Kappa Opioid Receptor Agonist Mesyl Sal B Attenuates Behavioral Sensitization to Cocaine with Fewer Aversive Side-Effects than Salvinorin A in Rodents
Abstract
1. Introduction
2. Results
2.1. Chemical Properties of Mesyl Sal B
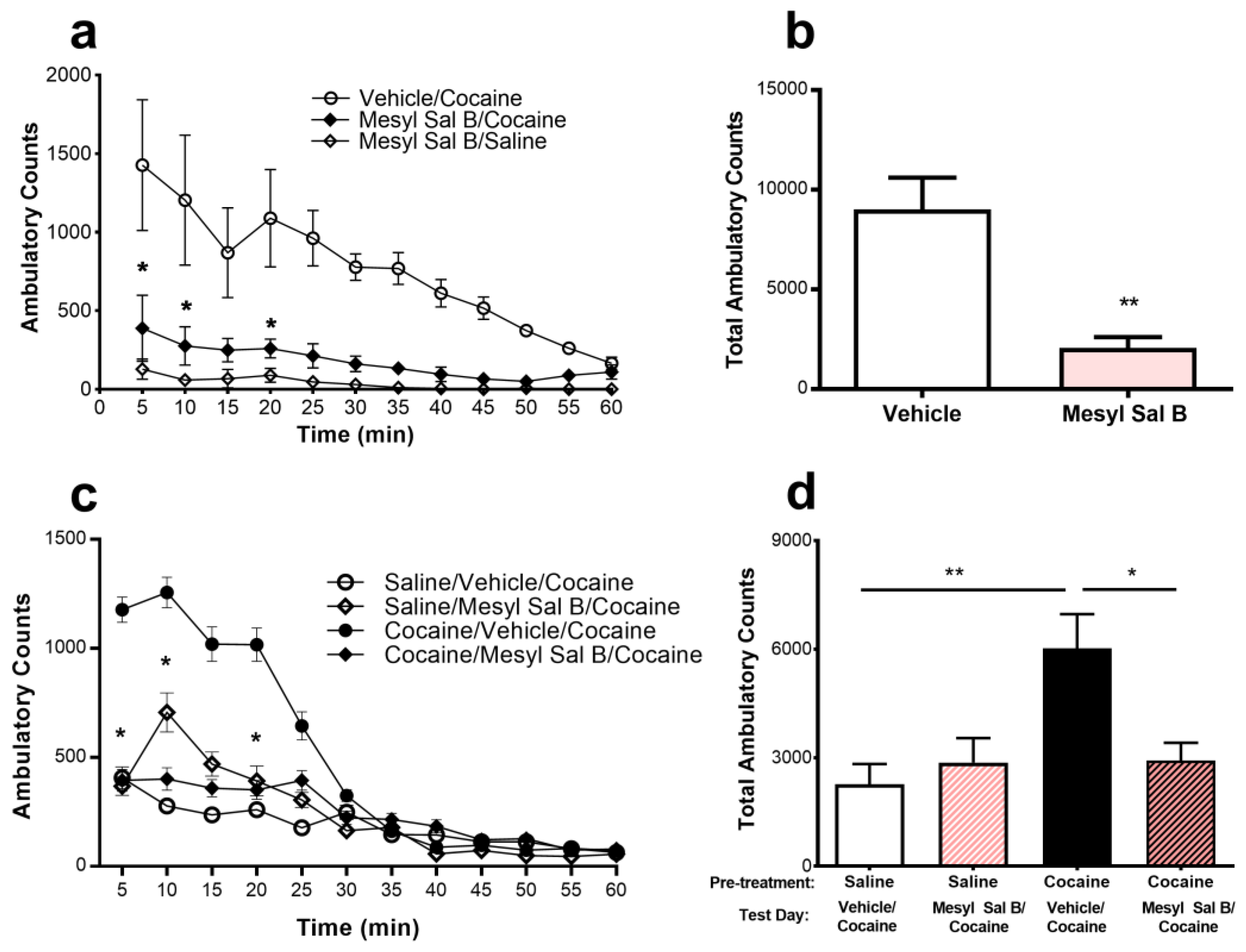
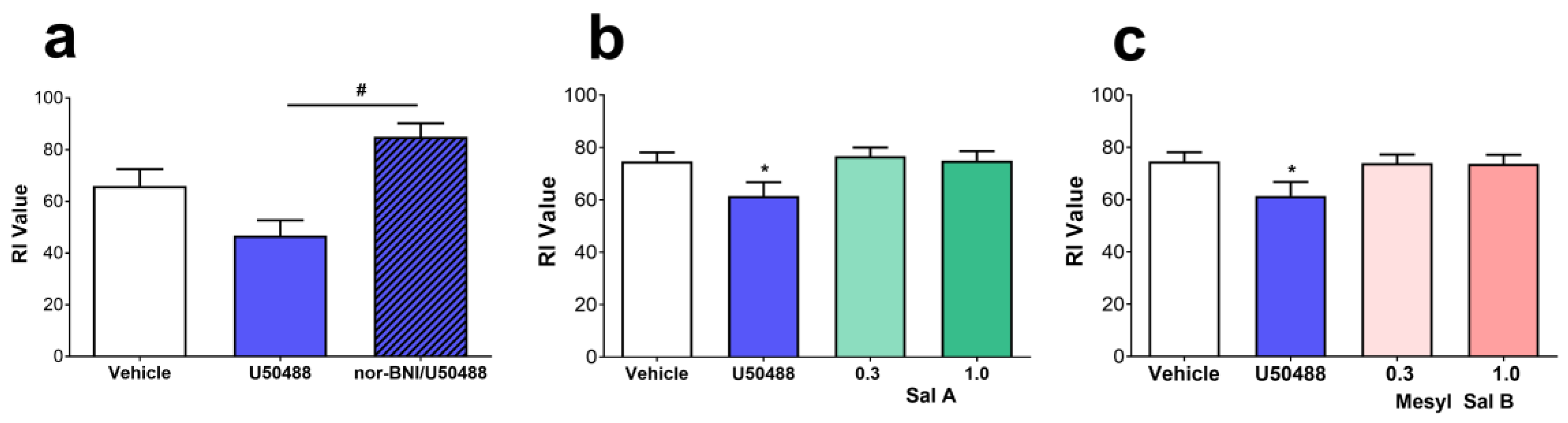
2.2. Anti-Cocaine Effects of Mesyl Sal B
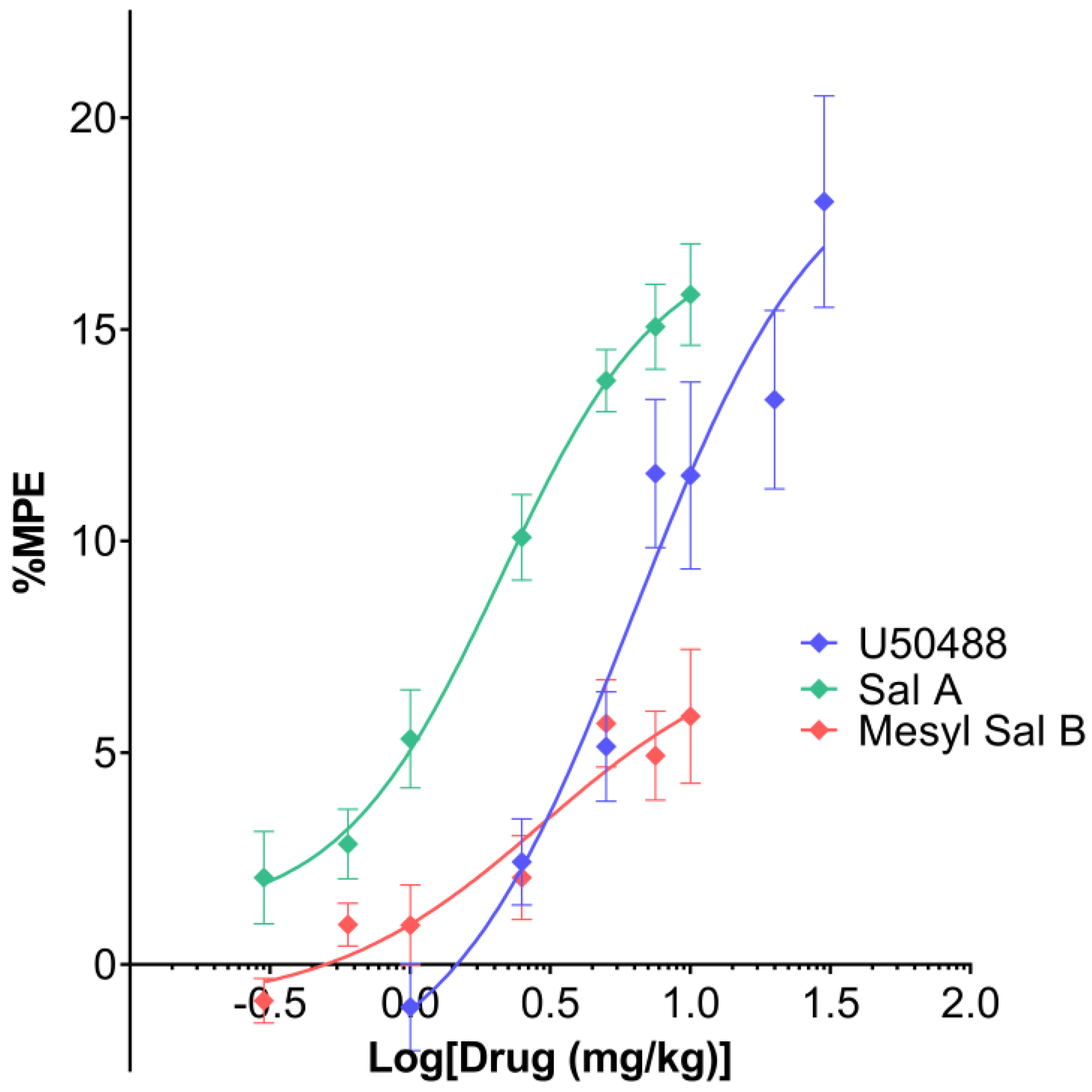
2.3. Thermal Antinociception
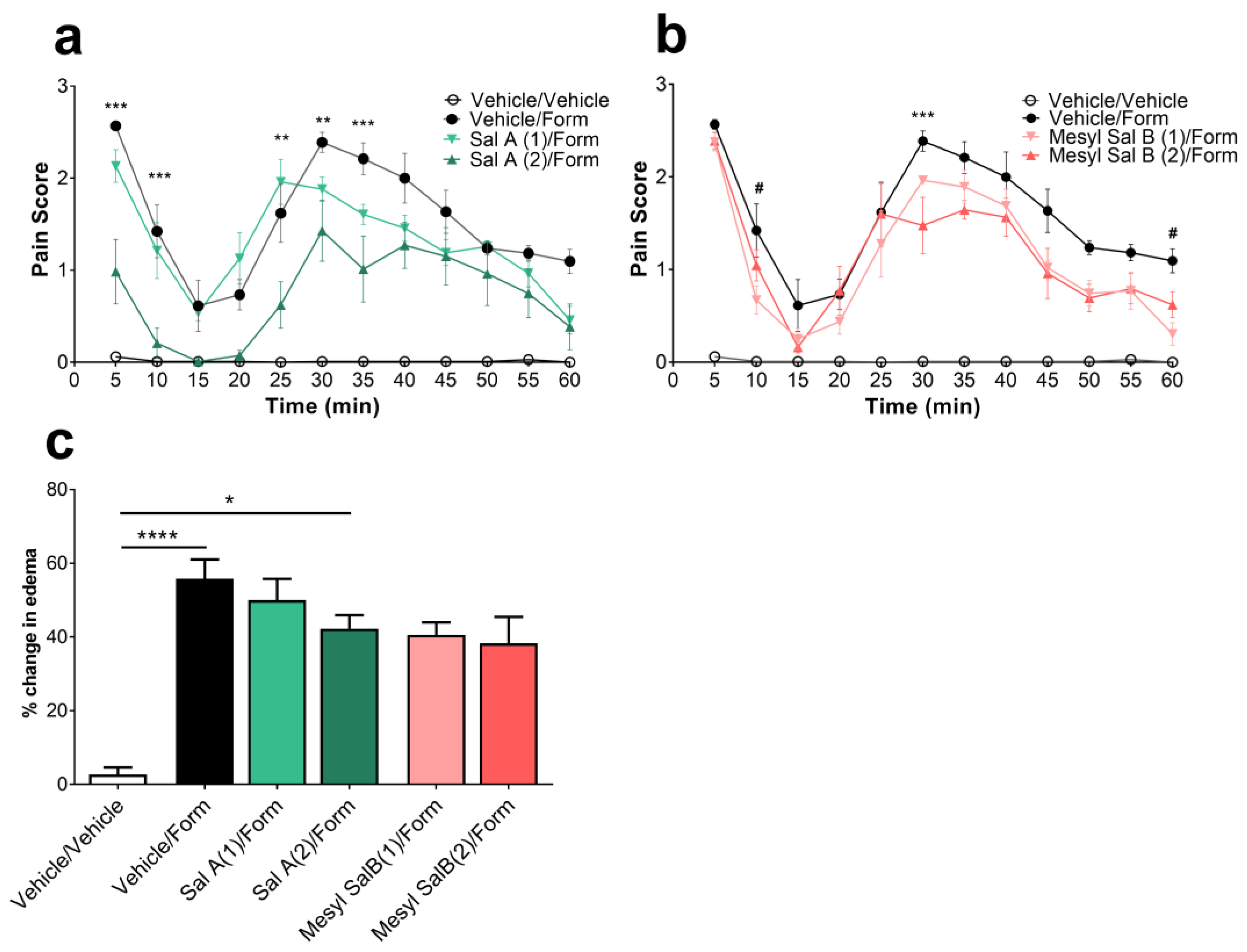
2.4. Effects of Mesyl Sal B on Intraplantar Formaldehyde Induced Inflammatory Pain
2.5. Side-Effect Profile of Mesyl Sal B
2.5.1. Effects of Mesyl Sal B on Sucrose Self-Administration
2.5.2. Novel Object Recognition
2.5.3. Motor Coordination
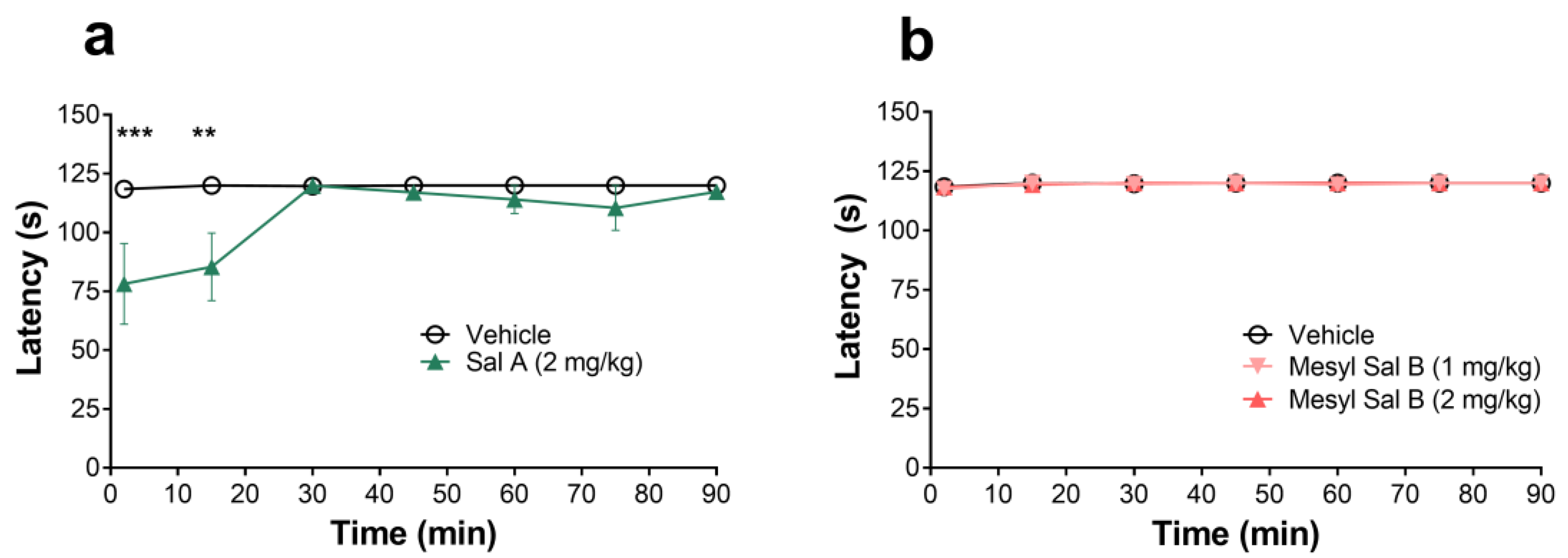
2.5.4. Aversion
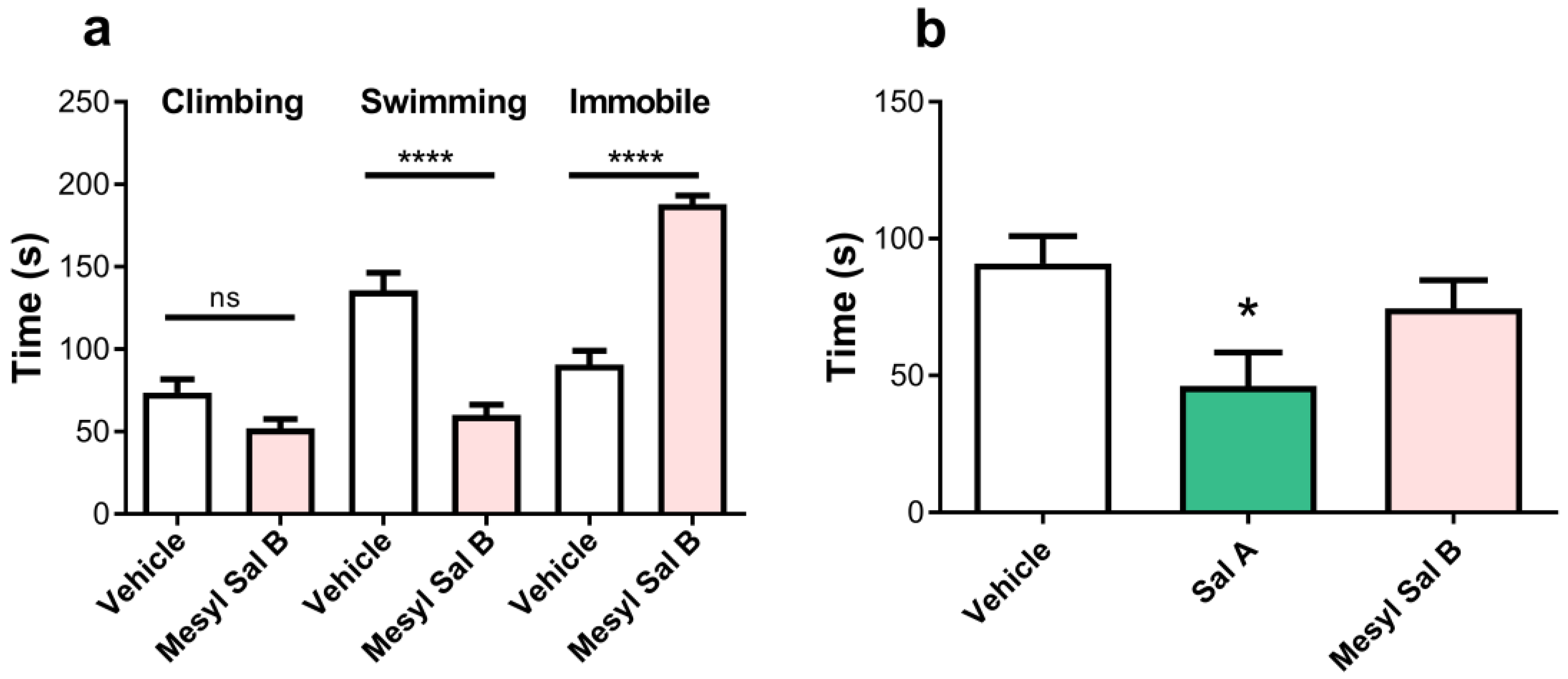
2.5.5. Depression and Anxiety
3. Discussion
3.1. The Cellular Signaling Profile of Mesyl Sal B
3.2. Anti-Cocaine Effects of Mesyl Sal B
3.3. Antinociceptive Effects of Mesyl Sal B
3.4. Side-Effect Profile of Mesyl Sal B
3.5. Limitations and Future Directions
4. Materials and Methods
4.1. Compounds and Drugs
4.2. Cellular Assays
4.3. Animals
4.4. Cocaine-Induced Hyperactivity and Behavioral Sensitization
4.5. Warm-Water Tail Withdrawal
4.6. Intraplantar Formaldehyde Inflammatory Pain Model
4.7. Rotarod Motor Coordination Tests
4.8. Sucrose Self-Administration
4.9. Novel Object Recognition
4.10. Conditioned Taste Aversion
4.11. Conditioned Place Aversion
4.12. Forced Swim Test
4.13. Elevated Plus Maze
4.14. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Koob, G.F.; Volkow, N.D. Neurocircuitry of addiction. Neuropsychopharmacology 2010, 35, 217–238. [Google Scholar] [CrossRef] [PubMed]
- Koob, G.F.; Le Moal, M. Neurobiological mechanisms for opponent motivational processes in addiction. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008, 363, 3113–3123. [Google Scholar] [CrossRef] [PubMed]
- Kuzmin, A.V.; Semenova, S.; Gerrits, M.A.; Zvartau, E.E.; Van Ree, J.M. Kappa-opioid receptor agonist U50,488H modulates cocaine and morphine self-administration in drug-naive rats and mice. Eur. J. Pharmacol. 1997, 321, 265–271. [Google Scholar] [CrossRef]
- Glick, S.D.; Maisonneuve, I.M.; Raucci, J.; Archer, S. Kappa opioid inhibition of morphine and cocaine self-administration in rats. Brain Res. 1995, 681, 147–152. [Google Scholar] [CrossRef]
- Vanderschuren, L.J.M.J.; Schoffelmeer, A.N.M.; Wardeh, G.; De Vries, T.J. Dissociable effects of the κ-opioid receptor agonists bremazocine, U69593, and U50488H on locomotor activity and long-term behavioral sensitization induced by amphetamine and cocaine. Psychopharmacology 2000, 150, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.L.; Gerdes, R.M.; D’Addario, C.; Izenwasser, S. Kappa opioid agonists alter dopamine markers and cocaine-stimulated locomotor activity. Behav. Pharmacol. 2001, 12, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Shippenberg, T.S.; LeFevour, A.; Heidbreder, C. kappa-Opioid receptor agonists prevent sensitization to the conditioned rewarding effects of cocaine. J. Pharmacol. Exp. Ther. 1996, 276, 545–554. [Google Scholar] [PubMed]
- Heidbreder, C.A.; Babovic-Vuksanovic, D.; Shoaib, M.; Shippenberg, T.S. Development of behavioral sensitization to cocaine: Influence of kappa opioid receptor agonists. J. Pharmacol. Exp. Ther. 1995, 275, 150–163. [Google Scholar] [PubMed]
- Schenk, S.; Partridge, B.; Shippenberg, T.S. U69593, a kappa-opioid agonist, decreases cocaine self-administration and decreases cocaine-produced drug-seeking. Psychopharmacology 1999, 144, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Schenk, S.; Partridge, B.; Shippenberg, T.S. Reinstatement of extinguished drug-taking behavior in rats: Effect of the kappa-opioid receptor agonist, U69593. Psychopharmacology 2000, 151, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Heidbreder, C.A.; Shippenberg, T.S. U-69593 prevents cocaine sensitization by normalizing basal accumbens dopamine. Neuroreport 1994, 5, 1797–1800. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.C.; Zapata, A.; Justice, J.B., Jr.; Vaughan, R.A.; Sharpe, L.G.; Shippenberg, T.S. Kappa-opioid receptor activation modifies dopamine uptake in the nucleus accumbens and opposes the effects of cocaine. J. Neurosci. 2000, 20, 9333–9340. [Google Scholar] [CrossRef] [PubMed]
- Chefer, V.I.; Czyzyk, T.; Bolan, E.A.; Moron, J.; Pintar, J.E.; Shippenberg, T.S. Endogenous kappa-opioid receptor systems regulate mesoaccumbal dopamine dynamics and vulnerability to cocaine. J. Neurosci. 2005, 25, 5029–5037. [Google Scholar] [CrossRef] [PubMed]
- Kivell, B.; Uzelac, Z.; Sundaramurthy, S.; Rajamanickam, J.; Ewald, A.; Chefer, V.; Jaligam, V.; Bolan, E.; Simonson, B.; Annamalai, B.; et al. Salvinorin A regulates dopamine transporter function via a kappa opioid receptor and ERK1/2-dependent mechanism. Neuropharmacology 2014, 86, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Margolis, E.B.; Lock, H.; Chefer, V.I.; Shippenberg, T.S.; Hjelmstad, G.O.; Fields, H.L. Kappa opioids selectively control dopaminergic neurons projecting to the prefrontal cortex. Proc. Natl. Acad. Sci. USA 2006, 103, 2938–2942. [Google Scholar] [CrossRef] [PubMed]
- Todtenkopf, M.S.; Marcus, J.F.; Portoghese, P.S.; Carlezon, W.A., Jr. Effects of kappa-opioid receptor ligands on intracranial self-stimulation in rats. Psychopharmacology 2004, 172, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, A.; Brantl, V.; Herz, A.; Emrich, H.M. Psychotomimesis mediated by kappa opiate receptors. Science 1986, 233, 774–776. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.L.; Strain, E.C.; Abreu, M.E.; Bigelow, G.E. Enadoline, a selective kappa opioid agonist: Comparison with butorphanol and hydromorphone in humans. Psychopharmacology 2001, 157, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Mello, N.K.; Negus, S.S. Interactions between kappa opioid agonists and cocaine. Preclinical studies. Ann. N. Y. Acad. Sci. 2000, 909, 104–132. [Google Scholar] [CrossRef] [PubMed]
- Mucha, R.F.; Herz, A. Preference conditioning produced by opioid active and inactive isomers of levorphanol and morphine in rat. Life Sci. 1986, 38, 241–249. [Google Scholar] [CrossRef]
- Bals-Kubik, R.; Ableitner, A.; Herz, A.; Shippenberg, T.S. Neuroanatomical sites mediating the motivational effects of opioids as mapped by the conditioned place preference paradigm in rats. J. Pharmacol. Exp. Ther. 1993, 264, 489–495. [Google Scholar] [PubMed]
- White, K.L.; Robinson, J.E.; Zhu, H.; DiBerto, J.F.; Polepally, P.R.; Zjawiony, J.K.; Nichols, D.E.; Malanga, C.J.; Roth, B.L. The G protein-biased kappa-opioid receptor agonist RB-64 is analgesic with a unique spectrum of activities in vivo. J. Pharmacol. Exp. Ther. 2015, 352, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Brust, T.F.; Morgenweck, J.; Kim, S.A.; Rose, J.H.; Locke, J.L.; Schmid, C.L.; Zhou, L.; Stahl, E.L.; Cameron, M.D.; Scarry, S.M.; et al. Biased agonists of the kappa opioid receptor suppress pain and itch without causing sedation or dysphoria. Sci. Signal. 2016, 9, ra117. [Google Scholar] [CrossRef] [PubMed]
- Simonson, B.; Morani, A.S.; Ewald, A.W.; Walker, L.; Kumar, N.; Simpson, D.; Miller, J.H.; Prisinzano, T.E.; Kivell, B.M. Pharmacology and anti-addiction effects of the novel kappa opioid receptor agonist Mesyl Sal B, a potent and long-acting analogue of salvinorin A. Br. J. Pharmacol. 2015, 172, 515–531. [Google Scholar] [CrossRef] [PubMed]
- Jamshidi, R.J.; Jacobs, B.A.; Sullivan, L.C.; Chavera, T.A.; Saylor, R.M.; Prisinzano, T.E.; Clarke, W.P.; Berg, K.A. Functional selectivity of kappa opioid receptor agonists in peripheral sensory neurons. J. Pharmacol. Exp. Ther. 2015, 355, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Albert-Vartanian, A.; Boyd, M.R.; Hall, A.L.; Morgado, S.J.; Nguyen, E.; Nguyen, V.P.; Patel, S.P.; Russo, L.J.; Shao, A.J.; Raffa, R.B. Will peripherally restricted kappa-opioid receptor agonists (pKORAs) relieve pain with less opioid adverse effects and abuse potential? J. Clin. Pharm. Ther. 2016, 41, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Guo, D.; Cassel, J.A.; Daubert, J.D.; Dehaven, R.N.; Dehaven-Hudkins, D.L.; Gauntner, E.K.; Gottshall, S.L.; Greiner, S.L.; Koblish, M.; et al. Synthesis and evaluation of novel peripherally restricted kappa-opioid receptor agonists. Bioorg. Med. Chem. Lett. 2005, 15, 1091–1095. [Google Scholar] [CrossRef] [PubMed]
- Roth, B.L.; Baner, K.; Westkaemper, R.; Siebert, D.; Rice, K.C.; Steinberg, S.; Ernsberger, P.; Rothman, R.B. Salvinorin A: A potent naturally occurring nonnitrogenous kappa opioid selective agonist. Proc. Natl. Acad. Sci. USA 2002, 99, 11934–11939. [Google Scholar] [CrossRef] [PubMed]
- Chavkin, C.; Sud, S.; Jin, W.; Stewart, J.; Zjawiony, J.K.; Siebert, D.J.; Toth, B.A.; Hufeisen, S.J.; Roth, B.L. Salvinorin A, an active component of the hallucinogenic sage salvia divinorum is a highly efficacious kappa-opioid receptor agonist: Structural and functional considerations. J. Pharmacol. Exp. Ther. 2004, 308, 1197–1203. [Google Scholar] [CrossRef] [PubMed]
- Carlezon, W.A., Jr.; Beguin, C.; DiNieri, J.A.; Baumann, M.H.; Richards, M.R.; Todtenkopf, M.S.; Rothman, R.B.; Ma, Z.; Lee, D.Y.; Cohen, B.M. Depressive-like effects of the kappa-opioid receptor agonist salvinorin A on behavior and neurochemistry in rats. J. Pharmacol. Exp. Ther. 2006, 316, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Baker, L.E.; Panos, J.J.; Killinger, B.A.; Peet, M.M.; Bell, L.M.; Haliw, L.A.; Walker, S.L. Comparison of the discriminative stimulus effects of salvinorin A and its derivatives to U69,593 and U50,488 in rats. Psychopharmacology 2009, 203, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, Y.; Xu, W.; Lee, D.Y.; Ma, Z.; Rawls, S.M.; Cowan, A.; Liu-Chen, L.Y. 2-Methoxymethyl-salvinorin B is a potent kappa opioid receptor agonist with longer lasting action in vivo than salvinorin A. J. Pharmacol. Exp. Ther. 2008, 324, 1073–1083. [Google Scholar] [CrossRef] [PubMed]
- Morani, A.S.; Kivell, B.; Prisinzano, T.E.; Schenk, S. Effect of kappa-opioid receptor agonists U69593, U50488H, spiradoline and salvinorin A on cocaine-induced drug-seeking in rats. Pharmacol. Biochem. Behav. 2009, 94, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Ewald, A.W.M.; Bosch, P.J.; Culverhouse, A.; Crowley, R.S.; Neuenswander, B.; Prisinzano, T.E.; Kivell, B.M. The C-2 derivatives of salvinorin A, ethoxymethyl ether Sal B and beta-tetrahydropyran Sal B, have anti-cocaine properties with minimal side effects. Psychopharmacology 2017, 234, 2499–2514. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.D.; Schmidt, M.S.; Butelman, E.R.; Harding, W.W.; Tidgewell, K.; Murry, D.J.; Kreek, M.J.; Prisinzano, T.E. Pharmacokinetics of the plant-derived kappa-opioid hallucinogen salvinorin A in nonhuman primates. Synapse 2005, 58, 208–210. [Google Scholar] [CrossRef] [PubMed]
- Butelman, E.R.; Prisinzano, T.E.; Deng, H.; Rus, S.; Kreek, M.J. Unconditioned behavioral effects of the powerful kappa-opioid hallucinogen salvinorin A in nonhuman primates: Fast onset and entry into cerebrospinal fluid. J. Pharmacol. Exp. Ther. 2009, 328, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Prisinzano, T.E. Psychopharmacology of the hallucinogenic sage Salvia divinorum. Life Sci. 2005, 78, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Butelman, E.R.; Caspers, M.; Lovell, K.M.; Kreek, M.J.; Prisinzano, T.E. Behavioral effects and central nervous system levels of the broadly available kappa-agonist hallucinogen salvinorin A are affected by P-glycoprotein modulation in vivo. J. Pharmacol. Exp. Ther. 2012, 341, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Kane, B.E.; Nieto, M.J.; McCurdy, C.R.; Ferguson, D.M. A unique binding epitope for salvinorin A, a non-nitrogenous kappa opioid receptor agonist. FEBS J. 2006, 273, 1966–1974. [Google Scholar] [CrossRef] [PubMed]
- Kane, B.E.; McCurdy, C.R.; Ferguson, D.M. Toward a structure-based model of salvinorin A recognition of the kappa-opioid receptor. J. Med. Chem. 2008, 51, 1824–1830. [Google Scholar] [CrossRef] [PubMed]
- Harding, W.W.; Tidgewell, K.; Byrd, N.; Cobb, H.; Dersch, C.M.; Butelman, E.R.; Rothman, R.B.; Prisinzano, T.E. Neoclerodane diterpenes as a novel scaffold for mu opioid receptor ligands. J. Med. Chem. 2005, 48, 4765–4771. [Google Scholar] [CrossRef] [PubMed]
- Munro, T.A.; Rizzacasa, M.A.; Roth, B.L.; Toth, B.A.; Yan, F. Studies toward the pharmacophore of salvinorin A, a potent kappa opioid receptor agonist. J. Med. Chem. 2005, 48, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Munro, T.A.; Duncan, K.K.; Xu, W.; Wang, Y.; Liu-Chen, L.Y.; Carlezon, W.A., Jr.; Cohen, B.M.; Beguin, C. Standard protecting groups create potent and selective kappa opioids: Salvinorin B alkoxymethyl ethers. Bioorg. Med. Chem. 2008, 16, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- Beguin, C.; Richards, M.R.; Li, J.G.; Wang, Y.; Xu, W.; Liu-Chen, L.Y.; Carlezon, W.A., Jr.; Cohen, B.M. Synthesis and in vitro evaluation of salvinorin A analogues: Effect of configuration at C(2) and substitution at C(18). Bioorg. Med. Chem. Lett. 2006, 16, 4679–4685. [Google Scholar] [CrossRef] [PubMed]
- Prisinzano, T.E.; Rothman, R.B. Salvinorin A analogs as probes in opioid pharmacology. Chem. Rev. 2008, 108, 1732–1743. [Google Scholar] [CrossRef] [PubMed]
- Prevatt-Smith, K.M.; Prisinzano, T.E. New therapeutic potential for psychoactive natural products. Nat. Prod. Rep. 2010, 27, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Morani, A.S.; Ewald, A.; Prevatt-Smith, K.M.; Prisinzano, T.E.; Kivell, B.M. The 2-methoxy methyl analogue of salvinorin A attenuates cocaine-induced drug seeking and sucrose reinforcements in rats. Eur. J. Pharmacol. 2013, 720, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Prevatt-Smith, K.M.; Lovell, K.M.; Simpson, D.S.; Day, V.W.; Douglas, J.T.; Bosch, P.; Dersch, C.M.; Rothman, R.B.; Kivell, B.; Prisinzano, T.E. Potential Drug Abuse Therapeutics Derived from the Hallucinogenic Natural Product Salvinorin A. MedChemComm 2011, 2, 1217–1222. [Google Scholar] [CrossRef] [PubMed]
- Tidgewell, K.; Harding, W.W.; Lozama, A.; Cobb, H.; Shah, K.; Kannan, P.; Dersch, C.M.; Parrish, D.; Deschamps, J.R.; Rothman, R.B.; et al. Synthesis of salvinorin A analogues as opioid receptor probes. J. Nat. Prod. 2006, 69, 914–918. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Crowley, R.S.; Ben, K.; Prisinzano, T.E.; Kreek, M.J. Synergistic blockade of alcohol escalation drinking in mice by a combination of novel kappa opioid receptor agonist Mesyl Salvinorin B and naltrexone. Brain Res. 2017, 1662, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Grignaschi, G.; Burbassi, S.; Zennaro, E.; Bendotti, C.; Cervo, L. A single high dose of cocaine induces behavioral sensitization and modifies mRNA encoding GluR1 and GAP-43 in rats. Eur. J. Neurosci. 2004, 20, 2833–2837. [Google Scholar] [CrossRef] [PubMed]
- Kalivas, P.W.; Stewart, J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res. Brain Res. Rev. 1991, 16, 223–244. [Google Scholar] [CrossRef]
- Heidbreder, C.A.; Thompson, A.C.; Shippenberg, T.S. Role of extracellular dopamine in the initiation and long-term expression of behavioral sensitization to cocaine. J. Pharmacol. Exp. Ther. 1996, 278, 490–502. [Google Scholar] [PubMed]
- Kalivas, P.W.; Pierce, R.C.; Cornish, J.; Sorg, B.A. A role for sensitization in craving and relapse in cocaine addiction. J. Psychopharmacol. 1998, 12, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Di Chiara, G.; Imperato, A. Opposite effects of mu and kappa opiate agonists on dopamine release in the nucleus accumbens and in the dorsal caudate of freely moving rats. J. Pharmacol. Exp. Ther. 1988, 244, 1067–1080. [Google Scholar] [PubMed]
- Kalivas, P.W.; Duffy, P. Effect of acute and daily cocaine treatment on extracellular dopamine in the nucleus accumbens. Synapse 1990, 5, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Kalivas, P.W.; Duffy, P. Time course of extracellular dopamine and behavioral sensitization to cocaine. I. Dopamine axon terminals. J. Neurosci. 1993, 13, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Reith, M.E.; Li, M.Y.; Yan, Q.S. Extracellular dopamine, norepinephrine, and serotonin in the ventral tegmental area and nucleus accumbens of freely moving rats during intracerebral dialysis following systemic administration of cocaine and other uptake blockers. Psychopharmacology 1997, 134, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Cadoni, C.; Solinas, M.; Di Chiara, G. Psychostimulant sensitization: Differential changes in accumbal shell and core dopamine. Eur. J. Pharmacol. 2000, 388, 69–76. [Google Scholar] [CrossRef]
- Robinson, T.E.; Berridge, K.C. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Res. Rev. 1993, 18, 247–291. [Google Scholar] [CrossRef]
- Robinson, T.E.; Berridge, K.C. Incentive-sensitization and addiction. Addiction 2001, 96, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Robinson, T.E.; Berridge, K.C. Addiction. Annu. Rev. Psychol. 2003, 54, 25–53. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Butelman, E.R.; Schlussman, S.D.; Ho, A.; Kreek, M.J. Effects of the plant-derived hallucinogen salvinorin A on basal dopamine levels in the caudate putamen and in a conditioned place aversion assay in mice: Agonist actions at kappa opioid receptors. Psychopharmacology 2005, 179, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Fantegrossi, W.E.; Kugle, K.M.; Valdes, L.J., 3rd; Koreeda, M.; Woods, J.H. Kappa-opioid receptor-mediated effects of the plant-derived hallucinogen, salvinorin A, on inverted screen performance in the mouse. Behav. Pharmacol. 2005, 16, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Butelman, E.R.; Rus, S.; Prisinzano, T.E.; Kreek, M.J. The discriminative effects of the κ-opioid hallucinogen salvinorin A in nonhuman primates: Dissociation from classic hallucinogen effects. Psychopharmacology 2010, 210, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Ebner, S.R.; Roitman, M.F.; Potter, D.N.; Rachlin, A.B.; Chartoff, E.H. Depressive-like effects of the kappa opioid receptor agonist salvinorin A are associated with decreased phasic dopamine release in the nucleus accumbens. Psychopharmacology 2010, 210, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Henderson-Redmond, A.; Czachowski, C. Effects of systemic opioid receptor ligands on ethanol- and sucrose seeking and drinking in alcohol-preferring (P) and Long Evans rats. Psychopharmacology 2014, 231, 4309–4321. [Google Scholar] [CrossRef] [PubMed]
- Carey, A.N.; Lyons, A.M.; Shay, C.F.; Dunton, O.; McLaughlin, J.P. Endogenous kappa opioid activation mediates stress-induced deficits in learning and memory. J. Neurosci. 2009, 29, 4293–4300. [Google Scholar] [CrossRef] [PubMed]
- Pellow, S.; Chopin, P.; File, S.E.; Briley, M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J. Neurosci. Methods 1985, 14, 149–167. [Google Scholar] [CrossRef]
- Valdes, L.J.; Diaz, J.L.; Paul, A.G. Ethnopharmacology of ska Maria Pastora (Salvia divinorum, Epling and Jativa-M.). J. Ethnopharmacol. 1983, 7, 287–312. [Google Scholar] [CrossRef]
- Valdes, L.J.; Butler, W.M.; Hatfield, G.M.; Paul, A.G.; Koreeda, M. Divinorin A, a psychotropic terpenoid, and divinorin B from the hallucinogenic Mexican mint, Salvia divinorum. J. Org. Chem. 1984, 49, 4716–4720. [Google Scholar] [CrossRef]
- Morani, A.S.; Schenk, S.; Prisinzano, T.E.; Kivell, B.M. A single injection of a novel kappa opioid receptor agonist salvinorin A attenuates the expression of cocaine-induced behavioral sensitization in rats. Behav. Pharmacol. 2012, 23, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Fichna, J.; Dicay, M.; Lewellyn, K.; Janecka, A.; Zjawiony, J.K.; MacNaughton, W.K.; Storr, M.A. Salvinorin A has antiinflammatory and antinociceptive effects in experimental models of colitis in mice mediated by KOR and CB1 receptors. Inflamm. Bowel Dis. 2012, 18, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Guida, F.; Luongo, L.; Aviello, G.; Palazzo, E.; De Chiaro, M.; Gatta, L.; Boccella, S.; Marabese, I.; Zjawiony, J.K.; Capasso, R.; et al. Salvinorin A reduces mechanical allodynia and spinal neuronal hyperexcitability induced by peripheral formalin injection. Mol. Pain 2012, 8, 60. [Google Scholar] [CrossRef] [PubMed]
- John, T.F.; French, L.G.; Erlichman, J.S. The antinociceptive effect of salvinorin A in mice. Eur. J. Pharmacol. 2006, 545, 129–133. [Google Scholar] [CrossRef] [PubMed]
- McCurdy, C.R.; Sufka, K.J.; Smith, G.H.; Warnick, J.E.; Nieto, M.J. Antinociceptive profile of salvinorin A, a structurally unique kappa opioid receptor agonist. Pharmacol. Biochem. Behav. 2006, 83, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Paton, K.F.; Kumar, N.; Crowley, R.S.; Harper, J.L.; Prisinzano, T.E.; Kivell, B.M. The analgesic and anti-inflammatory effects of Salvinorin A analogue beta-tetrahydropyran Salvinorin B in mice. Eur. J. Pain 2017, 21, 1039–1050. [Google Scholar] [CrossRef] [PubMed]
- Braida, D.; Donzelli, A.; Martucci, R.; Capurro, V.; Sala, M. Learning and memory impairment induced by salvinorin A, the principal ingredient of Salvia divinorum, in wistar rats. Int. J. Toxicol. 2011, 30, 650–661. [Google Scholar] [CrossRef] [PubMed]
- Crowley, R.S.; Riley, A.P.; Sherwood, A.M.; Groer, C.E.; Shivaperumal, N.; Biscaia, M.; Paton, K.; Schneider, S.; Provasi, D.; Kivell, B.M.; et al. Synthetic studies of neoclerodane diterpenes from Salvia divinorum: Identification of a potent and centrally acting mu opioid analgesic with reduced abuse liability. J. Med. Chem. 2016, 59, 11027–11038. [Google Scholar] [CrossRef] [PubMed]
- Riley, A.P.; Groer, C.E.; Young, D.; Ewald, A.W.; Kivell, B.M.; Prisinzano, T.E. Synthesis and kappa-opioid receptor activity of furan-substituted salvinorin A analogues. J. Med. Chem. 2014, 57, 10464–10475. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, A.M.; Crowley, R.S.; Paton, K.F.; Biggerstaff, A.; Neuenswander, B.; Day, V.W.; Kivell, B.M.; Prisinzano, T.E. Addressing structural flexibility at the A-ring on Salvinorin A: Discovery of a potent kappa opioid agonist with enhanced metabolic stability. J. Med. Chem. 2017, 60, 3866–3878. [Google Scholar] [CrossRef] [PubMed]
- Dogra, S.; Yadav, P.N. Biased agonism at kappa opioid receptors: Implication in pain and mood disorders. Eur. J. Pharmacol. 2015, 763, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Schattauer, S.S.; Kuhar, J.R.; Song, A.; Chavkin, C. Nalfurafine is a G-protein biased agonist having significantly greater bias at the human than rodent form of the kappa opioid receptor. Cell Signal. 2017, 32, 59–65. [Google Scholar] [CrossRef] [PubMed]
- DiMattio, K.M.; Ehlert, F.J.; Liu-Chen, L.Y. Intrinsic relative activities of kappa opioid agonists in activating Galpha proteins and internalizing receptor: Differences between human and mouse receptors. Eur. J. Pharmacol. 2015, 761, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Aviello, G.; Borrelli, F.; Guida, F.; Romano, B.; Lewellyn, K.; De Chiaro, M.; Luongo, L.; Zjawiony, J.K.; Maione, S.; Izzo, A.A.; et al. Ultrapotent effects of salvinorin A, a hallucinogenic compound from Salvia divinorum, on LPS-stimulated murine macrophages and its anti-inflammatory action in vivo. J. Mol. Med. (Berl) 2011, 89, 891–902. [Google Scholar] [CrossRef] [PubMed]
- Maillet, E.L.; Milon, N.; Heghinian, M.D.; Fishback, J.; Schurer, S.C.; Garamszegi, N.; Mash, D.C. Noribogaine is a G-protein biased kappa-opioid receptor agonist. Neuropharmacology 2015, 99, 675–688. [Google Scholar] [CrossRef] [PubMed]
- Bruchas, M.R.; Land, B.B.; Chavkin, C. The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res. 2010, 1314, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Weiss, F.; Markou, A.; Lorang, M.T.; Koob, G.F. Basal extracellular dopamine levels in the nucleus accumbens are decreased during cocaine withdrawal after unlimited-access self-administration. Brain Res. 1992, 593, 314–318. [Google Scholar] [CrossRef]
- Negus, S.S.; Mello, N.K.; Portoghese, P.S.; Lin, C.E. Effects of kappa opioids on cocaine self-administration by rhesus monkeys. J. Pharmacol. Exp. Ther. 1997, 282, 44–55. [Google Scholar] [PubMed]
- Mello, N.K.; Negus, S.S. Effects of kappa opioid agonists on cocaine- and food-maintained responding by rhesus monkeys. J. Pharmacol. Exp. Ther. 1998, 286, 812–824. [Google Scholar] [PubMed]
- Beguin, C.; Richards, M.R.; Wang, Y.; Chen, Y.; Liu-Chen, L.Y.; Ma, Z.; Lee, D.Y.; Carlezon, W.A., Jr.; Cohen, B.M. Synthesis and in vitro pharmacological evaluation of salvinorin A analogues modified at C(2). Bioorg. Med. Chem. Lett. 2005, 15, 2761–2765. [Google Scholar] [CrossRef] [PubMed]
- Sipols, A.J.; Bayer, J.; Bennett, R.; Figlewicz, D.P. Intraventricular insulin decreases kappa opioid-mediated sucrose intake in rats. Peptides 2002, 23, 2181–2187. [Google Scholar] [CrossRef]
- Jewett, D.C.; Grace, M.K.; Jones, R.M.; Billington, C.J.; Portoghese, P.S.; Levine, A.S. The kappa-opioid antagonist GNTI reduces U50,488-, DAMGO-, and deprivation-induced feeding, but not butorphanol- and neuropeptide Y-induced feeding in rats. Brain Res. 2001, 909, 75–80. [Google Scholar] [CrossRef]
- Sandin, J.; Nylander, I.; Georgieva, J.; Schott, P.A.; Ogren, S.O.; Terenius, L. Hippocampal dynorphin B injections impair spatial learning in rats: A kappa-opioid receptor-mediated effect. Neuroscience 1998, 85, 375–382. [Google Scholar] [CrossRef]
- Castellano, C.; Libri, V.; Ammassari-Teule, M. The amygdala mediates the impairing effect of the selective kappa-opioid receptor agonist U-50,488 on memory in CD1 mice. Behav. Brain Res. 1988, 30, 259–263. [Google Scholar] [CrossRef]
- Paris, J.J.; Reilley, K.J.; McLaughlin, J.P. Kappa Opioid Receptor-Mediated Disruption of Novel Object Recognition: Relevance for Psychostimulant Treatment. J. Addict. Res. Ther. 2011, S4. [Google Scholar] [CrossRef] [PubMed]
- Schindler, A.G.; Li, S.; Chavkin, C. Behavioral stress may increase the rewarding valence of cocaine-associated cues through a dynorphin/kappa-opioid receptor-mediated mechanism without affecting associative learning or memory retrieval mechanisms. Neuropsychopharmacology 2010, 35, 1932–1942. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.C.; Tully, T. CREB and the formation of long-term memory. Curr. Opin. Neurobiol. 1996, 6, 264–268. [Google Scholar] [CrossRef]
- Bruchas, M.R.; Chavkin, C. Kinase cascades and ligand-directed signaling at the kappa opioid receptor. Psychopharmacology 2010, 210, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Schoffelmeer, A.N.; Rice, K.C.; Jacobson, A.E.; Van Gelderen, J.G.; Hogenboom, F.; Heijna, M.H.; Mulder, A.H. Mu-, delta- and kappa-opioid receptor-mediated inhibition of neurotransmitter release and adenylate cyclase activity in rat brain slices: Studies with fentanyl isothiocyanate. Eur. J. Pharmacol. 1988, 154, 169–178. [Google Scholar] [CrossRef]
- Shippenberg, T.S.; Herz, A. Place preference conditioning reveals the involvement of D1-dopamine receptors in the motivational properties of mu- and kappa-opioid agonists. Brain Res. 1987, 436, 169–172. [Google Scholar] [CrossRef]
- Shippenberg, T.S.; Bals-Kubik, R.; Herz, A. Examination of the neurochemical substrates mediating the motivational effects of opioids: Role of the mesolimbic dopamine system and D-1 vs. D-2 dopamine receptors. J. Pharmacol. Exp. Ther. 1993, 265, 53–59. [Google Scholar] [PubMed]
- Tejeda, H.A.; Counotte, D.S.; Oh, E.; Ramamoorthy, S.; Schultz-Kuszak, K.N.; Backman, C.M.; Chefer, V.; O’Donnell, P.; Shippenberg, T.S. Prefrontal cortical kappa-opioid receptor modulation of local neurotransmission and conditioned place aversion. Neuropsychopharmacology 2013, 38, 1770–1779. [Google Scholar] [CrossRef] [PubMed]
- Laman-Maharg, A.R.; Copeland, T.; Sanchez, E.O.; Campi, K.L.; Trainor, B.C. The long-term effects of stress and kappa opioid receptor activation on conditioned place aversion in male and female California mice. Behav. Brain Res. 2017, 332, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Sufka, K.J.; Loria, M.J.; Lewellyn, K.; Zjawiony, J.K.; Ali, Z.; Abe, N.; Khan, I.A. The effect of Salvia divinorum and Mitragyna speciosa extracts, fraction and major constituents on place aversion and place preference in rats. J. Ethnopharmacol. 2014, 151, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Braida, D.; Limonta, V.; Capurro, V.; Fadda, P.; Rubino, T.; Mascia, P.; Zani, A.; Gori, E.; Fratta, W.; Parolaro, D.; et al. Involvement of kappa-opioid and endocannabinoid system on Salvinorin A-induced reward. Biol. Psychiatry 2008, 63, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Braida, D.; Capurro, V.; Zani, A.; Rubino, T.; Vigano, D.; Parolaro, D.; Sala, M. Potential anxiolytic- and antidepressant-like effects of salvinorin A, the main active ingredient of Salvia divinorum, in rodents. Br. J. Pharmacol. 2009, 157, 844–853. [Google Scholar] [CrossRef] [PubMed]
- Detke, M.J.; Rickels, M.; Lucki, I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology 1995, 121, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Harden, M.T.; Smith, S.E.; Niehoff, J.A.; McCurdy, C.R.; Taylor, G.T. Antidepressive effects of the kappa-opioid receptor agonist salvinorin A in a rat model of anhedonia. Behav. Pharmacol. 2012, 23, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, H.; Ebata, T.; Takamori, K.; Muramatsu, T.; Nakamoto, H.; Suzuki, H. Effect of a novel kappa-receptor agonist, nalfurafine hydrochloride, on severe itch in 337 haemodialysis patients: A Phase III, randomized, double-blind, placebo-controlled study. Nephrol. Dial. Transplant. 2010, 25, 1251–1257. [Google Scholar] [CrossRef] [PubMed]
- Seki, T.; Awamura, S.; Kimura, C.; Ide, S.; Sakano, K.; Minami, M.; Nagase, H.; Satoh, M. Pharmacological properties of TRK-820 on cloned mu-, delta- and kappa-opioid receptors and nociceptin receptor. Eur. J. Pharmacol. 1999, 376, 159–167. [Google Scholar] [CrossRef]
- Ueno, Y.; Mori, A.; Yanagita, T. One year long-term study on abuse liability of nalfurafine in hemodialysis patients. Int. J. Clin. Pharmacol. Ther. 2013, 51, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Nakao, K.; Hirakata, M.; Miyamoto, Y.; Kainoh, M.; Wakasa, Y.; Yanagita, T. Nalfurafine hydrochloride, a selective kappa opioid receptor agonist, has no reinforcing effect on intravenous self-administration in rhesus monkeys. J. Pharmacol. Sci. 2016, 130, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Butelman, E.R.; Mandau, M.; Tidgewell, K.; Prisinzano, T.E.; Yuferov, V.; Kreek, M.J. Effects of salvinorin A, a kappa-opioid hallucinogen, on a neuroendocrine biomarker assay in nonhuman primates with high kappa-receptor homology to humans. J. Pharmacol. Exp. Ther. 2007, 320, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Munro, T.A.; Huang, X.P.; Inglese, C.; Perrone, M.G.; Van’t Veer, A.; Carroll, F.I.; Beguin, C.; Carlezon, W.A., Jr.; Colabufo, N.A.; Cohen, B.M.; et al. Selective kappa opioid antagonists nor-BNI, GNTI and JDTic have low affinities for non-opioid receptors and transporters. PLoS ONE 2013, 8, e70701. [Google Scholar] [CrossRef] [PubMed]
- Dubuisson, D.; Dennis, S.G. The formalin test: A quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain 1977, 4, 161–174. [Google Scholar] [CrossRef]
- Walf, A.A.; Frye, C.A. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat. Protoc. 2007, 2, 322–328. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
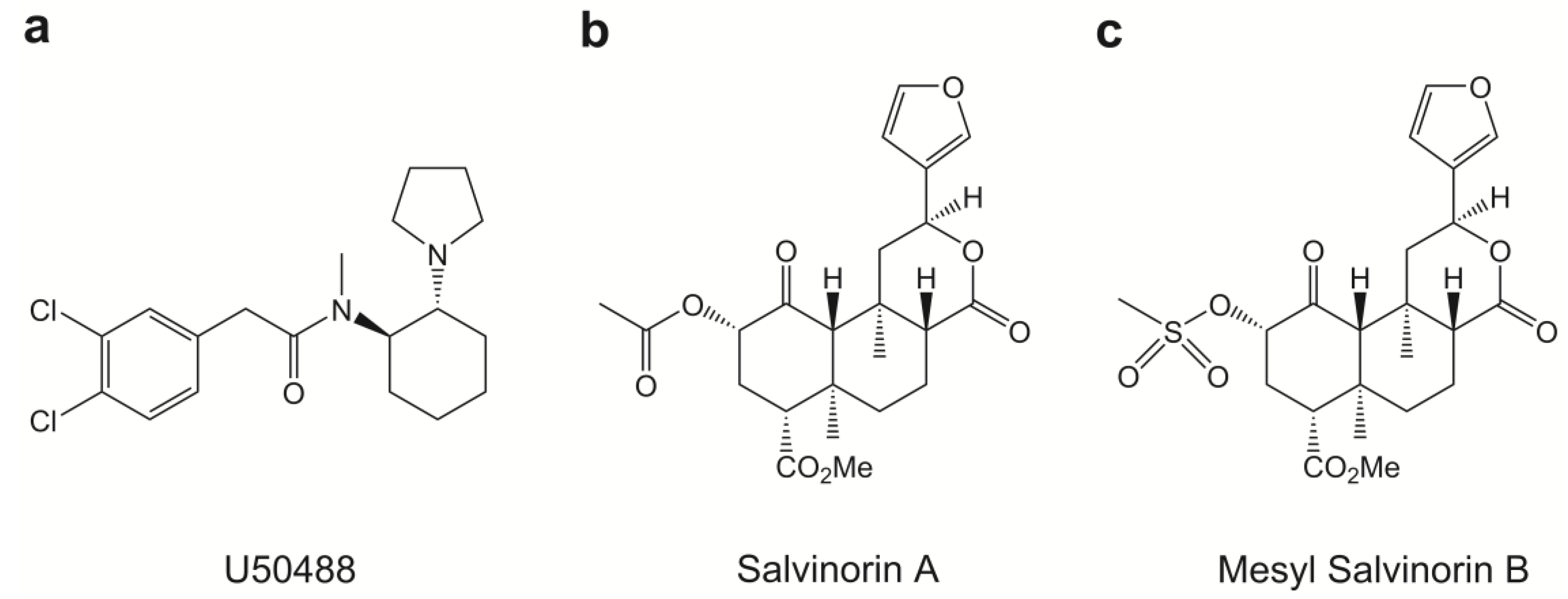


| Compound | Inhibition of Forskolin-Induced cAMP Accumulation (HitHunterTM) | β-Arrestin Recruitment (PathHunterTM) | |||||
|---|---|---|---|---|---|---|---|
| Potency (nM) | Potency (SEM) | Efficacy (%) | Potency (nM) | Potency (SEM) | Efficacy (%) | Bias Factor | |
| U50488 | 0.23 | 0.094 | 99.5 | 162.2 | 43.4 | 102.6 | - |
| Sal A | 0.03 | 0.013 | 98.7 | 248.5 | 66.5 | 94.9 | 0.35 |
| Mesyl Sal B | 0.12 | 0.049 | 101.2 | 236.0 | 44.3 | 90.2 | 0.61 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kivell, B.M.; Paton, K.F.; Kumar, N.; Morani, A.S.; Culverhouse, A.; Shepherd, A.; Welsh, S.A.; Biggerstaff, A.; Crowley, R.S.; Prisinzano, T.E. Kappa Opioid Receptor Agonist Mesyl Sal B Attenuates Behavioral Sensitization to Cocaine with Fewer Aversive Side-Effects than Salvinorin A in Rodents. Molecules 2018, 23, 2602. https://doi.org/10.3390/molecules23102602
Kivell BM, Paton KF, Kumar N, Morani AS, Culverhouse A, Shepherd A, Welsh SA, Biggerstaff A, Crowley RS, Prisinzano TE. Kappa Opioid Receptor Agonist Mesyl Sal B Attenuates Behavioral Sensitization to Cocaine with Fewer Aversive Side-Effects than Salvinorin A in Rodents. Molecules. 2018; 23(10):2602. https://doi.org/10.3390/molecules23102602
Chicago/Turabian StyleKivell, Bronwyn M., Kelly F. Paton, Nitin Kumar, Aashish S. Morani, Aimee Culverhouse, Amy Shepherd, Susan A. Welsh, Andrew Biggerstaff, Rachel S. Crowley, and Thomas E. Prisinzano. 2018. "Kappa Opioid Receptor Agonist Mesyl Sal B Attenuates Behavioral Sensitization to Cocaine with Fewer Aversive Side-Effects than Salvinorin A in Rodents" Molecules 23, no. 10: 2602. https://doi.org/10.3390/molecules23102602
APA StyleKivell, B. M., Paton, K. F., Kumar, N., Morani, A. S., Culverhouse, A., Shepherd, A., Welsh, S. A., Biggerstaff, A., Crowley, R. S., & Prisinzano, T. E. (2018). Kappa Opioid Receptor Agonist Mesyl Sal B Attenuates Behavioral Sensitization to Cocaine with Fewer Aversive Side-Effects than Salvinorin A in Rodents. Molecules, 23(10), 2602. https://doi.org/10.3390/molecules23102602






