Fermentation: A Boon for Production of Bioactive Compounds by Processing of Food Industries Wastes (By-Products)
Abstract
1. Introduction
1.1. Bioactive Compounds
- Releasing from the food matrix
- Integration with bile-salt making micelles
- Assimilation and absorption by epithelial cells, and finally
- Incorporation into the chylomicrons with secretion into lymphatic system
Synthesis and Purpose in Plants
1.2. Types of Bioactive Compounds
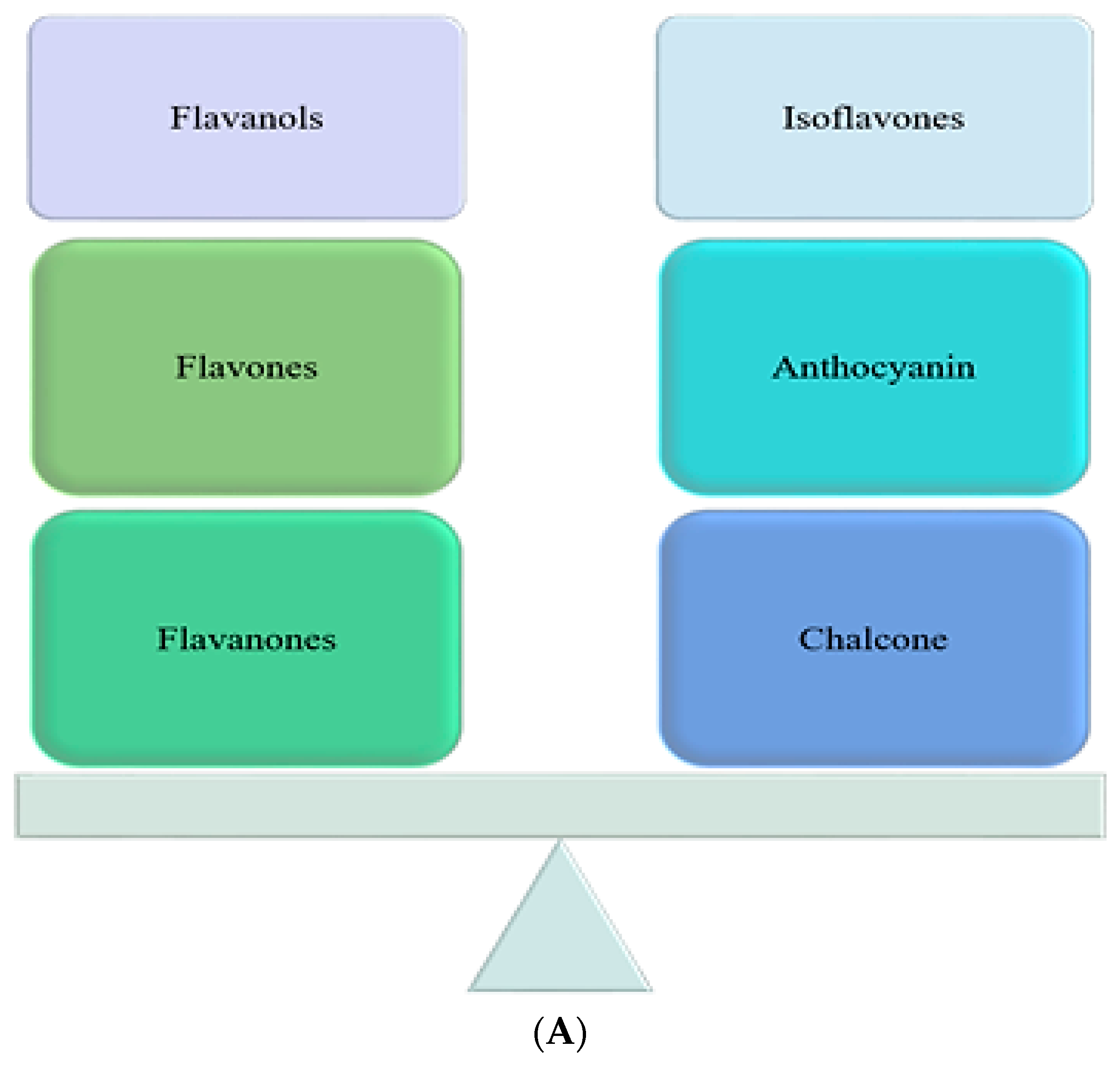
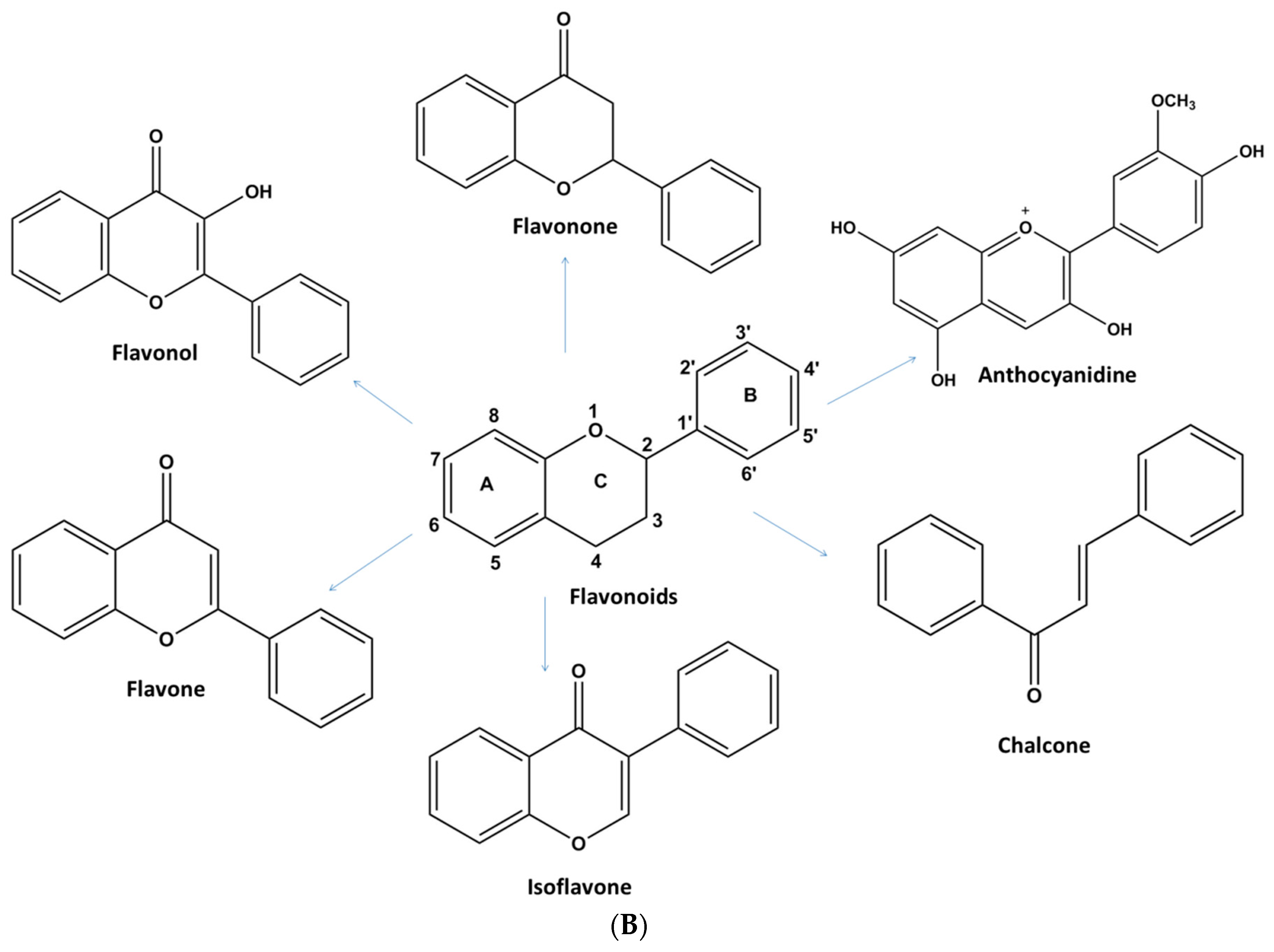
1.2.1. Flavonoids
Flavonols
Flavones
Flavonones
Isoflavones
Anthocyanins
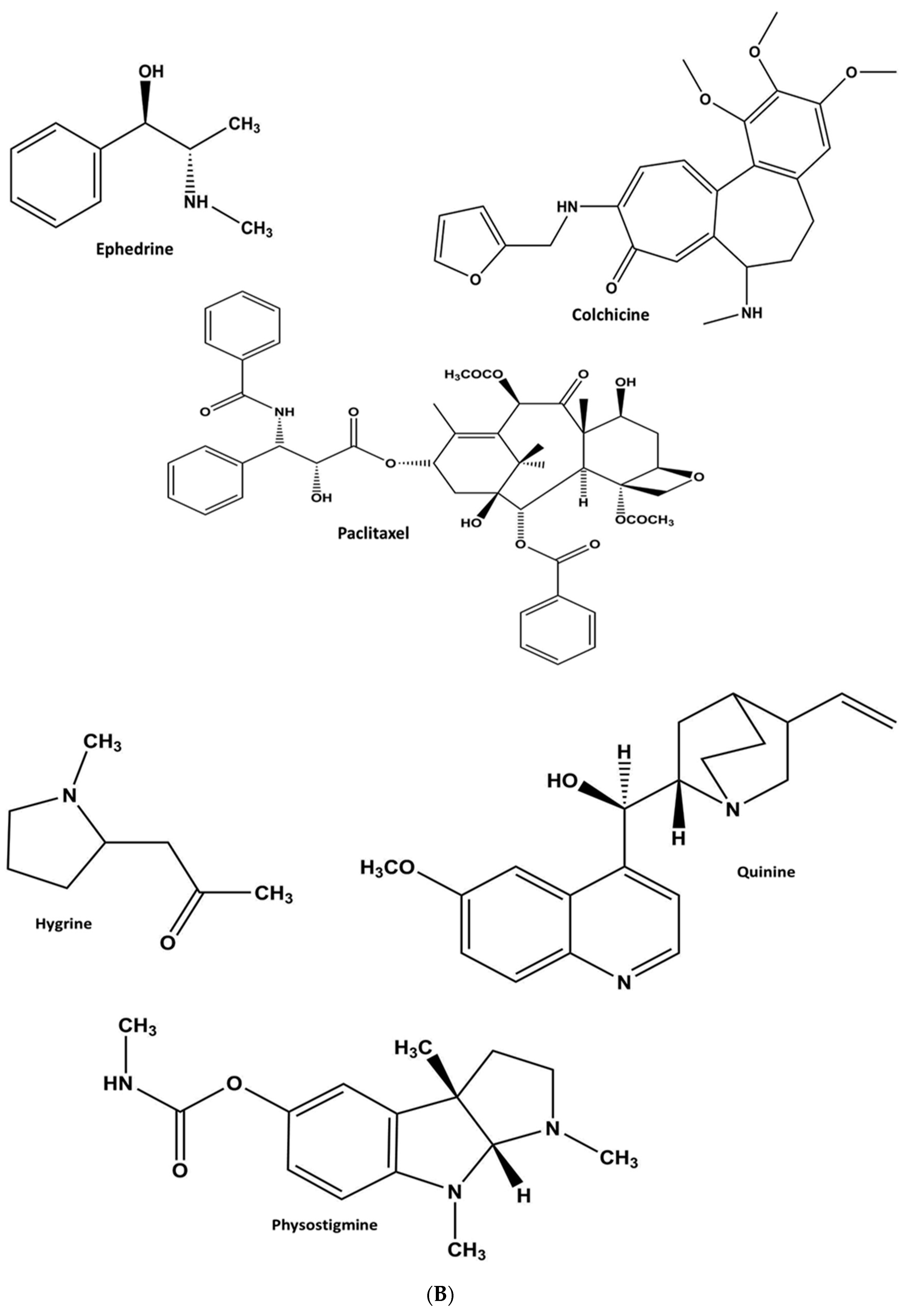
1.2.2. Alkaloids
Heterocyclic Alkaloids
Pyrrole
Quinoline
Indole or Benzopyrole
Non-Heterocyclic Alkaloids
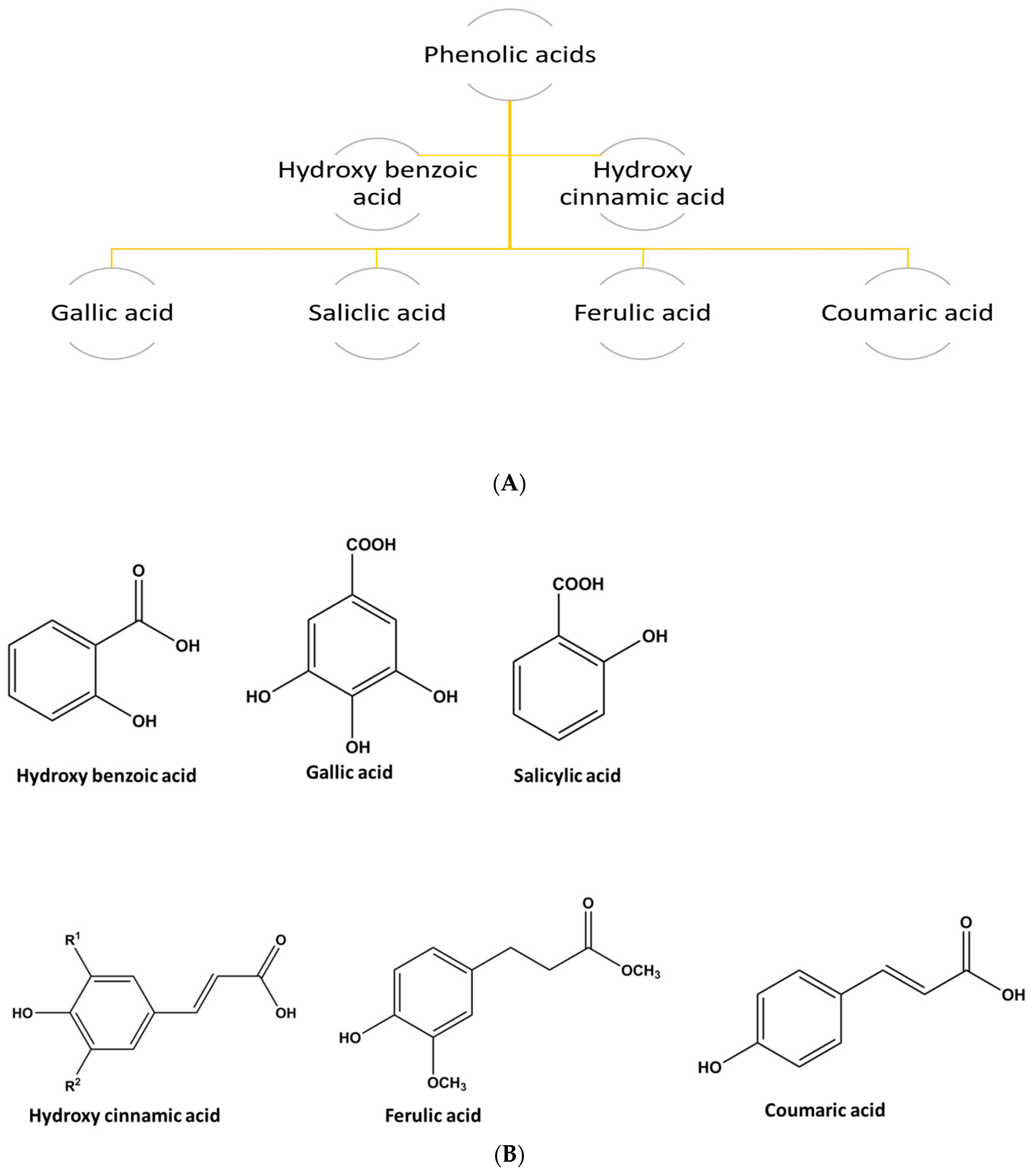
1.2.3. Phenolic Acids
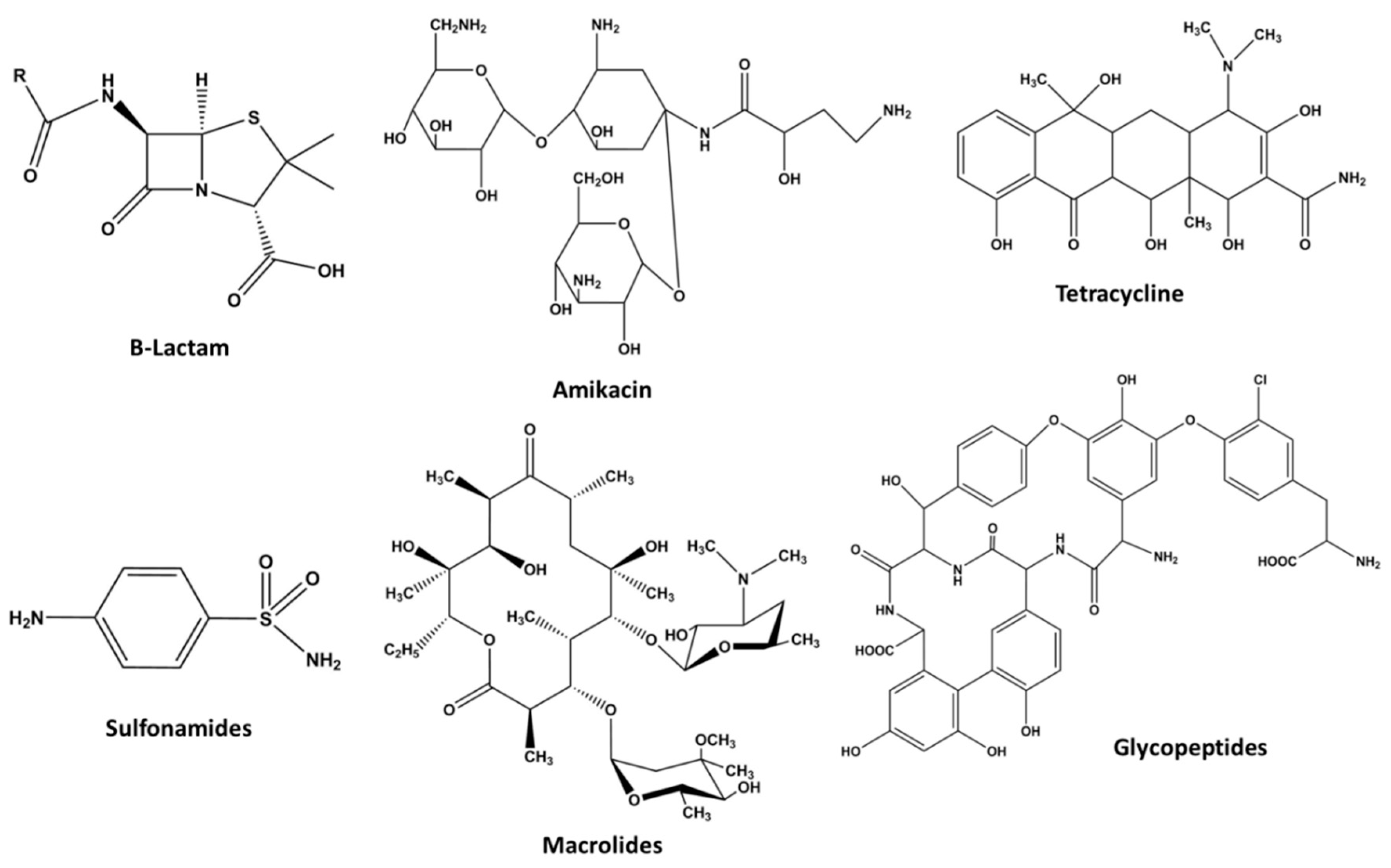
1.2.4. Antibiotics
β-Lactams
Aminoglycosides
Tetracycline
Sulfonamides
Macrolides
Glycopeptides
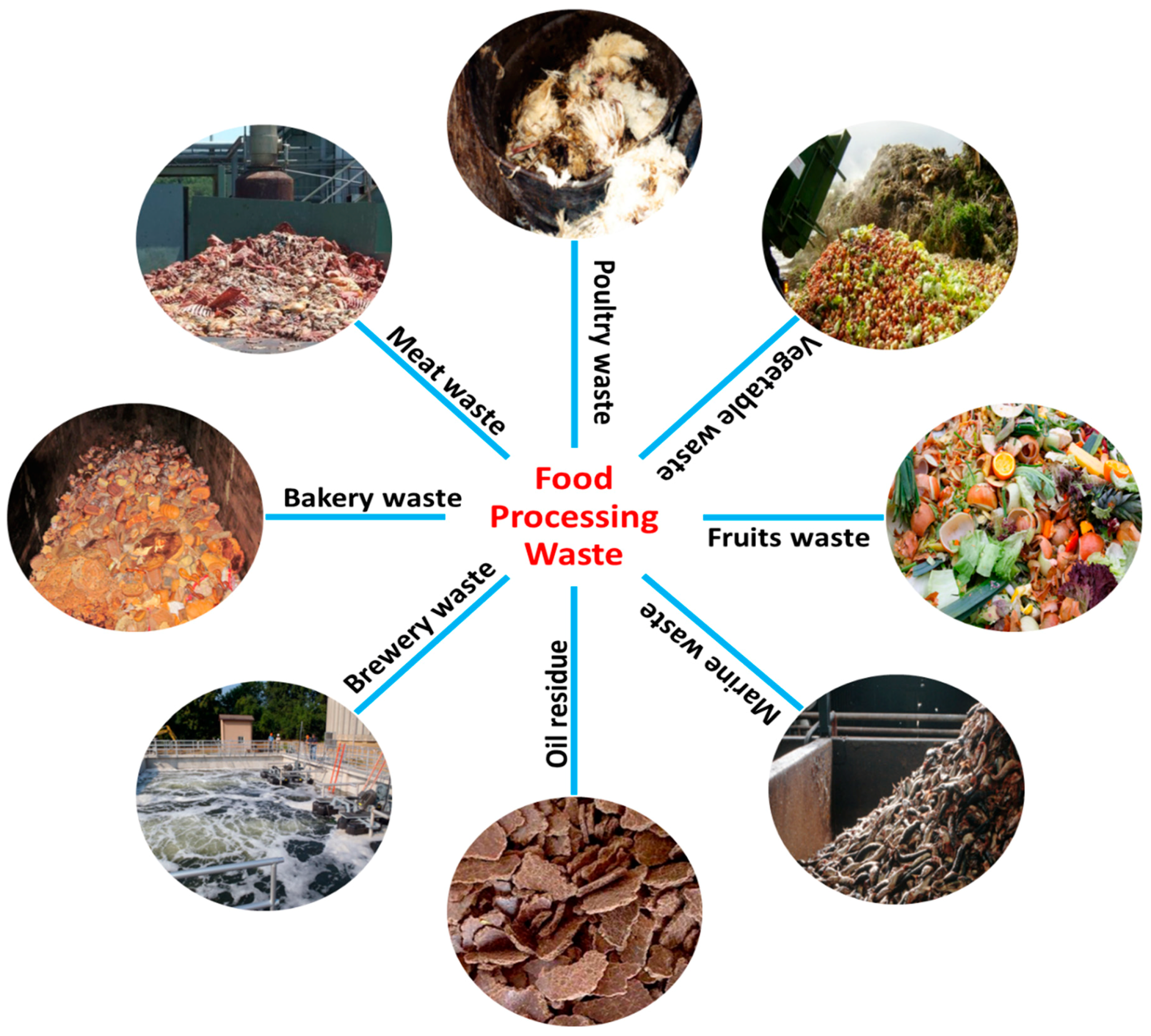
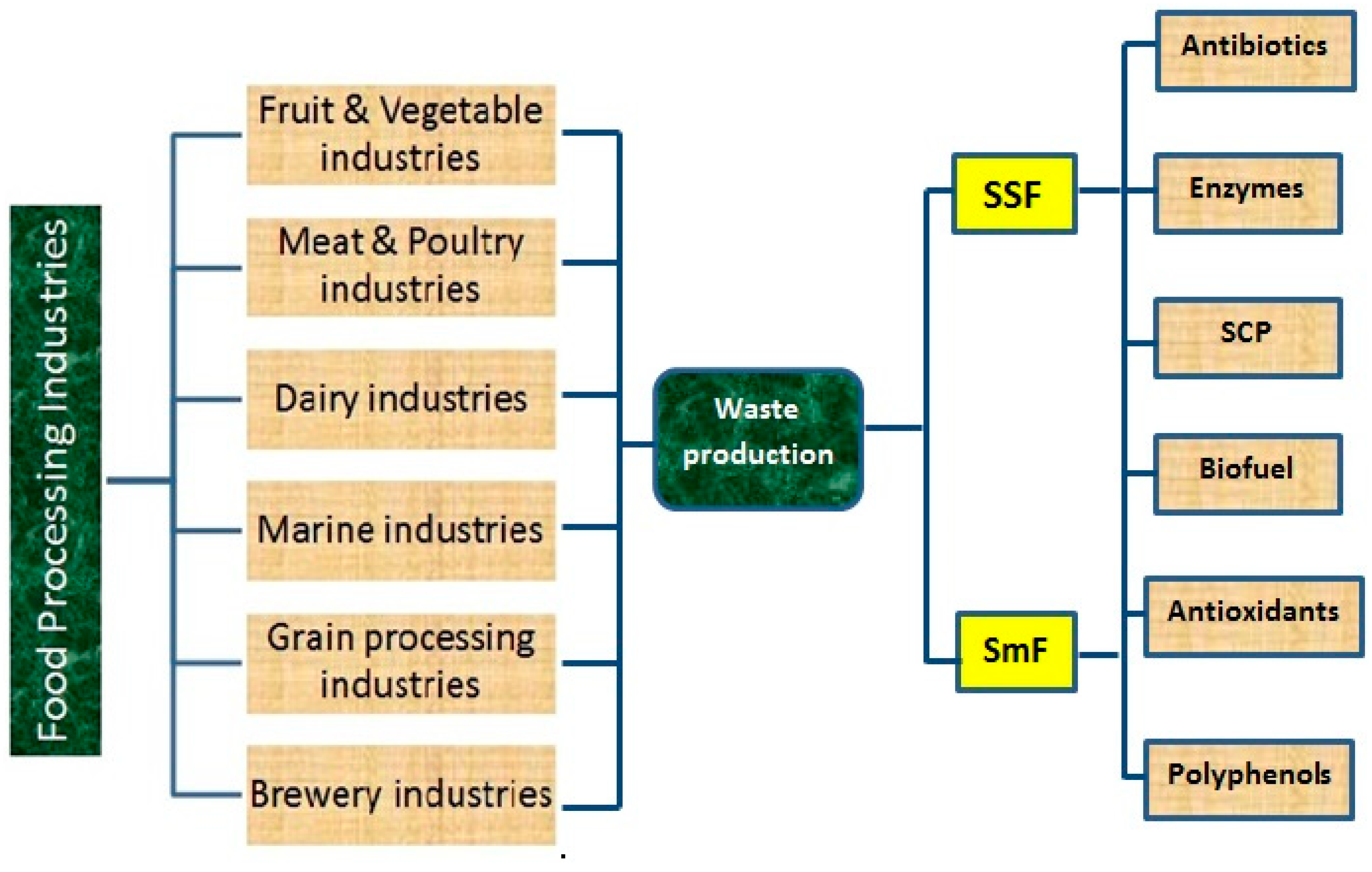
2. Food Processing Industries and Their By-Products
2.1. Fruit & Vegetable Processing Industries
2.2. Meat & Poultry Industries
2.3. Dairy Industries
2.4. Marine Industries
2.5. Grain Processing Industries
2.6. Brewery Industries
3. Fermentation Processes
3.1. Solid State Fermentation
3.2. Sub Merged/Liquid Fermentation
4. Uses of Fermentation for the Production of Bioactive/Value Added Compounds
5. Concluding Interpretations and Future Approaching
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Martins, S.; Mussatto, S.I.; Martínez-Avila, G.; Montañez-Saenz, J.; Aguilar, C.N.; Teixeira, J.A. Bioactive phenolic compounds: Production and extraction by solid-state fermentation. A review. Biotechnol. Adv. 2011, 29, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Carbonell-Capella, J.M.; Buniowska, M.; Barba, F.J.; Esteve, M.J.; Frígola, A. Analytical methods for determining bioavailability and bioaccessibility of bioactive compounds from fruits and vegetables: A review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 155–171. [Google Scholar] [CrossRef]
- Porrini, M.; Riso, P. Factors influencing the bioavailability of antioxidants in foods: A critical appraisal. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 647–650. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef] [PubMed]
- Carbonell-Capella, J.M.; Barba, F.J.; Esteve, M.J.; Frígola, A. High pressure processing of fruit juice mixture sweetened with Stevia rebaudiana Bertoni: Optimal retention of physical and nutritional quality. Innov. Food Sci. Emerg. Technol. 2013, 18, 48–56. [Google Scholar] [CrossRef]
- Joana Gil-Chávez, G.; Villa, J.A.; Fernando Ayala-Zavala, J.; Basilio Heredia, J.; Sepulveda, D.; Yahia, E.M.; González-Aguilar, G.A. Technologies for extraction and production of bioactive compounds to be used as nutraceuticals and food ingredients: An overview. Compr. Rev. Food Sci. Food Saf. 2013, 12, 5–23. [Google Scholar] [CrossRef]
- Parada, J.; Aguilera, J.M. Food microstructure affects the bioavailability of several nutrients. J. Food Sci. 2007, 72, R21–R32. [Google Scholar] [CrossRef] [PubMed]
- EUFIC (The European Food Information Council). 2010. Available online: https://www.eufic.org/en/food-safety/article/food-safety (accessed on 18 August 2018).
- McClements, D.J.; Xiao, H. Excipient foods: Designing food matrices that improve the oral bioavailability of pharmaceuticals and nutraceuticals. Food Funct. 2014, 5, 1320–1333. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, R.; Kulkarni, G.T.; Pawar, V.K. Phytosomes: An approach to increase the bioavailability of plant extracts. Int. J. Pharm. Pharm. Sci. 2011, 3, 1–3. [Google Scholar]
- De Souza, R.J.; Mente, A.; Maroleanu, A.; Cozma, A.I.; Ha, V.; Kishibe, T.; Anand, S.S. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: Systematic review and meta-analysis of observational studies. BMJ 2015, 351. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, J.P.; Serrano, J.; Tabernero, M.; Arranz, S.; Díaz-Rubio, M.E.; García-Diz, L.; Goñi, I.; Saura-Calixto, F. Effects of grape antioxidant dietary fiber in cardiovascular disease risk factors. Nutrition 2008, 24, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.N.; Shin, J.G.; Jang, H.D. Antioxidant and antidiabetic activity of Dangyuja (Citrus grandis Osbeck) extract treated with Aspergillus saitoi. Food Chem. 2009, 117, 35–41. [Google Scholar] [CrossRef]
- Ham, S.S.; Kim, S.H.; Moon, S.Y.; Chung, M.J.; Cui, C.B.; Han, E.K.; Chung, C.K.; Choe, M. Antimutagenic effects of subfractions of Chaga mushroom (Inonotus obliquus) extract. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2009, 672, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Parvathy, K.S.; Negi, P.S.; Srinivas, P. Antioxidant, antimutagenic and antibacterial activities of curcumin-β-diglucoside. Food Chem. 2009, 115, 265–271. [Google Scholar] [CrossRef]
- Duhan, J.S.; Bhardwaj, M.; Surekha, S. Free radical-scavenging and antimutagenic potential of acetone, chloroform and methanol extracts of leaves of Argemone maxicana. Int. J. Pharma. Biosci. 2011, 2, B455–B464. [Google Scholar]
- Duhan, J.S.; Bhardwaj, M.; Surekha, S. Free radical-scavenging and antimutagenic potential of acetone, chloroform and methanol extracts of fruit of Argemone maxicana. Afr. J. Biotechnol. 2011, 10, 8654–8661. [Google Scholar]
- Salar, R.K.; Seasotia, L.; Rohilla, S.K. Evaluation of antioxidant and radical scavenging property of Ficus bengalensis L. applying various spectroscopic and spin trapping methods. J. Biol. Act. Prod. Nat. 2011, 1, 248–261. [Google Scholar]
- Correia, A.L.; Bissell, M.J. The tumor microenvironment is a dominant force in multidrug resistance. Drug Resist. Updates 2012, 15, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Foo, L.Y. Identification and quantification of major polyphenols in apple pomace. Food Chem. 1997, 59, 187–194. [Google Scholar] [CrossRef]
- Foo, L.Y.; Lu, Y. Isolation and identification of procyanidins in apple pomace. Food Chem. 1999, 64, 511–518. [Google Scholar]
- Wolfe, K.; Wu, X.; Liu, R.H. Antioxidant activity of apple peels. J. Agric. Food Chem. 2003, 51, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Feng, X.; Yang, D.; Yi, C.; Qiu, X. Pi-pi stacking of the aromatic groups in lignosulfonates. Bioresources 2012, 7, 1145–1156. [Google Scholar]
- Kanazawa, K.; Sakakibara, H. High content of dopamine, a strong antioxidant, in cavendish banana. J. Agric. Food Chem. 2000, 48, 844–848. [Google Scholar] [CrossRef] [PubMed]
- Someya, S.; Yoshiki, Y.; Okubo, K. Antioxidant compounds from bananas (Musa cavendish). Food Chem. 2002, 79, 351–354. [Google Scholar] [CrossRef]
- González-Montelongo, R.; Lobo, M.G.; González, M. Antioxidant activity in banana peel extracts: Testing extraction conditions and related bioactive compounds. Food Chem. 2010, 119, 1030–1039. [Google Scholar] [CrossRef]
- Durga, M.; Nathiya, S.; Devasena, T. Multifarious actions of dietary flavonoids–implications in cancer and cataract. Int. J. Pharm. Biol. Sci. 2014, 5, 404–416. [Google Scholar]
- Coll, M.D.; Coll, L.; Laencina, J.; Tomas-Barberan, F.A. Recovery of flavonons from wastes of industrially processed lemons. Z. Lebensm. Unters. Forsch. A 1998, 206, 404–407. [Google Scholar] [CrossRef]
- Shrikhande, A.J. Wine by-products with health benefits. Food Res. Int. 2000, 33, 469–474. [Google Scholar] [CrossRef]
- Negro, C.; Tommasi, L.; Miceli, A. Phenolic compounds and antioxidant activity from red grape marc extracts. Bioresour. Technol. 2003, 87, 41–44. [Google Scholar] [CrossRef]
- Maier, T.; Schieber, A.; Kammerer, D.R.; Carle, R. Residues of grape (Vitis vinifera L.) seed oil production as a valuable source of phenolic antioxidants. Food Chem. 2009, 112, 551–559. [Google Scholar] [CrossRef]
- Lee, H.S.; Wicker, L. Anthocyanin pigments in the skin of lychee fruit. J. Food Sci. 1991, 56, 466–468. [Google Scholar] [CrossRef]
- Duan, X.; Jiang, Y.; Su, X.; Zhang, Z.; Shi, J. Antioxidant properties of anthocyanins extracted from litchi (Litchi chinenesis Sonn.) fruit pericarp tissues in relation to their role in the pericarp browning. Food Chem. 2007, 101, 1365–1371. [Google Scholar] [CrossRef]
- Arogba, S.S. Mango (Mangifera indica) kernel: Chromatographic analysis of the tannin, and stability study of the associated polyphenol oxidase activity. J. Food Comp. Anal. 2000, 13, 149–156. [Google Scholar] [CrossRef]
- Puravankara, D.; Boghra, V.; Sharma, R.S. Effect of antioxidant principles isolated from mango (Mangifera indica L.) seed kernels on oxidative stability of buffalo ghee (butter-fat). J. Sci. Food Agric. 2000, 80, 522–526. [Google Scholar] [CrossRef]
- Choo, Y.M.; Yap, S.C.; Ooi, C.K.; Ma, A.N.; Goh, S.H.; Ong, A.S.H. Recovered oil from palm-pressed fiber: A good source of natural carotenoids, vitamin E, and sterols. J. Am. Oil Chem. Soc. 1996, 73, 599–602. [Google Scholar] [CrossRef]
- Tan, I.A.W.; Ahmad, A.L.; Hameed, B.H. Adsorption of basic dye using activated carbon prepared from oil palm shell: Batch and fixed bed studies. Desalination 2008, 225, 13–28. [Google Scholar] [CrossRef]
- Gil, M.I.; Tomás-Barberán, F.A.; Hess-Pierce, B.; Holcroft, D.M.; Kader, A.A. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J. Agric. Food Chem. 2000, 48, 4581–4589. [Google Scholar] [CrossRef] [PubMed]
- Noda, Y.; Kaneyuki, T.; Mori, A.; Packer, L. Antioxidant activities of pomegranate fruit extract and its anthocyanidins: Delphinidin, cyanidin, and pelargonidin. J. Agric. Food Chem. 2002, 50, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Chantaro, P.; Devahastin, S.; Chiewchan, N. Production of antioxidant high dietary fiber powder from carrot peels. LWT Food Sci. Technol. 2008, 41, 1987–1994. [Google Scholar] [CrossRef]
- Zeyada, N.N.; Zeitoum, M.A.M.; Barbary, O.M. Utilization of some vegetables and fruit waste as natural antioxidants. Alex J. Food Sci. Technol. 2008, 5, 1–11. [Google Scholar]
- Marti, R.; Rosello, S.; Cebolla-Cornejo, J. Tomato as a source of carotenoids and polyphenols targeted to cancer prevention. Cancers 2016, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, J.M.; Croft, K.D. Dietary flavonoids: Effects on endothelial function and blood pressure. J. Sci. Food Agric. 2006, 86, 2492–2498. [Google Scholar] [CrossRef]
- Sainvitu, P.; Nott, K.; Richard, G.; Blecker, C.; Jérôme, C.; Wathelet, J.P.; Deleu, M. Structure, properties and obtention routes of flaxseed lignan secoisolariciresinol, a review. Biotechnol. Agron. Soc. Environ. 2012, 16, 115–124. [Google Scholar]
- Perretti, G.; Miniati, E.; Montanari, L.; Fantozzi, P. Improving the value of rice by-products by SFE. J. Supercrit. Fluid. 2003, 26, 63–71. [Google Scholar] [CrossRef]
- Oliveira, M.S.; Feddern, V.; Kupski, L.; Cipolatti, E.P.; Badiale-Furlong, E.; Souza-Soares, L.A. Changes in lipid, fatty acids and phospholipids composition of whole rice bran after solid-state fungal fermentation. Bioresour. Technol. 2011, 102, 8335–8338. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, B.; Cao, Y.; Tian, Y.; Li, X. Optimisation of ultrasound-assisted extraction of phenolic compounds from wheat bran. Food Chem. 2008, 106, 804–810. [Google Scholar] [CrossRef]
- Rodriguez-Mateos, A.; Vauzour, D.; Krueger, C.G.; Shanmuganayagam, D.; Reed, J.; Calani, L.; Daniele Del, R.; Crozier, A. Bioavailability, bioactivity and impact on health of dietary flavonoids and related compounds: An update. Arch. Toxicol. 2014, 88, 1803–1853. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mateos, A.; Pino-García, R.D.; George, T.W.; Vidal-Diez, A.; Heiss, C.; Spencer, J.P. Impact of processing on the bioavailability and vascular effects of blueberry (poly) phenols. Mol. Nut. Food Res. 2014, 58, 1952–1961. [Google Scholar] [CrossRef] [PubMed]
- Baxter, H.; Harborne, J.B.; Moss, G.P. Phytochemical Dictionary: A Handbook of Bioactive Compounds from Plants; CRC Press: Boca Raton, FL, USA, 1998; p. 976. [Google Scholar]
- Dwivedi, S.; Malik, C.; Chhokar, V. Molecular Structure, Biological Functions, and Metabolic Regulation of Flavonoids. Plant Biotechnol. Recent Adv. Dev. 2017. [Google Scholar] [CrossRef]
- Bramati, L.; Aquilano, F.; Pietta, P. Unfermented rooibos tea: Quantitative characterization of flavonoids by HPLC−UV and determination of the total antioxidant activity. J. Agric. Food Chem. 2003, 51, 7472–7474. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zhao, M.; Sun-Waterhouse, D.; Zhuang, M.; Chen, H.; Feng, M.; Lin, L. Absorption and desorption behaviour of the flavonoids from Glycyrrhiza glabra L. leaf on macroporous adsorption resins. Food Chem. 2015, 168, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Nidhi, S.; Gahlawat, S.K.; Lather, V. Flavonoids: A nutraceutical and its role as anti-inflammatory and anticancer agent. Plant Biotechnol. Recent Adv. Dev. 2017. [Google Scholar] [CrossRef]
- Paredes, A.; Alzuru, M.; Mendez, J.; Rodríguez-Ortega, M. Anti-Sindbis activity of flavanones hesperetin and naringenin. Biol. Pharm. Bull. 2003, 26, 108–109. [Google Scholar] [CrossRef] [PubMed]
- Duhan, J.S.; Rana, A.; Sadh, P.K.; Saharan, P. Antimicrobial and free radical scavenging activity of selective medicinal plants combination. World J. Pharm. Pharmaceut. Sci. 2015, 4, 1202–1216. [Google Scholar]
- Duhan, J.S.; Bhardwaj, M.; Sadh, P.K.; Surekha. In vitro antimicrobial efficacy, free radical scavenging activity and antimutagenic potential of stem extract of Capparis decudua. World J. Pharm. Pharm. Sci. 2016, 5, 786–803. [Google Scholar]
- Tripoli, E.; La Guardia, M.; Giammanco, S.; Di Majo, D.; Giammanco, M. Citrus flavonoids: Molecular structure, biological activity and nutritional properties: A review. Food Chem. 2007, 104, 466–479. [Google Scholar] [CrossRef]
- Kishida, T.; Nashiki, K.; Izumi, T.; Ebihara, K. Soy isoflavonoid aglycons genistein and daidzein do not increase the cytochrome P-450 content of the liver microsomes of mice. J. Agric. Food Chem. 2000, 48, 3872–3875. [Google Scholar] [CrossRef] [PubMed]
- Cvejic, L.; Harding, R.; Churchward, T.; Turton, A.; Finlay, P.; Massey, D.; Guy, P. Laryngeal penetration and aspiration in individuals with stable COPD. Respirology 2011, 16, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Barnes, S. The biochemistry, chemistry and physiology of the isoflavones in soybeans and their food products. Lymphat. Res. Biol. 2010, 8, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Huang, Y.; Tang, Y. Genetic and metabolic engineering of isoflavonoid biosynthesis. Appl. Microbiol. Biotechnol. 2010, 86, 1293–1312. [Google Scholar] [CrossRef] [PubMed]
- Weng, C.J.; Yen, G.C. Flavonoids, a ubiquitous dietary phenolic subclass, exert extensive in vitro anti-invasive and in vivo anti-metastatic activities. Cancer Metast Rev. 2012, 31, 323–351. [Google Scholar] [CrossRef] [PubMed]
- Runge, F.F. Ueber einige produkte der steinko hlen destillation. Ann. Phys. 1834, 107, 65–78. [Google Scholar] [CrossRef]
- Bhardwaj, V.; Gumber, D.; Abbot, V.; Dhiman, S.; Sharma, P. Pyrrole: A resourceful small molecule in key medicinal hetero-aromatics. RSC Adv. 2015, 5, 15233–15266. [Google Scholar] [CrossRef]
- Wilkerson, W.W.; Copeland, R.A.; Covington, M.; Trzaskos, J.M. Antiinflammatory 4,5-Diarylpyrroles. 2. activity as a function of cyclooxygenase-2 inhibition. J. Med. Chem. 1995, 38, 3895–3901. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, J.; Lee, S.; Shin, Y.; Jung, W.; Kim, J.H.; Park, K.; Kim, K.; Cho, H.S.; Ro, S. A novel class of highly potent, selective, and non-peptidic inhibitor of Ras farnesyltransferase (FTase). Bioorg. Med. Chem. Lett. 2001, 11, 3069–3072. [Google Scholar] [CrossRef]
- Wurz, R.P.; Charette, A.B. Doubly activated cyclopropanes as synthetic precursors for the preparation of 4-nitro-and 4-cyano-dihydropyrroles and pyrroles. Org. Lett. 2005, 7, 2313–2316. [Google Scholar] [CrossRef] [PubMed]
- Baeyer, A. Uebere in condensations product von pyrrol mit aceton. Ber. Dtsch. Chem. Ges. 1886, 19, 2184–2185. [Google Scholar] [CrossRef]
- Horton, D.A.; Bourne, G.T.; Smythe, M.L. The combinatorial synthesis of bicyclic privileged structures or privileged substructures. Chem. Rev. 2003, 103, 893–930. [Google Scholar] [CrossRef] [PubMed]
- Joule, J.A.; Mills, K. Heterocyclic Chemistry, 4th ed.; For an Excellent Introduction to Chemistry and Reactivity of Indoles; Blackwell Science: Oxford, UK, 2000; ISBN 978-1-405-13300-5. [Google Scholar]
- Ekhlass, N. Synthesis (in vitro) antitumor and antimicrobial activity of some pyrazoline, pyridine, and pyrimidine derivatives linked to indole moiety. J. Am. Sci. 2010, 6, 463–471. [Google Scholar]
- Goleniowski, M.; Bonfill, M.; Cusido, R.; Palazón, J. Phenolic acids. Nat. Prod. 2013. [Google Scholar] [CrossRef]
- Giada, M.D.L.R. Food phenolic compounds: Main classes, sources and their antioxidant power. Oxid. Stress Chron. Degener. Dis. Role Antioxid. 2013. [Google Scholar] [CrossRef]
- Korzybski, T.; Kowszyk-Gindifer, Z.; Kurylowicz, W. Antibiotics: Origin, Nature and Properties; PWN-Polish Scientific Publishers: Warsaw, Poland, 2013. [Google Scholar]
- Dezfully, N.K.; Heidari, A. Natural bioactive compounds: Antibiotics. J. Fundam. Appl. Sci. 2016, 8, 674–684. [Google Scholar] [CrossRef]
- Hogg, S. Essential Microbiology; John Wiley & Sons: Manhattan, NY, USA, 2005; p. 481. [Google Scholar]
- Khan, F.A. Biotechnology in Medical Sciences; CRC Press: New York, NY, USA, 2014; p. 471. ISBN 978-1-4822-2368-2. [Google Scholar]
- Kuriyama, T.; Karasawa, T.; Williams, D.W. Antimicrobial Chemotherapy: Significance to Healthcare. In Biofilms in Infection Prevention and Control; Academic Press: Cambridge, MA, USA, 2014; pp. 209–244. [Google Scholar]
- Holten, K.B.; Onusko, E.M. Appropriate prescribing of oral beta-lactam antibiotics. Am. Fam. Phys. 2000, 62, 611–620. [Google Scholar]
- Petri, W.A. Penicillin, cephalosporins, and other β-lactam antibiotics. Side Effects Drugs Annu. 2006, 18, 258–267. [Google Scholar]
- Yao, J.D.C.; Moellering, R.C. Antibacterial agents. In Manual of Clinical Microbiology; Versalovic, J., Carroll, K.C., Funke, G., Jorgensen, J.H., Landry, M.L., Warnock, D.W., Eds.; ASM Press: Washington, DC, USA, 2011; pp. 1043–1081. [Google Scholar]
- Elander, R.P. Industrial production of beta-lactam antibiotics. Appl. Microbiol. Biotechnol. 2003, 61, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Kimball, R.N. Glossary of Biotechnology Terms; CRC Press: New York, NY, USA, 2002; p. 289. [Google Scholar]
- Drawz, S.M.; Bonomo, R.A. Three Decades of β-Lactamase Inhibitors. Clin. Microbiol. Rev. 2010, 23, 160–201. [Google Scholar] [CrossRef] [PubMed]
- Livermore, D.M. β-lactamases in laboratory and clinical resistance. Clin. Microbiol. Rev. 1995, 8, 557–584. [Google Scholar] [PubMed]
- Jacoby, G.A. Amp C β-lactamase. Clin. Microbiol. Rev. 2009, 22, 161–182. [Google Scholar] [CrossRef] [PubMed]
- Page, C.P.; Curtis, M.; Sutter, M.C.; Walker, M.; Hoffman, B. Drug and bacteria. In Integrated Pharmacology, 2nd ed.; Mosby: St. Louis, MO, USA, 2002; pp. 111–143. [Google Scholar]
- Gilbert, D.N. Aminoglycosides. In Principles and Practice of Infectious Diseases; Mandell, G.L., Bennett, J.E., Dolin, R., Eds.; Churchill Livingstone: New York, NY, USA, 1995; pp. 279–306. [Google Scholar]
- Mingeot-Leclercq, M.P.; Glupczynski, Y.; Tulkens, P.M. Aminoglycosides: Activity and resistance. Antimicrob. Agents Chemother. 1999, 43, 727–737. [Google Scholar] [PubMed]
- Rang, H.P.; Dale, M.M.; Ritter, J.M.; Moore, P.K. Pharmacologia; Churchill Livingstone: London, UK, 2003; pp. 635–647. [Google Scholar]
- Witte, W. Antibiotic resistance in gram-positive bacteria: Epidemiological aspects. J. Antimicrob. Chemother. 1999, 44, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chambers, H.F. Aminoglycosides. In Goodman and Gilman’s the Pharmacological Basis of Therapeutics; Brunton, L.L., Lazo, J.S., Parker, K.L., Eds.; McGraw-Hill: New York, NY, USA, 2006; pp. 1155–1172. [Google Scholar]
- Murray, P.R.; Rosenthal, K.S.; Pfaller, M.A. Medical Microbiology; Mosby: Louis Missouri, ST, USA, 2009; pp. 199–208. [Google Scholar]
- Masters, P.A.; O’Bryan, T.A.; Zurlo, J.; Miller, D.Q.; Joshi, N. Trimethoprim-sulfamethoxazole revisited. Arch. Int. Med. 2003, 163, 402–410. [Google Scholar] [CrossRef]
- Gilbert, D.N.; Moellering, R.C.; Eliopoulos, G.M.; Chambers, H.F.; Saag, M.S. The Sanford Guide to Antimicrobial Therapy; Antimicrobial Therapy: Montgomery, OH, USA, 2007; p. 204. [Google Scholar]
- Hayden, M.K. Insights into the epidemiology and control of infection with vancomycin-resistant enterococci. Clin. Infect. Dis. 2000, 31, 1058–1065. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, B.; Hanson, C.; Lomax, J.; Kitinoja, L.; Waite, R.; Searchinger, T. Reducing food loss and waste. World Res. Inst. 2013, 22. Available online: http://www.ecdc.net.cn/2013gssd-unep/REduCING%20FOOd%20LOSS%20ANd%20WAStE.pdf (accessed on 18 August 2018).
- Kameswari, S.B.; Velmurugan, B.; Thirumaran, K.; Ramanujam, R.A. Biomethanation of Vegetable Market Waste-Untapped Carbon Trading Opportunities. In Proceedings of the International Conference on Sustainable Solid Waste Management, Chennai, India, 5–7 September 2007; pp. 415–420. [Google Scholar]
- Ravi Kiran, G.; Suresh, K.P.; Sampath, K.T.; Giridhar, K.; Anandan, S. Modeling and Forecasting Livestock and Fish Feed Resources: Requirements and Availability in India, National Institute of Animal Nutrition and Physiology. Ph.D. Thesis, National Institute of Animal Nutrition & Physiology, Bangalore, India, 2012. [Google Scholar]
- Chen, J. Aquatic feed industry under tension in world and China’s grain supply and demand. Chin. Fish 2012, 6, 32–34. [Google Scholar]
- Pogorzelska-Nowicka, E.; Atanasov, A.G.; Horbańczuk, J.; Wierzbicka, A. Bioactive Compounds in Functional Meat Products. Molecules 2018, 23, 307. [Google Scholar] [CrossRef] [PubMed]
- Byers, T.; Nestle, M.; McTiernan, A.; Doyle, C.; Currie-Williams, A.; Gansler, T.; Thun, M. American Cancer Society 2001 Nutrition and Physical Activity Guidelines Advisory Committee. American Cancer Society guidelines on nutrition and physical activity for cancer prevention: Reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J. Clin. 2002, 52, 92–119. [Google Scholar] [PubMed]
- Alao, B.O.; Falowo, A.B.; Chulayo, A.; Muchenje, V. The Potential of Animal By-Products in Food Systems: Production, Prospects and Challenges. Sustainability 2017, 9, 1089. [Google Scholar] [CrossRef]
- Jayathilakan, K.; Sultana, K.; Radhakrishna, K.; Bawa, A.S. Utilization of byproducts and waste materials from meat, poultry and fish processing industries: A review. J. Food Sci. Technol. 2012, 49, 278–293. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Ghosh, R.; Cui, Z. High-resolution plasma protein fractionation using ultrafiltration. Desalination 2002, 144, 301–306. [Google Scholar] [CrossRef]
- Ghosh, R. Fractionation of biological macromolecules using carrier phase ultrafiltration. Biotechnol. Bioeng. 2001, 74, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.D.; Silvestre, M.P. Functional properties of bovine blood plasma intended for use as a functional ingredient in human food. LWT Food Sci. Technol. 2003, 36, 709–718. [Google Scholar] [CrossRef]
- Bah, C.S.; Bekhit, A.E.D.A.; Carne, A.; McConnell, M.A. Slaughterhouse blood: An emerging source of bioactive compounds. Compr. Rev. Food Sci. Food Saf. 2013, 12, 314–331. [Google Scholar] [CrossRef]
- Raghunath, B.V.; Punnagaiarasi, A.; Rajarajan, G.; Irshad, A.; Elango, A. Impact of dairy effluent on environment—A review. In Integrated Waste Management in India; Springer: Cham, Switzerland, 2016; pp. 239–249. [Google Scholar]
- Brião, V.B.; Tavares, C.R. Effluent generation by the dairy industry: Preventive attitudes and opportunities. Braz. J. Chem. Eng. 2007, 24, 487–497. [Google Scholar] [CrossRef]
- Watkins, M.; Nash, D. Dairy factory wastewaters, their use on land and possible environmental impacts—A mini review. Open Agric. J. 2010, 4, 1–9. [Google Scholar] [CrossRef]
- Qin, G.; Liu, C.C.; Richman, N.H.; Moncur, J.E. Aquaculture wastewater treatment and reuse by wind-driven reverse osmosis membrane technology: A pilot study on Coconut Island, Hawaii. Aquac. Eng. 2005, 32, 365–378. [Google Scholar] [CrossRef]
- Nurdiyana, H.; Mazlina, M.S. Optimization of protein extraction from fish waste using response surface methodology. J. Appl. Sci. 2009, 9, 3121–3125. [Google Scholar]
- Michail, M.; Vasiliadou, M.; Zotos, A. Partial purification and comparison of precipitation techniques of proteolytic enzymes from trout (Salmo gairdnerii) heads. Food Chem. 2006, 97, 50–55. [Google Scholar] [CrossRef]
- Helkar, P.B.; Sahoo, A.K.; Patil, N.J. Review: Food industry by-products used as a functional food ingredients. Int. J. Waste Res. 2016, 6, 1–6. [Google Scholar]
- Sadh, P.K.; Chawla, P.; Duhan, J.S. Fermentation approach on phenolic, antioxidants and functional properties of peanut press cake. Food Biosci. 2018, 22, 113–120. [Google Scholar] [CrossRef]
- Kliopova, I.; Staniškis, J.K.; Petraškienė, V. Solid recovered fuel production from biodegradable waste in grain processing industry. Waste Manag. Res. 2013, 31, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, B.K.; Gowen, A.; McKenna, B. (Eds.) Processing, quality and nutraceutical applications. In Pulse Foods; Academic Press: San Diego, CA, USA, 2011; ISBN 978-0-12-382018-1. [Google Scholar]
- Olajire, A.A. The brewing industry and environmental challenges. J. Clean. Prod. 2012. [Google Scholar] [CrossRef]
- Mathias, T.R.; De Mello, P.P.M.; Servulo, E.F.C. Solid wastes in brewing process: A review. J. Brew. Distill. 2014, 5, 1–9. [Google Scholar]
- Thomas, K.R.; Rahman, P.K.S.M. Brewery wastes. Strategies for sustainability. A review. Asp. Appl. Biol. 2006, 80, 147–153. [Google Scholar]
- Subramaniyam, R.; Vimala, R. Solid state and submerged fermentation for the production of bioactive substances: A comparative study. Int. J. Sci. Nat. 2012, 3, 480–486. [Google Scholar]
- Bhargav, S.; Panda, B.P.; Ali, M.; Javed, S. Solid-state fermentation: An overview. Chem. Biochem. Eng. Q. 2008, 22, 49–70. [Google Scholar]
- Pérez-Guerra, N.; Torrado-Agrasar, A.; López-Macias, C.; Pastrana, L. Main characteristics and applications of solid substrate fermentation. Electron. J. Environ. Agric. Food Chem. 2003, 2, 343–350. [Google Scholar]
- Singhania, R.R.; Sukumaran, R.K.; Patel, A.K.; Larroche, C.; Pandey, A. Advancement and comparative profiles in the production technologies using solid-state and submerged fermentation for microbial cellulases. Enzyme Microb. Technol. 2010, 46, 541–549. [Google Scholar] [CrossRef]
- Maulini-Duran, C.; Abraham, J.; Rodríguez-Pérez, S.; Cerda, A.; Jiménez-Peñalver, P.; Gea, T. Gaseous emissions during the solid state fermentation of different wastes for enzyme production at pilot scale. Bioresour. Technol. 2015, 179, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Madhumithah, C.G.; Krithiga, R.; Sundaram, S.; Sasikumar, C.S.; Guhathakurta, S.; Cherian, K.M. Utilization of vegetable wastes for production of protease by solid state fermentation using Aspergillus niger. World J. Agric. Sci. 2011, 7, 550–555. [Google Scholar]
- Dhanasekaran, D.; Lawanya, S.; Saha, S.; Thajuddin, N.; Panneerselvam, A. Production of single cell protein from pineapple waste using yeast. Innov. Rom. Food Biotechnol. 2011, 8, 26–32. [Google Scholar]
- Jamal, P.; Akbar, I.; Yumi, Z.; Irwandi, J. Process development for maximum lycopene production from selected fruit waste and its antioxidant and antiradical activity. J. Food Process. Technol. 2016, 7, 576–581. [Google Scholar] [CrossRef]
- Schmidt, C.G.; Gonçalves, L.M.; Prietto, L.; Hackbart, H.S.; Furlong, E.B. Antioxidant activity and enzyme inhibition of phenolic acids from fermented rice bran with fungus Rizhopus oryzae. Food Chem. 2014, 146, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Knob, A.; Fortkamp, D.; Prolo, T.; Izidoro, S.C.; Almeida, J.M. Agro-residues as Alternative for Xylanase Production by Filamentous Fungi. Biol. Res. 2014, 9, 5338–53773. [Google Scholar]
- Vidyalakshmi, R.; Paranthaman, R.; Indhumathi, J. Amylase production on submerged fermentation by Bacillus spp. World J. Chem. 2009, 4, 89–91. [Google Scholar]
- Favela-Torres, E.; Volke-Sepúlveda, T.; Viniegra-González, G. Hydrolytic Depolymerising Pectinases. Food Technol. Biotechnol. 2006, 44, 221–227. [Google Scholar]
- Beg, Q.K.; Bhushan, B.; Kapoor, M.; Hoondal, G.S. Enhanced production of a thermostable xylanase from Streptomyces sp. QG-11-3 and its application in biobleaching of eucalyptus kraft pulp. Enzyme. Microb. Technol. 2000, 27, 459–466. [Google Scholar] [CrossRef]
- Debing, J.; Peijun, L.; Stagnitti, F.; Xianzhe, X.; Li, L. Pectinase production by solid fermentation from Aspergillus niger by a new prescription experiment. Ecotoxicol. Environ. Saf. 2006, 64, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Biz, A.; Finkler, A.T.J.; Pitol, L.O.; Medina, B.S.; Krieger, N.; Mitchell, D.A. Production of pectinases by solid-state fermentation of a mixture of citrus waste and sugarcane bagasse in a pilot-scale packed-bed bioreactor. Biochem. Eng. J. 2016, 111, 54–62. [Google Scholar] [CrossRef]
- Buyukkileci, A.O.; Lahore, M.F.; Tari, C. Utilization of orange peel, a food industrial waste, in the production of exo-polygalacturonase by pellet forming Aspergillus sojae. Bioprocess Biosyst. Eng. 2015, 38, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Nema, N.; Alamir, L.; Mohammad, M. Production of cellulase from Bacillus cereus by submerged fermentation using corn husks as substrates. Int. Food Res. J. 2015, 22, 1831–1836. [Google Scholar]
- Acourene, S.; Amourache, L.; Benchabane, A.; Djaafri, K. Utilisation of date wastes as substrate for the production of α-amylase. Int. Food Res. J. 2013, 20, 1367–1372. [Google Scholar]
- Budihal, S.R.; Agsar, D. Exploration of Agrowastes for the Production of Cellulase by Streptomyces DSK29 under Submerged and Solid State Systems. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 681–689. [Google Scholar]
- Yang, S.S.; Jang, H.D.; Liew, C.M.; Du Preez, J.C. Protein enrichment of sweet potato residue by solid-state cultivation with mono-and co-cultures of amylolytic fungi. World J. Microbiol. Biotechnol. 1993, 9, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Khan, S.S.; Ahmed, Z.; Tanveer, A. Production of single cell protein from Saccharomyces cerevisiae by utilizing fruit wastes. Nanobiotechnol. Univ. 2010, 1, 127–132. [Google Scholar]
- Mensah, J.K.; Twumasi, P. Use of pineapple waste for single cell protein (SCP) production and the effect of substrate concentration on the yield. J. Food Process Eng. 2017, 40, e12478. [Google Scholar] [CrossRef]
- Gaur, S.; Mathur, N.; Singh, A.; Bhatnagar, P. Characterization of dairy waste and its utilization as substrate for the production of single cell protein. J. Biotechnol. Biochem. 2017, 3, 73–78. [Google Scholar]
- Hossain, A.B.; Fazliny, A.R. Creation of alternative energy by bio-ethanol production from pineapple waste and the usage of its properties for engine. Afr. J. Microbiol. Res. 2010, 4, 813–819. [Google Scholar]
- Dhabekar, A.; Chandak, A. Utilization of banana peels and beet waste for alcohol production. Asiatic J. Biotech. Res. 2010, 1, 8–13. [Google Scholar]
- Swain, M.R.; Ray, R.C. Optimization of cultural conditions and their statistical interpretation for production of indole-3-acetic acid by Bacillus subtilis CM5 using cassava fibrous residue. J. Sci. Ind. Res. 2008, 67, 622–628. [Google Scholar]
- Chutmanop, J.; Chuichulcherm, S.; Chisti, Y.; Srinophakun, P. Protease production by Aspergillus oryzae in solid-state fermentation using agroindustrial substrates. J. Chem. Technol. Biotechnol. 2008, 83, 1012–1018. [Google Scholar] [CrossRef]
- Mathias, T.R.D.S.; Fernandes de Aguiar, P.; de Almeida e Silva, J.B.; Moretzsohn de Mello, P.P.; Camporese Sérvulo, E.F. Brewery Waste Reuse for Protease Production by Lactic Acid Fermentation. Food Technol. Biotechnol. 2017, 55, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Maghsoodi, V.; Kazemi, A.; Nahid, P.; Yaghmaei, S.; Sabzevari, M.A. Alkaline protease production by immobilized cells using B. licheniformis. Sci. Iran. 2013, 20, 607–610. [Google Scholar]
- Castro, R.J.S.; Ohara, A.; Nishide, T.G.; Bagagli, M.P.; Dias, F.F.G.; Sato, H.H. A versatile system based on substrate formulation using agro industrial wastes for protease production by Aspergillus niger under solid state fermentation. Biocatal. Agric. Biotechnol. 2015, 4, 678–684. [Google Scholar]
- Hitha, C.S.; Hima, C.S.; Yogesh, B.J.; Bharathi, S.; Sekar, K.V. Microbial utilization of dairy waste for lactic acid production by immobilized bacterial isolates on sodium alginate beads. Int. J. Pure Appl. Biosci. 2014, 2, 55–60. [Google Scholar]
- Ranjit, C.; Srividya, S. Lactic acid production from free and Polyurethane immobilized cells of Rhizopus oryzae MTCC 8784 by direct hydrolysis of starch and agro-industrial waste. Int. Food Res. J. 2016, 23, 2646–2652. [Google Scholar]
- Németh, Á.R.O.N.; Kaleta, Z.O.L.T.Á.N. Complex utilization of dairy waste (whey) in Biorefinery. WSEAS Trans. Environ. Dev. 2015, 11, 80–88. [Google Scholar]
- Vidhyalakshmi, R.; Vallinachiyar, C.; Radhika, R. Production of Xanthan from Agro-Industrial Waste. J. Adv. Sci. Res. 2012, 3, 56–59. [Google Scholar]
- Yalemtesfa, B.; Alemu, T.; Santhanam, A. Solid substrate fermentation and conversion of orange waste in to fungal biomass using Aspergillus niger KA-06 and Chaetomium Spp KC-06. Afr. J. Microbiol. Res. 2010, 4, 1275–1281. [Google Scholar]
- Sousa, B.A.; Correia, R.T.P. Phenolic content, antioxidant activity and antiamylolytic activity of extracts obtained from bioprocessed pineapple and guava wastes. Braz. J. Chem. Eng. 2012, 29, 25–30. [Google Scholar] [CrossRef]
- Dulf, F.V.; Vodnar, D.C.; Socaciu, C. Effects of solid-state fermentation with two filamentous fungi on the total phenolic contents, flavonoids, antioxidant activities and lipid fractions of plum fruit (Prunus domestica L.) by-products. Food chem. 2016, 209, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Verotta, L.; Panzella, L.; Antenucci, S.; Calvenzani, V.; Tomay, F.; Petroni, K.; Caneva, E.; Napolitano, A. Fermented pomegranate wastes as sustainable source of ellagic acid: Antioxidant properties, anti-inflammatory action, and controlled release under simulated digestion conditions. Food Chem. 2018, 246, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Dulf, F.V.; Vodnar, D.C.; Dulf, E.H.; Pintea, A. Phenolic compounds, flavonoids, lipids and antioxidant potential of apricot (Prunus armeniaca L.) pomace fermented by two filamentous fungal strains in solid state system. Chem. Cent. J. 2017. [Google Scholar] [CrossRef] [PubMed]
- Ajila, C.M.; Brar, S.K.; Verma, M.; Tyagi, R.D.; Valéro, J.R. Solid-state fermentation of apple pomace using Phanerocheate chrysosporium–liberation and extraction of phenolic antioxidants. Food Chem. 2011, 126, 1071–1080. [Google Scholar] [CrossRef]
- Vastrad, B.M.; Neelagund, S.E. Optimization and production of neomycin from different agro industrial wastes in solid state fermentation. Int. J. Pharm. Sci. Drug Res. 2011, 3, 104–111. [Google Scholar]
- Okorie, P.C.; Asagbra, A.E. Production of oxytetracycline in solid state fermentation of groundnut shell. World J. Biotechnol. 2005, 4, 124–130. [Google Scholar]
- Okorie, P.; Asagbra, A. Oxytetracycline production by mix culture of Streptomyces rimosus and S. vendagensis in solid–state fermentation of cassava peels. J. Ind. Res. Technol. 2008, 2, 43–47. [Google Scholar]
- Yang, S.S.; Yuan, S.S. Oxytetracyeline production by Streptomyces rimosus in solid state fermentation of sweet potato residue. World J. Microbiol. Biotechnol. 1990, 6, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Ezejiofor, T.I.N.; Duru, C.I.; Asagbra, A.E.; Ezejiofor, A.N.; Orisakwe, O.E.; Afonne, J.O.; Obi, E. Waste to wealth: Production of oxytetracycline using Streptomyces species from household kitchen wastes of agricultural produce. Afr. J. Biotechnol. 2012, 11, 10115–10124. [Google Scholar]
- Vastrad, B.M.; Neelagund, S.E. Optimization of process parameters for rifamycin b production under solid state fermentation from Amycolatopsis mediterranean MTCC14. Int. J. Curr. Pharm. Res. 2012, 4, 101–108. [Google Scholar]
- El-Naggar, M.Y.; El-Assar, S.A.; Abdul-Gawad, S.M. Solid-state fermentation for the production of meroparamycin by Streptomyces sp. strain MAR01. J. Microbiol. Biotechnol. 2009, 19, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Radwan, H.H.; Alanazi, F.K.; Taha, E.I.; Dardir, H.A.; Moussa, I.M.; Alsarra, I.A. Development of a new medium containing date syrup for production of bleomycin by Streptomyces mobaraensis ATCC 15003 using response surface methodology. Afr. J. Biotechnol. 2010, 9, 5450–5459. [Google Scholar]
- Sukan, A.; Roy, I.; Keshavarz, T. Agro-industrial waste materials as substrates for the production of poly (3-hydroxybutyric acid). J. Biomater. Nanobiotechnol. 2014, 5, 229–240. [Google Scholar] [CrossRef]
- Sharma, A.; Gupta, V.; Khan, M.; Balda, S.; Gupta, N.; Capalash, N.; Sharma, P. Flavonoid-rich agro-industrial residues for enhanced bacterial laccase production by submerged and solid-state fermentation. 3 Biotech 2017, 7, 200. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, G.S.; Kaur, S.; Brar, S.K. In-vitro decolorisation of recalcitrant dyes through an ecofriendly approach using lac-case from Trametes versicolor grown on brewer’s spent grain. Int. Biodeterior. Biodegrad. 2012, 72, 67–75. [Google Scholar] [CrossRef]
- Klaic, R.; Sallet, D.; Foletto, E.L.; Jacques, R.J.; Guedes, J.V.; Kuhn, R.C.; Mazutti, M.A. Optimization of solid-state fermentation for bioherbicide production by Phoma sp. Braz. J. Chem. Eng. 2017, 34, 377–384. [Google Scholar] [CrossRef]
- Dhillon, G.S.; Rosine, G.M.L.; Kaur, S.; Hegde, K.; Brar, S.K.; Drogui, P.; Verma, M. Novel biomaterials from citric acid fermentation as biosorbents for removal of metals from waste chromated copper arsenate wood leachates. Int. Biodeterior. Biodegrad. 2017, 119, 147–154. [Google Scholar] [CrossRef]
- Dursun, D.; Dalgıç, A.C. Optimization of astaxanthin pigment bioprocessing by four different yeast species using wheat wastes. Biocatal. Agric. Biotechnol. 2016, 7, 1–6. [Google Scholar] [CrossRef]
- Eryılmaz, E.B.; Dursun, D.; Dalgıç, A.C. Multiple optimization and statistical evaluation of astaxanthin production utilizing olive pomace. Biocatal. Agric. Biotechnol. 2016, 7, 224–227. [Google Scholar] [CrossRef]
- Haque, M.A.; Kachrimanidou, V.; Koutinas, A.; Lin, C.S.K. Valorization of bakery waste for biocolorant and enzyme production by Monascus purpureus. J. Biotechnol. 2016, 231, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Chandra, P.; Arora, D.S. Production of Antioxidant Bioactive Phenolic Compounds by Solid-State Fermentation on Agro-residues Using Various Fungi Isolated from Soil. Asian J. Biotechnol. 2016, 8, 8–15. [Google Scholar] [CrossRef]
- Vijayaraghavan, P.; Vincent, S.G.P.; Arasu, M.V.; Al-Dhabi, N.A. Bioconversion of agro-industrial wastes for the production of fibrinolytic enzyme from Bacillus halodurans IND18: Purification and biochemical characterization. Electron. J. Biotechnol. 2016, 20, 1–8. [Google Scholar] [CrossRef]
- Pili, J.; Danielli, A.; Nyari, N.L.; Zeni, J.; Cansian, R.L.; Backes, G.T.; Valduga, E. Biotechnological potential of agro-industrial waste in the synthesis of pectin lyase from Aspergillus brasiliensis. Food Sci. Technol. Int. 2017. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.S.; Vidhale, N.N. Protease production by Fusarium oxysporum in solid-state fermentation using rice bran. Am. J. Microbiol. Res. 2013, 1, 45–47. [Google Scholar]
- Ali, S.R.; Anwar, Z.; Irshad, M.; Mukhtar, S.; Warraich, N.T. Bio-synthesis of citric acid from single and co-culture-based fermentation technology using agro-wastes. J. Radiat. Res. Appl. Sci. 2016, 9, 57–62. [Google Scholar] [CrossRef]
- Das, R.K.; Brar, S.K.; Verma, M. A fermentative approach towards optimizing directed biosynthesis of fumaric acid by Rhizopus oryzae 1526 utilizing apple industry waste biomass. Fungal Biol. 2015, 119, 1279–1290. [Google Scholar] [CrossRef] [PubMed]
- Das, R.K.; Brar, S.K.; Verma, M. Potential use of pulp and paper solid waste for the bio-production of fumaric acid through submerged and solid state fermentation. J. Clean. Prod. 2016, 112, 4435–4444. [Google Scholar] [CrossRef]
- Rane, A.N.; Baikar, V.V.; Ravi Kumar, V.; Deopurkar, R.L. Agro-industrial wastes for production of biosurfactant by Bacillus subtilis ANR 88 and its application in synthesis of silver and gold nanoparticles. Front. Microbiol. 2017. [Google Scholar] [CrossRef]
- Chakraborty, K.; Raychaudhuri, U.; Chakraborty, R. Optimization of bioprocess parameters for wine from household vegetable waste production by employing response surface methodology. Int. Food Res. J. 2017, 24, 48–55. [Google Scholar]
- Vintila, T.; Dragomirescu, M.; Jurcoane, S.; Caprita, R.; Maiu, M. Production of cellulase by submerged and solid-state cultures and yeasts selection for conversion of lignocellulose to ethanol. Rom. Biotechnol. Lett. 2009, 14, 4275–4281. [Google Scholar]
- Grover, S.; Kathuria, R.S.; Kaur, M. Energy values and technologies for non woody biomass: As a clean source of energy. Ind. J. Sci. Technol. 2012, 1, 10–14. [Google Scholar] [CrossRef]
- Maldonado, M.C.; Strasser de Saad, A.M. Production of pectin estrase and polygalactouronase by Aspergillus niger in submerged and solid state systems. J. Ind. Microbiol. Biotechnol. 1998, 20, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Lun, O.K.; Wai, T.B.; Ling, L.S. Pineapple cannery waste as a potential substrate for microbial biotranformation to produce vanillic acid and vanillin. Int. Food Res. J. 2014, 21, 953–958. [Google Scholar]
- Huang, Y.P.; Lai, H.M. Bioactive compounds and antioxidative activity of colored rice bran. J. Food Drug Anal. 2016, 24, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Razak, D.L.A.; Rashid, N.Y.A.; Jamaluddin, A.; Sharifudin, S.A.; Kahar, A.A.; Long, K. Cosmeceutical potentials and bioactive compounds of rice bran fermented with single and mix culture of Aspergillus oryzae and Rhizopus oryzae. J. Saudi Soc. Agric. Sci. 2017, 16, 127–134. [Google Scholar]
- Godoy, M.G.; Gutarra, M.L.E.; Castro, A.M.; Machado, O.L.T.; Freire, D.M.G. Adding value to a toxic residue from the biodiesel industry: Production of two distinct pool of lipases from Penicillium simplicissimum in castor bean waste. J. Ind. Microbiol. Biotechnol. 2011, 38, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Joshi, C.; Mathur, P.; Khare, S.K. Degradation of phor-bol esters by Pseudomonas aeruginosa PseA during solid-state fermentation of deoiled Jatropha curcas seed cake. Bioresour. Technol. 2011, 102, 4815–4819. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, C.; Meng, X.; Yan, Y. Biodiesel synthesis directly catalyzed by the fermented solid of Burkholderia cenocepacia via solid state fermentation. Fuel Process. Technol. 2016, 106, 303–309. [Google Scholar] [CrossRef]
- Ferrarezi, A.L.; Ohe, T.H.K.; Borges, J.P.; Brito, R.R.; Siqueira, M.R.; Vendramini, P.H., Jr.; Quilles, J.C.; Nunesa, C.D.C.C.; Bonilla-Rodriguez, G.O.; Boscoloa, M.; et al. Production and characterization of lipases and immobilization of whole cell of the thermophilic Thermomucor indicae seudaticae N31 for trans esterification reaction. J. Mol. Catal. B Enzym. 2014, 107, 106–113. [Google Scholar] [CrossRef]
- Khiyami, M.; Aboseide, B.; Pometto, A., III. Influence of complex nutrient sources: Dates syrup and dates Pits on Lactococcus lactis growth and nisin production. J. Biotechnol. 2008. [Google Scholar] [CrossRef]
- Chawla, P.; Bhandari, L.; Sadh, P.K.; Kaushik, R. Impact of Solid-State Fermentation (Aspergillus oryzae) on Functional Properties and Mineral Bioavailability of Black-Eyed Pea (Vigna unguiculata) Seed Flour. Cereal Chem. 2017, 94, 437–442. [Google Scholar] [CrossRef]
- Sadh, P.K.; Duhan, S.; Duhan, J.S. Agro-industrial wastes and their utilization using solid state fermentation: A review. Bioresour. Bioprocess. 2018. [Google Scholar] [CrossRef]
- Sadh, P.K.; Saharan, P.; Duhan, J.S. Bio-augmentation of antioxidants and phenolic content of Lablab purpureus by solid state fermentation with GRAS filamentous fungi. Res. Effic. Technol. 2017, 3, 285–292. [Google Scholar] [CrossRef]
- Oloyede, O.O.; James, S.; Ocheme, B.O.; Chinma, C.E.; Akpa, V.E. Effects of fermentation time on the functional and pasting properties of defatted Moringa oleifera seed flour. Food Sci. Nutr. 2016, 4, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.H.; Cho, S.J. Improvement of bioactivity of soybean meal by solid-state fermentation with Bacillus amyloliquefaciens versus Lactobacillus spp. and Saccharomyces cerevisiae. LWT Food Sci. Technol. 2016, 68, 619–625. [Google Scholar] [CrossRef]
- Singh, H. Functional properties of milk proteins. Ref. Modul. Food Sci. 2011. [Google Scholar] [CrossRef]
- Bhandari, L.; Sodhi, N.S.; Chawla, P. Effect of acidified methanol modification on physico chemical properties of black-eyed pea (Vigna unguiculata) starch. Int. J. Food Prop. 2016, 19, 2635–2648. [Google Scholar] [CrossRef]
- Shilpashree, B.G.; Arora, S.; Chawla, P.; Tomar, S.K. Effect of succinylation on physicochemical and functional properties of milk protein concentrate. Food Res. Int. 2015, 72, 223–230. [Google Scholar] [CrossRef]
- Shilpashree, B.G.; Arora, S.; Sharma, V.; Chawla, P.; Vakkalagadda, R. Changes in physicochemical and functional properties of whey protein concentrate upon succinylation. Int. J. Dairy Technol. 2016, 68, 1–9. [Google Scholar] [CrossRef]
- Guan, G.; Zhang, Z.; Ding, H.; Li, M.; Shi, D.; Zhu, M.; Xia, L. Enhanced degradation of lignin in corn stalk by combined method of Aspergillus oryzae solid state fermentation and H2O2 treatment. Biomass Bioenergy 2015, 81, 224–233. [Google Scholar] [CrossRef]
- Sadh, P.K.; Saharan, P.; Duhan, S.; Duhan, J.S. Bio-enrichment of phenolics and antioxidant activity of combination of Oryza sativa and Lablab purpureus fermented with GRAS filamentous fungi. Res. Effic. Technol. 2017, 3, 347–352. [Google Scholar] [CrossRef]
- Sadh, P.K.; Chawla, P.; Bhandari, L.; Duhan, J.S. Bio-enrichment of functional properties of peanut oil cakes by solid state fermentation using Aspergillus oryzae. J. Food Meas. Charact. 2018, 12, 622–633. [Google Scholar] [CrossRef]
- Xiao, Y.; Xing, G.; Rui, X.; Li, W.; Chen, X.; Jiang, M.; Dong, M. Effect of solid-state fermentation with Cordyceps militaris SN-18 on physicochemical and functional properties of chickpea (Cicer arietinum L.) flour. LWT Food Sci. Technol. 2015, 63, 1317–1324. [Google Scholar] [CrossRef]
- Darwish, G.A.; Bakr, A.A.; Abdallah, M.M.F. Nutritional value upgrading of maize stalk by using Pleurotus ostreatus and Saccharomyces cerevisiae in solid state fermentation. Ann. Agric. Sci. 2012, 57, 47–51. [Google Scholar] [CrossRef]
- Kaushik, R.; Swami, N.; Sihag, M.; Ray, A. Isolation, characterization of wheat gluten and its regeneration properties. J. Food Sci. Technol. 2015, 52, 5930–5937. [Google Scholar] [CrossRef] [PubMed]
- Shilpashree, B.G.; Arora, S.; Chawla, P.; Vakkalagadda, R.; Sharma, A. Succinylation of sodium caseinate and its effect on physicochemical and functional properties of protein. LWT Food Sci. Technol. 2015, 64, 1270–1277. [Google Scholar] [CrossRef]
- Sadh, P.K.; Chawla, P.; Bhandari, L.; Kaushik, R.; Duhan, J.S. In vitro assessment of bio-augmented minerals from peanut oil cakes fermented by through Caco-2 cells. J. Food Sci. Technol. 2017, 11, 3640–3649. [Google Scholar] [CrossRef] [PubMed]
- Chafle, S.; Parmar, V.; Biya, S. Utilization of vegetable and fruit waste for bio-energy generation. J. Autom. Control Eng. 2014, 2, 143–145. [Google Scholar] [CrossRef]
- Joshi, V.K.; Sandhu, D.K. Preparation and evaluation of animal feed of an animal feed byproduct produced by solid-state fermentation of apple pomace. Bioresour. Technol. 1996, 56, 251–255. [Google Scholar] [CrossRef]
- Del-campo, I.; Alegria, I.; Zazpe, M.; Echeverria, M.; Echeverria, I. Diluted acid hydrolysis pretreatment of agri-food wastes for bioethanol production. Ind. Crops Prod. 2006, 24, 214–221. [Google Scholar] [CrossRef]
- Zheng, Y.; Pan, N.Z.; Zhang, R.; Donghai, W.D. Enzymatic saccharification of dilute acid pretreated salinecrops for fermentable sugar production. Appl. Energy 2009, 86, 2459–2465. [Google Scholar] [CrossRef]
- Kumar, A.; Sadh, P.K.; Kha, S.; Dhuan, J.S. Bio-ethanol production from sweet potato using co-culture of saccharolytic molds (Aspergillus spp.) and Saccharomyces cerevisiae MTCC170. J. Adv. Biotechnol. 2016, 6, 822–828. [Google Scholar] [CrossRef]
- Akin-Osanaiye, B.C.; Nzelibe, H.C.; Agbaji, A.S. Ethanol Production from Carica papaya Fruit Waste. Asian J. Biochem. 2008, 3, 188–193. [Google Scholar] [CrossRef]
- Ergun, S.O.; Urek, R.O. Production of ligninolytic enzymes by solid state fermentation using Pleurotus ostreatus. Annal. Agrar. Sci. 2017, 15, 273–277. [Google Scholar] [CrossRef]
- Dhillon, G.S.; Brar, S.K.; Kaur, S.; Verma, M. Screening of agro-industrial wastes for citric acid bioproduction by Aspergillus niger NRRL 2001 through solid state fermentation. J. Sci. Food Agric. 2013, 93, 1560–1567. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.S.; Garlapati, V.K.; Banerjee, R. Optimization of laccase production using response surface methodology coupled with differential evolution. New Biotechnol. 2011, 28, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Karp, S.G.; Faraco, V.; Amore, A.; Birolo, L.; Giangrande, C.; Soccol, V.T.; Pandey, A.; Soccol, C.R. Characterization of laccaseiso forms produced by Pleurotus ostreatus in solid state fermentation of sugarcane bagasse. Bioresour. Technol. 2012, 114, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, S.; Soni, R.; Kaur, J.; Soni, S.K. Unravelling the capability of Pyrenophora phaeocomes S-1 for the production of ligno-hemicellulolytic enzyme cocktail and simultaneous bio-delignification of rice straw for enhanced enzymatic saccharification. Bioresour. Technol. 2016, 222, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Soccol, C.R.; da Costa, E.S.F.; Letti, L.A.J.; Karp, S.G.; Woiciechowski, A.L.; de Souza Vandenberghe, L.P. Recent developments and innovations in solid state fermentation. Biotechnol. Res. Innov. 2017, 1, 52–71. [Google Scholar] [CrossRef]
- Nagar, S.; Mittal, A.; Kumar, D.; Kumar, L.; Kuhad, R.C.; Gupta, V.K. Hyper production of alkali stable xylanase in lesser duration by Bacillus pumilus SV-85S using wheat bran under solid state fermentation. New Biotechnol. 2011, 28, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Joshi, A.; Kashyap, R.; Khanna, S. Production of xylanase by Promicromonospora spp. MARS with rice straw under non sterile conditions. Process. Biochem. 2011, 46, 1614–1618. [Google Scholar] [CrossRef]
- Duhan, J.S.; Kumar, A.; Tanwar, S.K. Bioethanol production from starchy part of tuberous plant (potato) using Saccharomyces cerevisiae MTCC-170. Afr. J. Microbiol. Res. 2013, 7, 5253–5260. [Google Scholar]
- Pirota, R.D.P.B.; Tonelotto, M.; Da Silva Delabona, P.; Fonseca, R.F.; Paixão, D.A.A.; Baleeiro, F.C.F.; Farinas, C.S. Enhancing xylanases production by a new Amazon Forest strain of Aspergillus oryzae using solid-state fermentation under controlled operation conditions. Ind. Crops Prod. 2013, 45, 465–471. [Google Scholar] [CrossRef]
- Irfan, M.; Nadeem, M.; Syed, Q. One-factor-at-a-time (OFAT) optimization of xylanase production from Trichoderma viride-IR05 in solid-state fermentation. J. Radiat. Res. Appl. Sci. 2014, 7, 317–326. [Google Scholar] [CrossRef]
- Pandey, A.K.; Edgard, G.; Negi, S. Optimization of concomitant production of cellulase and xylanase from Rhizopus oryzae SN5 through EVOP-factorial design technique and application in sorghum stover based bioethanol production. Renew. Energ. 2016, 98, 51–56. [Google Scholar] [CrossRef]
- Duhan, J.S.; Mehta, K.; Sadh, P.K.; Saharan, P. Bio-enrichment of phenolics and free radicals scavenging activity of wheat (WH-711) fractions by solid state fermentation with Aspergillus oryzae. Afr. J. Biochem. Res. 2016, 10, 12–19. [Google Scholar]
- Cervero, J.M.; Skovgaard, P.A.; Felby, C.; Sorensen, H.R.; Jorgensen, H. Enzymatic hydrolysis and fermentation of palm kernel press cake for production of bioethanol. Enzyme Microb. Technol. 2010, 46, 177–184. [Google Scholar] [CrossRef]
- Hashem, M.; Darwish, S.M.I. Production of bioethanol and associated by-products from potato starch residue stream by Saccharomyces cerevisiae. Biomass Bioenergy 2010, 3, 1–7. [Google Scholar] [CrossRef]
- Oliveira, R.; Oliveira, V.; Aracava, K.K.; da Costa Rodrigues, C.E. Effects of the extraction conditions on the yield and composition of rice bran oil extracted with ethanol—A response surface approach. Food Bioprod. Process. 2012, 90, 22–31. [Google Scholar] [CrossRef]
- Zhang, H.J.; Zhang, H.; Wang, L.; Guo, X.N. Preparation and functional properties of rice bran proteins from heat-stabilized defatted rice bran. Food Res. Int. 2012, 47, 359–363. [Google Scholar] [CrossRef]
- Xia, N.; Wang, J.; Yang, X.; Yin, S.; Qi, J.; Hu, L.; Zhou, X. Preparation and characterization of protein from heat stabilized rice bran using hydrothermal cooking combined with amylase pretreatment. J. Food Eng. 2012, 110, 95–101. [Google Scholar] [CrossRef]
- Jamaluddin, A.; Rashid, N.Y.A.; Razak, D.L.A.; Sharifudin, S.A.; Long, K. Effect of fungal fermentation on tyrosinase and elastase inhibition activity in rice bran. Agric. Agric. Sci. Procedia 2014, 2, 252–256. [Google Scholar] [CrossRef]
- Saharan, P.; Sadh, P.K.; Duhan, J.S. Comparative assessment of effect of fermentation on phenolics, flavanoids and free radical scavenging activity of commonly used cereals. Biocatal. Agric. Biotechnol. 2017, 12, 236–240. [Google Scholar] [CrossRef]
- Sadh, P.K.; Saharan, P.; Duhan, J.S. Bioaugmentation of phenolics and antioxidant activity of Oryza sativa by solid state fermentation with Aspergillus spp. Int. Food Res. J. 2017, 24, 1160–1166. [Google Scholar]
- Schmidt, C.G.; Furlong, E.B. Effect of particle size and ammonium sulfate concentration on rice bran fermentation with the fungus Rhizopus oryzae. Bioresour. Technol. 2012, 123, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Saykhedkar, S.S.; Singhal, R.S. Solid-state fermentation for production of griseofulvin on rice bran using Penicillium griseofulvum. Biotechnol. Progr. 2004, 20, 1280–1284. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.T.; Kaneko, M.; Hirata, M.; Toorisaka, E.; Hano, T. Utilization of rice bran as nutrient source for fermentative lactic acid production. Bioresour. Technol. 2008, 99, 3659–3664. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lu, J.; Yang, Z.; Han, L.; Tan, T. Utilization of white rice bran for production of lactic acid. Biomass Bioenergy 2012, 39, 53–58. [Google Scholar] [CrossRef]
- Watanabe, M.; Makino, M.; Kaku, N.; Koyama, M.; Nakamura, K.; Sasano, K. Fermentative L−(+)−lactic acid production from non-sterilized rice washing drainage containing rice bran by a newly isolated lactic acid bacteria without any additions of nutrients. J. Biosci. Bioeng. 2013, 115, 449–452. [Google Scholar] [CrossRef] [PubMed]
- Oshoma, C.E.; Ikenebomeh, M.J. Production of Aspergillus niger biomass from rice bran. Pak. J. Nutr. 2005, 4, 32–36. [Google Scholar]
- Rajesh, M.J.; Rajesh, L.; Abachire, L.W. Optimization of solid state fermentation conditions for the production of cellulase by using Trichoderma reesei. Eur. J. Appl. Eng. Sci. Res. 2012, 1, 196–200. [Google Scholar]
- Grover, A.; Maninder, A.; Sarao, L.K. Production of fungal amylase and cellulase enzymes via solid state fermentation using Aspergillus oryzae and Trichoderma reesei. Int. J. Adv. Res. Technol. 2013, 2, 108–124. [Google Scholar]
- Chinma, C.E.; Ilowefah, M.; Muhammad, K. Optimization of Rice Bran Fermentation Conditions Enhanced by Baker’s Yeast for Extraction of Protein Concentrate. Niger. Food J. 2014, 32, 126–132. [Google Scholar] [CrossRef]
- Santa, H.S.D.; Santa, O.R.D.; Brand, D.; Vandenberghe, L.P.D.S.; Soccol, C.R. Spore production of Beauveria bassiana from agro-industrial residues. Braz. Arch. Biol. Technol. 2005, 48, 51–60. [Google Scholar] [CrossRef]
- Pandey, A.; Soccol, C.R.; Mitchell, D. New developments in solid state fermentation: I-bioprocesses and products. Process Biochem. 2000, 35, 1153–1169. [Google Scholar] [CrossRef]
- Khiyami, M.A.; Al-Fadual, S.M.; Bahklia, A.H. Polyhydroxyalkanoates production via Bacillus plastic composite support (PCS) biofilm and date palm syrup. J. Med. Plant. Res. 2011, 5, 3312–3320. [Google Scholar]
- Chauhan, K.; Trivedi, U.; Patel, K.C. Statistical screening of medium components by Plackett–Burman design for lactic acid production by Lactobacillus sp. KCP01 using date juice. Bioresour. Technol. 2007, 98, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Salah, R.B.; Jaouadi, B.; Bouaziz, A.; Chaari, K.; Blecker, C.; Derrouane, C.; Besbes, S. Fermentation of date palm juice by curdlan gum production from Rhizobium radiobacter ATCC 6466™: Purification, rheological and physico-chemical characterization. LWT Food Sci. Technol. 2011, 44, 1026–1034. [Google Scholar] [CrossRef]
- Moosavi-Nasab, M.; Yousefi, A. Biotechnological production of cellulose by Gluconacetobacter xylinus from agricultural waste. Iran J. Biotechnol. 2011, 9, 94–101. [Google Scholar]
- Davati, N.; Hamidi, E.Z.; Shoja, A.S. Study on producing possibility of amino acids from date palm wastes by two mutant Corynebacterium glutamicum CECT690 & CECT77. FSCT 2007, 4, 55–64. [Google Scholar]
- Tavakkoli, M.; Hamidi-Esfahani, Z.; Azizi, M.H. Optimization of Corynebacterium glutamicum glutamic acid production by response surface methodology. Food Bioprocess Technol. 2012, 5, 92–99. [Google Scholar] [CrossRef]
- Cheok, C.Y.; Mohd Adzahan, N.; Abdul Rahman, R.; Zainal Abedin, N.H.; Hussain, N.; Sulaiman, R.; Chong, G.H. Current trends of tropical fruit waste utilization. Crit. Rev. Food Sci. Nutr. 2018, 58, 335–361. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |

| Food Sources | Bioactive Compounds | References |
|---|---|---|
| Apple | Epicatechin, catechins, chlorogenic acid, hydroxycinnamates, phloretin glycosides, quercitin glycosides, procyanidins, anthocyanins | [20,21,22] |
| Avocado | Epicatechin, catechin, gallic acid, chlorogenic acid, cyanidin 3-glucoside, homogentisic acid | [23] |
| Banana | Gallocatechin, anthocyanins, delphindin, cyaniding, catecholamine | [24,25,26] |
| Berries | Cyanidin, delphinidin, malvidin | [27] |
| Citrus | Hesperidin, naringin, eriocitrin, narirutin | [27,28] |
| Grapes | Cinnamic acid, coumaric acid, caffeic acid, ferulic acid, chlorogenic acid, neochlorogenic acid, p-hydroxybenzoic acid, protocatechuic acid, vanillic acid, gallic acid, proanthocyanidins, quercetin, resvaratrol, pullulan | [27,29,30,31] |
| Guava | Catechin, cyanidin 3-glucoside, galangin, gallic acid, homogentisic acid, kaempferol | [23] |
| Litchi | Cyanidin-3-glucosides, cyanidin-3-rutonoside, malvidin-3-glucoside, gallic acid, epicatechin-3-gallate | [32,33] |
| Mango | Gallic acid, ellagic acid, gallates, gallotannins, condensed tannins | [34,35] |
| Olives | Cyanidin, delphinidin, malvidin | [27] |
| Palm | Tocopherols, tocotrienols, sterols, and squalene, phenolic antioxidants | [36,37] |
| Pomegranate | Gallic acid, cyanidin-3,5-diglucoside, cyanidin-3-diglucoside, delphinidin-3,5-diglucoside | [38,39] |
| Carrot | Phenols, beta-carotene | [40] |
| Celery | Cyanidin, delphinidin, malvidin | [27] |
| Cucumber | Chlorophyll, pheophytin, phellandrene, caryophyllene | [41] |
| Onion | Quercetin, rutin | [27] |
| Tomato | Carotenoids | [42] |
| Parsley | Apigenin, luteolin, quercetin | [27] |
| Spinach | Apigenin; luteolin | [43] |
| Chenopodium | Apigenin; luteolin | [43] |
| Barley | β-Glucan | [44] |
| Rice | γ-Oryzanol, bran oil | [45,46] |
| Wheat | Phenolic acids, antioxidants | [47] |
| Beans | Daidzen, glycindin | [27] |
| Dark chocolate | Epicatechin | [48] |
| Green tea | (−)-epigallocatechin, (+)-gallocatechin, (−)epicatechin-3-O-gallate | [49] |
| Bioactive Compounds | Substrate | Microorganism | Fermentation Process | References |
|---|---|---|---|---|
| Single cell protein | Sweet potato, banana skin, orange peel, mango waste and pineapple peel; Dairy waste | Saccharomyces sp., Saccharomyces cerevisiae, Candida tropicalis, Lactobacillus acidophilus | Solid state fermentation; Liquid fermentation | [142,143,144,145] |
| Bioethanol | pineapple waste, banana waste | Saccharomyces cerevisiae, | Solid state fermentation | [146,147] |
| Indole-3-acetic acid | Cassava fibrous residue | Bacillus subtilis | Solid state fermentation | [148] |
| Protease production | Rice bran, Brewery waste (brewer’s spent grain, hottrub and residual brewer’s yeast); Soybean meal; Wheat bran, cotton seed meal and orange peel. | Lactobacillus delbrueckii ssp.; Bacillus licheniformis; Aspergillus niger | Liquid fermentation; Solid state fermentation | [149,150,151,152] |
| Lactic acid production | Dairy waste; rice bran, wheat bran, ragi bran, rice starch water, tea waste, sugar cane bagasse, groundnut and coconut oil cakes | Lactobacillus sp.; R. oryzae MTCC 8784 | Fed batch fermentation | [153,154] |
| Ergosterol | Dairy waste (whey) | Cryptococcus albidus sp. Aerius | Liquid fermentation | [155] |
| Xanthan | Potato peel | Xanthomonas citri | Solid state fermentation | [156] |
| Protein | Orange peel | Chaetomium spp. (KC-06) and Aspergillus niger | Solid state fermentation | [157] |
| Phenolic content | Guava and pineapple waste; Peanut waste (peanut press cake); Rice bran; plum pomaces and brandy distillery wastes; pomegranate wastes | Rhizopus oligosporus: Aspergillus awamori; Rhizopus oryzae; Aspergillus niger and Rhizopus oligosporus; Punica granatum | Solid state fermentation | [117,158,159,160] |
| Antioxidants | Peanut waste (peanut press cake); apricot pomace; Apple pomace | Aspergillus awamori; Aspergillus niger (ATCC-6275) and Rhizopus oligosporus (ATCC-22959); Phanerocheate chrysosporium | Solid state fermentation | [117,161,162] |
| Neomycin | Apple pomace, cotton seed meal, soy bean powder and wheat bran | Streptomyces fradiae NCIM 2418 | Solid state fermentation | [163] |
| Oxytetracycline | Groundnut shell, Sweet potato residues, Cassava peels, cocoyam peels | Streptomyces Rimosus, S. vendagensis, S. speibonae | Solid state fermentation | [164,165,166,167] |
| Rifamycin | Coconut oil cake, groundnut oil cake, ground nut shell and rice husk | Amycolatopsis Mediterranean | Solid state fermentation | [168] |
| Meroparamycin | Rice, wheat bran, quaker, bread, and ground corn | Streptomyces sp. strain MAR01 | Solid state fermentation | [169] |
| Bleomycin | Date syrup | Streptomyces mobaraensis ATCC | Fermentation | [170] |
| Poly(3-Hyrdroxybutyric Acid) | Orange peel | Bacillus subtilis | Batch fermentation | [171] |
| Laccase | Peels of citrus fruits, soybean meal, tofu dreg, Brewer’s spent grain | Rheinheimera sp., Lysinibacillus sp., Trametes versicolor | Sub merged fermentation; Solid state fermentation | [172,173] |
| Bioherbicide | Soybean bran, bagasse and corn steep liquor | Phoma sp. | Solid state fermentation | [174] |
| Biosorbents | Apple pomace | Aspergillus niger | Solid state fermentation | [175] |
| Astaxanthin (pigment) | Wheat waste; olive pomace; bakery waste | Yamadazyma guilliermondii, Yarrowia lipolytica; Xantophylomyces dendrorhous, Sporidiobolus salmonicolor; Monascus purpureus | Solid state fermentation | [176,177,178] |
| Bioactive phenolic compounds | Wheat straw, Rice straw, Corn cob, Pea pod, Sugarcane baggase | Aspergillus fumigatus, A. terreus, A. wentii, Penicillium citrinum, P. granulatum, P. expansum | Solid state fermentation | [179] |
| Fibrinolytic enzyme | Banana peel, black gram husk, paddy straw, rice bran, and wheat bran | Bacillus halodurans IND18 | Solid state fermentation | [180] |
| Pectin lyase | corn steep liquor and orange peel | Aspergillus brasiliensis | Sub merged fermentation | [181] |
| Citric acid | Apple pomace, brewer’s spent grain, citrus waste, sphagnum peat moss; peanut shell | Aspergillus niger NRRL 2001; Aspergillus ornatus and Alternaria alternata | Solid state fermentation | [182,183] |
| Fumaric acid | Apple pomace; pulp and paper solid waste | Rhizopus oryzae 1526 | Solid state fermentation; Sub merged fermentation | [184,185] |
| Biosurfactant | Potato peels, orange peels, banana peels, and bagasse | Bacillus subtilis ANR 88 | Solid state fermentation | [186] |
| Wine (antioxidant rich) | Potato, pumpkin and carrot peels | Saccharomyces cerevisiae (NCIM 3206) | Liquid fermentation | [187] |
| Cellulase | Wheat bran, Rice bran, Corn husks | Trichoderma viride, Bacillus cereus | Sub merged fermentation | [188,189] |
| Lycopene | Tomato waste | Aspergillus niger | Solid state fermentation | [130] |
| Polygalactouronase | Wheat bran, Coffee pulp | Aspergillus niger | Solid state fermentation | [190] |
| Vanillic acid and vanillin | Pineapple canary waste | A. niger I-1472 and Pycnoporus cinnabarinus MUCL 39533 | Liquid fermentation | [191] |
| Proanthocyanidins, anthocynidins, phenolic acids, vitamin E and oryzanol | Rice bran | - | - | [192] |
| Ferulic, p-coumaric, sinapic and syringic | Rice bran | Aspergillus oryzae and Rhizopus oryzae | Solid state fermentation | [193] |
| Lipase | Castor bean waste; Jatropha curcas seed cake; Sugarcane bagasse, sunflower seed and olive oil | Penicillium simplicissimum; Pseudomonas aeruginosa; Burk holderiacenocepacia; Thermomucorindicaeseudaticae | Solid state fermentation | [194,195,196,197] |
| Nisin | Date by product | Lactococcus lactis | Solid state fermentation | [198] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadh, P.K.; Kumar, S.; Chawla, P.; Duhan, J.S. Fermentation: A Boon for Production of Bioactive Compounds by Processing of Food Industries Wastes (By-Products). Molecules 2018, 23, 2560. https://doi.org/10.3390/molecules23102560
Sadh PK, Kumar S, Chawla P, Duhan JS. Fermentation: A Boon for Production of Bioactive Compounds by Processing of Food Industries Wastes (By-Products). Molecules. 2018; 23(10):2560. https://doi.org/10.3390/molecules23102560
Chicago/Turabian StyleSadh, Pardeep Kumar, Suresh Kumar, Prince Chawla, and Joginder Singh Duhan. 2018. "Fermentation: A Boon for Production of Bioactive Compounds by Processing of Food Industries Wastes (By-Products)" Molecules 23, no. 10: 2560. https://doi.org/10.3390/molecules23102560
APA StyleSadh, P. K., Kumar, S., Chawla, P., & Duhan, J. S. (2018). Fermentation: A Boon for Production of Bioactive Compounds by Processing of Food Industries Wastes (By-Products). Molecules, 23(10), 2560. https://doi.org/10.3390/molecules23102560





