstract
1. Introduction
2. Results and Discussion
2.1. Extraction of Non-Psychoactive Cannabinoids and Flavonoids from Hemp Inflorescences
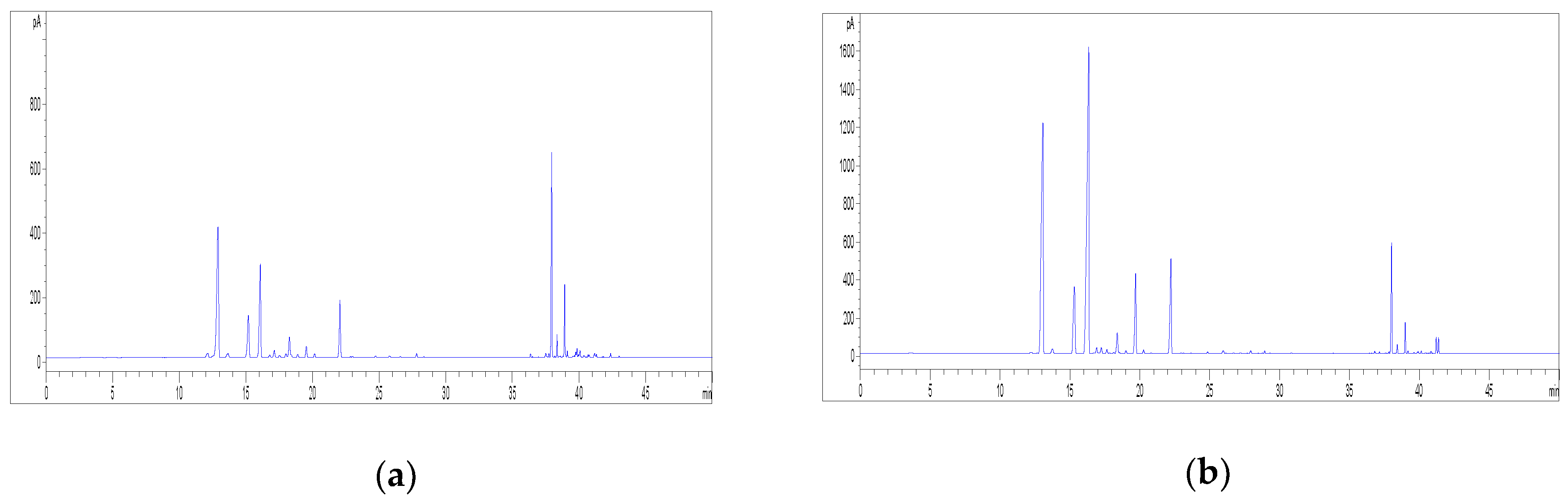
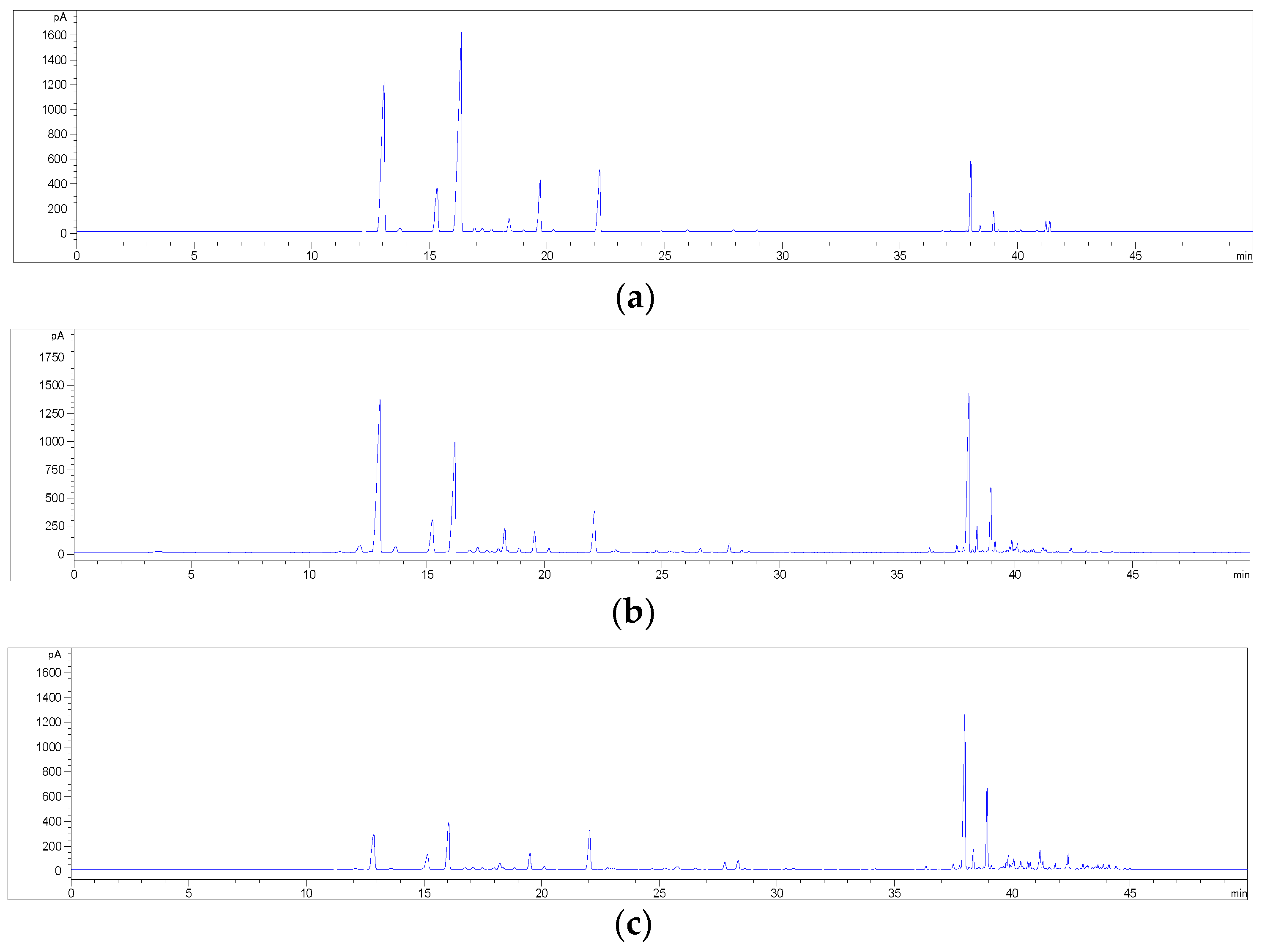
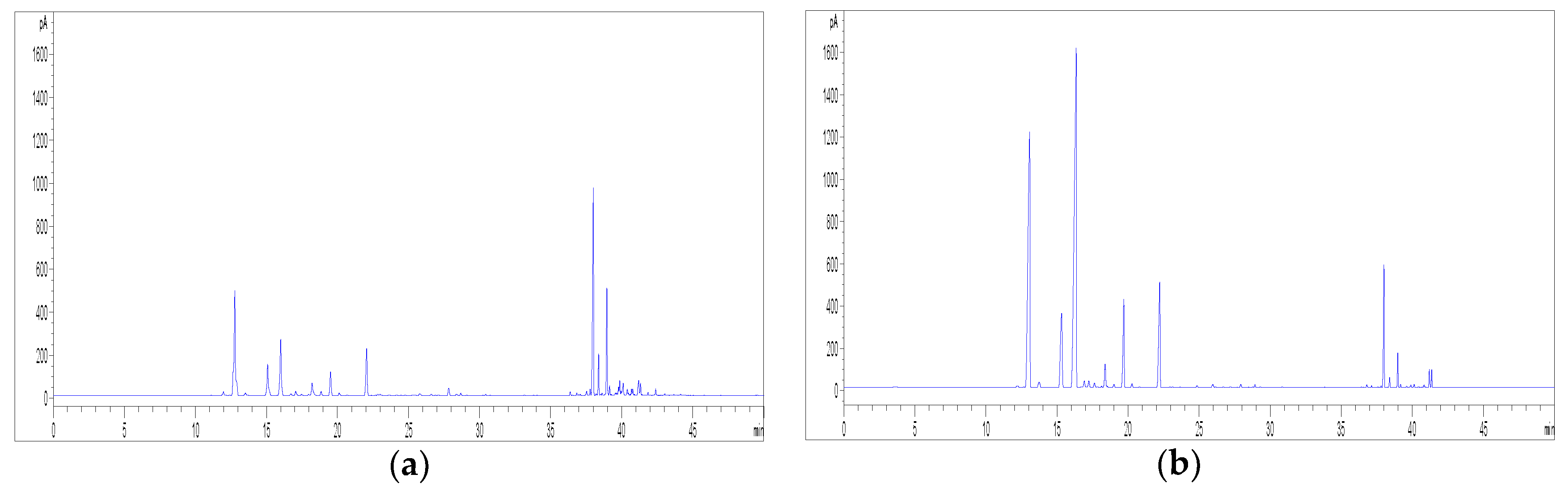
2.2. HPLC Methods for the Analysis of Non-Psychoactive Cannabinoids and Flavonoids in Hemp Extracts
2.3. Identification of Non-Psychoactive Cannabinoids and Flavonoids in Hemp Extracts
2.4. Validation Data of the HPLC Methods
2.5. Quantitative Analysis of Non-Psychoactive Cannabinoids in Hemp by HPLC-UV/DAD
2.6. Quantitative Analysis of Flavonoids and Related Compounds in Hemp by HPLC-UV/DAD
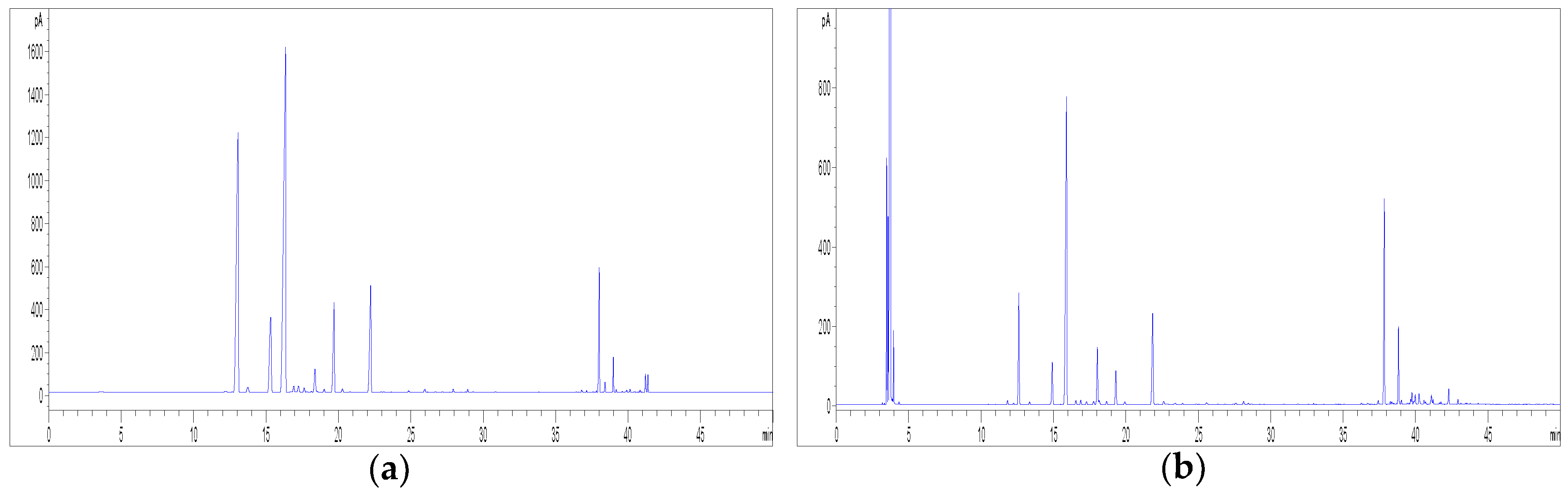
2.7. Extraction of Volatile Compounds from Hemp by HS-SPME
2.8. GC Methods for the Analysis of Volatile Compounds from Hemp
3. Materials and Methods
3.1. Chemicals and Solvents
3.2. Hemp Plant Material
3.3. Sample Preparation
3.3.1. Extraction of Non-Psychoactive Cannabinoids
3.3.2. Extraction of Flavonoids and Related Compounds
3.3.3. Extraction of Volatile Compounds
3.4. HPLC-UV/DAD Conditions
3.4.1. HPLC-UV/DAD Analysis of Non-Psychoactive Cannabinoids
3.4.2. HPLC-UV/DAD Analysis of Flavonoids and Related Compounds
3.5. HPLC-ESI-MS and MS2 Conditions
3.6. HPLC-UV/DAD Method Validation
3.7. GC-FID Analysis of Volatile Compounds
3.8. GC-MS Analysis of Volatile Compounds
3.9. Qualitative and Semi-Quantitative Analysis of Volatile Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chandra, S.; Lata, H.; ElSohly, M.A. Cannabis sativa L.—Botany and Biotechnology, 1st ed.; Springer International Publishing AG: Cham, Switzerland, 2017; pp. ix–xi. ISBN 978-3-319-54563-9. [Google Scholar]
- Hartsel, J.A.; Eades, J.; Makriyannis, A. Cannabis sativa and hemp. In Nutraceuticals: Efficacy, Safety and Toxicity, 1st ed.; Gupta, R.C., Ed.; Elsevier Inc.: San Diego, CA, USA, 2016; pp. 735–754. ISBN 978-0-12-802147-7. [Google Scholar]
- Aizpurua-Olaizola, O.; Soydaner, U.; Öztürk, E.; Schibano, D.; Simsir, Y.; Navarro, P.; Etxebarria, N.; Usobiaga, A. Evolution of the cannabinoid and terpene content during the growth of Cannabis sativa plants from different chemotypes. J. Nat. Prod. 2016, 79, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Appendino, G.; Chianese, G.; Taglialatela-Scafati, O. Cannabinoids: Occurrence and medicinal chemistry. Curr. Med. Chem. 2011, 18, 1085–1099. [Google Scholar] [CrossRef] [PubMed]
- Rules for Direct Payments to Farmers under Support Schemes within the Framework of the Common Agricultural Policy and Repealing Council Regulation (EC) No 637/2008 and Council Regulation (EC) No 73/2009. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv:OJ.L_.2013.347.01.0608.01.ENG (accessed on 10 October 2018).
- Plant Variety Catalogues, Databases & Information Systems. Available online: https://ec.europa.eu/food/plant/plant_propagation_material/plant_variety_catalogues_databases_en (accessed on 18 August 2018).
- Cascio, M.G.; Pertwee, R.G.; Marini, P. The pharmacology and therapeutic potential of plant cannabinoids. In Cannabis sativa L.—Botany and Biotechnology, 1st ed.; Chandra, S., Lata, H., ElSohly, M.A., Eds.; Springer International Publishing AG: Cham, Switzerland, 2017; pp. 207–225. ISBN 978-3-319-54563-9. [Google Scholar]
- Allegrone, G.; Pollastro, F.; Magagnini, G.; Taglialatela-Scafati, O.; Seegers, J.; Koberle, A.; Werz, O.; Appendino, G. The bibenzyl canniprene inhibits the production of pro-inflammatory eicosanoids and selectively accumulates in some Cannabis sativa strains. J. Nat. Prod. 2017, 80, 731–734. [Google Scholar] [CrossRef] [PubMed]
- Brighenti, V.; Pellati, F.; Steinbach, M.; Maran, D.; Benvenuti, S. Development of a new extraction technique and HPLC method for the analysis of non-psychoactive cannabinoids in fibre-type Cannabis sativa L. (hemp). J. Pharm. Biomed. Anal. 2017, 143, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Calzolari, D.; Magagnini, G.; Lucini, L.; Grassi, G.; Appendino, G.B.; Amaducci, S. High-added value compounds from Cannabis threshing residues. Ind. Crops Prod. 2017, 108, 558–563. [Google Scholar] [CrossRef]
- Campos, A.C.; Brant, F.; Miranda, A.S.; Machado, F.S.; Teixeira, A.L. Cannabidiol increases survivals and promotes rescue of cognitive function in a murine model of cerebral malaria. Neuroscience 2015, 289, 166–180. [Google Scholar] [CrossRef] [PubMed]
- Gul, W.; Gul, S.W.; Radwan, M.M.; Wanas, A.S.; Mehmedic, Z.; Khan, I.I.; Sharaf, M.H.M.; ElSohly, M.A. Determination of 11 cannabinoids in biomass and extracts of different varieties of Cannabis using high-performance liquid chromatography. J. AOAC Int. 2015, 98, 1523–1528. [Google Scholar] [CrossRef] [PubMed]
- Peschel, W.; Politi, M. 1H-NMR and HPLC/DAD for Cannabis sativa L. chemotype distinction, extract profiling and specification. Talanta 2015, 140, 150–165. [Google Scholar] [CrossRef] [PubMed]
- Da Porto, C.; Decorti, D.; Natolino, A. Separation of aroma compounds from industrial hemp inflorescences (Cannabis sativa L.) by supercritical CO2 extraction and on-line fractionation. Ind. Crops Prod. 2014, 58, 99–103. [Google Scholar] [CrossRef]
- Bertoli, A.; Tozzi, S.; Pistelli, L.; Angelini, L.G. Fibre hemp inflorescences: From crop-residues to essential oil production. Ind. Crops Prod. 2010, 32, 329–337. [Google Scholar] [CrossRef]
- De Backer, B.; Debrus, B.; Lebrun, P.; Theunis, L.; Dubois, N.; Decock, L.; Verstraete, A.; Hubert, P.; Charlier, C. Innovative development and validation of an HPLC/DAD method for the qualitative and quantitative determination of major cannabinoids in Cannabis plant material. J. Chromatogr. B 2009, 877, 4115–4124. [Google Scholar] [CrossRef] [PubMed]
- Andre, C.M.; Hausman, J.F.; Guerriero, G. Cannabis sativa: The plant of the thousand and one molecule. Front. Plant. Sci. 2016, 7, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Thomas, B.F.; ElSohly, M.A. The Analytical Chemistry of Cannabis: Quality Assessment, Assurance and Regulation of Medicinal Marijuana and Cannabinoid Preparations, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 27–41. ISBN 9780128046463. [Google Scholar]
- ElSohly, M.A.; Slade, D. Chemical constituents of marijuana: The complex mixture of natural cannabinoids. Life Sci. 2005, 78, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Alexander, S.P.H. Therapeutic potential of cannabis-related drugs, Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 64, 157–166. [Google Scholar] [CrossRef]
- Campos, A.C.; Fogaça, M.V.; Sonego, A.B.; Guimarães, F.S. Cannabidiol, neuroprotection and neuropsychiatric disorders. Pharmacol. Res. 2016, 112, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Izzo, A.A.; Borrelli, F.; Capasso, R.; Di Marzo, V.; Mechoulam, R. Non-psychotropic plant cannabinoids: New therapeutic opportunities from an ancient herb. Trends Pharmacol. Sci. 2009, 30, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Corsi, L.; Pellati, F.; Brighenti, V.; Plessi, N.; Benvenuti, S. Chemical composition and in vitro neuroprotective activity of fibre-type Cannabis sativa L. (Hemp). Curr. Bioact. Compd. in press.
- Brenneisen, R. Chemistry and Analysis of Phytocannabinoids and other Cannabis constituents. In Forensic Science and Medicine: Marijuana and the Cannabinoids, 1st ed.; ElSohly, M.A., Ed.; Humana Press Inc.: Totowa, NJ, USA, 2007; pp. 17–49. ISBN 978-1-58829-456-2. [Google Scholar]
- Pollastro, F.; Minassi, A.; Fresu, L.G. Cannabis phenolics and their bioactivities. Curr. Med. Chem. 2018, 25, 1160–1185. [Google Scholar] [CrossRef] [PubMed]
- Wertz, O.; Seegers, J.; Schaible, A.M.; Weinigel, C.; Barz, D.; Koeberle, A.; Allegrone, G.; Pollastro, F.; Zampieri, L.; Grassi, G.; et al. Cannflavins from hemp sprouts, a novel cannabinoid-free hemp food product, target microsomial prostaglandin E2 synthase-1 and 5-lipoxygenase. PharmaNutrition 2014, 2, 53–60. [Google Scholar] [CrossRef]
- Calvi, L.; Pentimalli, D.; Panseri, S.; Giupponi, L.; Gelmini, F.; Beretta, G.; Vitali, D.; Bruno, M.; Zilio, E.; Pavlovic, R.; et al. Comprehensive quality evaluation of medical Cannabis sativa L. inflorescence and macerated oils based on HS-SPME coupled to GC–MS and LC-HRMS (q-exactive orbitrap®) approach. J. Pharm. Biomed. Anal. 2018, 150, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Marchini, M.; Charvoz, C.; Dujourdy, L.; Baldovini, N.; Filippi, J.-J. Multidimensional analysis of cannabis volatile constituents: Identification of 5,5-dimethyl-1-vinylbicyclo[2.1.1]hexane as a volatile marker of hashish, the resin of Cannabis sativa L. J. Chroamtogr. A 2014, 1370, 200–215. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.A.; ElSohly, M.A. The volatile oil composition of fresh and air-dried buds of Cannabis sativa. J. Nat. Prod. 1996, 59, 49–51. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.B. Taming THC: Potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br. J. Pharmacol. 2011, 163, 1344–1364. [Google Scholar] [CrossRef] [PubMed]
- Brglez Mojer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction methods, antioxidative action, bioavailability and anticarcinogenic effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef] [PubMed]
- Hanuš, L.O.; Meyer, S.M.; Muñoz, E.; Taglialatela-Scafati, O.; Appendino, G. Phytocannabinoids: A unified critical inventory. Nat. Prod. Rep. 2016, 33, 1357–1392. [Google Scholar] [CrossRef] [PubMed]
- Stolker, A.A.M.; van Schoonhoven, J.; de Vries, A.J.; Bolbedijk-Pastorova, I.; Vaes, W.H.J.; van den Berg, R. Determination of cannabinoids in Cannabis products using liquid chromatography-ion trap mass spectrometry. J. Chromatogr. A 2004, 1058, 143–151. [Google Scholar] [CrossRef]
- Demarque, D.P.; Crotti, A.E.M.; Vessecchi, R.; Lopes, J.L.C.; Lopes, N.P. Fragmentation reactions using electrospray ionization mass spectrometry: an important tool for the structural elucidation and characterization of synthetic and natural products. Nat. Prod. Rep. 2016, 33, 432–455. [Google Scholar] [CrossRef] [PubMed]
- Validation of Analytical Procedures: Text and Methodology Q2(R1). Available online: https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q2_R1/Step4/Q2_R1__Guideline.pdf (accessed on 12 September 2018).
Sample Availability: Samples of fibre-type hemp extracts are available from the authors. |
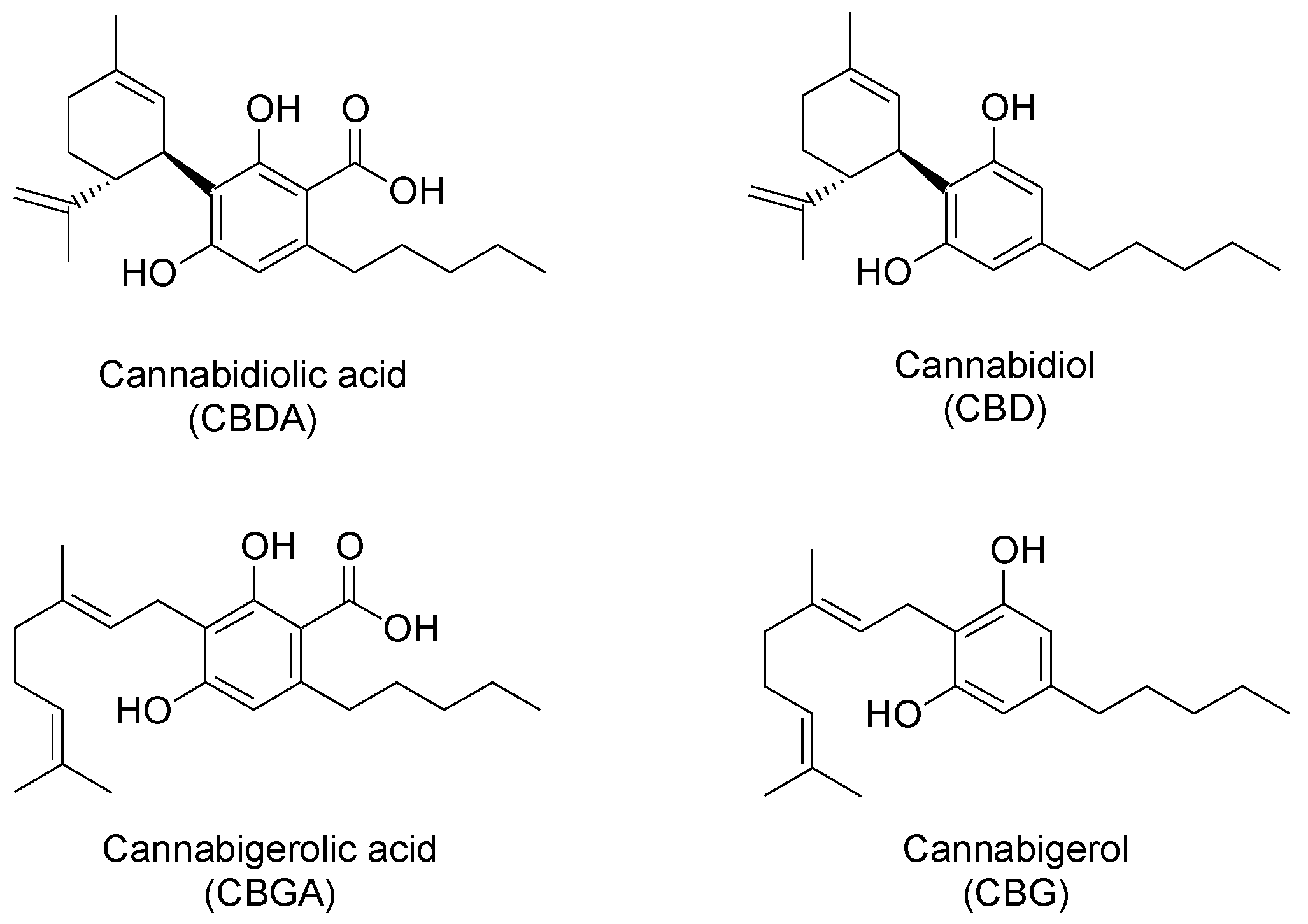
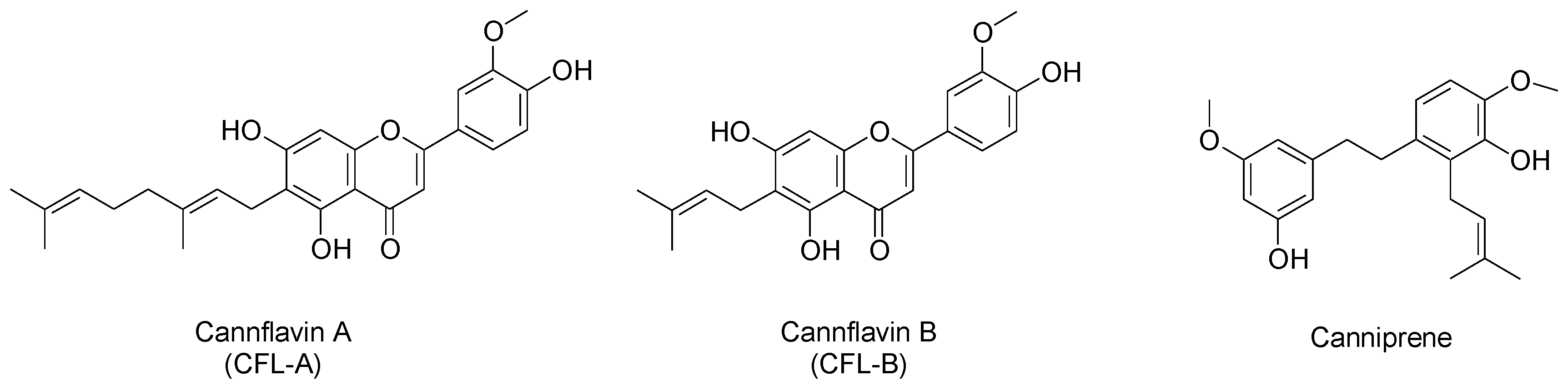
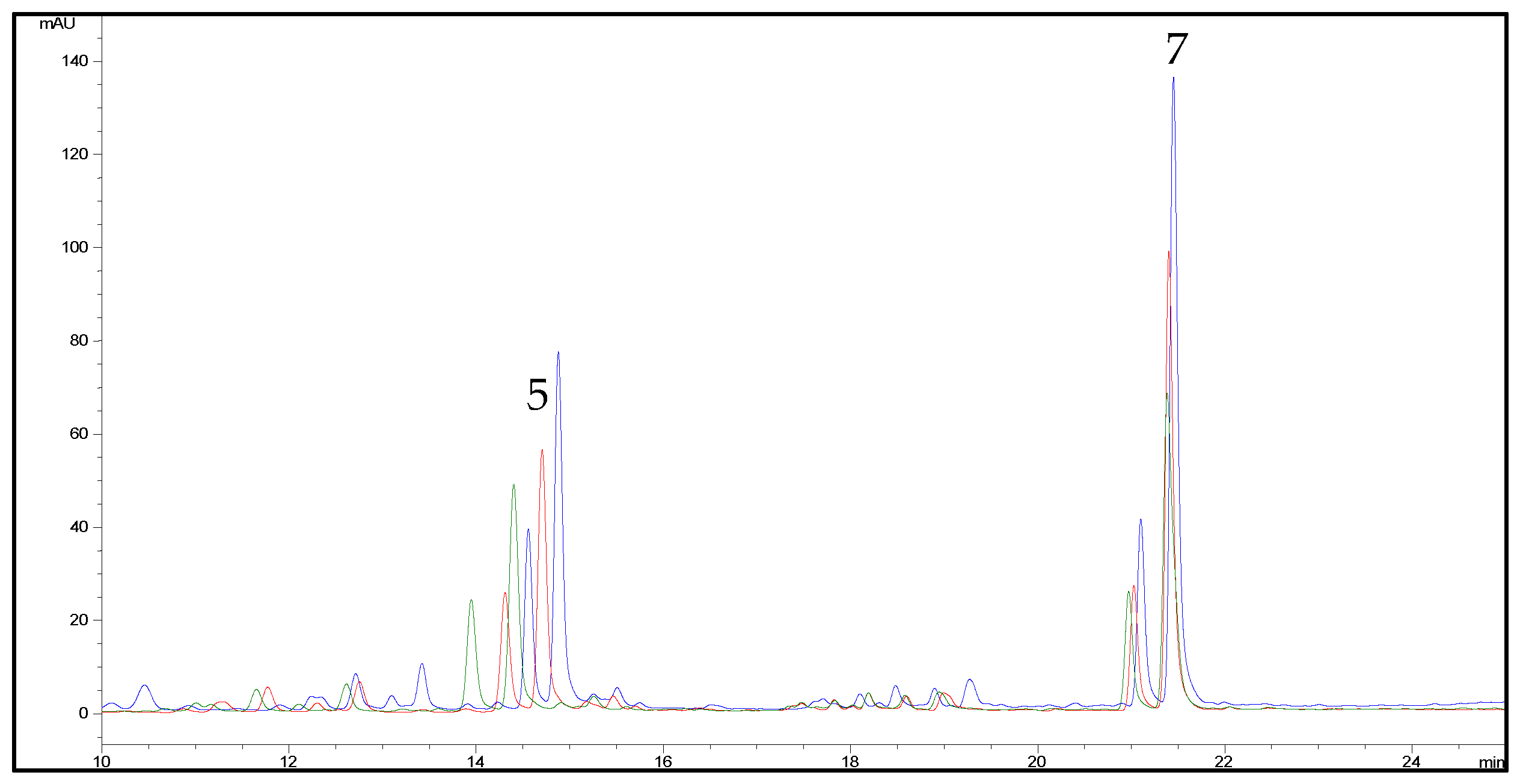
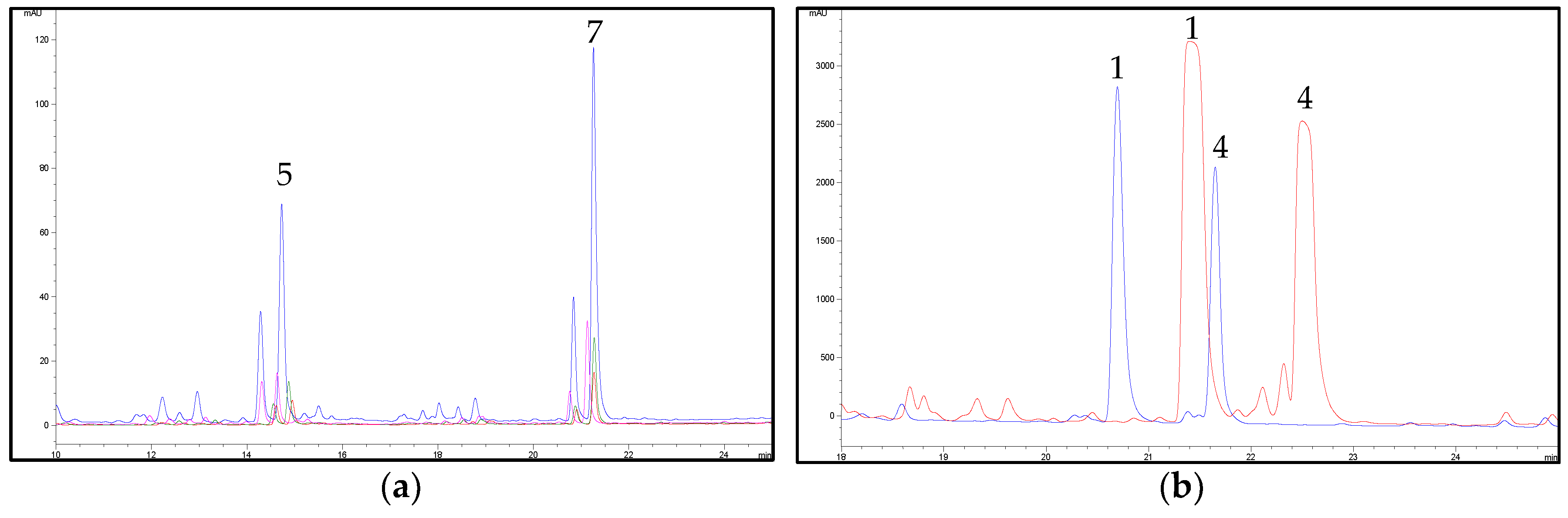
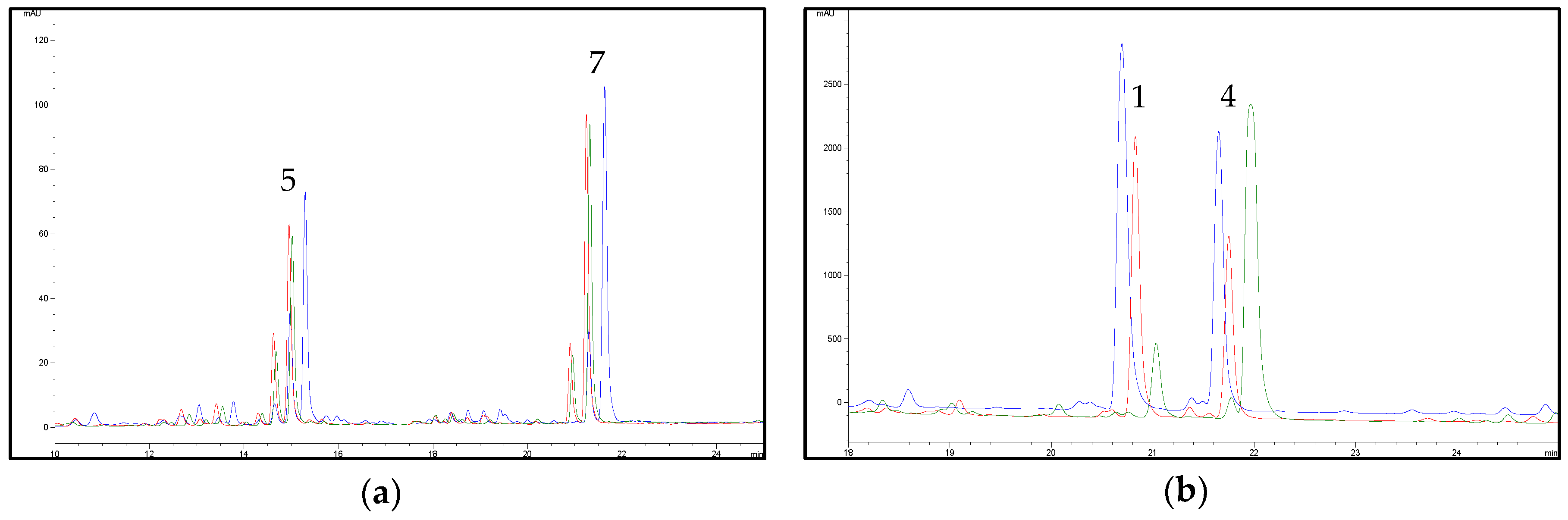
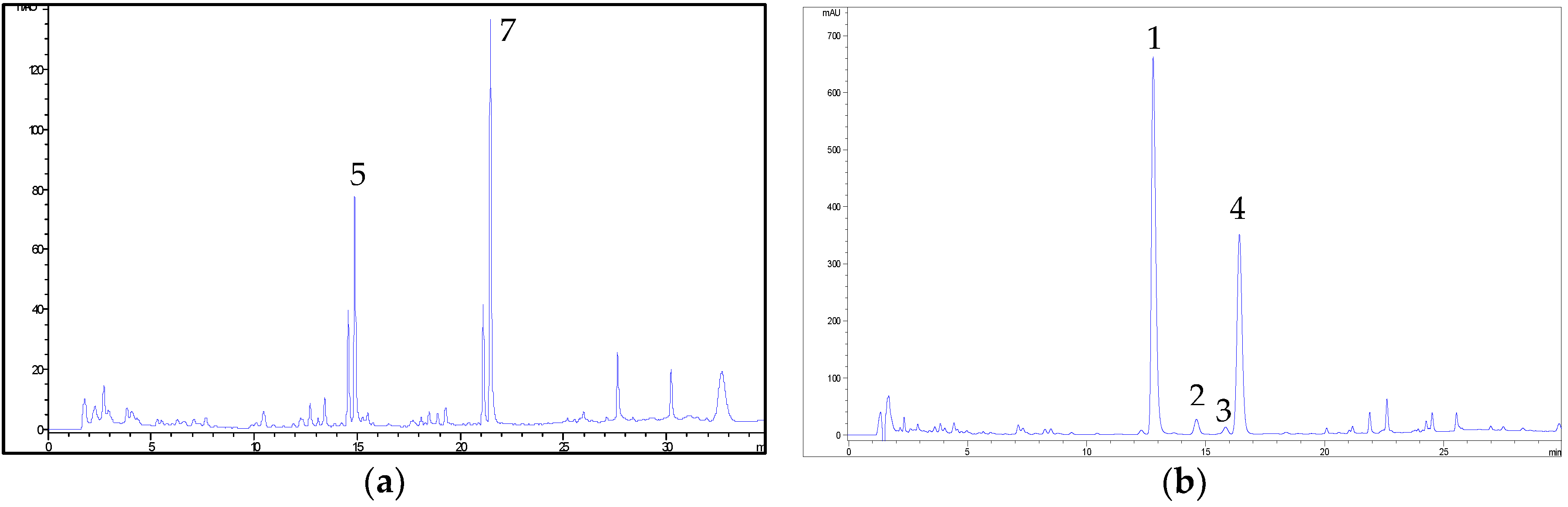
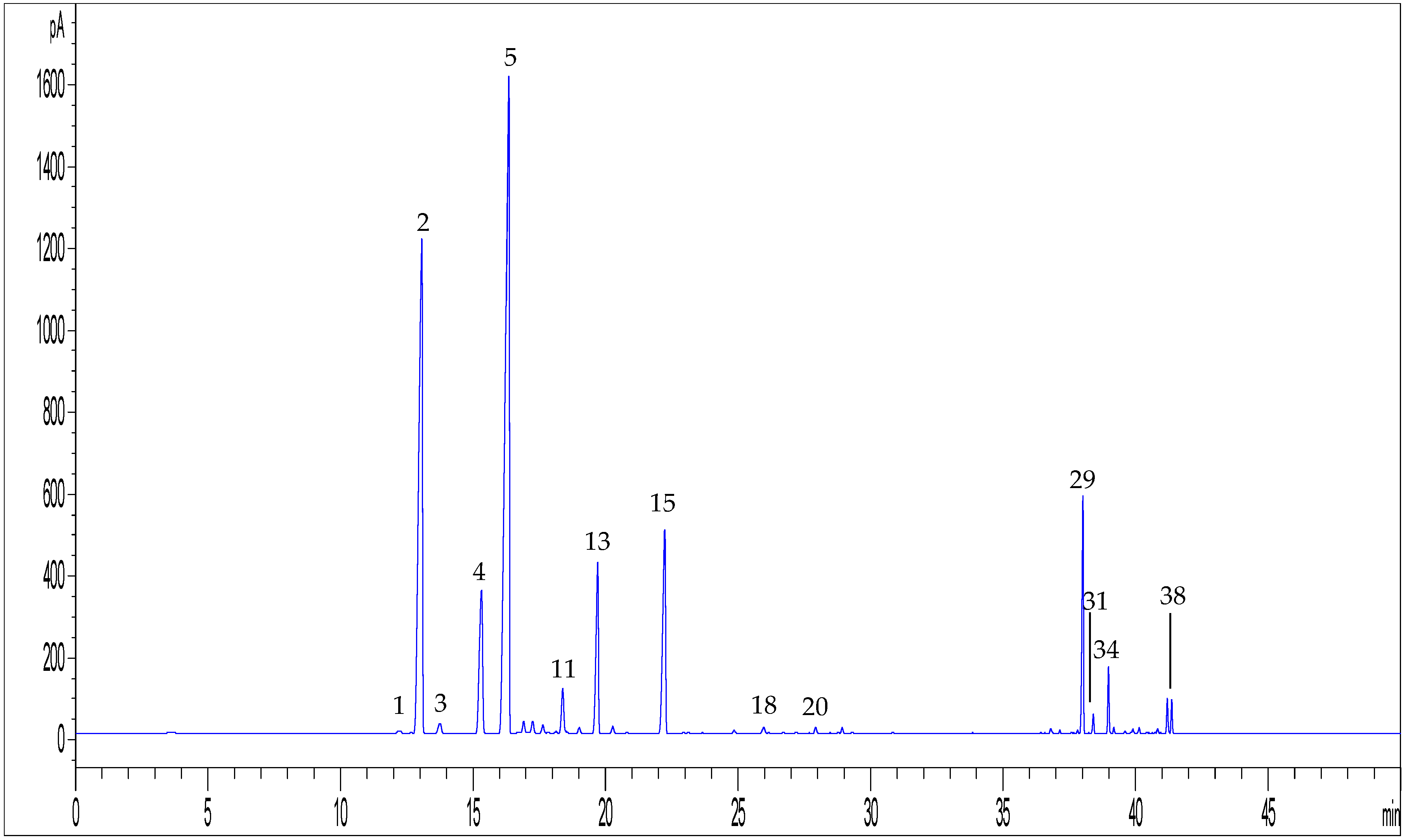
| Peak Number | Compound | tR (min) | UV λmax (nm) | MS (m/z) a | MS2 (m/z) a | MS (m/z) b | MS2 (m/z) b |
|---|---|---|---|---|---|---|---|
| 1 | CBDA | 13.8 | 225,269,307 | 359, 341 * | 219 (100), 261 (55), 285 (33), 232 (22) | 357 | 339 (100), 313 (9) |
| 2 | CBGA | 15.7 | 223,268,305 | 361, 343 * | 219 (100), 261 (49), 233 (19) | 359 | 341 (100), 359 (6) |
| 3 | CBG | 17.1 | 207,231sh,273 | 317 | 207 (100), 233 (40), 280 (10) | 315 | 271 (100), 204 (80), 247 (51), 297 (49) |
| 4 | CBD | 17.9 | 209,232sh,276 | 315 | 259 (100), 233 (26), 221 (24), 207 (24) | 313 | 245 (100), 210 (6) |
| 5 | CFL-B | 14.5 | 215,273,342 | 370 | 313 (100) | - | - |
| 6 | Canniprene | 16.9 | 204,280 | 343, 287 * | 255 (100), 227 (98), 269 (11) | 341 | 326 (100), 269 (84), 283 (43), 257 (12) |
| 7 | CFL-A | 21.1 | 214,274,342 | 437 | 313 (100) | - | - |
| Compound | C1 | C2 | C3 | C4 | C5 | C6 |
|---|---|---|---|---|---|---|
| CBDA | 22.6 ± 2.5 | 4.1 ± 1.7 | 36.4 ± 1.0 | 4.1 ± 0.1 | 3.7 ± 0.2 | 17.6 ± 0.4 |
| CBGA | 0.4 b | 0.2 ± 0.1 | 0.6 b | 0.2 b | <LOQ | 0.1 b |
| CBG | <LOQ | 0.3 ± 0.1 | <LOQ | 0.3 b | <LOQ | <LOQ |
| CBD | 1.9 ± 0.2 | 5.9 ± 0.4 | 6.3 ± 0.3 | 5.9 ± 0.1 | 8.6 ± 0.5 | 6.4 ± 0.4 |
| Compound | C1 | C2 | C3 | C4 | C5 | C6 |
|---|---|---|---|---|---|---|
| CFL-A | 163.4 ± 2.4 | 109.4 ± 5.7 | 318.9 ± 5.8 | 59.9 ± 3.4 | 89.5 ± 6.7 | 140.6 ± 12.5 |
| CFL-B | 100.9 ± 1.7 | 21.0 ± 2.6 | 412.2 ± 4.0 | 25.3 ± 0.2 | 61.4 ± 5.7 | 75.9 ± 6.6 |
| Canniprene | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| Peak Number | Compound | LRI Calc. | LRI Lit. c | % Relative Peak Area | |||||
|---|---|---|---|---|---|---|---|---|---|
| C1 | C2 | C3 | C4 | C5 | C6 | ||||
| 1 | α-Thujene | 932 | 911 | 3.7 ± 1.1 | 1.4 ± 1.0 | 0.2 b | 0.2 b | 0.5 ± 0.1 | - |
| 2 | α-Pinene | 939 | 932 | 20.1 ± 0.5 | 40.1 ± 2.3 | 26.7 b | 9.6 ± 0.4 | 15.9 ± 2.1 | 10.3 b |
| 3 | Camphene | 952 | 957 | 0.3 b | - | 0.5 b | 0.3 b | 0.3 b | 0.2 b |
| 4 | β-Pinene | 980 | 978 | 5.7 b | 9.3 ± 0.1 | 6.9 ± 0.1 | 2.9 ± 0.2 | 4.2 ± 0.3 | 3.7 b |
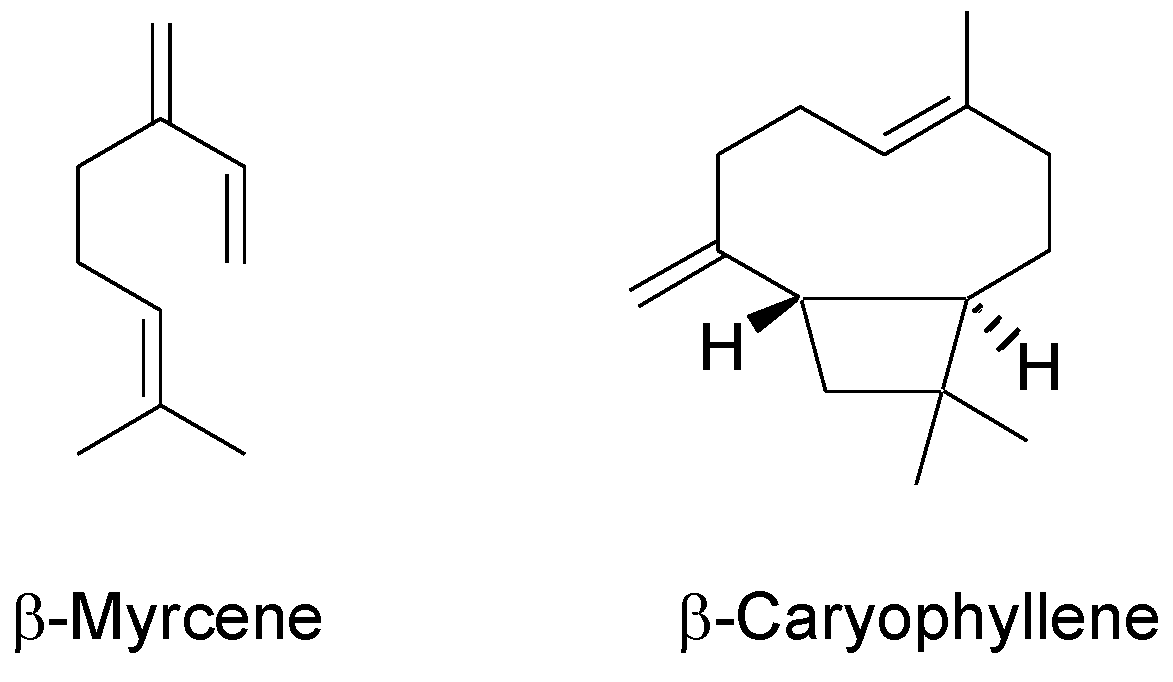
| 5 | β-Myrcene | 1000 | 992 | 28.7 ± 0.1 | 12.9 ± 0.1 | 36.9 ± 0.2 | 32.1 ± 3.3 | 6.9 ± 0.5 | 34.1 ± 1.1 |
| 6 | α-Phellandrene | 1009 | 1007 | 0.1 b | - | 0.4 b | 0.5 b | - | 0.3 b |
| 7 | Δ3-Carene | 1014 | 1010 | 1.3 b | 1.4 b | 0.5 b | 0.6 b | - | 0.3 b |
| 8 | α-Terpinene | 1020 | 1018 | - | - | 0.3 b | 0.4 b | - | 0.3 ± 0.1 |
| 9 | o-Cymene | 1022 | 1021 | 0.1 b | - | 0.1 b | 0.1 b | 1.3 ± 0.1 | - |
| 10 | p-Cymene | 1028 | 1026 | 0.2 b | - | 0.1 b | 0.5 ± 0.1 | 2.5 ± 0.1 | 0.8 ± 0.1 |
| 11 | Limonene | 1032 | 1035 | 2.4 b | 2.3 ± 0.1 | 1.6 b | 11.3 ± 0.5 | 3.5 ± 0.2 | 11.5 ± 0.3 |
| 12 | cis-Ocimene | 1042 | 1040 | 0.5 b | 0.7 b | 0.2 b | 0.6 ± 0.1 | 0.4 ± 0.1 | - |
| 13 | trans-Ocimene | 1053 | 1097 | 7.1 ± 0.3 | 0.6 b | 6.1 ± 0.2 | 5.2 ± 0.1 | 0.4 ± 0.1 | 0.5 b |
| 14 | γ-Terpinene | 1062 | 1062 | 0.2 b | - | 0.3 b | 0.4 b | 0.3 b | 0.5 b |
| 15 | α-Terpinolene | 1093 | 1088 | 3.9 ± 0.1 | - | 7.9 ± 0.1 | 12.0 ± 0.8 | 1.4 ± 0.1 | 4.9 ± 0.1 |
| 16 | Linalool | 1104 | 1104 | 0.6 b | - | 0.1 b | 0.3 b | 0.8 ± 0.1 | 0.4 b |
| 17 | 6-Camphenol | 1106 | 1110 | 0.3 b | - | - | 0.1 b | 1.2 ± 0.1 | - |
| 18 | neo-Alloocimene | 1133 | 1143 | 0.4 b | - | 0.1 b | 0.4 b | 1.1 ± 0.2 | - |
| 19 | Isopinocarveol | 1145 | 1139 | - | 0.6 b | - | - | 0.6 ± 0.1 | - |
| 20 | Terpinen-4-ol | 1181 | 1177 | 0.5 b | - | 0.2 b | 0.3 b | 3.3 ± 0.2 | 0.6 b |
| 21 | p-Cymen-8-ol | 1183 | 1181 | - | - | 0.1 b | - | 2.2 ± 0.2 | - |
| 22 | α-Terpineol | 1193 | 1195 | 0.1 b | - | - | 0.1 b | 0.7 ± 0.1 | 0.4 b |
| 23 | Eugenol | 1371 | 1373 | - | - | - | 0.3 ± 0.1 | - | - |
| 24 | α-Copaene | 1375 | 1376 | - | - | - | 0.8 ± 0.2 | - | 0.3 ± 0.1 |
| 25 | Geranyl acetate | 1379 | 1386 | - | - | - | 0.3 ± 0.1 | - | - |
| 26 | Ylangene | 1389 | 1406 | - | - | 0.1 b | - | - | - |
| 27 | Z,Z-α-Farnesene | 1415 | 1462 | 0.2 b | 0.7 b | - | 0.1 b | 1.2 b | 0.4 b |
| 28 | α-Santalene | 1423 | 1420 | - | - | 0.1 b | 0.1 b | 0.3 b | - |
| 29 | β-Caryopyllene | 1430 | 1428 | 13.4 ± 0.1 | 18.1 ± 0.5 | 5.2 ± 0.1 | 8.1 ± 2.0 | 22.6 ± 1.0 | 21.8 ± 0.7 |
| 30 | Z,E-α-Farnesene | 1437 | 1486 | - | - | - | 0.1 b | 2.2 b | - |
| 31 | α-Guaiene | 1444 | 1439 | 0.2 b | 0.5 b | 0.4 b | 0.9 ± 0.3 | - | - |
| 32 | Aromadendrene | 1446 | 1449 | 0.4 ± 0.1 | - | - | 0.3 ± 0.1 | - | - |
| 33 | β-Farnesene | 1460 | 1454 | 0.1 b | - | - | 1.8 ± 0.5 | 0.3 b | - |
| 34 | α-Humulene | 1464 | 1455 | 2.8 b | 4.6 ± 0.3 | 1.3 b | 2.1 ± 0.5 | 8.7 ± 0.6 | 5.6 ± 0.2 |
| 35 | Alloaromadendrene | 1471 | 1467 | 0.3 b | 0.6 b | 0.1 b | 0.3 ± 0.1 | 1.4 ± 0.2 | 0.2 b |
| 36 | Germacrene D | 1489 | 1480 | - | - | - | 0.1 b | 0.5 b | - |
| 37 | β-Selinene | 1493 | 1485 | 0.3 b | 0.6 b | - | 0.2 b | 0.5 ± 0.1 | 0.1 b |
| 38 | Valencene | 1496 | 1496 | 0.5 b | - | 0.1 b | 0.1 b | 1.4 ± 0.1 | 0.3 b |
| 39 | α-Selinene | 1505 | 1494 | 0.4 b | 0.4 b | 0.1 b | 0.1 b | 1.2 ± 0.1 | 0.2 b |
| 40 | γ-Cadinene | 1515 | 1514 | 0.3 b | - | - | 0.6 ± 0.2 | 0.4 b | - |
| 41 | δ-Cadinene | 1532 | 1530 | - | - | 0.1 b | 0.4 ± 0.1 | 0.4 b | - |
| 42 | α-Calacorene | 1547 | 1542 | - | - | - | 0.6 ± 0.1 | - | 0.2 b |
| 43 | Celina-3,7(11)-diene | 1554 | 1550 | - | - | 0.6 b | - | 0.3 b | - |
| 44 | Caryophyllene oxide | 1591 | 1583 | - | 0.6 b | 0.2 b | 0.1 b | 2.6 ± 0.4 | - |
| Total | 94.8 ± 0.9 | 95.4 ± 1.0 | 97.5 ± 1.3 | 95.3 ± 1.1 | 91.5 ± 1.6 | 97.9 ± 0.7 | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pellati, F.; Brighenti, V.; Sperlea, J.; Marchetti, L.; Bertelli, D.; Benvenuti, S. New Methods for the Comprehensive Analysis of Bioactive Compounds in Cannabis sativa L. (hemp). Molecules 2018, 23, 2639. https://doi.org/10.3390/molecules23102639
Pellati F, Brighenti V, Sperlea J, Marchetti L, Bertelli D, Benvenuti S. New Methods for the Comprehensive Analysis of Bioactive Compounds in Cannabis sativa L. (hemp). Molecules. 2018; 23(10):2639. https://doi.org/10.3390/molecules23102639
Chicago/Turabian StylePellati, Federica, Virginia Brighenti, Johanna Sperlea, Lucia Marchetti, Davide Bertelli, and Stefania Benvenuti. 2018. "New Methods for the Comprehensive Analysis of Bioactive Compounds in Cannabis sativa L. (hemp)" Molecules 23, no. 10: 2639. https://doi.org/10.3390/molecules23102639
APA StylePellati, F., Brighenti, V., Sperlea, J., Marchetti, L., Bertelli, D., & Benvenuti, S. (2018). New Methods for the Comprehensive Analysis of Bioactive Compounds in Cannabis sativa L. (hemp). Molecules, 23(10), 2639. https://doi.org/10.3390/molecules23102639